Biodiesel Production by Lipase-Catalyzed in Situ Transesterification of Rapeseed Oil Containing a High Free Fatty Acid Content with Ethanol in Diesel Fuel Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Rapeseed Quality and Its Oil Properties
2.3. Selection of Lipase for In Situ Transesterification Process
2.3.1. Thin-Layer Chromatography
2.3.2. Gas Chromatography
2.4. Optimization of the Enzymatic In Situ Transesterification Process
2.5. Fourier-Transform Infrared Spectroscopy
3. Results and Discussion
3.1. Selection of Lipase for In Situ Transesterification
3.2. Optimization of the Enzymatic In Situ Transesterification Process
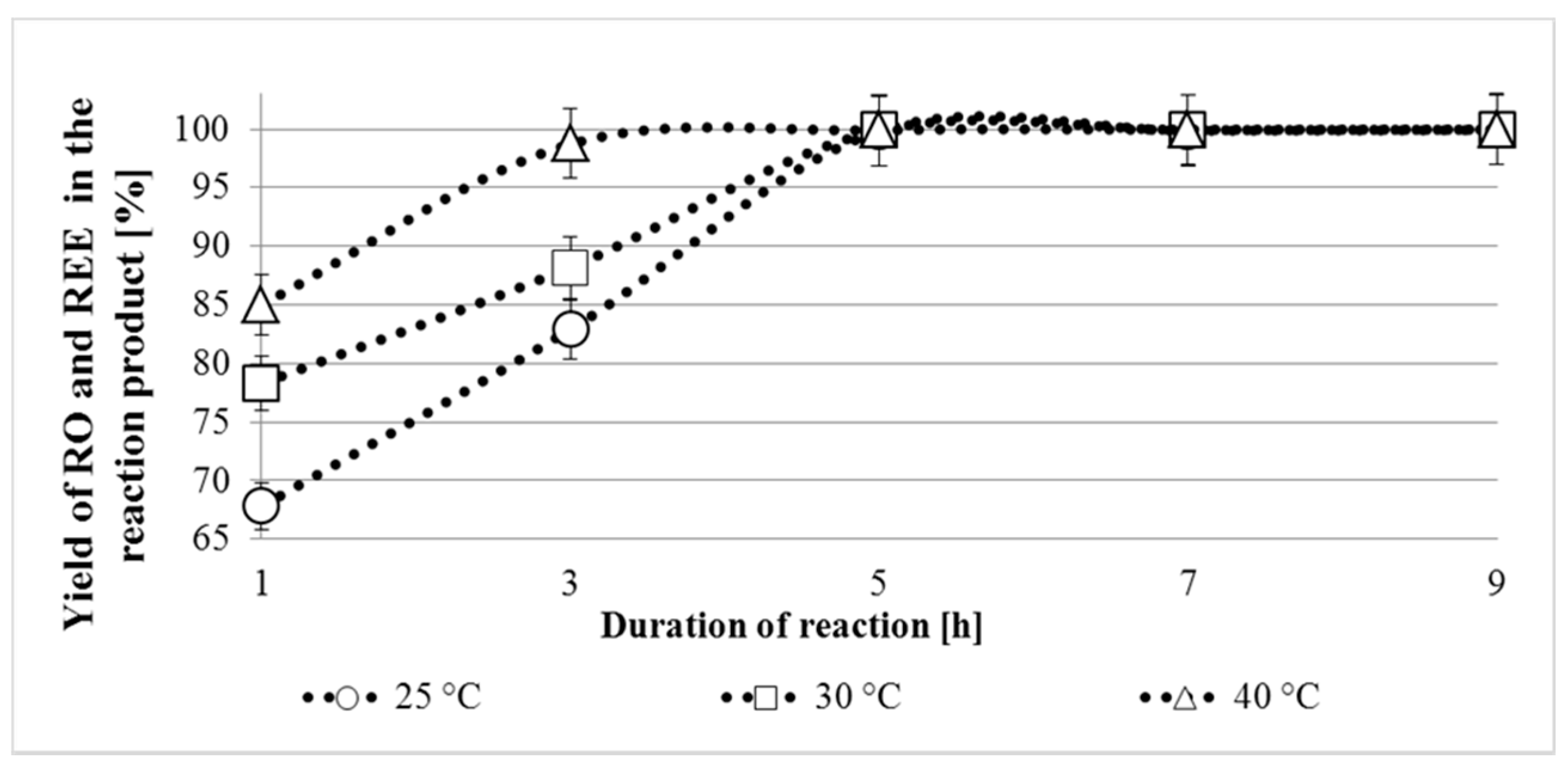
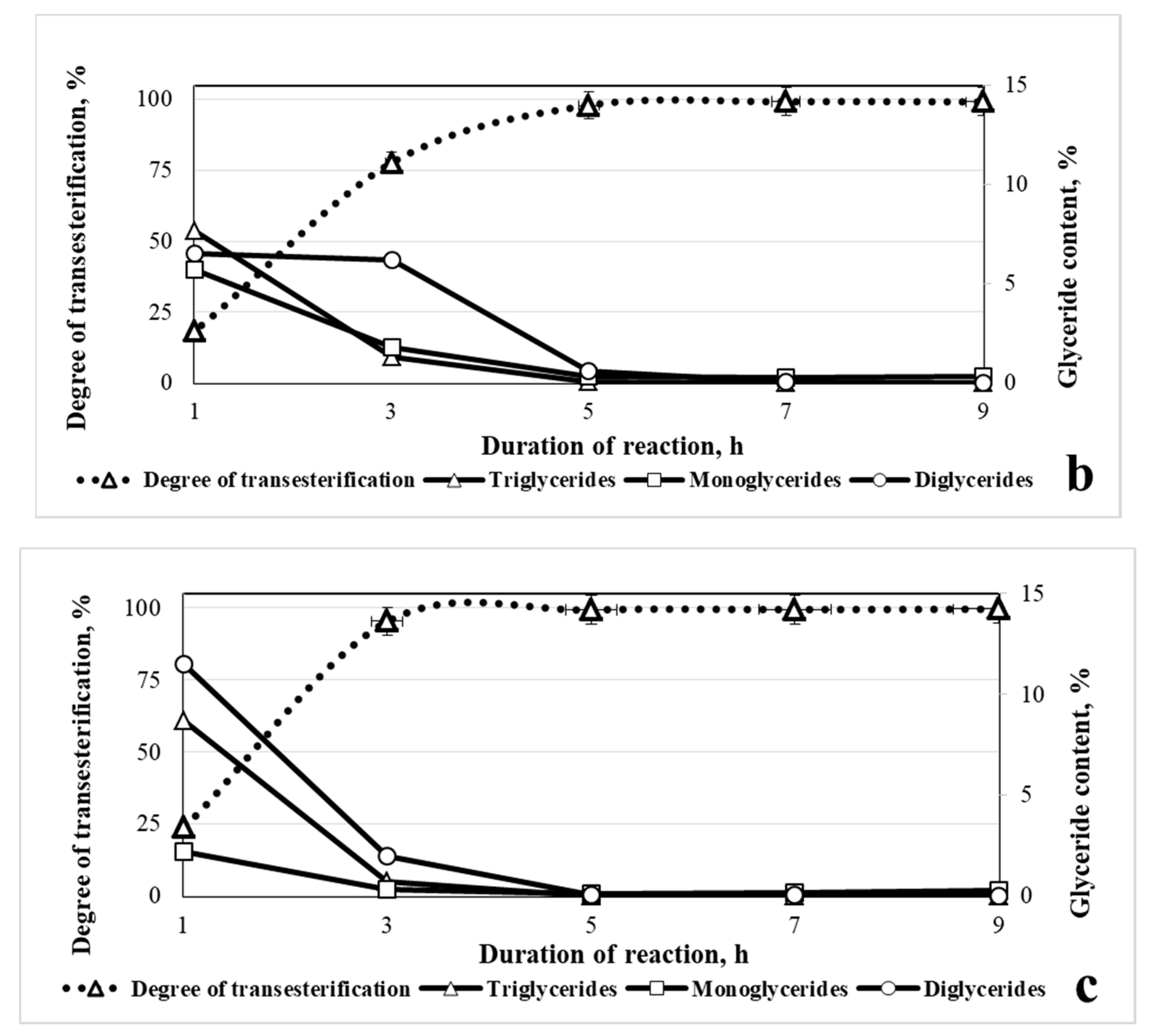
3.2.1. Optimal Temperature and Duration of Reaction
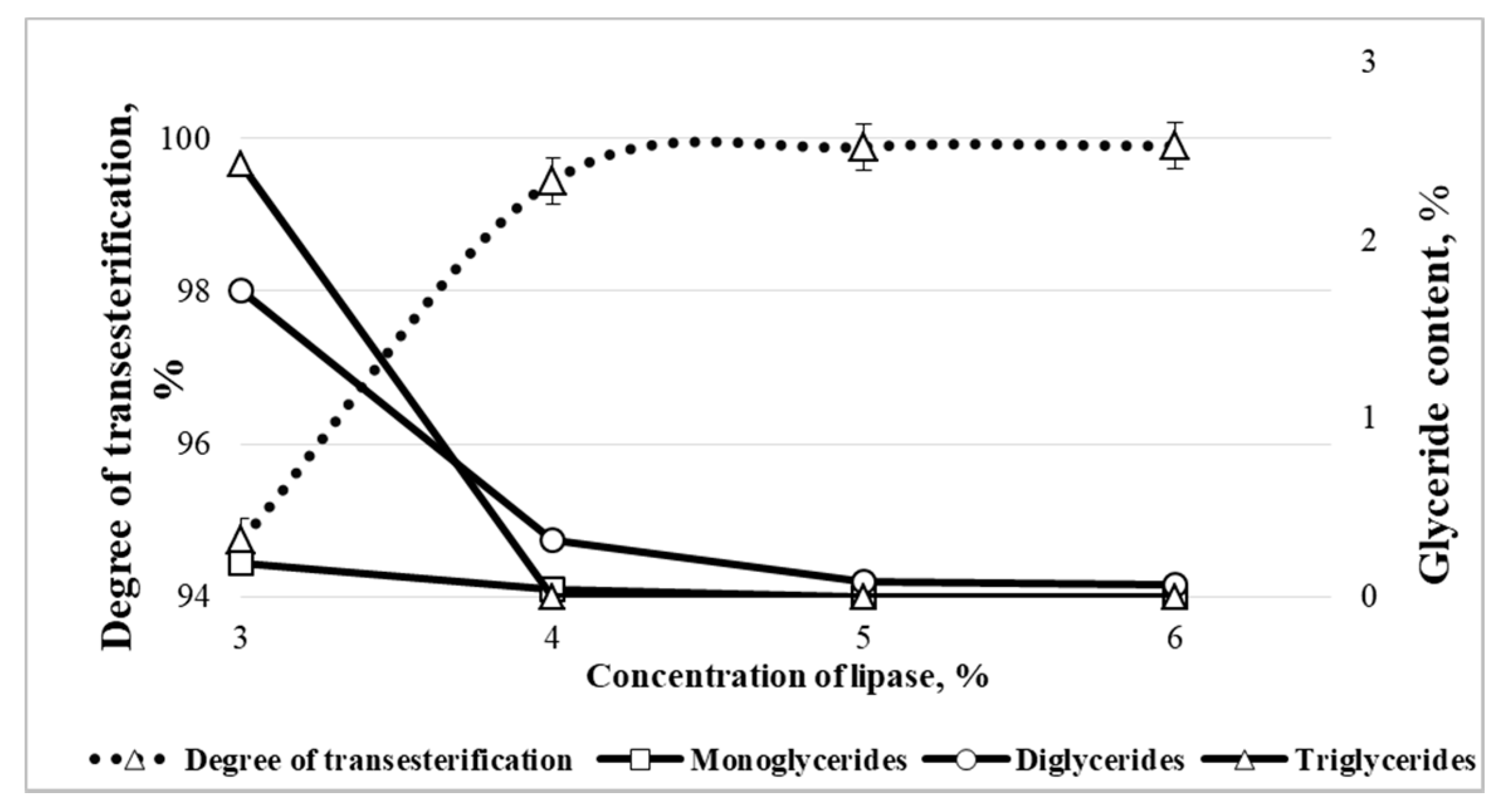
3.2.2. Optimal Lipase Concentration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurayama, F.; Yoshikawa, T.; Furusawa, T.; Bahadurc, N.M.; Handa, H.; Sato, M.; Suzuki, N. Microcapsule with a heterogeneous catalyst for the methanolysis of rapeseed oil. Bioresour. Technol. 2013, 135, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Go, A.W.; Sutanto, S.; Liu, Y.T.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.H. In situ transesterification of Jatropha curcas L. seeds in subcritical solvent system. J. Taiwan Inst. Chem. E 2014, 45, 1516–1522. [Google Scholar] [CrossRef]
- Avhad, M.R.; Sánchez, M.; Peña, E.; Bouaid, A.; Martínez, M.; Aracil, J.; Marchetti, J.M. Renewable production of value-added jojobyl alcohols and biodiesel using a naturally-derived heterogeneous green catalyst. Fuel 2016, 179, 332–338. [Google Scholar] [CrossRef]
- Patel, R.L.; Sankhavara, C.D. Biodiesel production from Karanja oil and its use in diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 71, 464–474. [Google Scholar] [CrossRef]
