Nanostructure Effect on Methane Adsorption Capacity of Shale with Type III Kerogen
Abstract
1. Introduction
2. Samples and Experiment
2.1. Sample Information
2.2. Experiments
2.2.1. Low-Temperature Carbon Dioxide Adsorption Experiment
2.2.2. Low-Temperature Nitrogen Adsorption Experiment
2.2.3. High-Pressure Mercury Injection Experiment (HPMI)
2.2.4. Methane Adsorption Experiment
3. Results
3.1. Results of the Low-Temperature Carbon Dioxide Adsorption Experiment
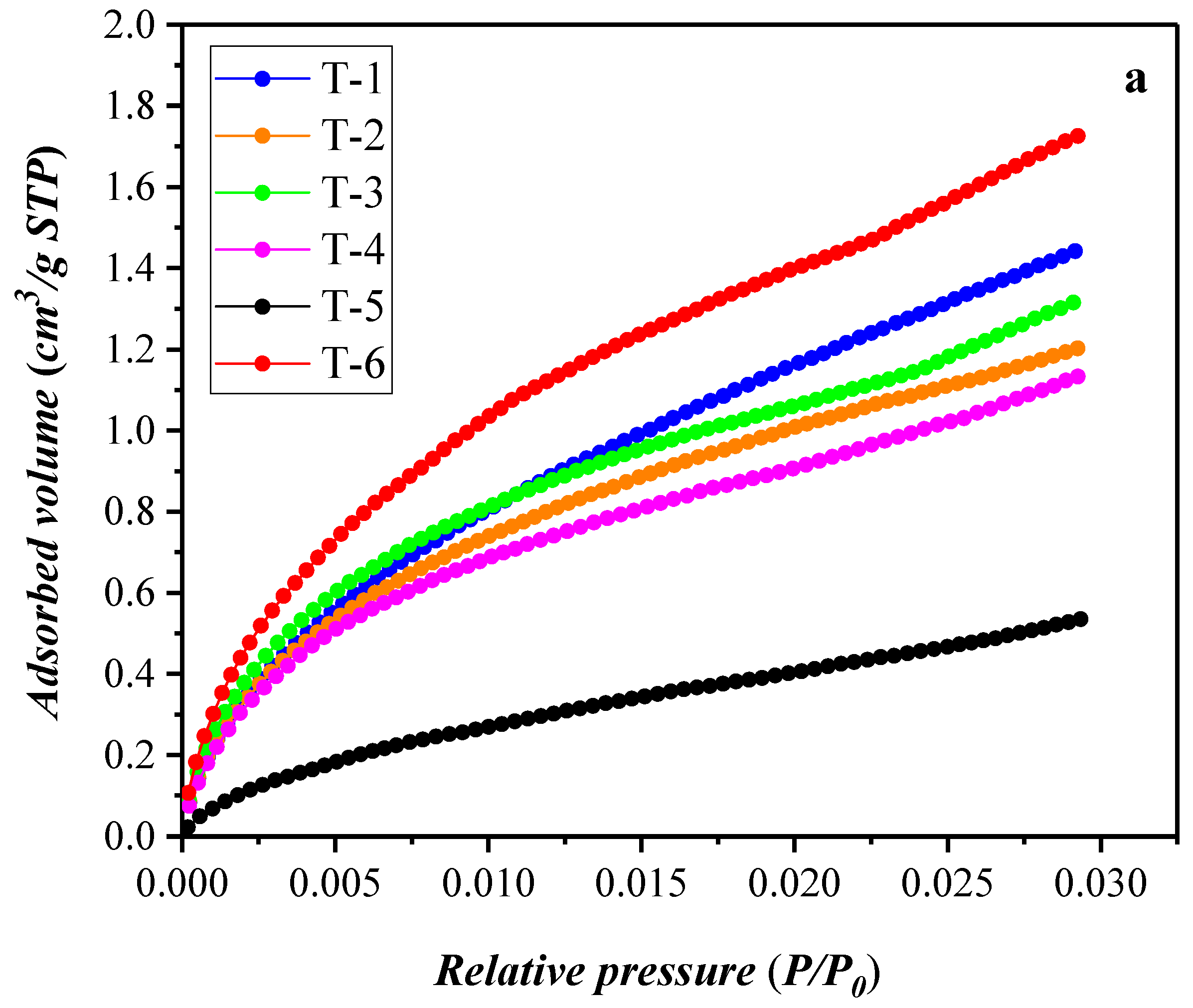
