Hydrogenation Ability of Mg-Li Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
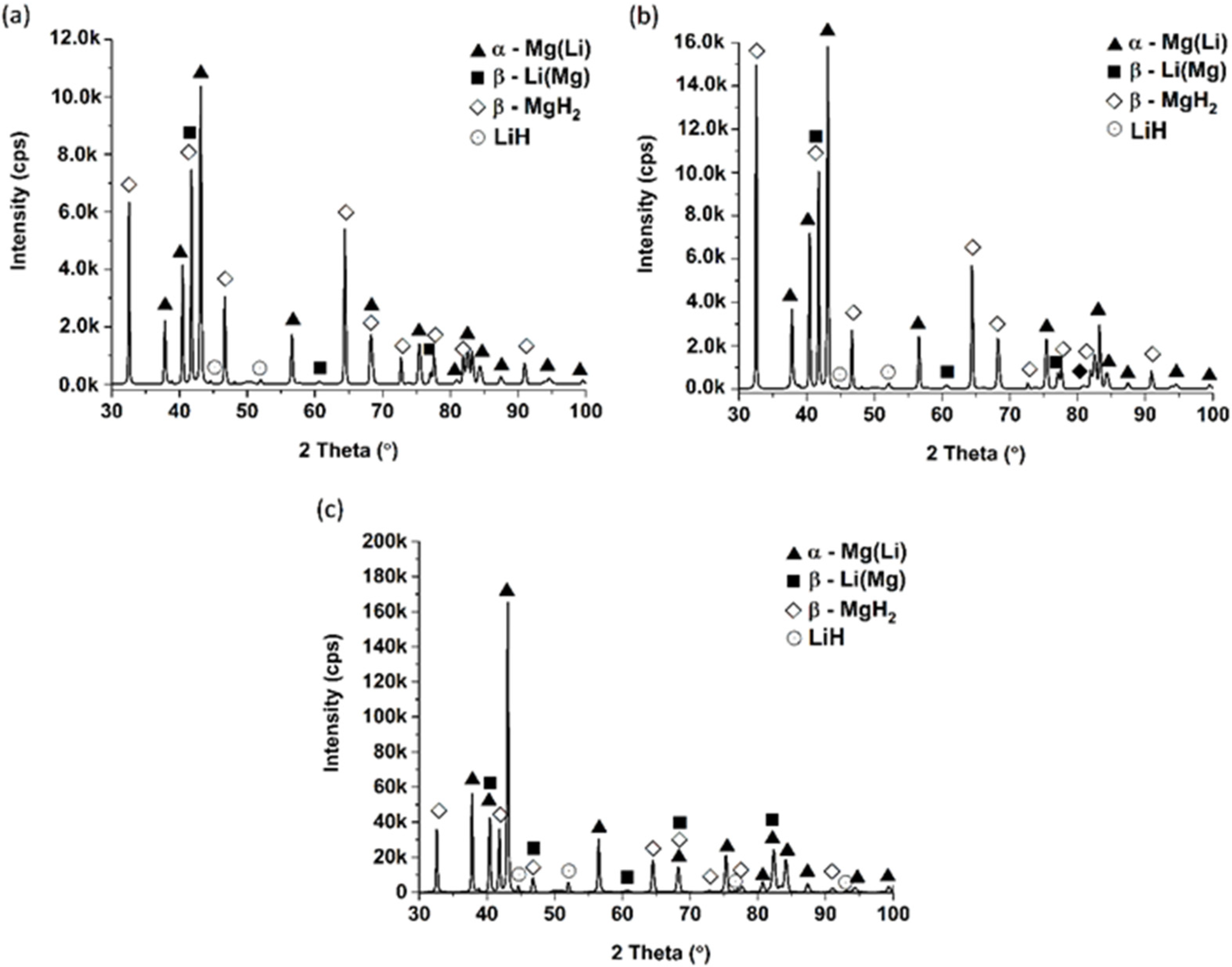
3.1. Phase Structure and Morphology of the Materials Before and After Hydrogenation
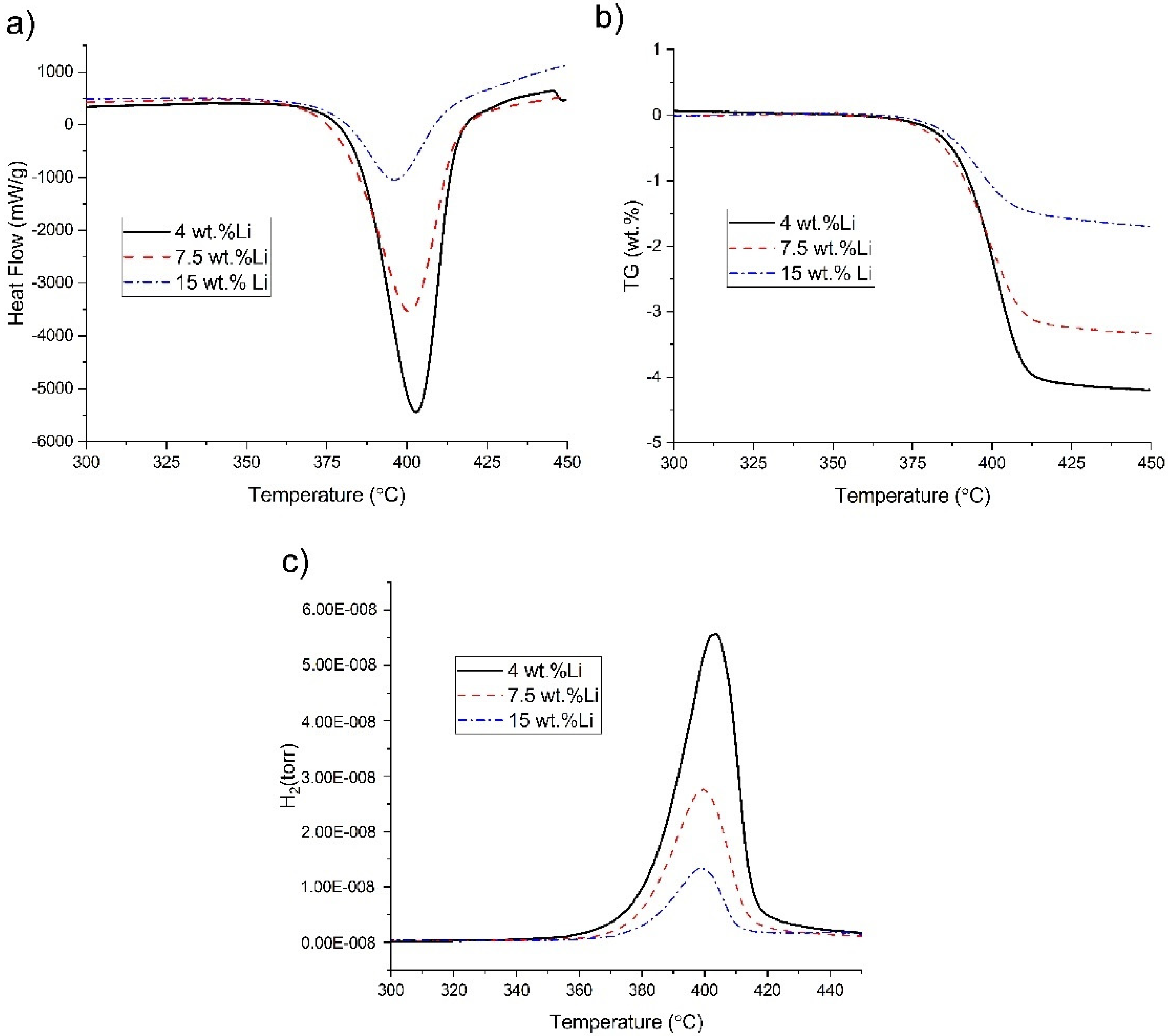
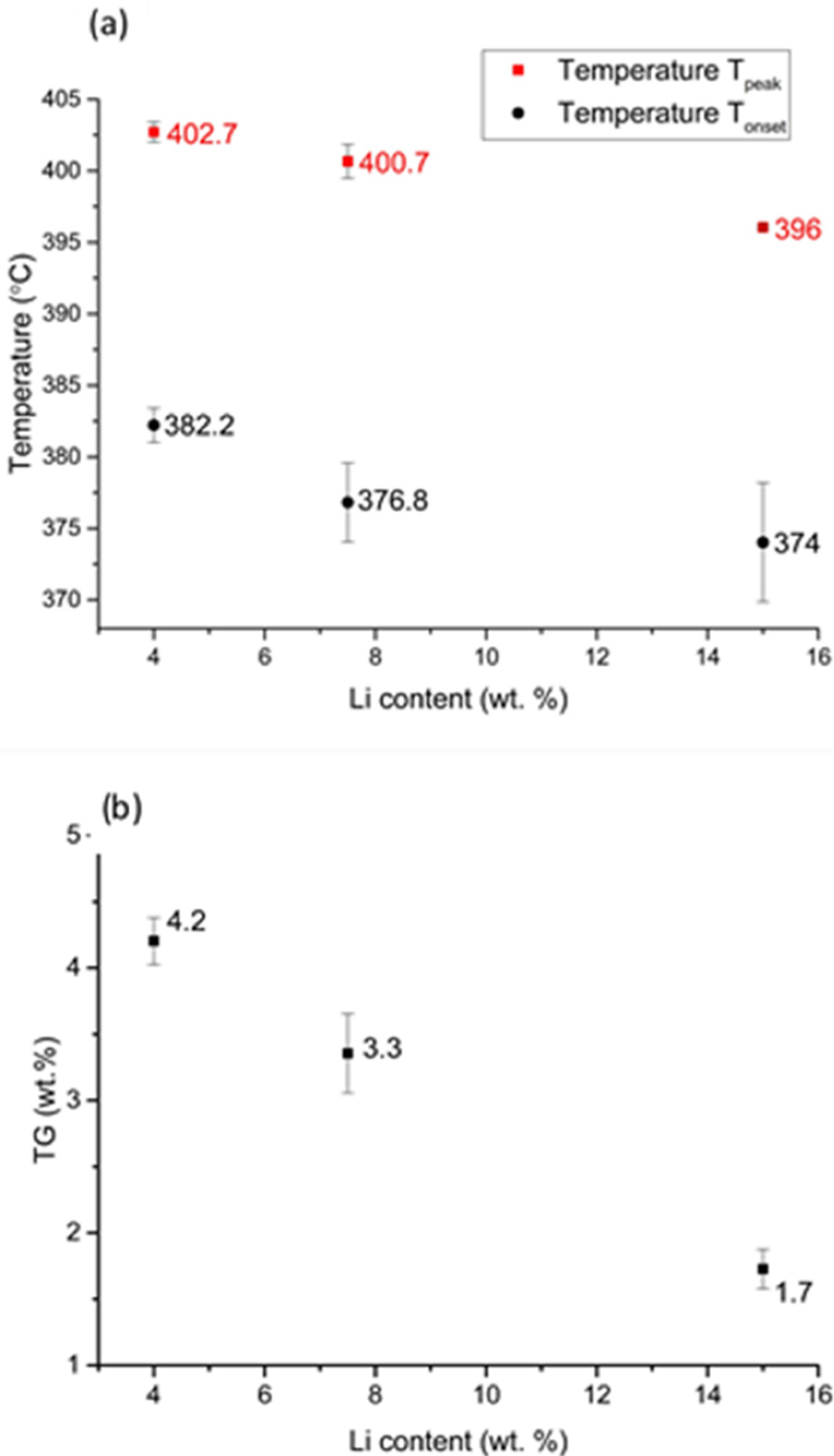
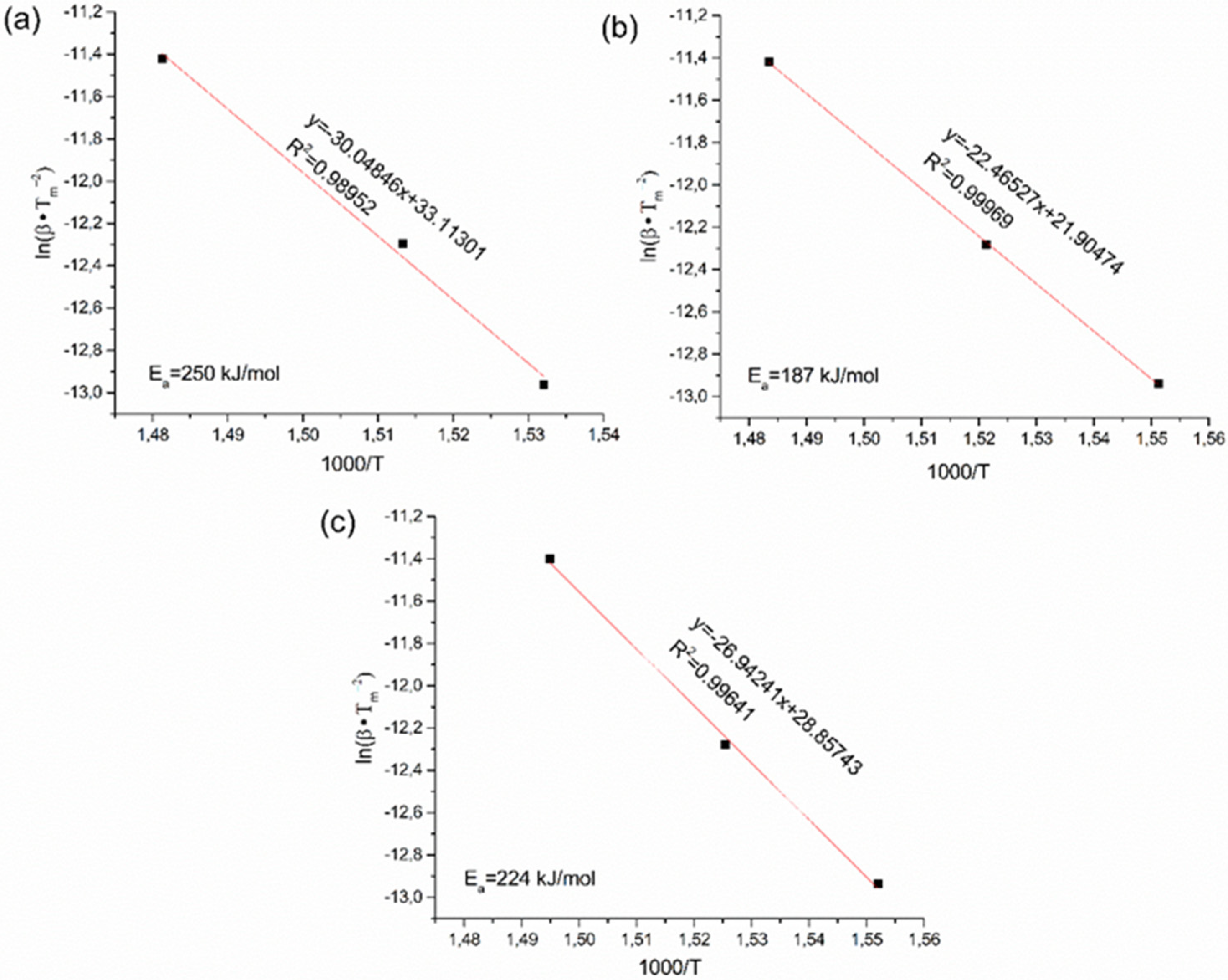
3.2. Phase Structure and Hydrogenation Properties of the Alloys with Different Lithium Contents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vajeeston, P.; Ravindran, P.; Fjellvåg, H. Predicting New Materials for Hydrogen Storage Application. Materials 2009, 2, 2296–2318. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, Y.; Ouyang, L.; Wang, H. Thermodynamic Tuning of Mg-Based Hydrogen Storage Alloys: A Review. Materials 2013, 6, 4654–4674. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Li, H.-W.; Yan, Y.; Orimo, S.-I.; Züttel, A.; Jensen, C.M. Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Møller, K.; Sheppard, D.; Ravnsbæk, D.; Buckley, C.; Akiba, E.; Li, H.-W.; Jensen, T. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Xueping, Z.; Jiaojiao, Z.; Shenglin, L.; Xuanhui, Q.; Ping, L.; Yanbei, G.; Weihua, L. A new solid material for hydrogen storage. Int. J. Hydrogen Energy 2015, 40, 10502–10507. [Google Scholar] [CrossRef]
