Application of Simultaneous Oil Extraction and Transesterification in Biodiesel Fuel Synthesis: A Review
Abstract
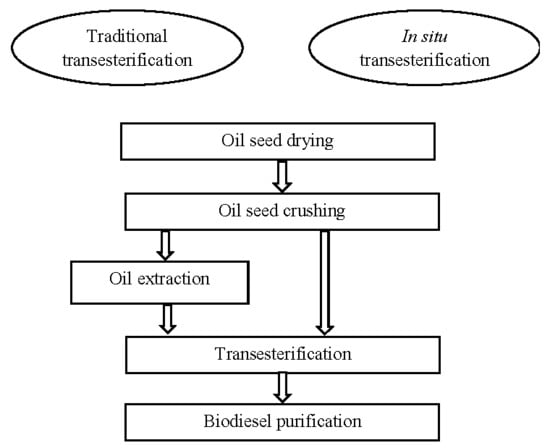
1. Introduction
2. Raw Materials and Their Preparation Methods
3. Chemical Catalysis for Simultaneous Oil Extraction and Transesterification
3.1. Influence of Alcohols/Solvents on Process Efficiency
3.2. Chemical Catalysts Used for Simultaneous Oil Extraction and Transesterification
3.3. Temperature of Simultaneous Oil Extraction and Transesterification
3.4. Duration of Simultaneous Oil Extraction and Transesterification
4. Enzymatic Simultaneous Oil Extraction and Transesterification Method
4.1. Catalysts for Enzymatic In Situ Transesterification
4.2. Alcohols and Solvents Used for Enzymatic In Situ Transesterification
4.3. Temperature of Enzymatic In Situ Transesterification
4.4. Duration of Enzymatic In Situ Transesterification
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lei, H.; Ding, X.; Zhang, H.; Chen, X.; Li, Y.; Zhang, H.; Wang, Z. In situ production of fatty acid methyl ester from low quality rice bran: An economical route for biodiesel production. Fuel 2010, 89, 1475–1479. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Sinkuniene, D.; Kazanceva, I.; Kazancev, K. Optimization of low quality rapeseed oil transesterification with butanol by applying the response surface methodology. Renew. Energy 2016, 87, 266–272. [Google Scholar] [CrossRef]
- Basha, S.A.; Gopal, K.R.; Jebaraj, S. A review on biodiesel production, combustion, emissions and performance. Renew. Sustain. Energy Rev. 2009, 13, 1628–1634. [Google Scholar] [CrossRef]
- Wang., R.; Zhou, W.W.; Hanna, M.A.; Zhang, Y.P.; Bhadury, P.S.; Wanga, Y.; Song, B.A.; Yang, S. Biodiesel preparation, optimization, and fuel properties from non-edible feedstock, Datura stramonium L. Fuel 2012, 91, 182–186. [Google Scholar] [CrossRef]
- Lin, C.Y.; Fan, C.L. Fuel properties of biodiesel produced from Camellia oleifera abel oil through supercritical-methanol transesterification. Fuel 2011, 90, 2240–2244. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, X.; Zhao, B.; Sun, J.; Wang, Y. Rapid in situ transesterification of sunflower oil. Ind Eng Chem Res. 2009, 48, 850–856. [Google Scholar] [CrossRef]
- Kaul, S.; Porwal, J.; Garg, M.O. Parametric study of jatropha seeds for biodiesel production by reactive extraction. J. Am. Oil Chem. Soc. 2010, 87, 903–908. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Gumbyte, M. Reactive extraction and fermental transesterification of rapeseed oil with butanol in diesel fuel media. Fuel Process. Technol. 2015, 138, 758–764. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Zakaria, R.; Harvey, A.P. Direct production of biodiesel from rapeseed by reactive extraction/insitu transesterification. Fuel Process. Technol. 2012, 102, 53–60. [Google Scholar] [CrossRef]
- Abo El-Enin, S.A.; Attia, N.K.; El-Ibiari, N.N.; El-Diwani, G.I.; El-Khatib, K.M. In-situ transesterification of rapeseed and cost indicators for biodiesel production. Renew. Sustain. Energy Rev. 2013, 18, 471–477. [Google Scholar] [CrossRef]