- No, S.Y. Inedible vegetable oils and their derivatives for alternative diesel fuels in CI engines: A review. Renew. Sustain. Energy Rev. 2011, 15, 131–149. [Google Scholar] [CrossRef]
- Dasari, S.R.; Borugadda, V.B.; Goud, V.V. Reactive extraction of castor seeds and storage stability characteristics of produced biodiesel. Process Saf. Environ. 2016, 100, 252–263. [Google Scholar] [CrossRef]
- Koutsouki, A.A.; Tegou, E.; Kontakos, S.; Kontominas, M.G.; Pomonis, P.J.; Manos, G. In situ transesterification of Cynara cardunculus L. seed oil via direct ultrasonication for the production of biodiesel. Fuel Process Technol. 2015, 134, 122–129. [Google Scholar] [CrossRef]
- Joshi, G.; Rawat, D.S.; Sharma, A.K.; Pandey, J.K. Microwave enhanced alcoholysis of non-edible (algal, jatropha and pongamia) oils using chemically activated egg shell derived CaO as heterogeneous catalyst. Bioresour. Technol. 2016, 219, 487–492. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Zhang, J.; Li, G. In situ reactive extraction of cottonseeds with methyl acetate for biodiesel production using magnetic solid acid catalysts. Bioresour. Technol. 2014, 174, 182–189. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, P.; Garg, S. Alkali transesterification of linseed oil for biodiesel production. Fuel 2013, 104, 553–560. [Google Scholar] [CrossRef]
- Behçet, R.; Oktay, H.; Çakmak, A.; Aydin, H. Comparison of exhaust emissions of biodiesel–diesel fuel blends produced from animal fats. Renew. Sustain. Energy Rev. 2015, 46, 157–165. [Google Scholar] [CrossRef]
- Attia, A.M.A.; Hassaneen, A.E. Influence of diesel fuel blended with biodiesel produced from waste cooking oil on diesel engine performance. Fuel 2016, 167, 316–328. [Google Scholar] [CrossRef]
