New Knowledge on the Performance of Supercritical Brayton Cycle with CO2-Based Mixtures
Abstract
1. Introduction
1.1. Background
1.2. Cycle Layouts and Performance Comparison
1.3. Current Status of CO2-Based Mixtures
- (1)
- For the studies of Jeong et al. [19,20] and Hu et al. [21], the inlet temperature of main compressor is always assumed to be 1 K above the critical temperature of corresponding mixture, namely Tc + 1. However, due to the fact that Tc is different for various mixtures, this assumption will cause the performance comparison of mixtures under different compressor inlet temperatures.
- (2)
- For the work of Vesely et al. [24,25] and Guo et al. [23], the range of employed gas fraction is too less. Although Baik and Lee [22] considered the whole fraction range 0–1, most of the employed additives belong to organic fluids, which may decompose in the high temperature. Furthermore, Baik and Lee [22] only analyzed the performance of simple Brayton cycle.
- (3)
- Cycle efficiency is mainly employed to evaluate the cycle performance of different CO2-based mixtures. Thus, more detailed performance comparisons are required.
1.4. Contribution of the Study
2. Cycle Layout and CO2-Based Mixtures
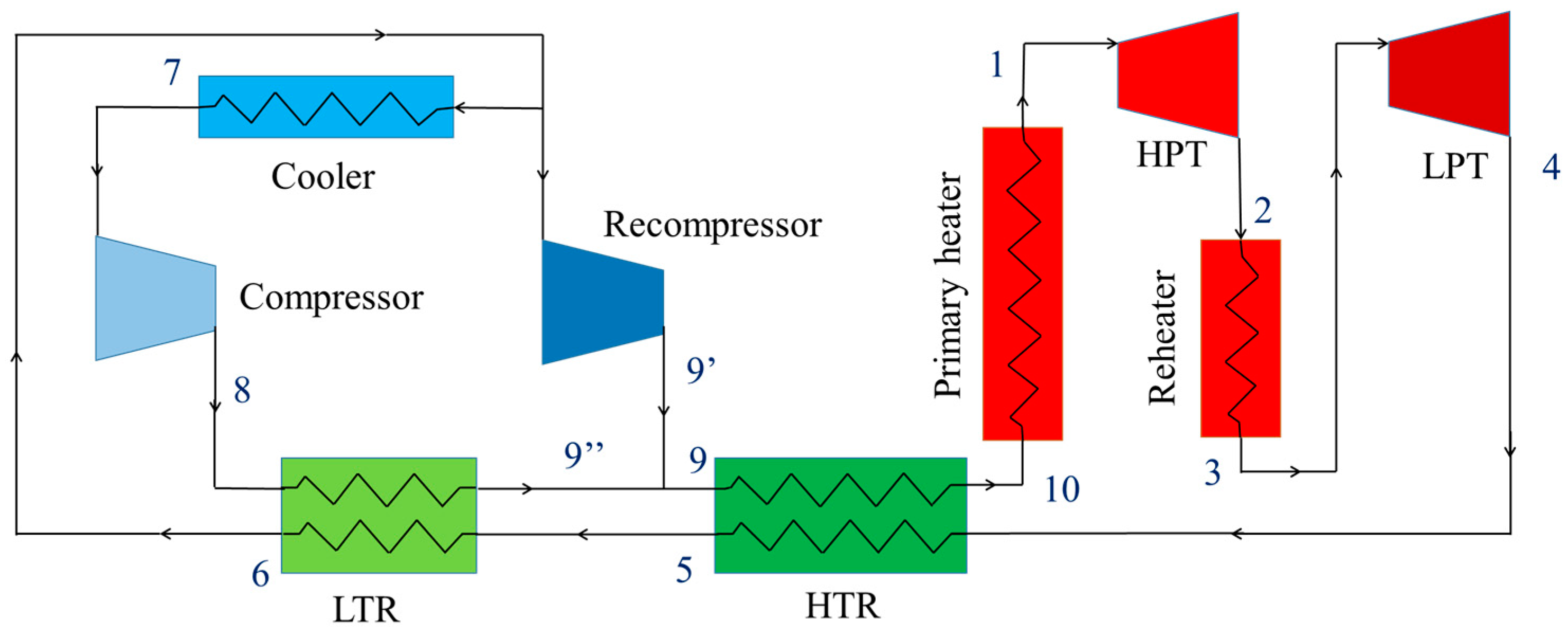
2.1. Cycle Layout
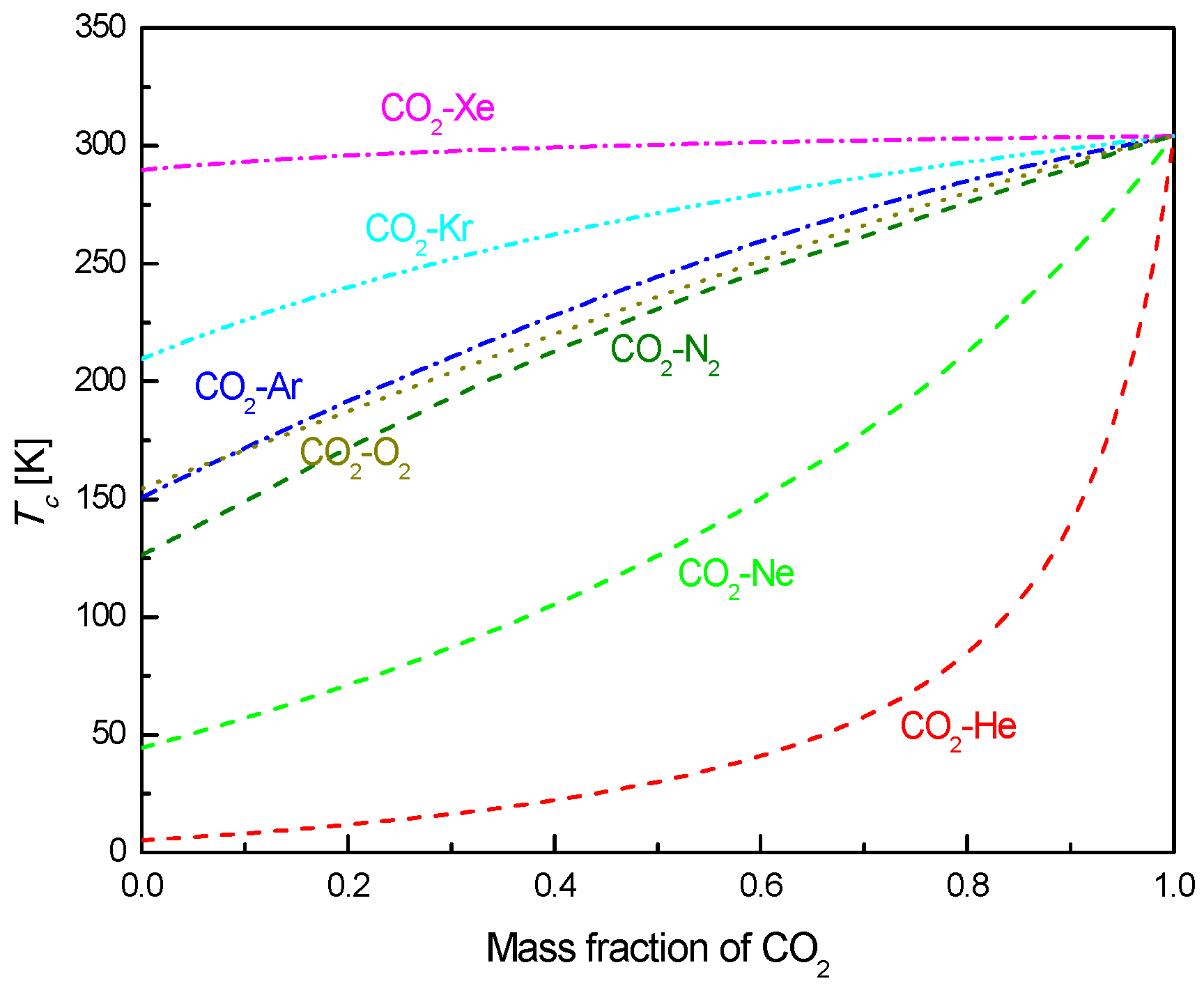
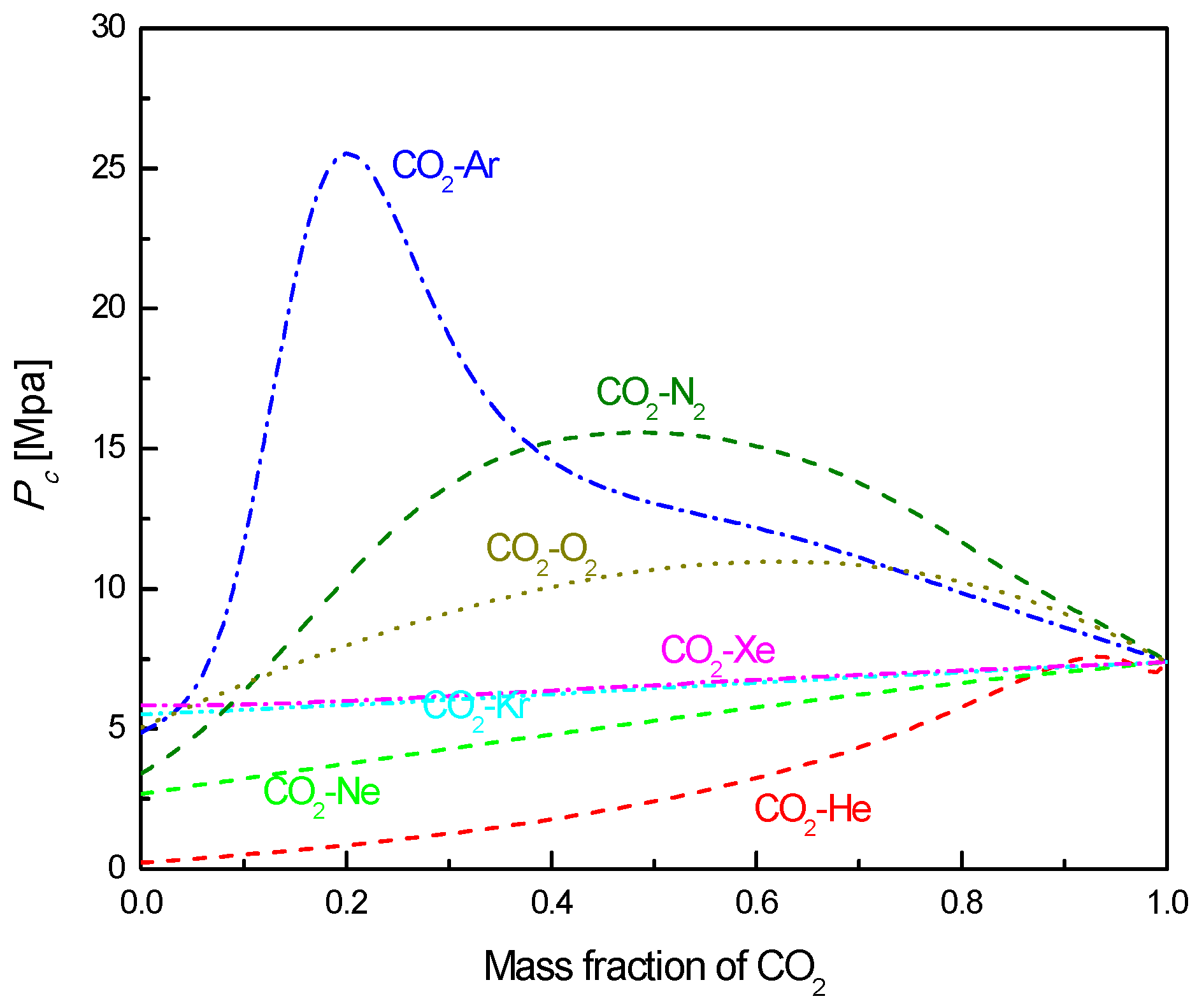
2.2. CO2-Based Mixtures
3. Thermodynamic Model and Validation
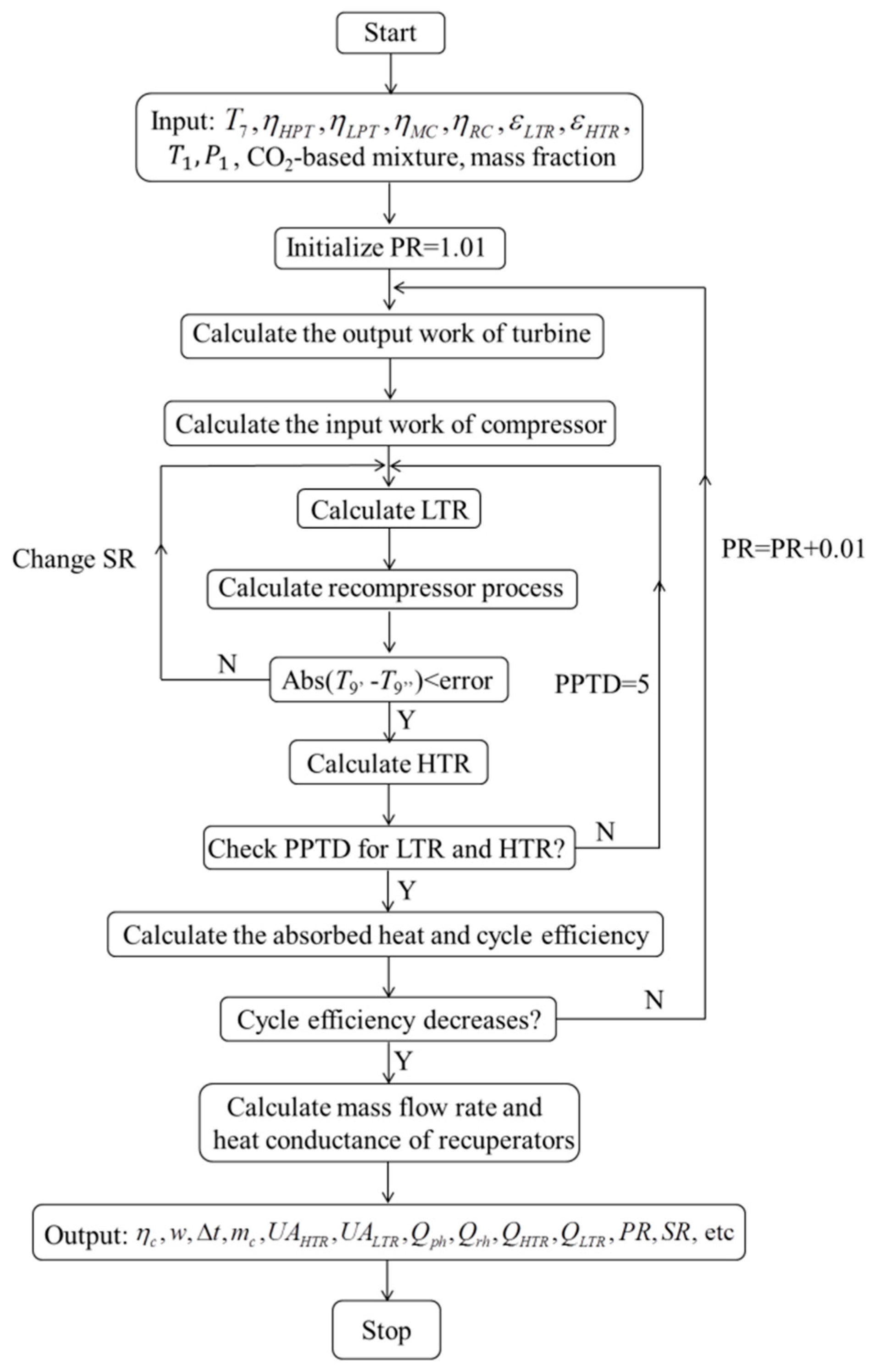
3.1. Model Establishment
- The recompression cycle is reached in steady state operating condition.