- Haagenson, D.M.; Brudvik, R.L.; Lin, H.; Wiesenborn, D.P. Implementing an in Situ alkaline transesterification method for canola biodiesel quality screening. J. Am. Oil Chem. Soc. 2010, 87, 1351–1358. [Google Scholar] [CrossRef]
- Siler-Marinkovic, S.; Tomasevic, A. Transesterification of sunflower oil in situ. Fuel 1998, 77, 1389–1391. [Google Scholar] [CrossRef]
- Harrington, K.J.; d’Arcy-Evans, C. Transesterification in situ of sunflower seed oil. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 314–318. [Google Scholar] [CrossRef]
- Haas, M.J.; Scott, K.M.; Marmer, W.N.; Foglia, T.A. In situ alkaline transesterification: An effective method for the production of fatty acid esters from vegetable oils. J. Am. Oil Chem. Soc. 2004, 81, 83–89. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Zhang, J.; Li, G. In situ reactive extraction of cottonseeds with methyl acetate for biodiesel production using magnetic solid acid catalysts. Bioresour. Technol. 2014, 174, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, F.; Liu, S.; Yun, Z. In situ alkaline transesterification of cottonseed oil for production of biodiesel and nontoxic cottonseed meal. Bioresour. Technol. 2008, 99, 9009–9012. [Google Scholar] [CrossRef]
- Shuit, S.H.; Lee, K.T.; Kamaruddin, A.H.; Yusup, S. Reactive extraction and in situ esterification of Jatropha curcas L. seeds for the production of biodiesel. Fuel 2010, 89, 527–530. [Google Scholar] [CrossRef]
- Shuit, S.H.; Lee, K.T.; Kamaruddin, A.H.; Yusup, S. Reactive extraction of Jatropha curcas L. seed for production of biodiesel: Process optimization study. Environ. Sci. Technol. 2010, 44, 4361–4367. [Google Scholar] [CrossRef]
- Kartika, I.A.; Yani, M.; Ariono, D.; Evon, P.; Rigal, L. Biodiesel production from jatropha seeds: Solvent extraction and in situ transesterification in a single step. Fuel 2013, 106, 111–117. [Google Scholar] [CrossRef]
- Chapagain, B.P.; Yehoshua, Y.; Wiesman, Z. Desert date (Balanites aegyptiaca) as an arid lands sustainable bioresource for biodiesel. Bioresour. Technol. 2009, 100, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Hincapié, G.; Mondragón, F.; López, D. Conventional and in situ transesterification of castor seed oil for biodiesel production. Fuel 2011, 90, 1618–1623. [Google Scholar] [CrossRef]
- Dasari, S.R.; Borugadda, V.B.; Goud, V.V. Reactive extraction of castor seeds and storage stability characteristics of produced biodiesel. Process. Saf. Environ. 2016, 100, 252–263. [Google Scholar] [CrossRef]
- Özgül, S.; Türkay, S. Variables affecting the yields of methyl esters derived from in situ esterification of rice bran oil. J. Am. Oil Chem. Soc. 2002, 79, 611–614. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, Q.; Knothe, G.; Lu, M. Direct transesterification of spent coffee grounds for biodiesel production. Fuel 2017, 199, 157–161. [Google Scholar] [CrossRef]
- Tuntiwiwattanapun, N.; Monono, E.; Wiesenborn, D.; Tongcumpou, C. In-situ transesterification process for biodiesel production using spent coffee grounds from the instant coffee industry. Ind. Crops Prod. 2017, 102, 23–31. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Lee, J.W. In-situ transesterification of wet spent coffee grounds for sustainable biodiesel production. Bioresour. Technol. 2016, 221, 55–60. [Google Scholar] [CrossRef]