- Tuntiwiwattanapuna, N.; Monono, E.; Wiesenborn, D.; Tongcumpou, C. In-situ transesterification process for biodiesel production using spent coffee grounds from the instant coffee industry. Ind. Crop. Prod. 2017, 102, 23–31. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, Q.; Knothe, G.; Lu, M. Direct transesterification of spent coffee grounds for biodiesel production. Fuel 2017, 199, 157–161. [Google Scholar] [CrossRef]
- Bolonio, D.; García-Martínez, M.J.; Ortega, M.F.; Lapuerta, M.; Canoira, L. Fatty acid ethyl esters (FAEEs) obtained from grapeseed oil: A fully renewable biofuel. Renew. Energy 2019, 132, 278–283. [Google Scholar] [CrossRef]
- Taniguchi, I.; Kagotani, K.; Kimura, Y. Microbial production of poly(hydroxyalkanoates) from waste edible oils. Green Chem. 2003, 5, 545–548. [Google Scholar] [CrossRef]
- Brunschwig, C.; Moussavou, W.; Blin, J. Use of bioethanol for biodiesel production. Prog. Energy Combust. 2012, 38, 283–301. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. Innovation in solid heterogeneous catalysis for the generation of economically viable and ecofriendly biodiesel: A review. Catal Rev. Sci. Eng. 2016, 58, 157–208. [Google Scholar] [CrossRef]
- Go, A.W.; Sutanto, S.; Ong, L.K.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.H. Developments in in-situ (trans) esterification for biodiesel production: A critical review. Renew. Sustain. Energy Rev. 2016, 60, 284–305. [Google Scholar] [CrossRef]
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Sim, J.H.; Kamaruddin, A.H.; Bhatia, S. Biodiesel (FAME) Productivity, Catalytic Efficiency and Thermal Stability of Lipozyme TL IM for Crude Palm Oil Transesterification with Methanol. J. Am. Oil Chem. Soc. 2010, 87, 1027–1034. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Gumbyte, M. Reactive extraction and fermental transesterification of rapeseed oil with butanol in diesel fuel media. Fuel Process Technol. 2015, 138, 758–764. [Google Scholar] [CrossRef]