- Pressure drops and heat losses in the pipes and heat exchangers are ignored.
- Recuperators are considered as counter-flow heat exchangers.
3.2. Model Validation
4. Results and Discussions
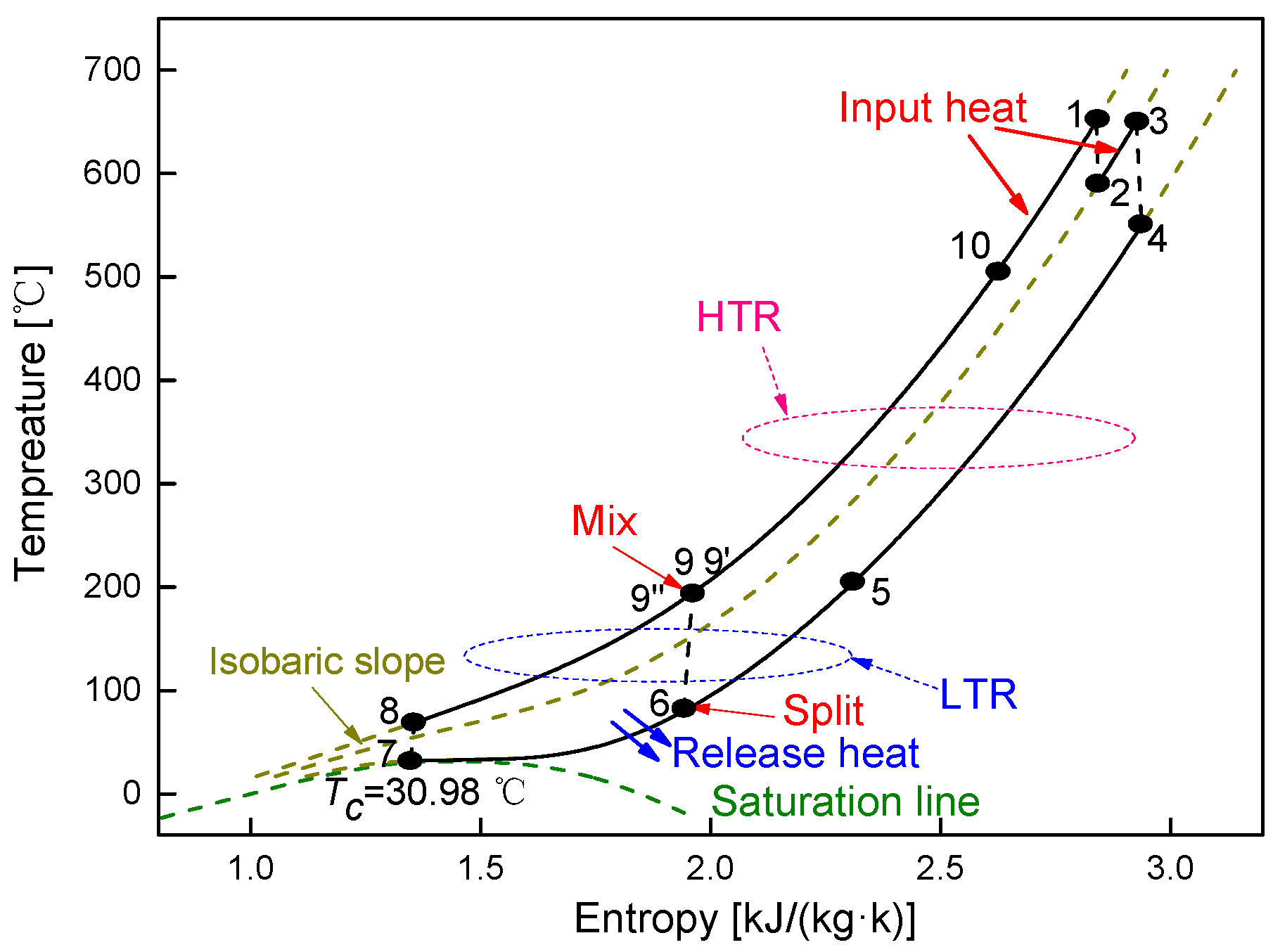
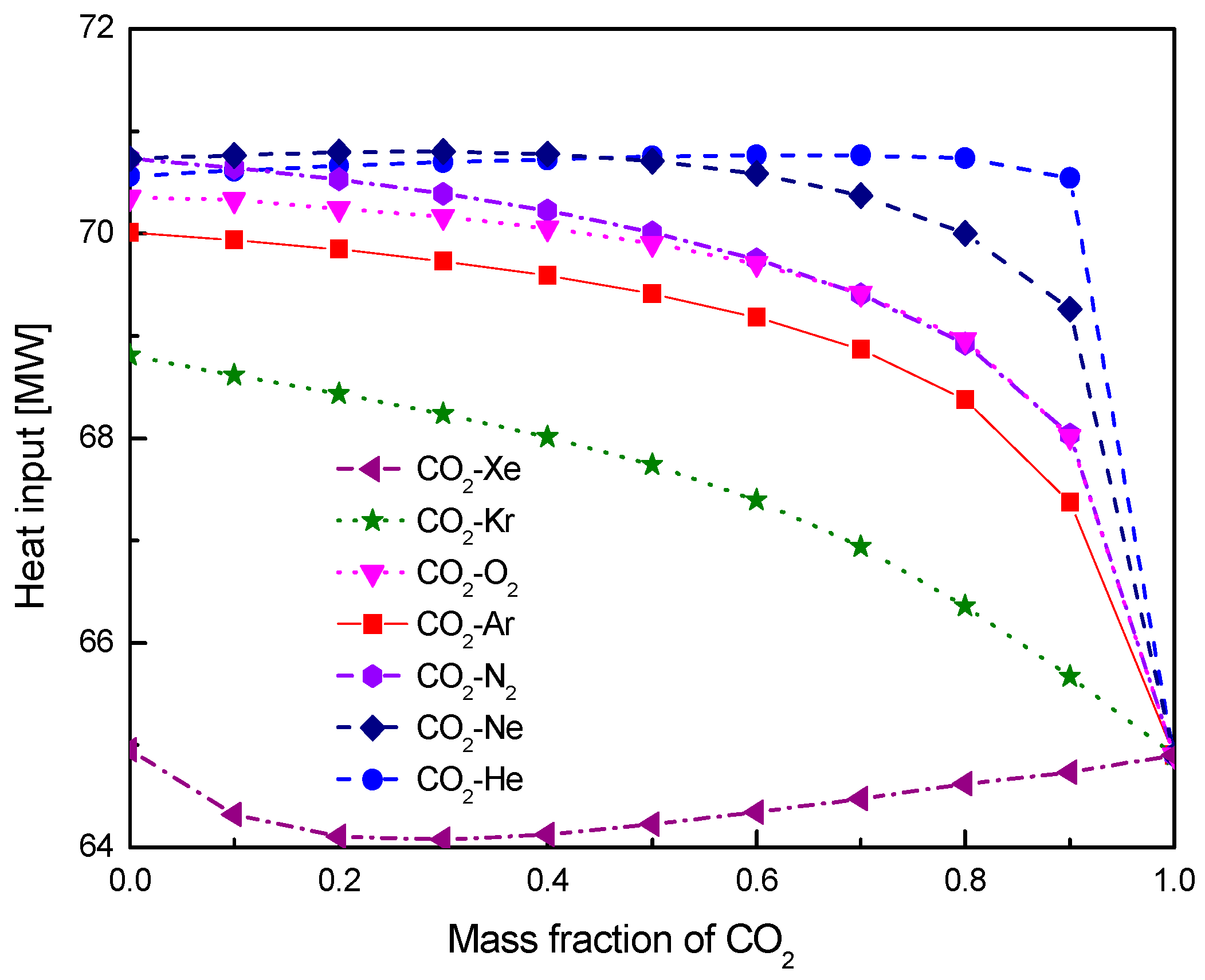
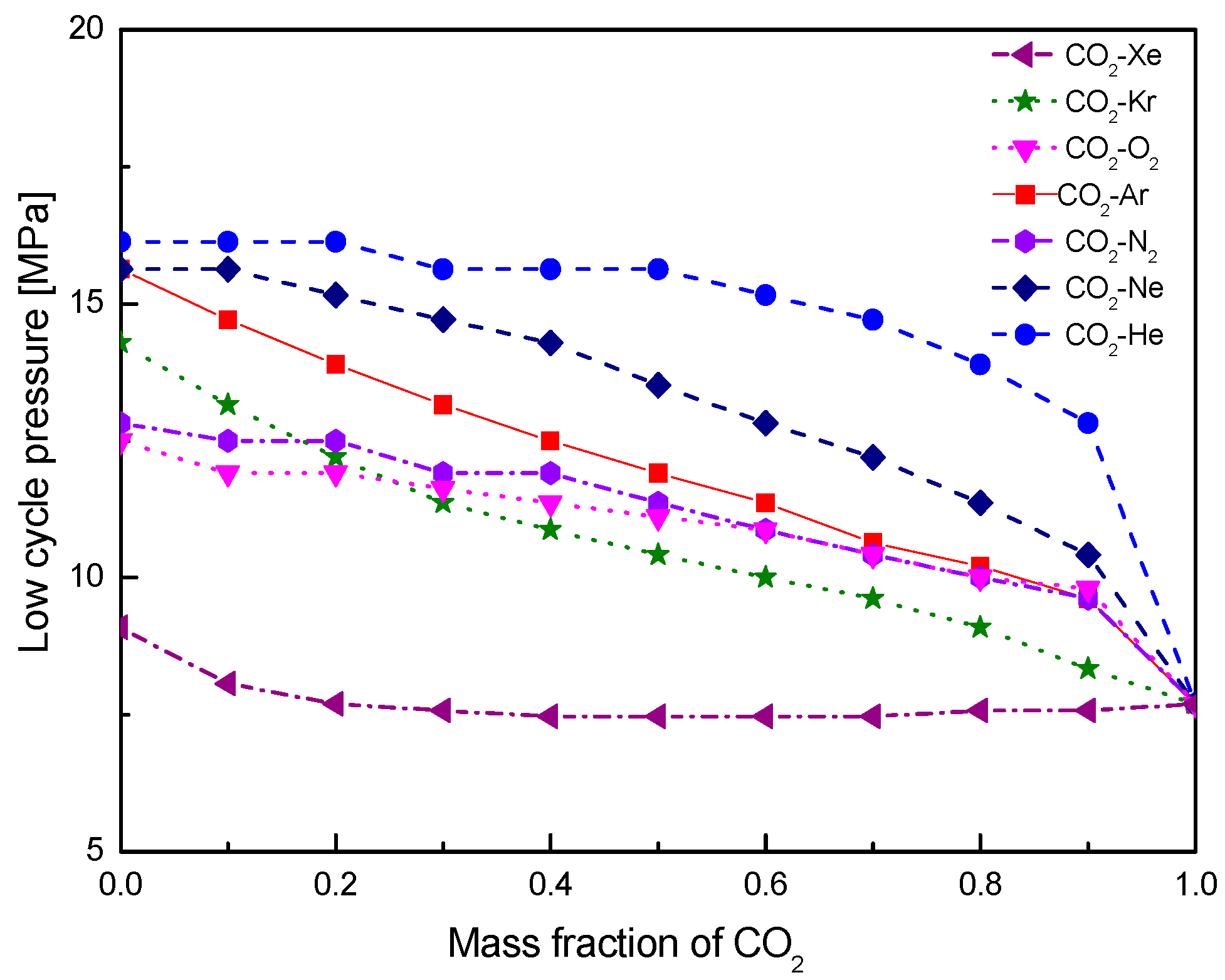
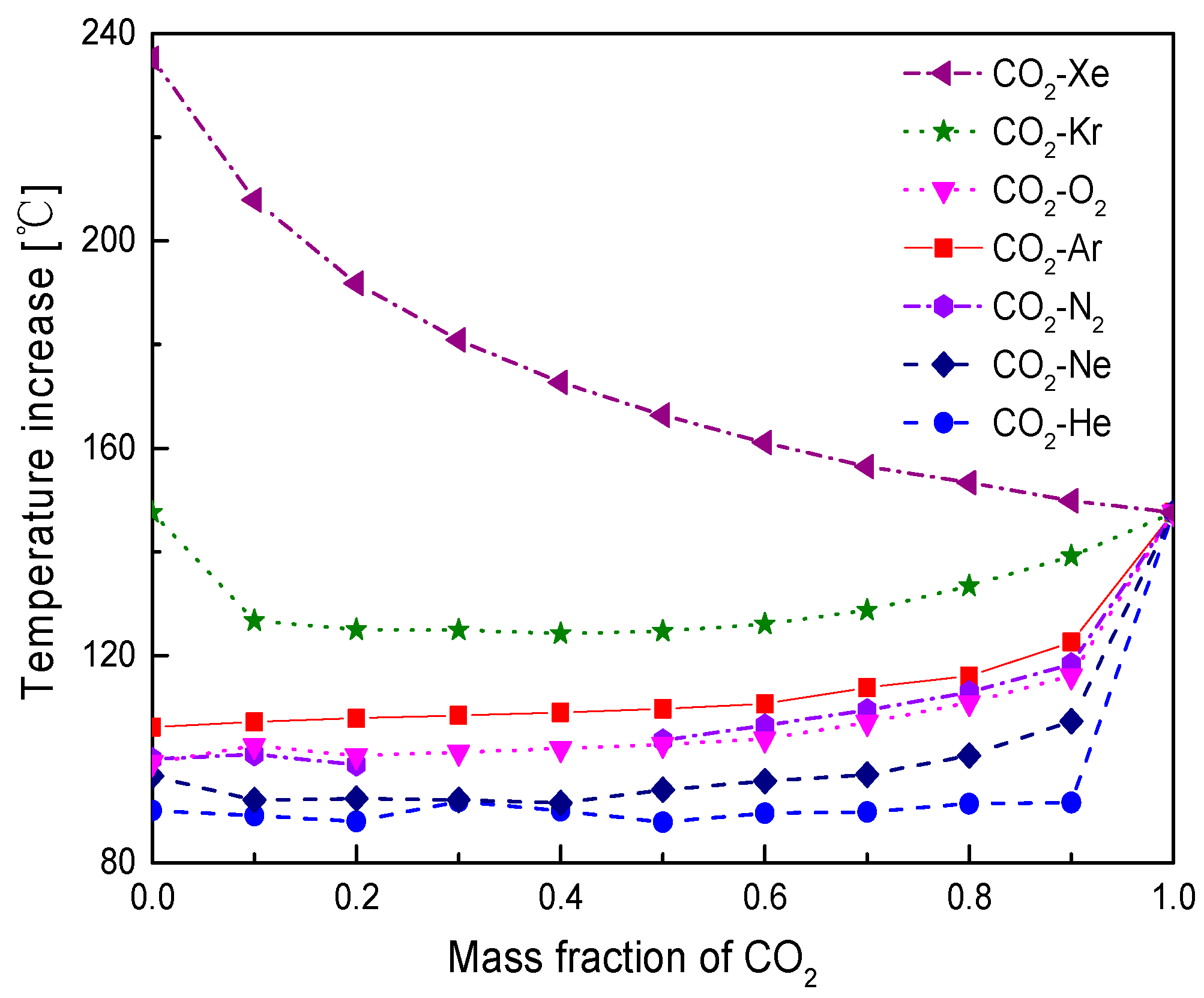
4.1. Cycle Performance Analysis
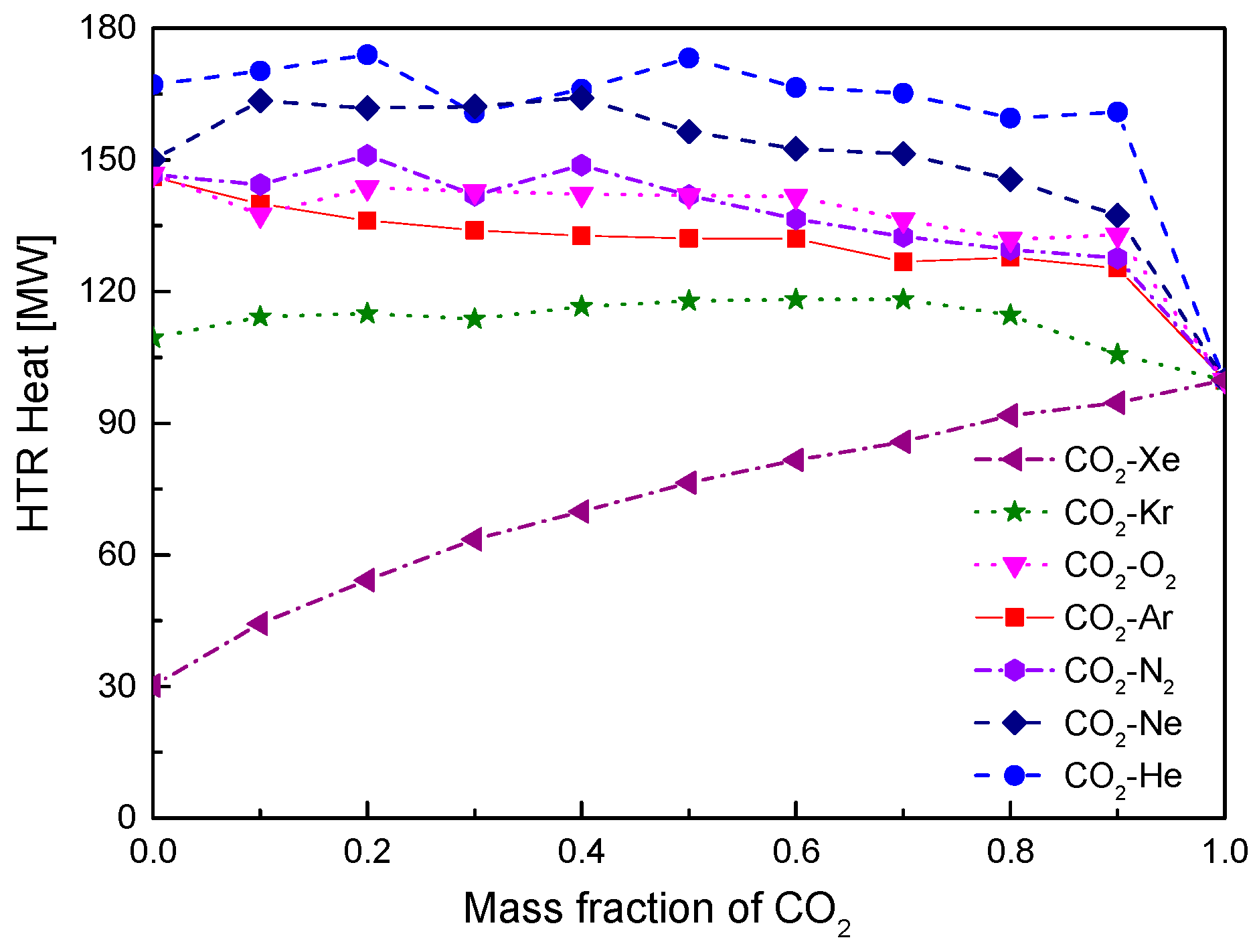