- Shiu, P.J.; Gunawan, S.; Hsieh, W.H.; Kasim, N.S.; Ju, Y.H. Biodiesel production from rice bran by a two-step in-situ process. Bioresour. Technol. 2010, 101, 3984–3989. [Google Scholar] [CrossRef]
- Sangaletti-Gerhard, N.; Cea, M.; Risco, V.; Navia, R. In situ biodiesel production from greasy sewage sludge using acid and enzymatic catalysts. Bioresour. Technol. 2015, 179, 63–70. [Google Scholar] [CrossRef]
- Choi, O.K.; Song, J.S.; Cha, D.K.; Lee, J.W. Biodiesel production from wet municipal sludge: Evaluation of in situ transesterification using xylene as a cosolvent. Bioresour. Technol. Vol. 2014, 166, 51–56. [Google Scholar] [CrossRef]
- Mondala, A.; Liang, K.; Toghiani, H.; Hernandez, R.; French, T. Biodiesel production by in situ transesterification of municipal primary and secondary sludges. Bioresour. Technol. 2009, 100, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Scott, K.; Foglia, T.; Mariner, W. The general applicability of in situ transesterification for the production of fatty acid esters from a variety of feedstocks. J. Am. Chem. Soc. 2007, 84, 963–970. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Robles-Medina, A.; Hita-Peña, E.; Jiménez-Callejón, M.J.; Estéban-Cerdán, L.; González-Moreno, P.A.; Molina-Grima, E. Biodiesel production from wet microalgal biomass by direct transesterification. Fuel 2015, 150, 14–20. [Google Scholar] [CrossRef]
- Li, Y.S.; Lian, S.A.; Tong, D.M.; Song, R.L.; Yang, W.Y.; Fan, Y.; Qing, R.W.; Hu, C.W. One-step production of biodiesel from Nannochloropsis sp. on solid base Mg-Zr catalyst. Appl. Energy 2011, 88, 3313–3317. [Google Scholar] [CrossRef]
- Xu, R.; Mi, Y. Simplifying the process of microalgal biodiesel production through in situ transesterification technology. J. Am. Oil Chem. Soc. 2011, 88, 91–99. [Google Scholar] [CrossRef]
- Johnson, M.B.; Wen, Z. Production of biodiesel fuel from microalga Schizochytrium limacinum by direct transesterificaton of algal biomass. Energy Fuel 2009, 23, 5179–5183. [Google Scholar] [CrossRef]
- Ehimem, E.A.; Sun, Z.F.; Carrington, C.G. Variables affecting the in situ transesterification of microalgae lipids. Fuel 2010, 89, 677–684. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar]
- Koberg, M.; Cohen, M.; Ben-Amotz, A.; Gedanke, A. Bio-diesel production directly from the microalgae biomass of Nannochloropsis by microwave and ultrasound radiation. Bioresour. Technol. 2011, 102, 4265–4269. [Google Scholar]
- Zhang, J.; Cui, C.; Chen, H.; Liu, J. The completion of esterification of free fatty acids in Zanthoxylum bungeanum seed oil with ethanol. Int. J. Green Energy 2014, 11, 822–832. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y.; Valero, J.R. Ultrasonication aided in-situ transesterification of microbial lipids to biodiesel. Bioresour. Technol. 2014, 169, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Ehinem, E.A.; Sun, Z.; Carrington, G.C. Use of ultrasound and co-solvents to improve the in situ transesterification of microalgae biomass. Procedia Environ. Sci. 2012, 15, 47–55. [Google Scholar]