- Robles-Medina, A.; González-Moreno, P.A.; Esteban-Cerdán, L.; Molina-Grima, E. Biocatalysis: Toward ever greener biodiesel production. Biotechnol. Adv. 2009, 27, 398–408. [Google Scholar] [CrossRef]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A review on FAME production processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Ganesan, D.; Rajendran, A.; Thangavelu, V. An overview on the recent advances in the transesterification of vegetable oils for biodiesel production using chemical and biocatalysts. Rev. Environ. Sci. Biotechol. 2009, 8, 367–394. [Google Scholar] [CrossRef]
- Vasudevan, P.; Fu, B. Environmentally sustainable biofuels: Advances in biodiesel research. Waste Biomass Valori. 2010, 1, 47–63. [Google Scholar] [CrossRef]
- Antczak, S.M.; Kubiak, A.; Antczak, T.; Bielecki, S. Enzymatic biodiesel synthesis—Key factors affecting efficiency of the process. Renew. Energy 2009, 34, 1185–1194. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikiene, E.; Gumbyte, M. Application of Simultaneous Oil Extraction and Transesterification in Biodiesel Fuel Synthesis: A Review. Energies 2020, 13, 2204. [Google Scholar] [CrossRef]
- Gumbyte, M.; Makareviciene, V.; Skorupskaite, V.; Sendzikiene, E.; Kondratavičius, M. Enzymatic microalgac oil transesterification with ethanol in mineral diesel fuel media. J. Renew. Sustain. Energy 2018, 10, 013105. [Google Scholar]
- Su, E.; You, P.; Wei, D. In situ lipase-catalyzed reactive extraction of oilseeds with short-chained dialkyl carbonates for biodiesel production. Bioresour. Technol. 2009, 100, 5813–5817. [Google Scholar] [CrossRef] [PubMed]
- Aguieiras, E.C.G.; Cavalcanti-Oliveira, E.D.; de Castro, A.M.; Langone, M.A.P.; Freire, D.M.G. Simultaneous Enzymatic Transesterification and Esterification of an Acid Oil Using Fermented Solid as Biocatalyst. J. Am. Oil Chem. Soc. 2017, 94, 551–558. [Google Scholar] [CrossRef]