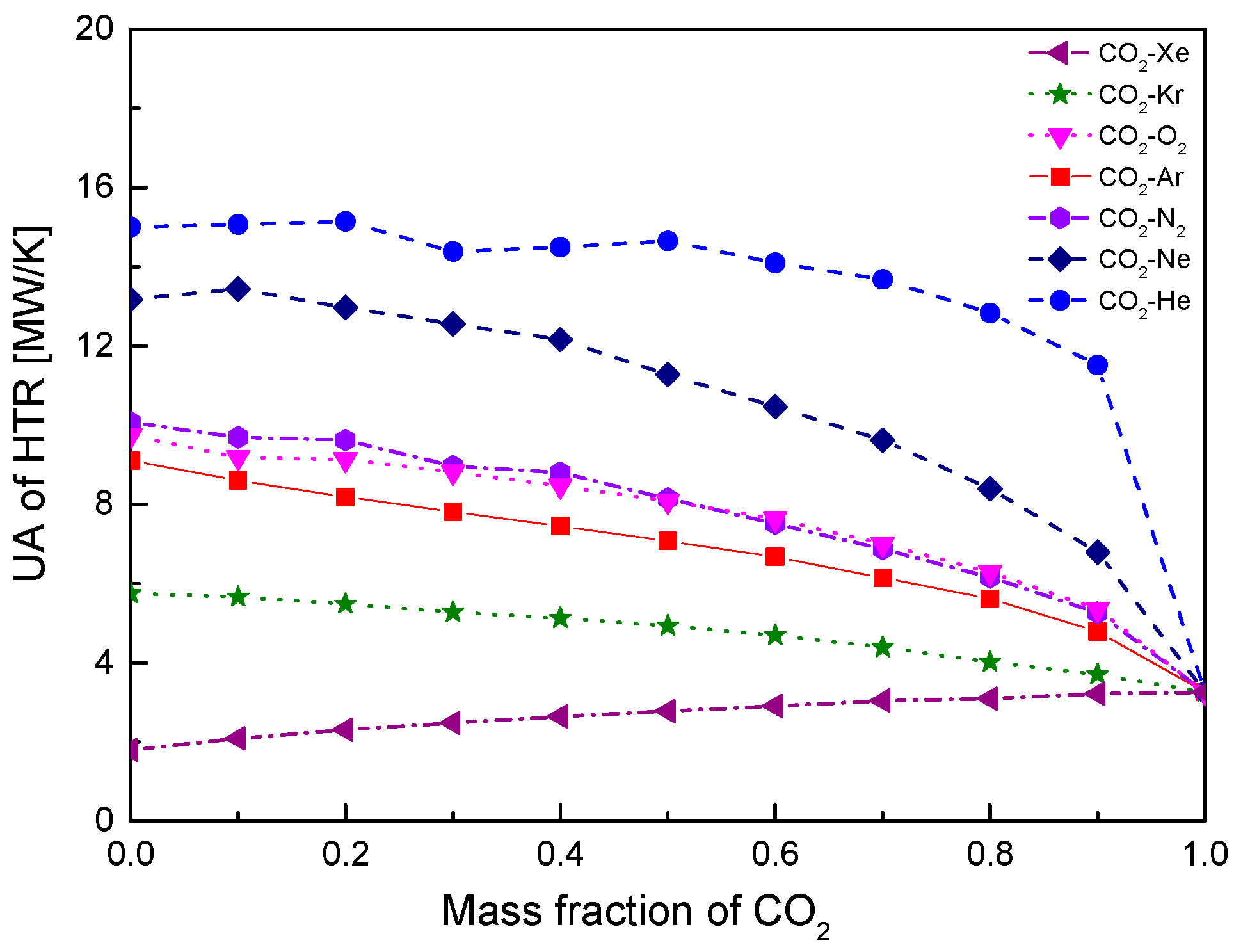
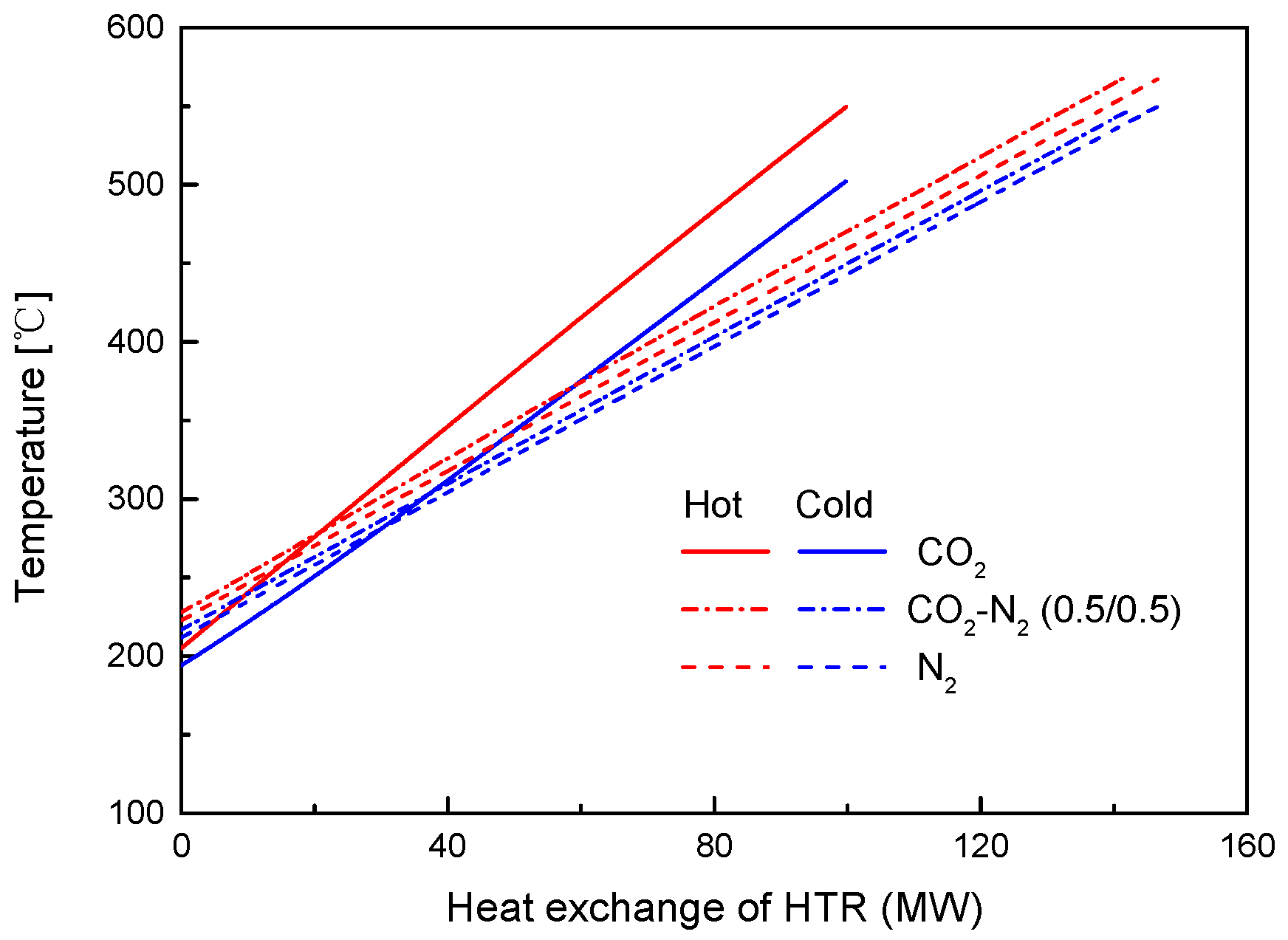
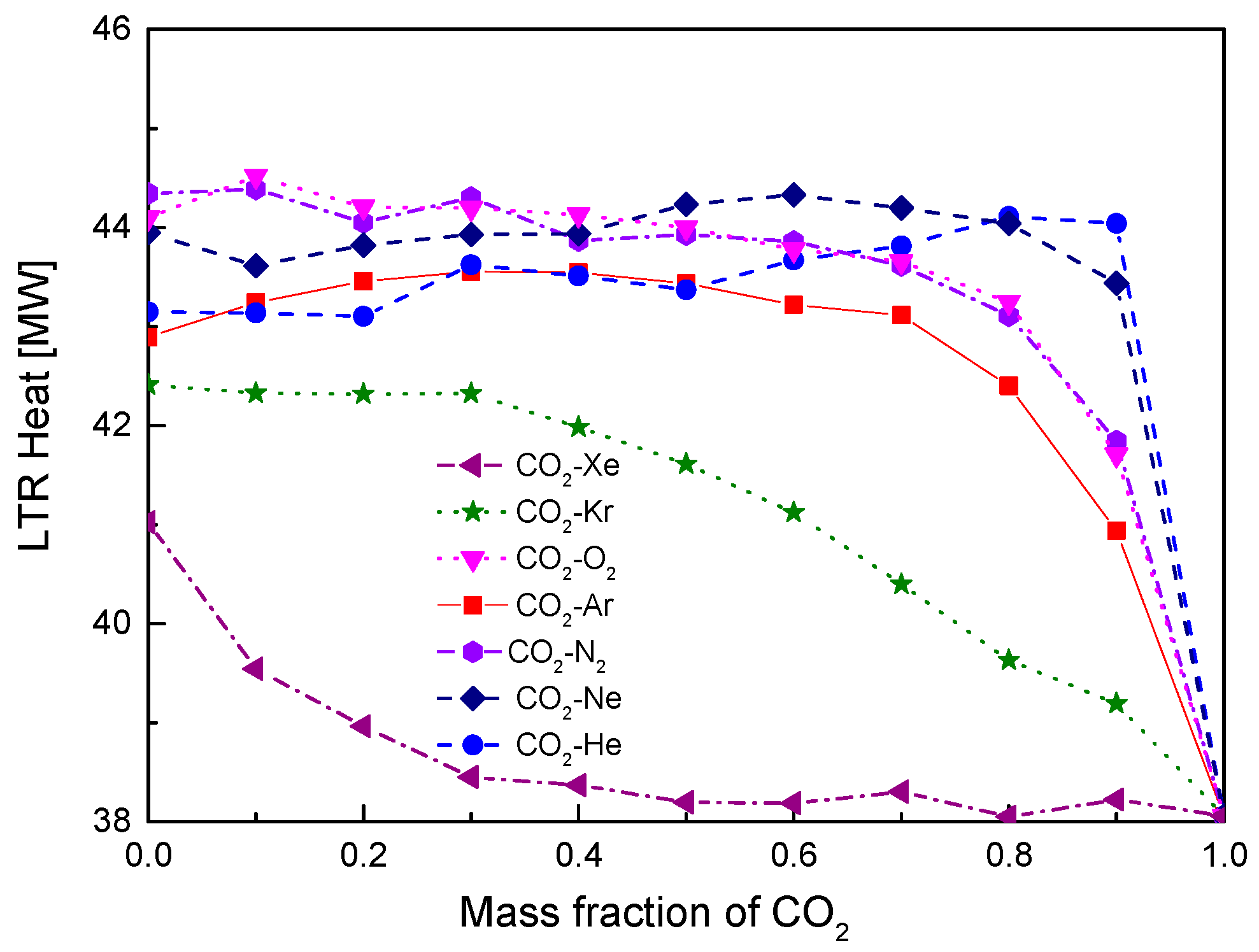
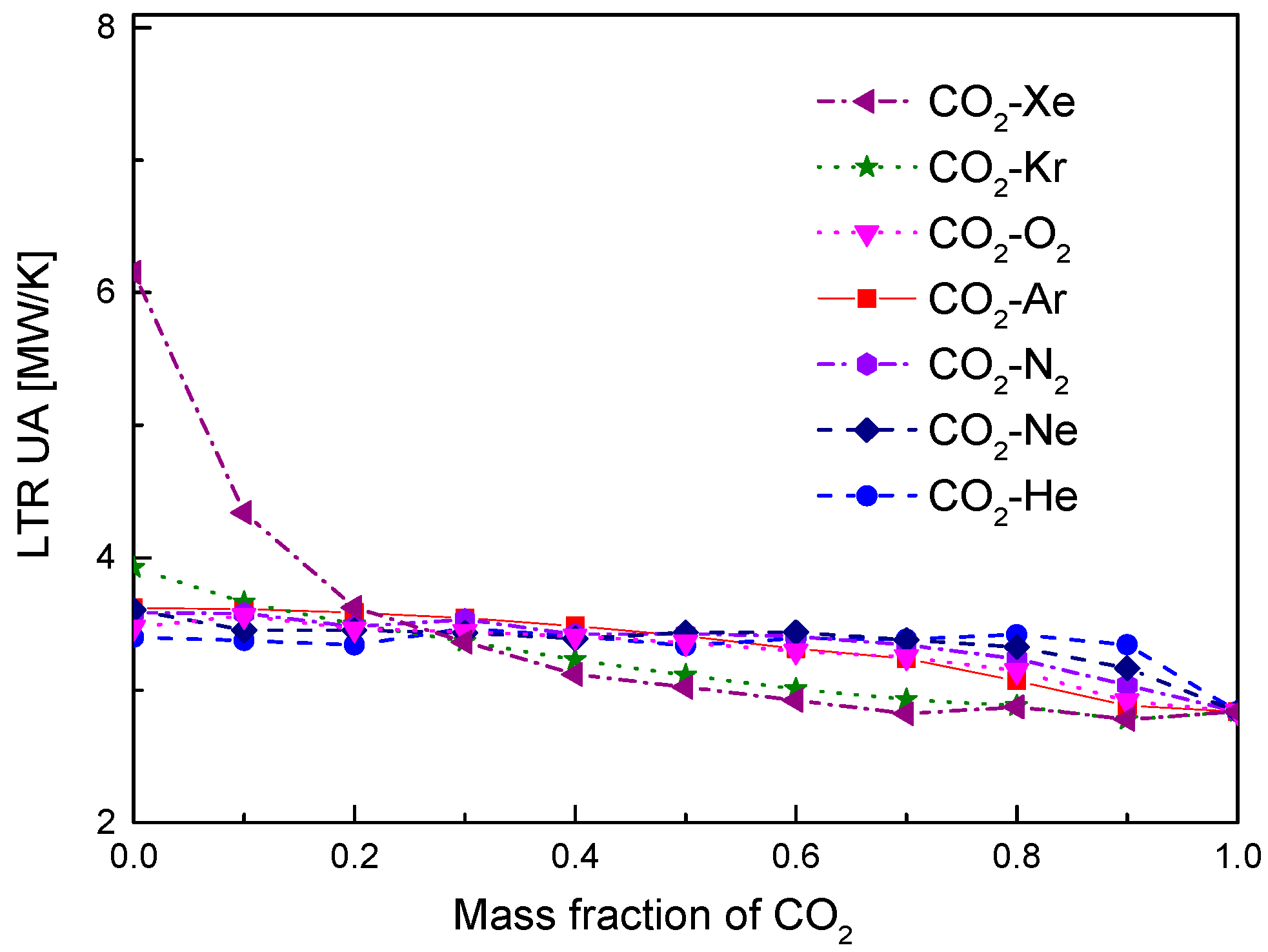
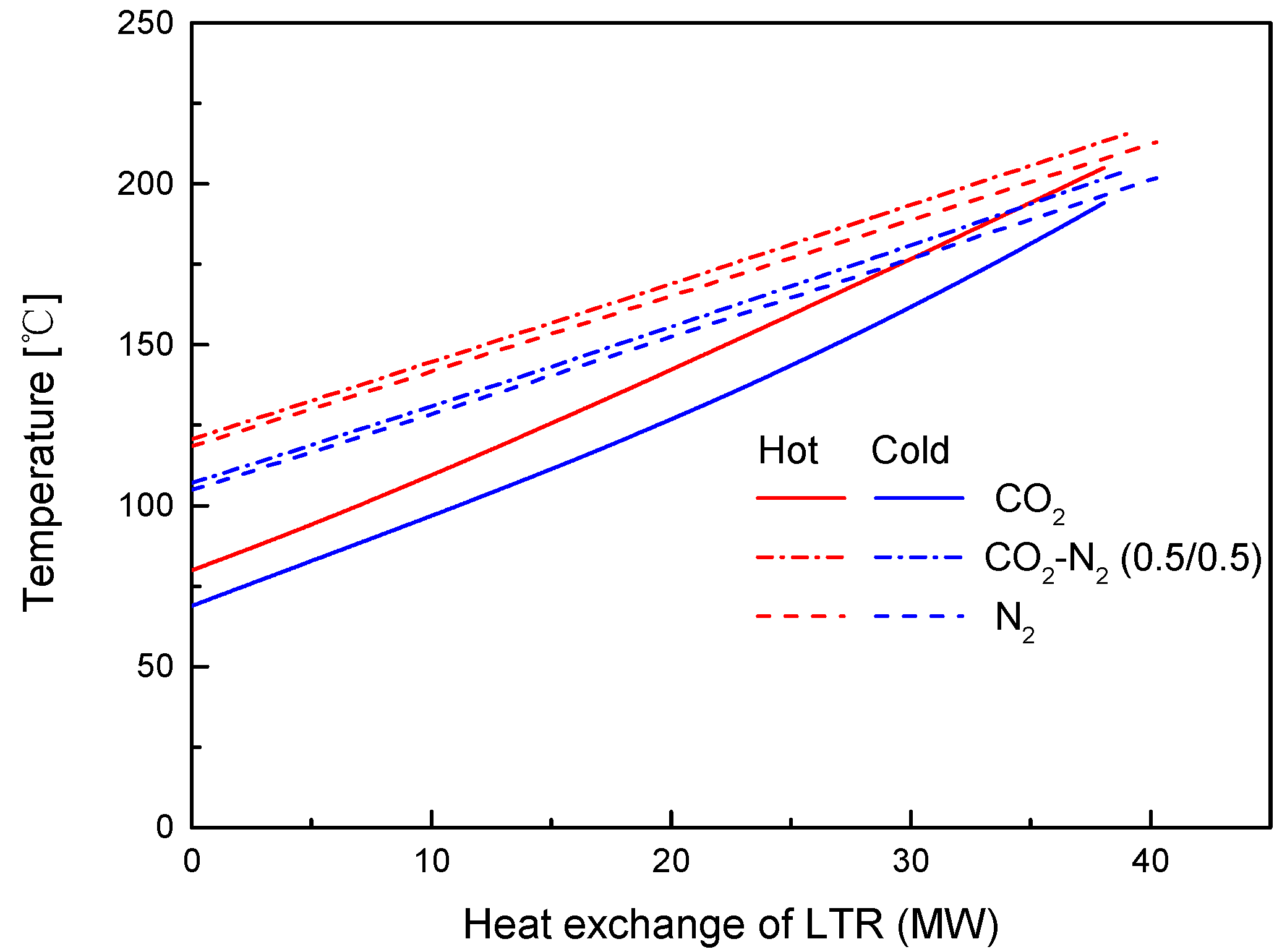
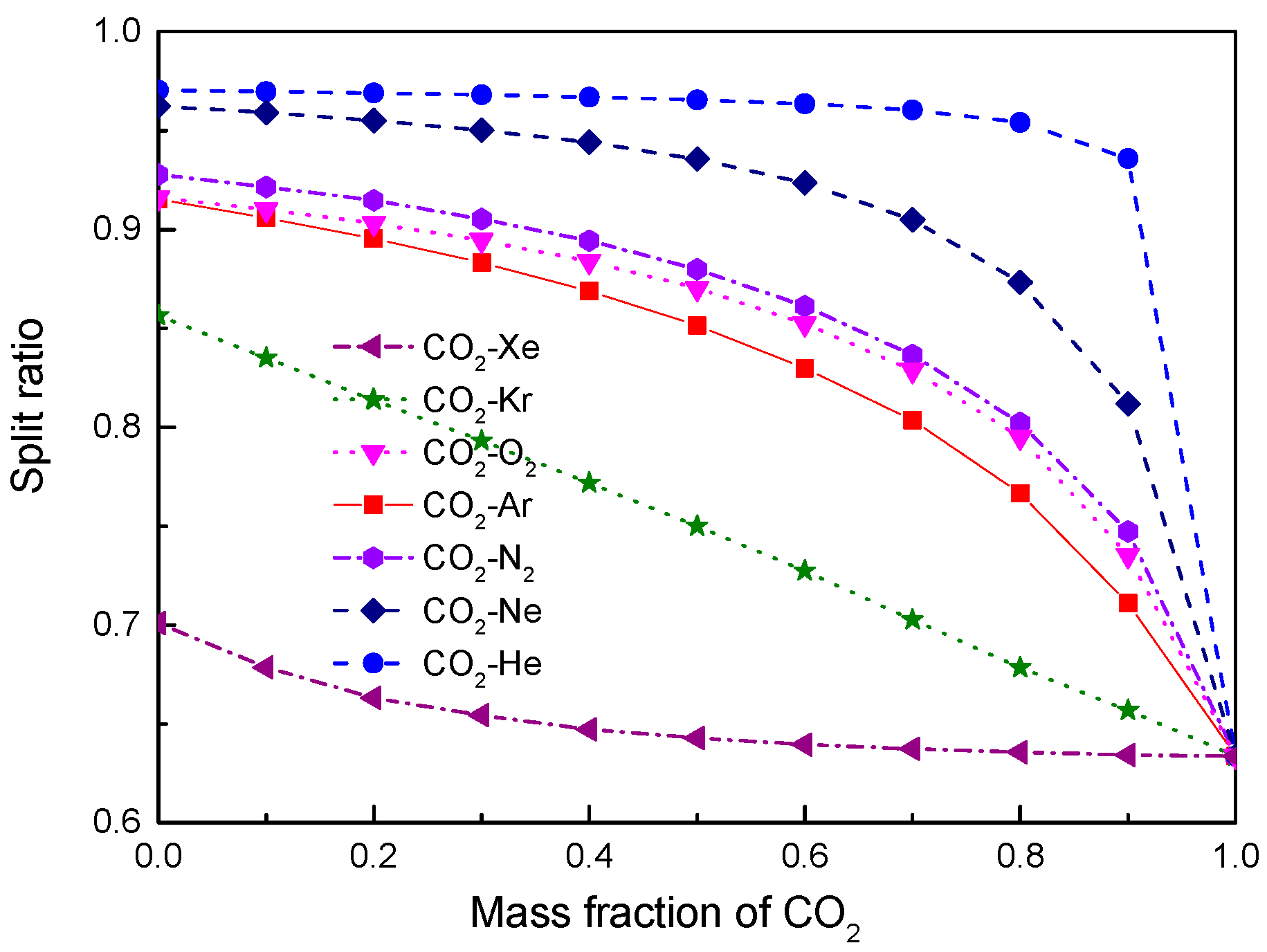
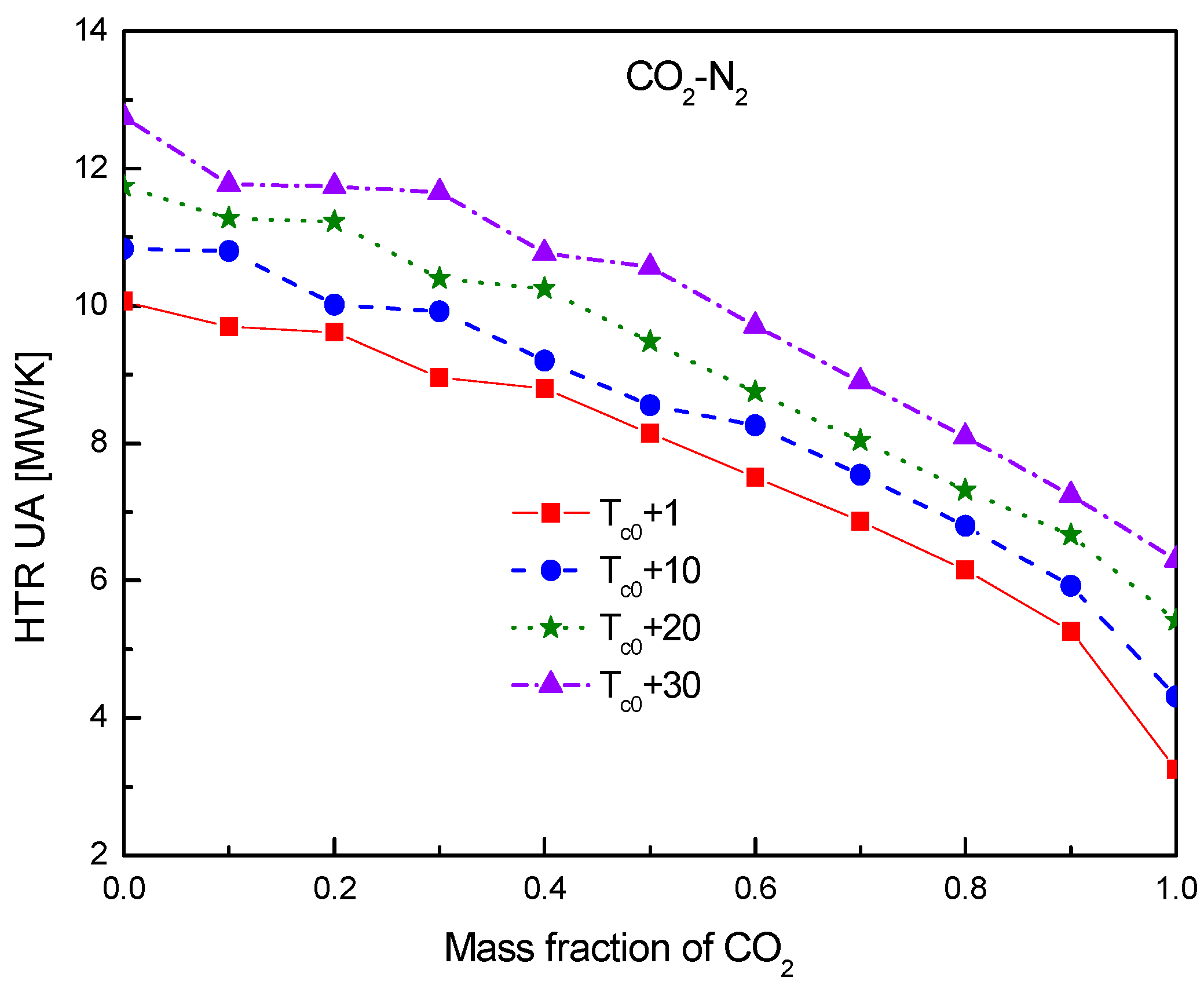
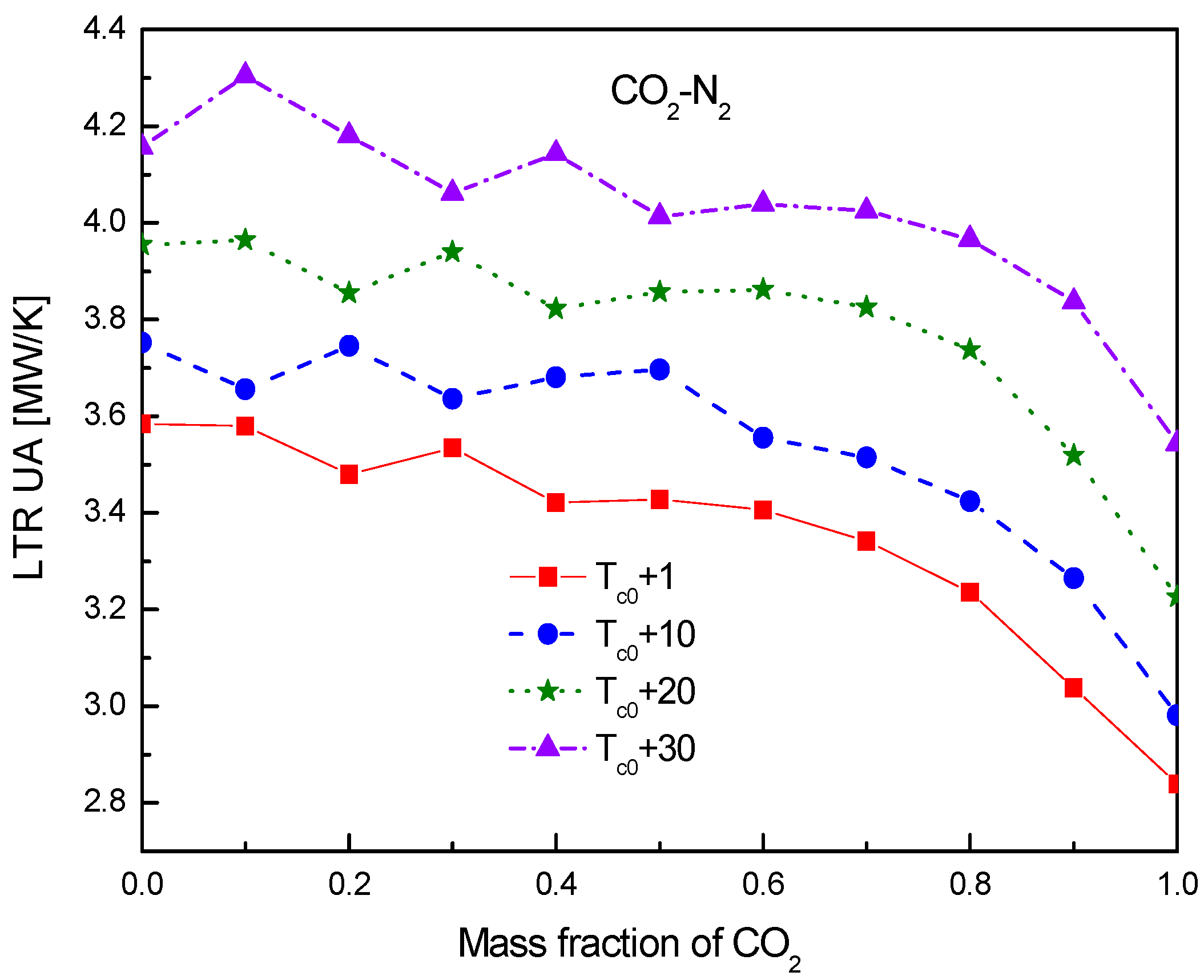
4.2. Recuperator Analysis
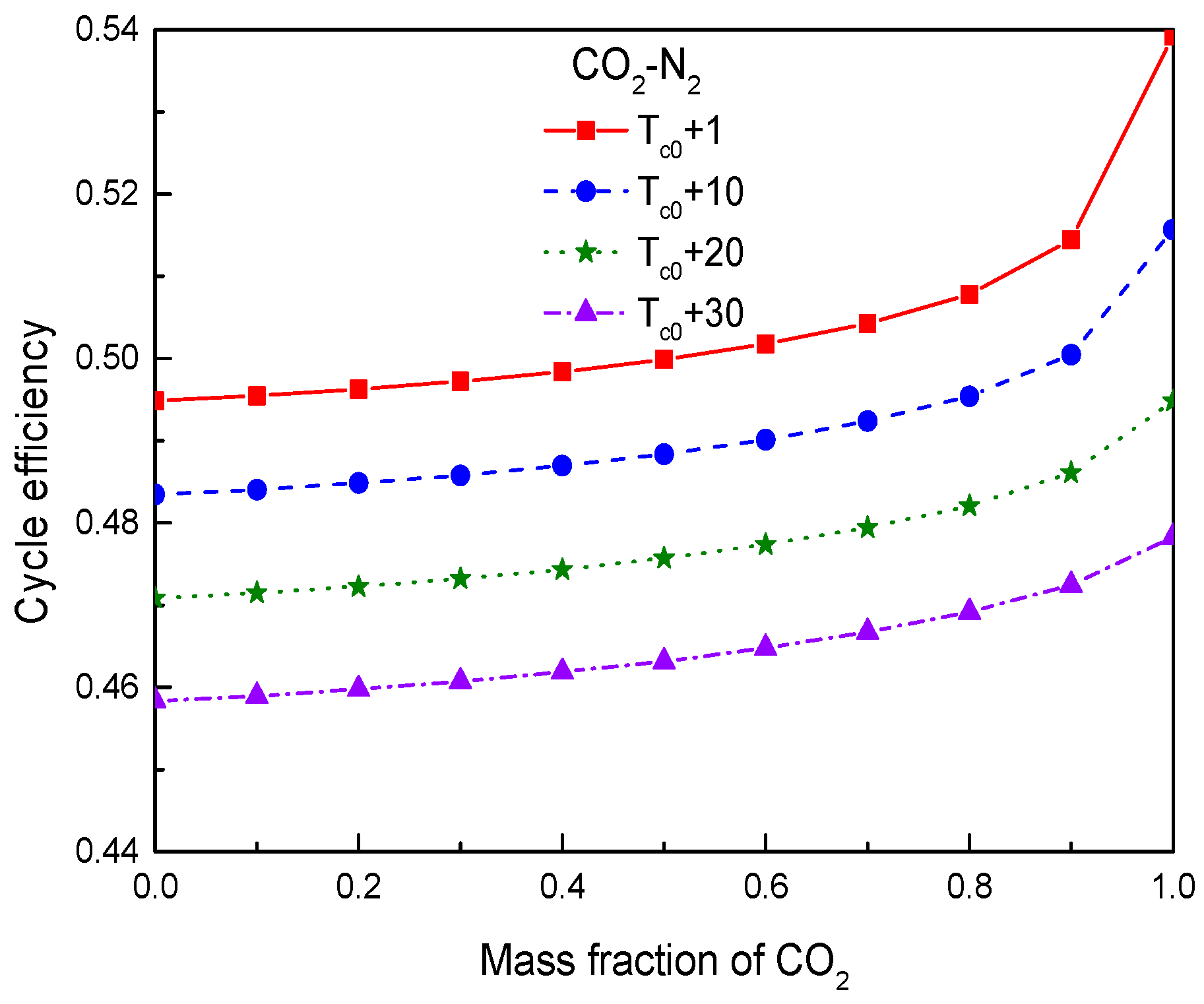
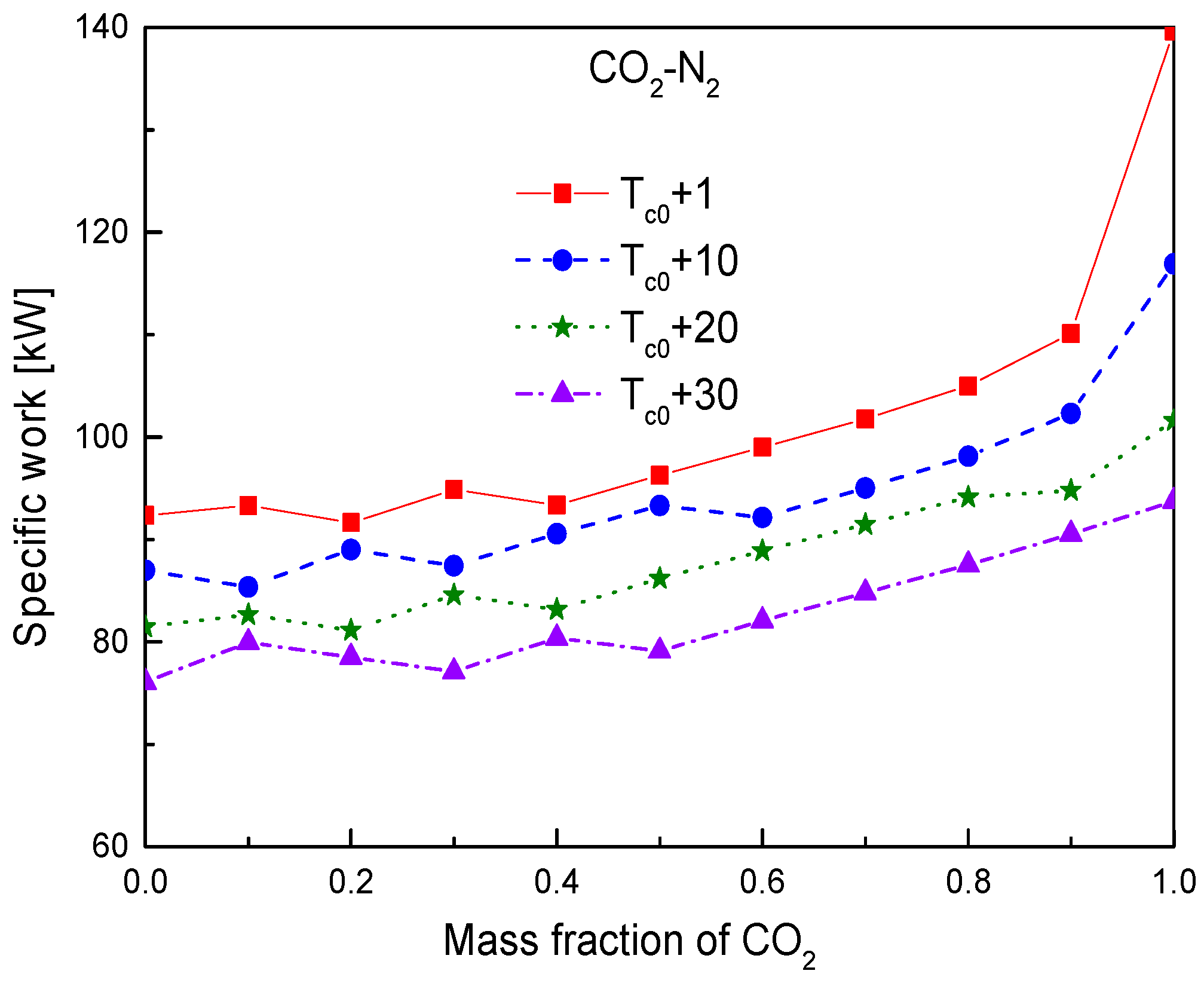