- Sung, M.; Han, J.I. Ultrasound-assisted in-situ transesterification of wet Aurantiochytrium sp. KRS 101 using potassium carbonate. Bioresour. Technol. 2018, 261, 117–121. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Kontominas, M.G.; Avlonitis, D.; Gergis, V. Transesterification of sunflower seed oil for the production of biodiesel: Effect of catalyst concentration and ultrasonication. In Proceedings of the 2006 IASME/WSEAS International Conference on Energy & Environmental Systems, Chalkida, Greece, 8–10 May 2006; pp. 425–429. [Google Scholar]
- Qian, J.; Yang, Q.; Sun, F.; He, M.; Chen, Q.; Yun, Z.; Qin, L. Cogeneration of biodiesel and nontoxic rapeseed meal from rapeseed through in-situ alkaline transesterification. Bioresour. Technol. 2013, 128, 8–13. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Z.K. Biodiesel production by direct methanolysis of oleaginous microbial biomass. Chem. Technol. Biotechnol. 2007, 82, 775–780. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, Z.; Wu, X.; Miao, X. Direct biodiesel production from wet microalgae biomass of Chlorella pyrenoidosa through in situ transesterification. Biomed. Res. Int. 2013, 2013, 930686. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Lee, J.G.M.; Harvey, A.P. Evaluation of FAME production from wet marine and freshwater microalgae by in situ transesterification. Biochem. Eng. 2013, 76, 83–89. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Uemura, Y.; Lam, M.K.; Mansor, N.; Lim, J.W. Revealing the effect of reaction parameters towards alkyl group distribution in in-situ transesterification of Chlorella vulgaris. Energy Convers. Manag. 2019, 185, 223–231. [Google Scholar] [CrossRef]
- Bruton, T.; Lyons, H.; Lerat, Y.; Stanley, M.; Rasmussen, M.B. A Reviewof the Potential of Marine Algae as a Source of Biofuel in Ireland, Report, Sustainable Energy Ireland. 2009. Available online: http://www.fao.org/uploads/media/0902_SEI_-_A_Review_of_the_Potential_of_Marine_Algae.pdf (accessed on 30 March 2020).
- Liu, J.; Liu, Y.; Wang, H.; Xue, S. Direct transesterification of fresh microalgal cells. Bioresour. Technol. 2015, 176, 284–287. [Google Scholar] [CrossRef]
- Tran, D.T.; Yeh, K.L.; Chen, C.L.; Chang, J.S. Enzymatic transesterification of microalgal oil from Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized Burkholderia lipase. Bioresour. Technol. 2012, 108, 119–127. [Google Scholar] [CrossRef]
- Nguyen, T.; Lam, M.K.; Uemura, Y.; Mansor, N.; Lim, J.W.; Show, P.L.; Tan, I.S.; Lim, S. High biodiesel yield from wet microalgae paste via in-situ transesterification: Effect of reaction parameters towards the selectivity of fatty acid esters. Fuel 2020, 272, 117718. [Google Scholar] [CrossRef]
- Kim, B.; Heo, H.Y.; Son, J.; Yang, J.; Chang, Y.K.; Lee, J.H.; Lee, J.W. Simplifying biodiesel production from microalgae via wet in situ transesterification: A review in current research and future prospects. Algal Res. 2019, 41, 101557. [Google Scholar] [CrossRef]
- Ozgul-Yucel, S.; Turkay, S. FA monoalkylester from rice bran oil by in situ transesterification. J. Am. Oil Chem. Soc. 2003, 81, 81–84. [Google Scholar] [CrossRef]
- Harvey, A.P.; Khurana, R.; Lee, J.G.M. In-situ Transesterification of Jatropha Seeds to Produce Biodiesel using Alcohol Mixture. In Proceedings of the 4th International Biofuels Conference, New Delhi, India, 1–2 February 2007. [Google Scholar]