- Jiang, Y.; Gu, H.; Zhou, L.; Cui, C.; Gao, J. Novel in situ batch reactor with a facile catalyst separation device for biodiesel production. Ind. Eng. Chem. Res. 2012, 51, 14935–14940. [Google Scholar] [CrossRef]
- Bajaj, A.; Lohan, P.; Jha, P.N.; Mehrotra, R. Biodiesel production through lipase catalyzed transesterification: An overview. J. Mol. Catal B Enzym. 2010, 62, 9–14. [Google Scholar] [CrossRef]
- Abo El-Enin, S.A.; Attia, N.K.; El-Ibiari, N.N.; El-Diwani, G.I.; El-Khatib, K.M. In-situ transesterification of rapeseed and cost indicators for biodiesel production. Renew. Sustain. Energy Rev. 2013, 18, 471–477. [Google Scholar] [CrossRef]
- Thliveros, P.; Kiran, E.U.; Web, C. Microbial biodiesel production by direct methanolysis of oleaginous biomass. Bioresour. Technol. 2014, 157, 181–187. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Hernández-Martín, E.; Otero, C. Different enzyme requirements for the synthesis of biodiesel: Novozym® 435 and Lipozyme® TL IM. Bioresour. Technol. 2008, 99, 277–286. [Google Scholar] [CrossRef]

| Property | Unit | Value | Standard |
|---|---|---|---|
| Oil content in seeds | % | 45 ± 2.0 | ISO 659 |
| Acid value | mg KOH/g | 2.63 ± 0.25 | ISO 660 |
| Moisture content in seeds | % | 6.3 ± 1.5 | ISO 665 |
| Fatty acid composition | % | ISO 5508 | |
| C14:1 | 0.04 ± 0.01 | ||
| C15:0 | 0.33 ± 0.06 | ||
| C16:0 | 3.73 ± 0.12 | ||
| C16:1 | 0.20 ± 0.01 | ||
| C18:0 | 1.80 ± 0.21 | ||
| C18:1 | 64.84 ± 1.03 | ||
| C18:2 | 18.47 ± 0.94 | ||
| C18:3 | 7.42 ± 0.66 | ||
| C20:0 | 0.61 ± 0.05 | ||
| C20:1 | 1.33 ± 0.02 | ||
| C22:0 | 0.43 ± 0.06 | ||
| C22:1 | 0.45 ± 0.02 | ||
| C24:0 | 0.19 ± 0.02 | ||
| C24:1 | 0.16 ± 0.01 |
| Lipase | Degree of Transesterification, % | Glyceride Content in Biodiesel Fuel, % | ||
|---|---|---|---|---|