- Hardy, B.; Tamburello, D.; Corgnal, C. Hydrogen storage adsorbent systems acceptability Envelope. Int. J. Hydrogen Energy 2018, 43, 19528–19539. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H.; Xu, L.; Li, S.; Wang, X.; Yan, M. Hydrogen storage properties of nano-CoB/CNTs catalyzed MgH2. J. Alloys Compd. 2018, 735, 642. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroglu, T.N. Current status of hydrogen energy. Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- Stavila, V.; Klebanoff, L. Metal Hydrides. In Fuel Cells: Data, Facts And Figures; Stolten, D., Samsun, R.C., Garland, N., Eds.; Wiley-VCH: Weinheim, Germany, 2016; Volume 16, pp. 149–161. [Google Scholar]
- Gosselin, C.; Huot, J. Hydrogenation Properties of TiFe Doped with Zirconium. Materials 2015, 8, 7864–7872. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, C.; Santos, D.; Huot, J. First hydrogenation enhancement in TiFe alloys for hydrogen storage. J. Phys. D Appl. Phys. 2017, 50. [Google Scholar] [CrossRef]
- Gosselin, C.; Huot, J. First Hydrogenation Enhancement in TiFe Alloys for Hydrogen Storage Doped with Yttrium. Metals 2019, 9, 242. [Google Scholar] [CrossRef]
- Manna, J.; Tougas, B.; Huot, J. First hydrogenation kinetics of Zr and Mn doped TiFe alloy after air exposure and reactivation by mechanical treatment. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Formation and properties of iron titanium hydride. Inorg. Chem. 1974, 13, 218–222. [Google Scholar] [CrossRef]
- Akiba, E.; Ibab, H. Hydrogen absorption by Laves phase related BCC solid solution. Intermetollics 1998, 6, 461–470. [Google Scholar] [CrossRef]