- Chamola, R.; Khan, M.F.; Raj, A.; Verma, M.; Jain, R. Response surface methodology based optimization of in situ transesterification of dry algae with methanol, H2SO4 and NaOH. Fuel 2019, 239, 511–520. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Louhasakul, Y. Industrial wastes as a promising renewable source for production of microbial lipid and direct transesterification of the lipid into biodiesel. Bioresour. Technol. 2013, 14, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Dianursanti, P.R.; Wijanarko, A. Utilization of n-hexane as co-solvent to increase biodiesel yield on direct transesterification reaction from marine microalgae. Procedia Environ. Sci. 2015, 23, 412–420. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Lee, J.G.M.; Harvey, A.P. Alkaline in situ transesterification of Chlorella vulgaris. Fuel 2012, 94, 544–550. [Google Scholar] [CrossRef]
- Ginting, M.S.A.; Azizan, M.T.; Yusup, S. Alkaline in situ ethanolysis of Jatropha curcas. Fuel 2012, 93, 82–85. [Google Scholar] [CrossRef]
- Martínez, A.; Mijangos, G.A.; Romero-Ibarra, I.C.; Hernández-Altamirano, R.; Mena-Cervante, V.Y. In-situ transesterification of Jatropha curcas L. seeds using homogeneous and heterogeneous basic catalysts. Fuel 2019, 235, 277–287. [Google Scholar]
- Kazemifard, S.; Nayebzadeh, H.; Saghatoleslami, N.; Safakish, E. Application of magnetic alumina-ferric oxide nanocatalyst supported by KOH for in-situ transesterification of microalgae cultivated in wastewater medium. Biomass Bioenergy 2019, 129, 105338. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Muthukumar, K. Direct transesterification of Oedogonium sp. oil be using immobilized isolated novel Bacillus sp. Lipase. J. Biosci. Bioeng. 2014, 117, 86–91. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Brooks, M.S.; Budge, S. Production of Biodiesel by Enzymatic Transesterification: Review. J. Biochem. Biotechnol. 2010, 6, 54–76. [Google Scholar] [CrossRef]
- Su, E.; You, P.; Wei, D. In situ lipase-catalyzed reactive extraction of oilseeds with short-chained dialkyl carbonates for biodiesel production. Bioresour. Technol. 2009, 100, 5813–5817. [Google Scholar] [CrossRef]
- Kojima, S.; Du, D.; Sato, M.; Park, E.Y. Efficient production of fatty acid methyl esterfrom waste activated bleaching earth using diesel oil as organic solvent. J. Biosci. Bioeng. 2004, 98, 420–424. [Google Scholar] [CrossRef]
- Ve’ras, I.C.; Silva, F.A.L.; Ferr´co-Gonzales, A.D.; Moreau, V.H. One-step enzymatic production of fatty acid ethyl ester from high-acidity waste feedstocks in solvent-free media. Bioresour. Technol. 2011, 102, 9653–9658. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Pinsirodom, P.; Nagao, T.; Yamauchi, A.; Kobayashi, T.; Nishida, Y.; Takagi, Y.; Shimada, Y. Conversion of acid oil by-produced in vegetable oil refining to biodiesel fuel by immobilized Candida antarctica lipase. J. Mol. Catal. B Enzym. 2007, 44, 99–105. [Google Scholar] [CrossRef]