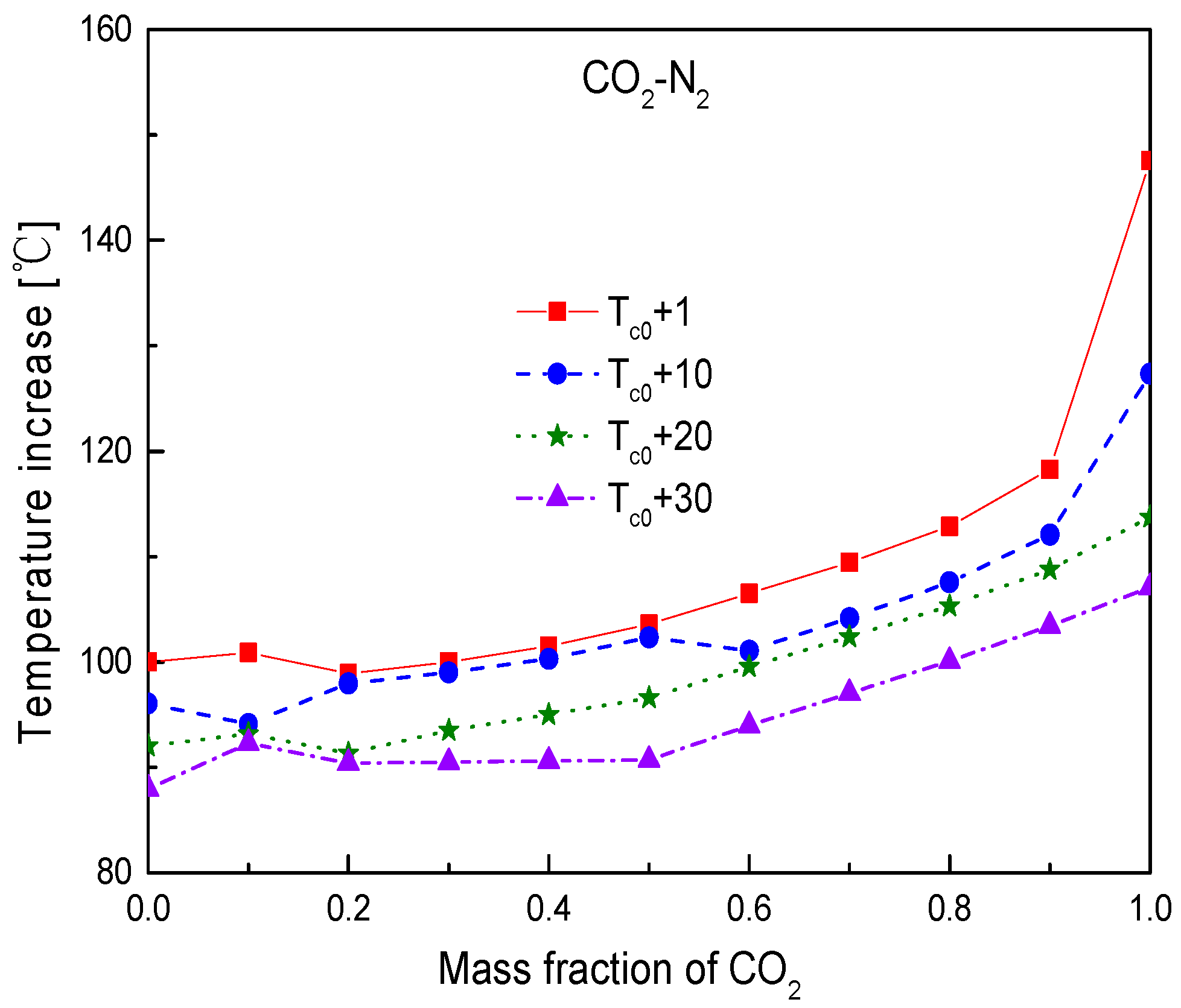
4.3. Effect of Compressor Inlet Temperature
5. Conclusions
- (1)
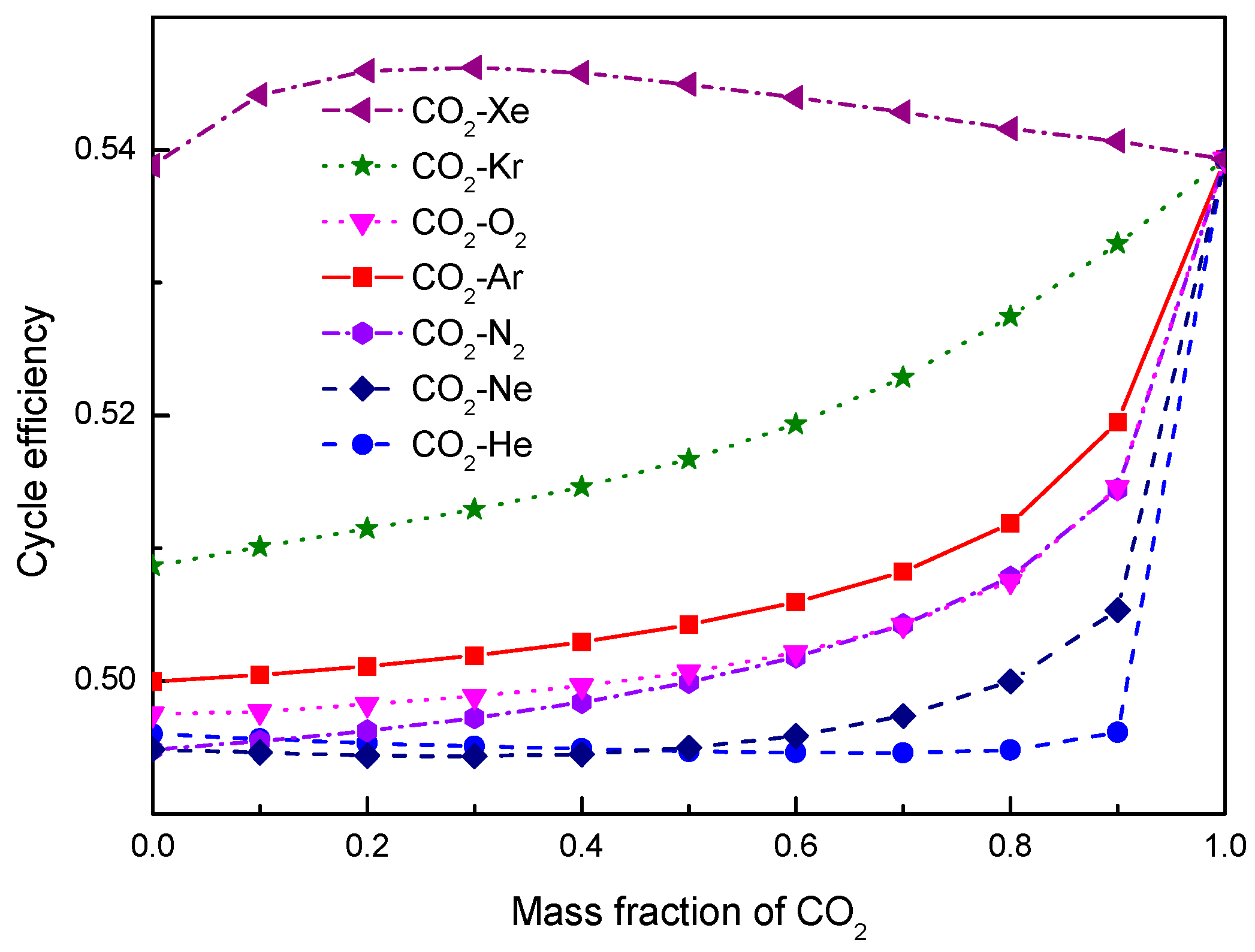
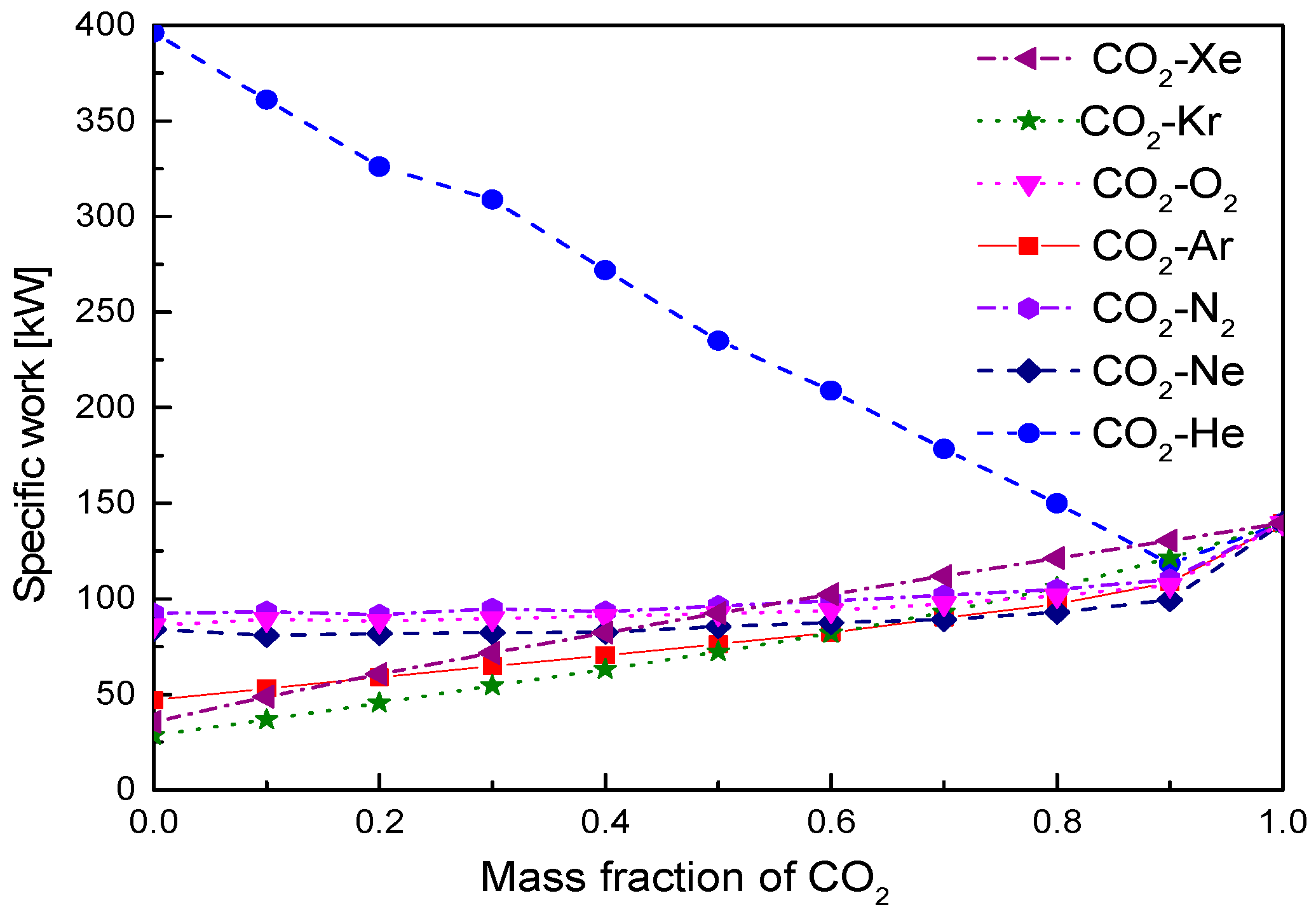
- Under the fixed compressor inlet temperature, except CO2-Xe and CO2-He, cycle efficiency, specific work and temperature increase in the primary heater for other mixtures are generally lower than those for pure