- Kumar, S.; Kojima, Y.; Kumar Dey, G. Thermodynamics and kinetics of hydrogen absorption–desorption of highly crystalline LaNi5. J. Therm. Anal. Calorim. 2018, 134, 889–894. [Google Scholar] [CrossRef]
- Pęska, M.; Dworecka-Wójcik, J.; Płociński, T.; Polański, M. The Influence of Cerium on the Hydrogen Storage Properties of La1-xCexNi5 Alloys. Energies 2020, 13, 1437. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, H.; Xiong, J.; Pan, S.; Luo, J. Microstructure, electromagnetic and microwave absorbing properties of plate-like LaCeNi powder. J. Mater. Sci. Mater. Electron. 2018, 29, 18030–18035. [Google Scholar] [CrossRef]
- An, X.H.; Gu, Q.F.; Zhang, J.Y.; Chen, S.L.; Yu, X.B.; Li, Q. Experimental investigation and thermodynamic reassessment of La–Ni and LaNi5–H systems. Calphad 2013, 40, 48–55. [Google Scholar] [CrossRef]
- Ngameni, R.; Mbemba, N.; Grigoriev, S.A.; Millet, P. Comparative analysis of the hydriding kinetics of LaNi5, La0.8Nd0.2Ni5 and La0.7Ce0.3Ni5 compounds. Int. J. Hydrogen Energy 2011, 36, 4178–4184. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, A.K.; Ramakrishna, K.; Srivastava, O.N. Investigations on the structural and hydrogenation characteristics of LaNi5, HoNi5, GdNi5, SmNi5, MmNi5, and CFMmNi4.5Al0.5 thin films. Int. J. Hydrogen Energy 1985, 10, 523–529. [Google Scholar] [CrossRef]
- Bouhadda, Y.; Djellab, S.; Bououdina, M.; Fenineche, N.; Boudouma, Y. Structural and elastic properties of LiBH4 for hydrogen storage applications. J. Alloys Compd. 2012, 534, 20–24. [Google Scholar] [CrossRef]