| Monoglycerides | Diglycerides | Triglycerides | ||
| Lipozyme RM IM | 84.40 ± 1.32 | 2.26 ± 0.15 | 0.50 ± 0.05 | 9.50 ± 1.17 |
| Lipozyme TL IM | 97.74 ± 0.62 | 0.91 ± 0.04 | 0.02 ± 0.01 | 0.00 ± 0.01 |
| Lipolase 100L | 50.82 ± 3.04 | 3.27 ± 0.08 | 8.40 ± 0.45 | 29.86 ± 3.28 |
| Lipozyme TL 100L | 30.10 ± 2.17 | 3.51 ± 0.11 | 10.97 ± 1.04 | 93.56 ± 1.43 |
| Lipex 100L | 73.35 ± 1.03 | 2.51 ± 0.08 | 5.56 ± 0.27 | 12.72 ± 1.04 |
| Concentration of Lipase, % | Yield of RO and REE in the Reaction Product, % |
|---|---|
| 3 | 99.81 ± 0.04 |
| 4 | 99.95 ± 0.02 |
| 5 | 99.92 ± 0.02 |
| 6 | 99.97 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santaraite, M.; Sendzikiene, E.; Makareviciene, V.; Kazancev, K. Biodiesel Production by Lipase-Catalyzed in Situ Transesterification of Rapeseed Oil Containing a High Free Fatty Acid Content with Ethanol in Diesel Fuel Media. Energies 2020, 13, 2588. https://doi.org/10.3390/en13102588
Santaraite M, Sendzikiene E, Makareviciene V, Kazancev K. Biodiesel Production by Lipase-Catalyzed in Situ Transesterification of Rapeseed Oil Containing a High Free Fatty Acid Content with Ethanol in Diesel Fuel Media. Energies. 2020; 13(10):2588. https://doi.org/10.3390/en13102588
Chicago/Turabian StyleSantaraite, Migle, Egle Sendzikiene, Violeta Makareviciene, and Kiril Kazancev. 2020. "Biodiesel Production by Lipase-Catalyzed in Situ Transesterification of Rapeseed Oil Containing a High Free Fatty Acid Content with Ethanol in Diesel Fuel Media" Energies 13, no. 10: 2588. https://doi.org/10.3390/en13102588
APA StyleSantaraite, M., Sendzikiene, E., Makareviciene, V., & Kazancev, K. (2020). Biodiesel Production by Lipase-Catalyzed in Situ Transesterification of Rapeseed Oil Containing a High Free Fatty Acid Content with Ethanol in Diesel Fuel Media. Energies, 13(10), 2588. https://doi.org/10.3390/en13102588