- Chen, J.; Wu, W. Regeneration of immobilized Candida antarctica lipase for transesterification. J. Biosci. Bioeng. 2003, 95, 466–469. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Samukawa, T.; Sugihara, A.; Noda, H.; Fukuda, H.; Tominaga, Y. Conversion of vegetable oil to biodiesel using immobilized Candida antarctica lipase. J. Am. Oil Chem. Soc. 1999, 76, 789–793. [Google Scholar] [CrossRef]
- Ochoa-Gómeza, J.R.; Gómez-Jiménez-Aberasturib, O.; Maestro-Madurgab, B.; Pesquera-Rodríguezb, A.; Ramírez-Lópezb, C.; Lorenzo-Ibarretab, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization. Appl. Catal. A Gen. 2009, 366, 315–324. [Google Scholar] [CrossRef]
- Jo, Y.J.; Lee, O.K.; Lee, E.Y. Dimethyl carbonate-mediated lipid extraction and lipase-catalyzed in situ transesterification for simultaneous preparation of fatty acid methyl esters and glycerol carbonate from Chlorella sp. KR-1 biomass. Bioresour. Technol. 2014, 158, 105–110. [Google Scholar] [CrossRef]
- Ceni, G.; Lerin, L.A.; Conto, J.F.; Brancher, V.; Silva, P.C.; Toniazzo, G.; Treichel, H.; de Oliveira, D.; Oliveira, J.V.; Oestreicher, E.G.; et al. Optimization of 1-glyceryl benzoate production by enzymatic transesterification in organic solvents. Enzyme Microb. Technol. 2010, 46, 107–112. [Google Scholar] [CrossRef]

| Raw Material | Alcohol Solvent | Reaction Conditions | Fatty Acid Esters Yield (%) | References |
|---|---|---|---|---|
| Jatropha. curcas L. seeds | Methanol/hexane | Catalyst H2SO4—15 wt% of seed, 60 °C, 24 h, methanol to seed ratio—7.5 mL/g, hexane -10 vol% of solvent. | 99.8 | [18] |
| Chlorella sp. | Methanol | Catalyst H2SO4 lipid molar ratio 0.35:1, 60 °C, 19 h, methanol to seed molar ratio—600:1. | 92 ± 2 | [48] |
| Chlorella vulgaris | Methanol | 200 powdered microalgae, catalyst H2SO4 (at 80.8 mol/mol of H+ to esterifiable lipid), 60 °C, 120 min. | 94.6 | [53] |
| Nannochloropsis | Methanol | Catalyst H2SO4 lipid molar ratio 0.35:1, 60 °C, 19 h, methanol to seed molar ratio—600:1. | 73 ± 5 | [48] |
| Microalgae lipids | Methanol | Microalgae lipids—15 mL, catalyst H2SO4—0.04 mL, 23 °C, 8 h, methanol—60 mL. | 91.3 | [37] |
| Dry water microalgae | Methanol | Catalyst H2SO4—3.361%, w/w, methanol/algae ratio—8:1, w/w, 50 °C, 60.4 min. | 89.58 | [57] |
| Rice bran | Methanol | Rice bran—50 g, catalyst H2SO4—7.5 mL, 65 °C, 1 h, alcohol– 200 mL. | 85.8 | [55] |
| Rice bran | Ethanol (96%) | Rice bran—50 g, catalyst H2SO4—7.5 mL, 78 °C, 1 h, alcohol—200 mL. | 76.4 | [55] |
| Rice bran | Ethanol (99.1%) | Rice bran—50 g, catalyst H2SO4—7.5 mL, 78 °C, 1 h, alcohol—200 mL. | 78.3 | [55] |
| Rice bran | Isopropanol | Rice bran—50 g, catalyst H2SO4—7.5 mL, 82 °C, 1 h, alcohol—200 mL. | 69.7 | [55] |
| Rice bran | n-Propanol | Rice bran—50 g, catalyst H2SO4—7.5 mL, 97 °C, 1 h, alcohol—200 mL. | 74.8 | [55] |
| Rice bran | n-Butanol | Rice bran—50 g, catalyst H2SO4—7.5 mL, 117 °C, 1 h, alcohol—200 mL. | 68 | [55] |
| Primary municipal sludge | Methanol | Catalyst H2SO4—5% (v/v) of seed, 75 °C, methanol to seed ratio—12:1 | 14.5 | [31] |
| Secondary municipal sludge | Methanol | Catalyst H2SO4—5% (v/v) of seed, 75 °C, methanol to seed ratio—12:1 | 2.5 | [31] |
| Raw Material | Alcohol Solvent | Reaction Conditions | Fatty Acid Esters Yield (%) | References |
|---|---|---|---|---|