- Xu, J.; Meng, R.; Cao, J.; Gu, X.; Song, W.-L.; Wang, W.; Chen, Z. Graphene-supported Pd catalysts for reversible hydrogen storage in LiBH4. J. Alloys Compd. 2013, 564, 84–90. [Google Scholar] [CrossRef]
- Suarez-Alcantara, K.; Tena-Garcia, J.R.; Guerrero-Ortiz, R. Alanates, a Comprehensive Review. Materials 2019, 12, 2724. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Ravnsbæk, D.B.; Grundlach, C.; Besenbacher, F.; Skibstede, J.; Jensen, T.R. A novel intermediate in the LiAlH4–LiNH2 hydrogen storage system. Dalton Trans 2014, 43, 3095–3103. [Google Scholar] [CrossRef] [PubMed]
- Cabo, M.S.; Garroni, S.; Pellicer, E.; Milanese, C.; Girella, A.; Marini, A.; Rossinyol, E.; Suriñach, S.; Baro, M.D. Hydrogen sorption performance of MgH2 doped with mesoporous nickel- and cobalt-based oxides. Int. J. Hydrogen Energy 2011, 36, 5400–5410. [Google Scholar] [CrossRef]
- Charbonnier, J.; de Rangoa, P.; Fruchart, D.; Miragliaa, S.; Pontonnier, L.; Rivoirard, S.; Skryabina, N.; Vulliet, P. Hydrogenation of transition element additives (Ti, V) during ball milling of magnesium hydride. J. Alloys Compd. 2004, 383, 205–208. [Google Scholar] [CrossRef]
- Li, Y.; Hu, F.; Luo, L.; Xu, J.; Zhao, Z.; Zhang, Y.; Zhao, D. Hydrogen storage of casting MgTiNi alloys. Catal. Today 2018, 318, 103–106. [Google Scholar] [CrossRef]