CO2. However, mass flow rate, heat input and low cycle pressure of these mixtures are generally higher than those of CO2. For the analyzed parameters, the order of mixtures almost coincides with the descending or ascending order of corresponding critical temperature. Performance curves of the considered mixtures locate between the curves of CO2-Xe and CO2-He. Furthermore, when the mass fraction is close to 1.0, there usually exists a sharp change of cycle performance for CO2-He, CO2-Ne, CO2-N2, CO2-Ar, and CO2-O2.
- (2)
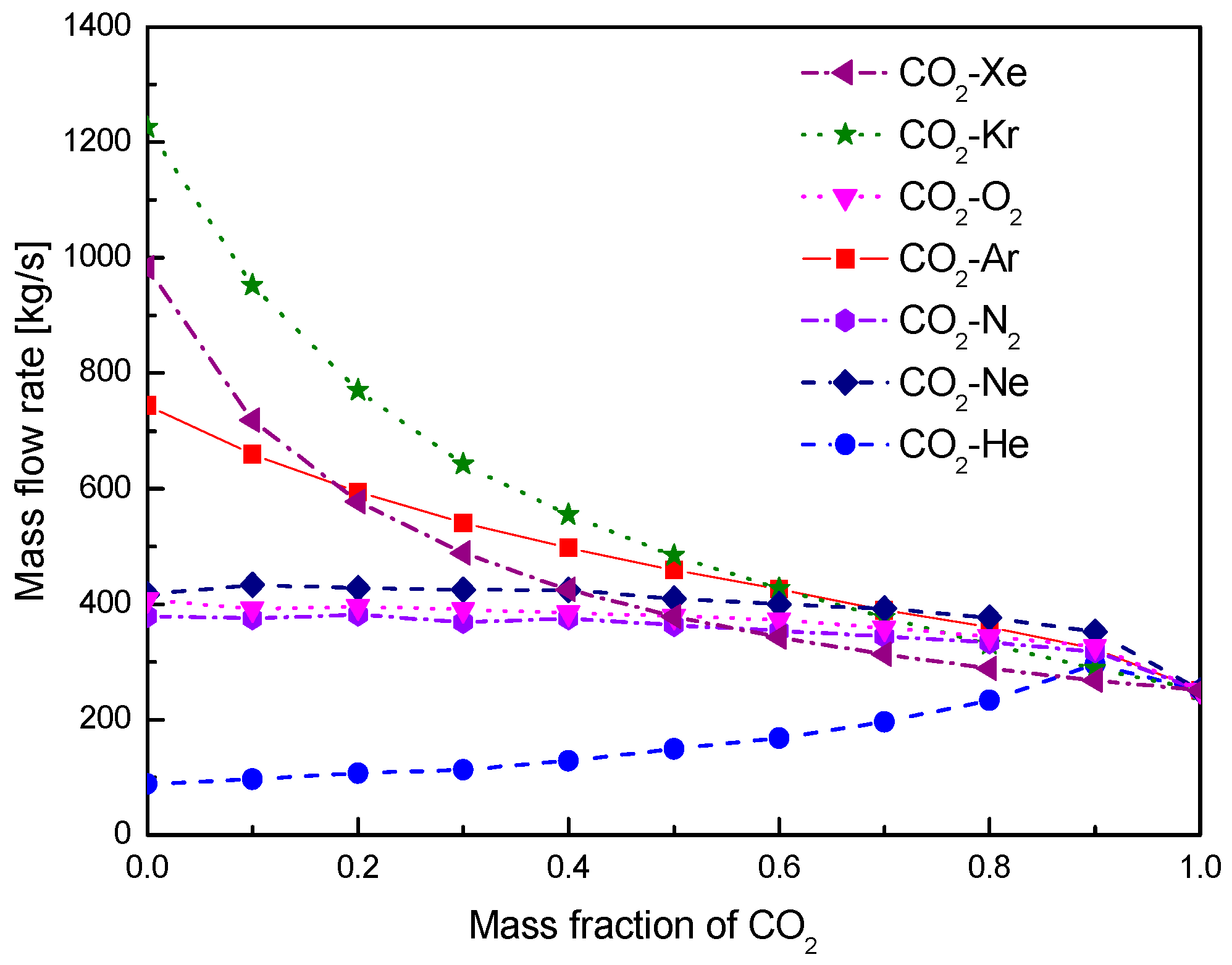
- For the recuperators of recompression cycle, compared with the LTR heat, the HTR heat of mixtures is larger. Except CO2-Xe, the transferred heat and the required conductance of HTR and LTR decrease with the increase of CO2 mass fraction. At high mass fractions of CO2, the considered mixtures satisfy the order: CO2-He > CO2-Ne > CO2-O2 > CO2-N2 > CO2-Ar > CO2-Kr > CO2-Xe. As for the split ratio, the larger the heat capacity difference of mixtures between hot and cold sides in LTR, the lower the split ratio. All mixtures have higher split ratio than pure CO2. The order of split ratio for mixtures almost coincides with the above sequence. In general, the curves of CO2-O2 and CO2-N2 are close to each other.