| Soybeans | Methanol | Catalyst NaOH, methanol/acylglycerols/NaOH molar ratio 543:1:2.0, 23 °C, 8 h. | 84 | [15] |
| Distillers dried grains with solubles (DDGS) | Methanol | Catalyst NaOH—0.4 N, 35 °C, 1.2 h, methanol to seed molar ratio—655:1. | 91.1 | [32] |
| Meat and bone meal (MBM) | Methanol | Catalyst NaOH—2.0 N, 35 °C, 0.2 h, methanol to seed molar ratio—550:1. | 9.3 | [32] |
| Jatropha seeds | Methanol/hexane | Catalyst KOH—0,075 mol/l in methanol, 60 °C, 4 h, methanol to seed molar ratio—6:1. | 87 | [20] |
| Rapeseed | Methanol | Catalyst NaOH—0.1 mol/l in methanol, 60 °C, 1 h, methanol to seed molar ratio—475:1. | 88.8 ± 0.1 | [10] |
| Cottonseed meal | Methanol | Catalyst NaOH—0.1 mol/l in methanol, 40 °C, 3 h, methanol to seed molar ratio—135:1. | 98 | [17] |
| Chlorella sp | Methanol | Catalyst NaOH and lipid molar ratio 0.35:1, 60 °C, 19 h, methanol to seed molar ratio—600:1. | 79 ± 2 | [47] |
| Jatropha curcas | Methanol/ethanol mix | Catalyst NaOH—0.02 N, 60 °C, 1 h, methanol to seed molar ratio—512:1. | 87 | [56] |
| Chlorella sp | Methanol | Catalyst CH3ONa to lipid molar ratio 0.35:1, 60 °C, 19 h, methanol to seed molar ratio—600:1. | 90 ± 2 | [47] |
| Dry water microalgae | Methanol | Catalyst NaOH—3.499%, w/w, methanol/algae ratio—8:1, w/w, 50 °C, 73.64 min. | 87.42 | [57] |
| Raw Material | Alcohol Solvent | Reaction Conditions | Fatty Acid Esters Yield (%) | References |
|---|---|---|---|---|
| Castor seeds (Ricinus communis L. red) | Ethanol | Seeds—20 g, catalyst H2SO4—1.0 wt%, 60 °C, 1 h, ethanol to oil molar ratio—40:1. After 1 h, catalyst KOH—1.0 wt%, 60 °C, 1 h, ethanol to oil molar ratio—20:1. | 95.3 | [22] |
| Castor seeds (BRS-149 nordestina) | Ethanol | Seeds—20 g, catalyst H2SO4—1.0 wt%, 60 °C, 1 h, ethanol to oil molar ratio—40:1. After 1 h, catalyst KOH—1.0 wt%, 60 °C, 1 h, ethanol to oil molar ratio—20:1. | 98.0 | [22] |
| Castor seeds (Ricinus communis L. red) | Ethanol/hexane | Seeds—20 g, catalyst H2SO4—1.0 wt%, 60 °C, 1 h, hexane 20% v/v, ethanol to oil molar ratio—40:1, After 1 h, catalyst KOH—1.0 wt%, 60 °C, 1 h, hexane 20% v/v, ethanol to oil molar ratio—20:1. | 95.6 | [22] |
| Castor seeds (BRS-149 nordestina) | Ethanol/hexane | Seeds—20 g, catalyst H2SO4—1.0 wt%, 60 °C, 1 h, hexane 20% v/v, ethanol to oil molar ratio—40:1, After 1 h, catalyst KOH—1.0 wt%, 60 °C, 1 h, hexane 20% v/v, ethanol to oil molar ratio—20:1. | 97.4 | [22] |
| Rice bran | Methanol/petroleum ether | I STEP Rice bran 50 g (18.6% oil content). Catalyst H2SO4—0.75 g, 3 h, methanol—75 mL, petroleum ether—150 mL. II STEP Catalyst NaOH—0.71 g, 3 h, methanol—75 mL, petroleum ether—150 mL. | 95.16 | [1] |
| Raw Material | Alcohol Solvent | Reaction Conditions | Fatty Acid Esters Yield (%) | References |
|---|---|---|---|---|
| Nannochloropsis sp. | Methanol/ methylene dichloride | Catalyst Mg–Zr—10 wt%, 65 °C, 4 h, 45 mL mixed 2:1(v/v) solvent /1 g dried microalgae powder | 2.6 | [34] |
| Nannochloropsis sp. | Methanol/ methylene dichloride | I STEP Catalyst Mg–Zr—10 wt%, 65 °C, 4 h, 45 mL mixed 2:1(v/v) solvent /1 g dried microalgae powderII STEP Catalyst Mg–Zr—10 wt%, 65 °C, 4 h, methanol to lipid molar ratio—10:1. | 22.2 | [34] |