- Witek, K.; Karczewski, K.; Karpowicz, M.; Polanski, M. Mg2FeH6 Synthesis Efficiency Map. Crystals 2018, 8, 94. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Thermal stability of Vale Inco nanonometric nickel as a catalytic additive for magnesium hydride (MgH2). Int. J. Hydrogen Energy 2009, 34, 8603–8610. [Google Scholar] [CrossRef]
- Okamoto, H.; Schlesinger, M.E. Li (Lithium) Binary Alloy Phase Diagrams. In ASM Handbook Volume 3: Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1992. [Google Scholar]
- Atkins, G.; Marya, M.; Olson, D.; Eliezer, D. Magnesium-Lithium Alloy Weldability: A Microstructural Characterization. Proc. TMS Miner. Metals Mater. Soc. 2004, 1, 37–41. [Google Scholar]
- Huajian, W.; Zhenzhen, S.; Jiaqi, D.; Hua, N.; Guangxu, L.; Wenlou, W.; Zhiqiang, L.; Jin, G. Catalytic effect of graphene on the hydrogen storage properties of Mg-Li alloy. Mater. Chem. Phys. 2018, 207, 221. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Zhou, W.; Xu, L.; Guo, J.; Lan, Z. Effects of in-situ formed Mg2Si phase on the hydrogen storage properties of Mg-Li solid solution alloys. Mater. Des. 2016, 111, 248–252. [Google Scholar] [CrossRef]
- Ji, H.; Wu, G.; Liu, W.; Liang, X.; Liao, G.; Ding, D. Microstructure characterization and mechanical properties of the as-cast and as-extruded Mg-xLi-5Zn-0.5Er (x = 8, 10 and 12 wt%) alloys. Mater. Charact. 2020, 159, 110008. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Mizera, J. The effect of MgNi2 intermetallic compound on nanostructurization and amorphization of Mg-Ni alloys processed by controlled mechanical milling. J. Alloys Compd. 2003, 354, 281–295. [Google Scholar] [CrossRef]