- (3)
- For the effect of compressor inlet temperature, the increase of inlet temperature will cause the decrease of cycle efficiency, specific work, and temperature difference in the primary heater. Heat conductance of recuperators will be increased. However, the obvious benefit of increasing inlet temperature is that it can increase the temperature difference between the working fluid and the cooling source, especially in hot or arid environments. This will greatly reduce the mass flow of cooling source and the required heat exchange area of gas cooler. Thus, how to determine the optimal inlet temperature of compressor is worth investigating by considering the thermodynamic and economic performances of working fluid simultaneously.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | |
| C | Heat Capacity, KJ/(kg∙K) |
| D | Density, kg/m3 |
| HTR | High temperature recuperator |
| HPT | High pressure turbine |
| h | Enthalpy, kJ/kg |
| LTR | Low temperature recuperator |
| LPT | Low pressure turbine |
| m | Mass flow rate, kg/s |
| M | Molar mass, g/mol |
| P | Pressure, MPa |
| PPTD | Pinch point temperature difference, °C |
| Q | Heat, kJ |
| S-CO2 | Supercritical CO2 |
| SFR | Sodium-cooled fast reactor |
| SR | Split ratio |
| T | Temperature, K |
| W | Work, kW |
| UA | Heat conductance, MW/K |
| Greeks | |
| ε | Effectiveness of recuperator |
| Efficiency | |
| Δ | Difference |
| Subscripts | |
| 1, 2,…10 | State points of recompression cycle |
| b | Boiling temperature |
| c | Cycle/critical temperature |
| c0 | Critical temperature of CO2 |
| HPT | High pressure turbine |
| HTR | High temperature recuperator |
| LPT | Low pressure turbine |
| LTR | Low temperature recuperator |
| MC | Main Compressor |
| net | Net work |
| p | Heat capacity |
| ph | Primary heater |
| RC | Recompressor |
| rh | Reheater |
| s | Equal entropy |
References
- Dudley, B. BP statistical review of world energy. In Proceedings of the World Petroleum Congress, London, UK, 30 September 2019. [Google Scholar]
- Wang, J.; Lund, P.D.; Zhu, H. Thermal performance analysis of a direct-heated recompression supercritical carbon dioxide Brayton cycle using solar concentrators. Energies 2019, 12, 4358. [Google Scholar] [CrossRef]
- Manente, G.; Costa, M. On the Conceptual Design of Novel Supercritical CO2 Power Cycles for Waste Heat Recovery. Energies 2020, 13, 370. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Huang, D. Supercritical CO2 Brayton cycle: A state-of-the-art review. Energy 2019, 189, 115900. [Google Scholar] [CrossRef]
- Wang, X.R.; Li, X.X.; Li, Q.B.; Liu, L.; Liu, C. Performance of a solar thermal power plant with direct air-cooled supercritical carbon dioxide Brayton cycle under off-design conditions. Appl. Energy 2020, 261, 114359. [Google Scholar] [CrossRef]
- Ma, T.; Li, M.J.; Xu, J.L.; Cao, F. Thermodynamic analysis and performance prediction on dynamic response characteristic of PCHE in 1000 MW S-CO2 coal fired power plant. Energy 2019, 175, 123–138. [Google Scholar] [CrossRef]
- Yu, H.; Hartanto, D.; Moon, J.; Kim, Y. A conceptual study of a supercritical CO2-cooled Micro Modular Reactor. Energies 2015, 8, 13938–13952. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Xie, Y. Investigation into off-design performance of a s-CO2 turbine based on concentrated Solar Power. Energies 2018, 11, 3014. [Google Scholar] [CrossRef]
- Ma, Y.G.; Morosuk, T.; Luo, J.; Liu, M.; Liu, J. Superstructure design and optimization on supercritical carbon dioxide cycle for application in concentrated solar power plant. Energy Convers. Manag. 2020, 206, 112290. [Google Scholar] [CrossRef]
- Crespi, F.; Gavagnin, G.; Sánchez, D.; Martinez, G.S. Supercritical carbon dioxide cycles for power generation: A review. Appl. Energy 2017, 195, 152–183. [Google Scholar] [CrossRef]
- Linares, J.I.; Montes, M.J.; Cantizano, A.; Sanchez, C. A novel supercritical CO2 recompression Brayton power cycle for power tower concentrating solar plants. Appl. Energy 2020, 263, 114644. [Google Scholar] [CrossRef]
- Crespi, F.; Sánchez, D.; Rodríguez, J.M.; Gavagnin, G. A thermo-economic methodology to select sCO2 power cycles for CSP applications. RENEW Energy 2020, 147, 2905–2912. [Google Scholar] [CrossRef]
- Wang, K.; He, Y.L.; Zhu, H.H. Integration between supercritical CO2 Brayton cycles and molten salt solar power towers: A review and a comprehensive comparison of different cycle layouts. Appl. Energy 2017, 195, 819–836. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ma, Z.; Neises, T.W.; Wagner, M.J. Thermodynamic study of advanced supercritical carbon dioxide power cycles for concentrating solar power systems. J. Sol. Energy Eng. 2013, 135, 041007. [Google Scholar] [CrossRef]
- Zhu, H.H.; Wang, K.; He, Y.L. Thermodynamic analysis and comparison for different direct-heated supercritical CO2 Brayton cycles integrated into a solar thermal power tower system. Energy 2017, 140, 144–157. [Google Scholar] [CrossRef]
- Moisseytsev, A.; Sienicki, J.J. Investigation of alternative layouts for the super-critical carbon dioxide Brayton cycle for a sodium-cooled fast reactor. Nucl. Eng. Des. 2009, 239, 1362–1371. [Google Scholar] [CrossRef]
- Kulhánek, M.; Dostal, V. Thermodynamic analysis and comparison of supercritical carbon dioxide cycles. In Proceedings of the Supercritical CO2 Power Cycle Symposium, Boulder, CO, USA, 24–25 May 2011. [Google Scholar]
- Wright, S.A.; Conboy, T.M.; Ames, D.E.; Lewis, T.G. CO2-Based Mixtures as Working Fluids for Geothermal Turbines; AC04-94AL85000; Sandia National Laboratories: Livermore, CA, USA, January 2012.
- Jeong, W.S.; Jeong, Y.H. Performance of supercritical Brayton cycle using CO2-based binary mixture at varying critical points for SFR applications. Nucl. Eng. Des. 2013, 262, 12–20. [Google Scholar] [CrossRef]
- Jeong, W.S.; Lee, J.I.; Jeong, Y.H. Potential improvements of supercritical recompression CO2 Brayton cycle by mixing other gases for power conversion system of a SFR. Nucl. Eng. Des. 2011, 241, 2128–2137. [Google Scholar] [CrossRef]
- Hu, L.; Chen, D.; Huang, Y.; Li, L.; Cao, Y.; Yuan, D.; Wang, J.; Pan, L. Investigation on the performance of the supercritical Brayton cycle with CO2-based binary mixture as working fluid for an energy transportation system of a nuclear reactor. Energy 2015, 89, 874–886. [Google Scholar] [CrossRef]
- Baik, S.; Lee, J.I. Preliminary study of supercritical CO2 mixed with gases for power cycle in warm environments. In Proceedings of the ASME Turbo Expo; ASME Digital Library: New York, NY, USA, 2018. [Google Scholar]
- Guo, J.Q.; Li, M.J.; Xu, J.L.; Yan, J.; Wang, K. Thermodynamic performance analysis of different supercritical Brayton cycles using CO2-based binary mixtures in the molten salt solar power tower systems. Energy 2019, 173, 785–798. [Google Scholar] [CrossRef]
- Vesely, L.; Dostal, V.; Stepanek, J. Effect of gaseous admixtures on cycles with supercritical carbon dioxide. In Proceedings of the ASME Turbo Expo; ASME Digital Library: New York, NY, USA, 2016. [Google Scholar]
- Vesely, L.; Manikantachari, K.R.V.; Vasu, S.; Kapat, J.; Dostal, V.; Martin, S. Effect of impurities on compressor and cooler in supercritical CO2 cycles. J. Energy Resour. Technol. 2019, 141, 012003. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, M.; Yan, J.; Liu, J. Performance investigation of a novel closed Brayton cycle using supercritical CO2-based mixture as working fluid integrated with a LiBr absorption chiller. Appl. Therm. Eng. 2018, 141, 531–547. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP; Version 10.0; National Institute of Standards and Technology, Standard Reference Data Program: Gaithersburg, MD, USA, 2018.
- Neises, T.; Turchi, C. A comparison of supercritical carbon dioxide power cycle configurations with an emphasis on CSP applications. Energy Procedia 2014, 49, 1187–1196. [Google Scholar] [CrossRef]
| Authors | Year | Mixtures | Tc/Mole Fraction | Inlet Temperature of Compressor | Performance Parameters |
|---|---|---|---|---|---|
| Jeong et al. [20] | 2011 | CO2-N2 CO2-O2 CO2-He CO2-Ar | 292 K–304 K | Tc + 1 | cycle efficiency |
| Conboy et al. [18] | 2012 | CO2-He CO2-Ne | 0.9/0.1 | — | Compatibility; compressor flow |
| CO2-methane CO2-butane | 0.81/0.19; 0.92/0.08 | ||||
| Jeong et al. [19] | 2013 | CO2-Xe CO2-Kr CO2-N2 CO2-O2 CO2-Ar | 292 K–304 K | Tc + 1 | cycle efficiency |
| Hu et al. [21] | 2015 | CO2-O2 CO2-He CO2-Ar CO2-Kr | 284.15 K–304.15 K | Tc + 1 | heat transfer; mass flow rate; cycle power; cycle efficiency |
| CO2-butane CO2-cyclohexane | 304.15 K–324.15 K | ||||
| Vesely et al. [24] | 2016 | CO2-He CO2-H2 CO2-O2 CO2-H2S CO2-N2 CO2-Ar | impurity: 0–0.05 | 307.15 K | cycle efficiency |
| Baik and Lee [22] | 2018 | CO2-SF6 CO2-R123 CO2-R134a CO2-R32 CO2-toluene | CO2: 0–1 | 304.15 K–313.15 K | cycle efficiency |
| Ma et al. [26] | 2018 | CO2-Kr | Kr: 0–0.48 | — | cycle efficiency; cooling capacity |
| Vesely et al. [25] | 2019 | CO2-He CO2-CO CO2-O2 CO2-N2 CO2-Ar CO2-Xe | impurity: 0–0.10 | 307.15 K–313.15 K | cycle power; heat transfer |
| Guo et al. [23] | 2019 | CO2-butane | 0.95/0.05 | 313.15 K–333.15 K | cycle efficiency; exery efficiency |
| CO2-Xe | 0.7/0.3 | 303.15 K–333.15 K |
| Pure Fluid | M [g/mol] | Tb [K] | Tc [K] | Pc [MPa] | Dc [kg/m3] | Cp [KJ/(kg∙K)] | |
|---|---|---|---|---|---|---|---|
| Tc0 + 150, 7.5 Mpa | Tc0 + 150, 25 Mpa | ||||||
| CO2 | 44.01 | 194.69 | 304.13 | 7.38 | 467.60 | 1.14 | 1.55 |
| Xe | 131.29 | 165.05 | 289.73 | 5.84 | 1102.90 | 0.21 | 0.32 |
| Kr | 83.80 | 119.73 | 209.48 | 5.53 | 909.21 | 0.28 | 0.34 |
| O2 | 32.00 | 90.19 | 154.58 | 5.04 | 436.14 | 1.00 | 1.08 |
| Ar | 39.95 | 87.30 | 150.69 | 4.86 | 535.60 | 0.55 | 0.61 |
| N2 | 28.01 | 77.36 | 126.19 | 3.40 | 313.30 | 1.09 | 1.16 |
| Ne | 20.18 | 27.10 | 44.49 | 2.68 | 481.91 | 1.04 | 1.05 |
| He | 4.00 | 4.22 | 5.20 | 0.23 | 69.58 | 5.19 | 5.18 |
| Design Parameters | Value Range | Reference Value |
|---|---|---|
| Turbine efficiency | 0.93 | 0.93 |
| Compressor efficiency | 0.89 | 0.89 |
| Heat exchanger effectiveness | 0.97 | 0.97 |
| Temperature difference in heat exchanger | ≥5 °C | ≥5 °C |
| Turbine inlet temperature | 650 °C | 650 °C |
| Compressor inlet temperature | Tc0 + 1–Tc0 + 30 | Tc0 + 1 |
| Upper pressure | 25 MPa | 25 MPa |
| Net power output | 35 MW | 35 MW |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, A.; Su, W.; Zhao, L.; Lin, X.; Zhou, N. New Knowledge on the Performance of Supercritical Brayton Cycle with CO2-Based Mixtures. Energies 2020, 13, 1741. https://doi.org/10.3390/en13071741
Yu A, Su W, Zhao L, Lin X, Zhou N. New Knowledge on the Performance of Supercritical Brayton Cycle with CO2-Based Mixtures. Energies. 2020; 13(7):1741. https://doi.org/10.3390/en13071741
Chicago/Turabian StyleYu, Aofang, Wen Su, Li Zhao, Xinxing Lin, and Naijun Zhou. 2020. "New Knowledge on the Performance of Supercritical Brayton Cycle with CO2-Based Mixtures" Energies 13, no. 7: 1741. https://doi.org/10.3390/en13071741
APA StyleYu, A., Su, W., Zhao, L., Lin, X., & Zhou, N. (2020). New Knowledge on the Performance of Supercritical Brayton Cycle with CO2-Based Mixtures. Energies, 13(7), 1741. https://doi.org/10.3390/en13071741