| Nannochloropsis sp. | Methanol/ methylene dichloride | Catalyst Mg–Zr—10 65 °C, 4 h, 45 mL mixed 3:1(v/v) solvent /1 g dried microalgae powder | 28 | [34] |
| Jatropha curcas L. | Methanol | Catalyst—Na2ZrO3 —5 wt%, 65 °C, 8 h, 1:65M ratio of oil/methanol | 99.9 | [62] |
| Microalgae mixed culture (dry) | Methanol | Magnetic nano catalyst Fe2O3–Al2O3—4 wt%, 65 °C, 6 h, 12 mL/g of methanol-to-dry biomass | 95.6 | [63] |
| Raw Material | Alcohol Solvent | Reaction Conditions | Fatty Acid Esters Yield (%) | References |
|---|---|---|---|---|
| Jatropha curcas L. seed | Dimethyl carbonate | Catalyst Novozym 435—10 wt%, 50 °C, 24 h, dimethyl carbonate to oil ratio—10:1 | 95.5 | [66] |
| Jatropha curcas L. seed | Diethyl carbonate | Catalyst Novozym 435—10 wt%, 50 °C, 24 h, diethyl carbonate to oil ratio—10:1 | 94.5 | [66] |
| Pistacia chinensis Bunge seed | Dimethyl carbonate | Catalyst Novozym 435—10 wt%, 50 °C, 24 h, dimethyl carbonate to oil ratio—10:1 | 89.6 | [68] |
| Pistacia chinensis Bunge seed | Diethyl carbonate | Catalyst Novozym 435—10 wt% of seed, reaction temperature –50 °C, reaction duration—24 h, diethyl carbonate to oil ratio—10:1 | 90.7 | [66] |
| Microalgae Chlorella vulgaris ESP-31 | Methanol/ hexane | Catalyst Burkholderia lipase—1203.11 U/g, lipase was immobilized on alkyl-grafted nanocomposites (Fe3O4–SiO2), 40 °C, water content—71.39 wt%, 48 h, methanol to oil molar ratio—67.93:1, hexane content –80.57 wt%. | 97.3 | [52] |
| Greasy sewage sludge | Methanol | Catalyst Novozym ® 435—10% in mass fraction of sludge (wet weight base), 40 °C, 24 h, methanol to sludge ratio—4 mL/g | 52 | [29] |
| Waste activated bleaching earth | Methanol | Catalyst Candida cylindracea—10 wt%, 37 °C, 96 h, methanol to oil ratio—4:1 | 55 | [67] |
| Waste activated bleaching earth | Methanol/ hexane | Catalyst Candida cylindracea—10 wt%, 37 °C, 7 h, methanol to oil ratio—4:1, FAME content in sample 10% (w/w) | 100 | [67] |
| Waste activated bleaching earth | Methanol/ mineral diesel | Catalyst Candida cylindracea—10 wt%, 37 °C, 2–3 h, methanol to oil ratio—4:1, FAME content in sample 10% (w/w) | 100 | [67] |
| Rapeseeds | Butanol/mineral diesel | Catalyst Lipozyme RMIM—5.2 wt%, mineral diesel to oil ratio (w/w)—9:1, 40 °C, 19.6 h, butanol to oil ratio—31:1 | 97.6 | [8] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makareviciene, V.; Sendzikiene, E.; Gumbyte, M. Application of Simultaneous Oil Extraction and Transesterification in Biodiesel Fuel Synthesis: A Review. Energies 2020, 13, 2204. https://doi.org/10.3390/en13092204
Makareviciene V, Sendzikiene E, Gumbyte M. Application of Simultaneous Oil Extraction and Transesterification in Biodiesel Fuel Synthesis: A Review. Energies. 2020; 13(9):2204. https://doi.org/10.3390/en13092204
Chicago/Turabian StyleMakareviciene, Violeta, Egle Sendzikiene, and Milda Gumbyte. 2020. "Application of Simultaneous Oil Extraction and Transesterification in Biodiesel Fuel Synthesis: A Review" Energies 13, no. 9: 2204. https://doi.org/10.3390/en13092204
APA StyleMakareviciene, V., Sendzikiene, E., & Gumbyte, M. (2020). Application of Simultaneous Oil Extraction and Transesterification in Biodiesel Fuel Synthesis: A Review. Energies, 13(9), 2204. https://doi.org/10.3390/en13092204