- Polanski, M.; Nawra, D.; Zasada, D. Mg2FeH6 synthesized from plain steel and magnesium hydride. J. Alloys Compd. 2019, 776, 1029–1040. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Y.; Yang, S. De-alloying Behavior of Mg–Al alloy in Sulphuric Acid and Acetic Acid Aqueous Solutions. Materials 2019, 12, 2046. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef] [PubMed]
- Varin, R.A.; Czujko, T.; Chiu, C.; Wronski, Z. Particle size effects on the desorption properties of nanostructured magnesium dihydride (MgH2) synthesized by controlled reactive mechanical milling (CRMM). J. Alloys Compd. 2006, 424, 356–364. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Chiu, C.; Pulz, R.; Wronski, Z.S. Synthesis of nanocomposite hydrides for solid-state hydrogen storage by controlled mechanical milling techniques. J. Alloys Compd. 2009, 483, 252–255. [Google Scholar] [CrossRef]
- Wang, L.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Direct and reversible hydrogen storage of lithium hydride (LiH) nanoconfined in high surface area graphite. Int. J. Hydrogen Energy 2016, 41, 8088–18094. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Hydrogen Storage Characteristics of Commercial Mg and MgH2. In Nanomaterials for Solid State Hydrogen Storage; Springer Science: New York, NY, USA, 2009; pp. 87–102. [Google Scholar]
- Varin, R.A.; Jang, M.; Czujko, T.; Wronski, Z.S. The effect of ball milling under hydrogen and argon on the desorption properties of MgH2 covered with a layer of Mg(OH)2. J. Alloys Compd. 2010, 493, L29–L32. [Google Scholar] [CrossRef]



| Sample | Average Particle Diameter from Volume-Weighted Size Distribution Dv (µm) | Median Diameter Dmed (µm) |
|---|---|---|
| Mg 4 wt.% Li | 23.5 | 71.1 |
| Mg 7.5 wt.% Li | 37.0 | 86.3 |
| Mg 15 wt.% Li | 40.4 | 74.3 |
| Sample | Theoretical Hydrogen Capacity in MgH2 (wt.%) | Measured Hydrogen Capacity in MgH2 (wt.%) | Yield of Mg Hydrogenation Reaction (%) |
|---|---|---|---|
| Mg 4.0 wt.% Li | 7.3 | 4.2 | 57.5 |
| Mg 7.5 wt.% Li | 7.0 | 3.3 | 47.1 |
| Mg 15.0 wt.% Li | 6.5 | 1.7 | 26.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pęska, M.; Czujko, T.; Polański, M. Hydrogenation Ability of Mg-Li Alloys. Energies 2020, 13, 2080. https://doi.org/10.3390/en13082080
Pęska M, Czujko T, Polański M. Hydrogenation Ability of Mg-Li Alloys. Energies. 2020; 13(8):2080. https://doi.org/10.3390/en13082080
Chicago/Turabian StylePęska, Magda, Tomasz Czujko, and Marek Polański. 2020. "Hydrogenation Ability of Mg-Li Alloys" Energies 13, no. 8: 2080. https://doi.org/10.3390/en13082080
APA StylePęska, M., Czujko, T., & Polański, M. (2020). Hydrogenation Ability of Mg-Li Alloys. Energies, 13(8), 2080. https://doi.org/10.3390/en13082080