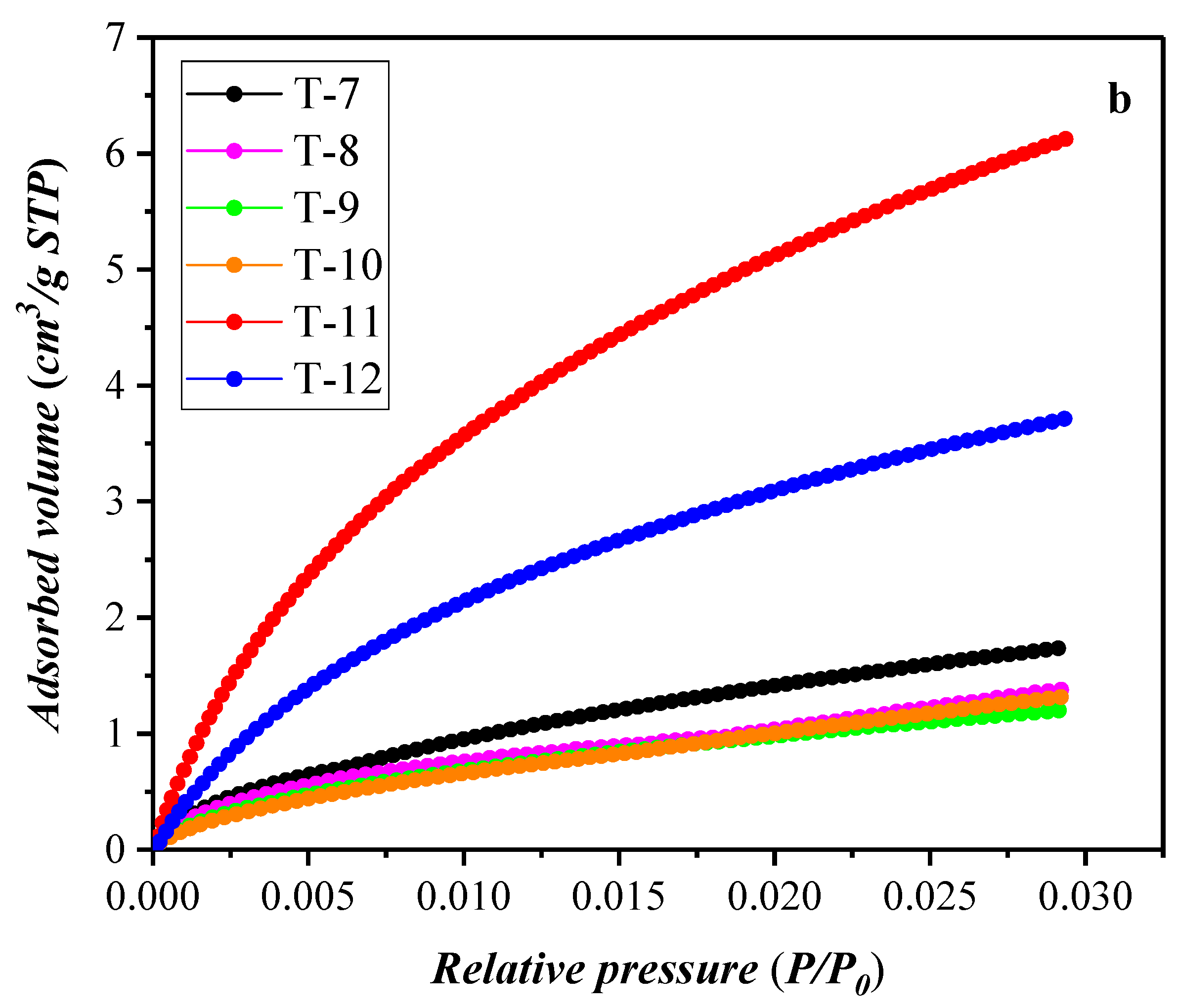
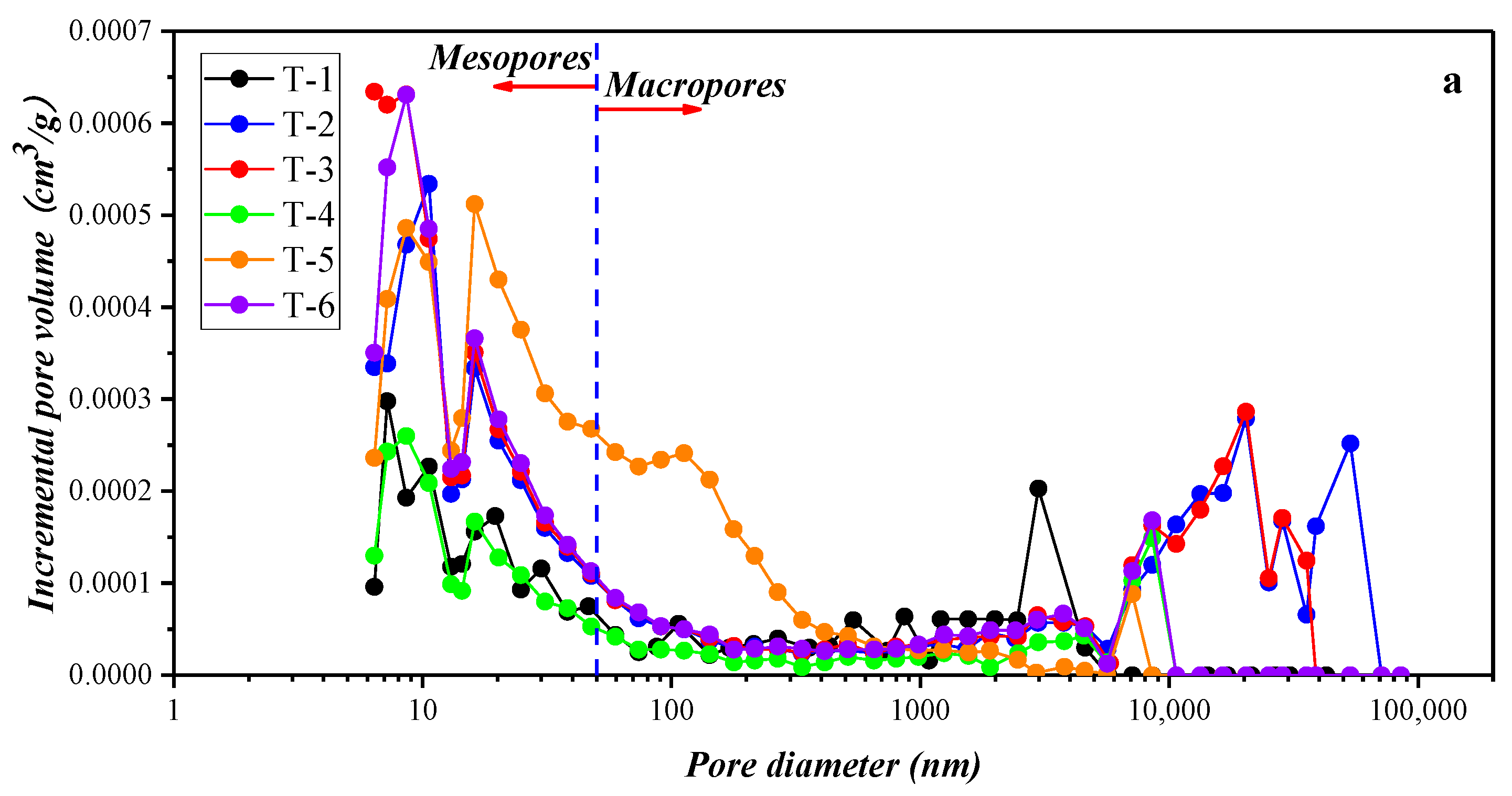
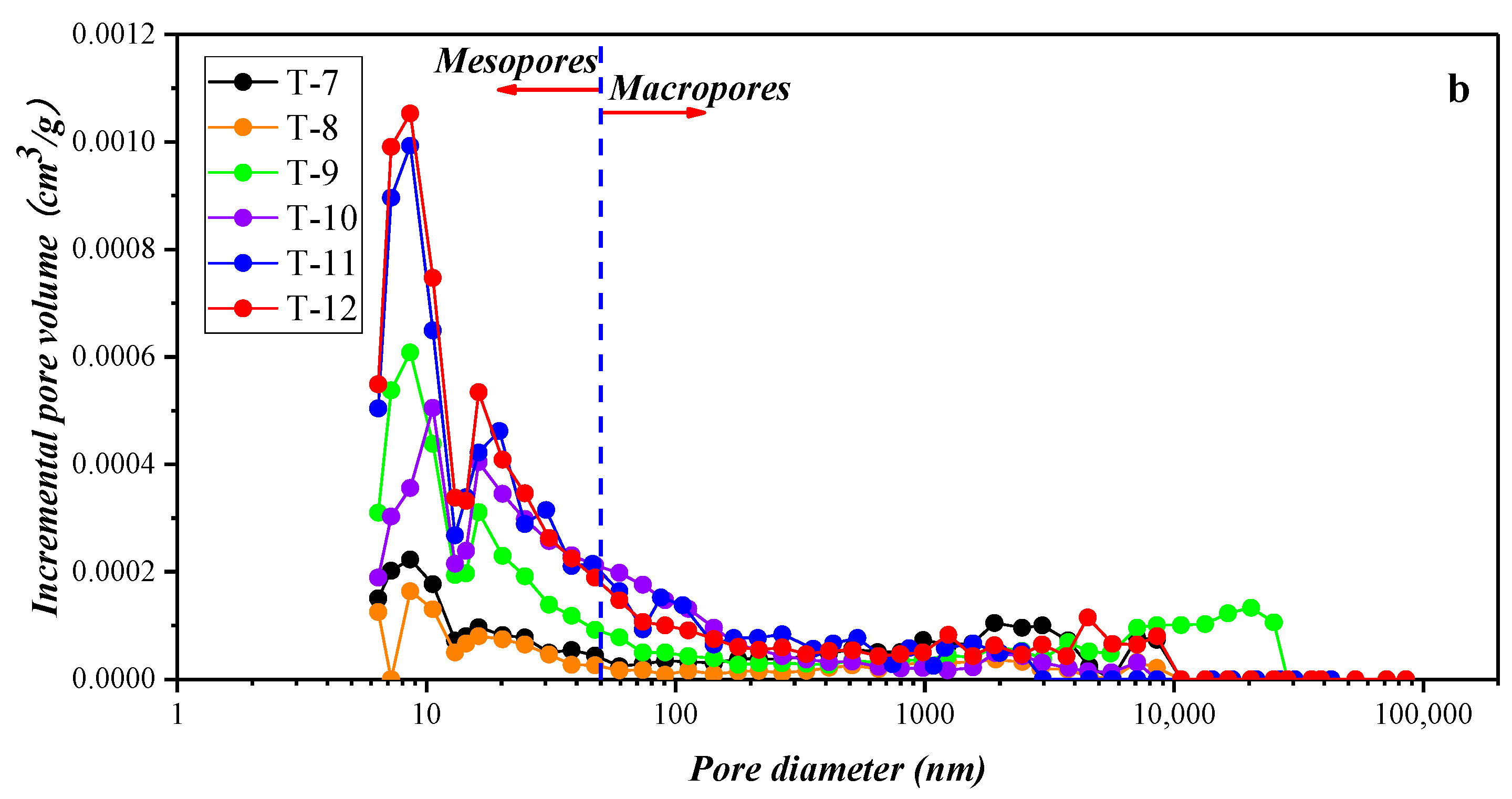
3.2. Results of the Low-Temperature Nitrogen Adsorption Experiment
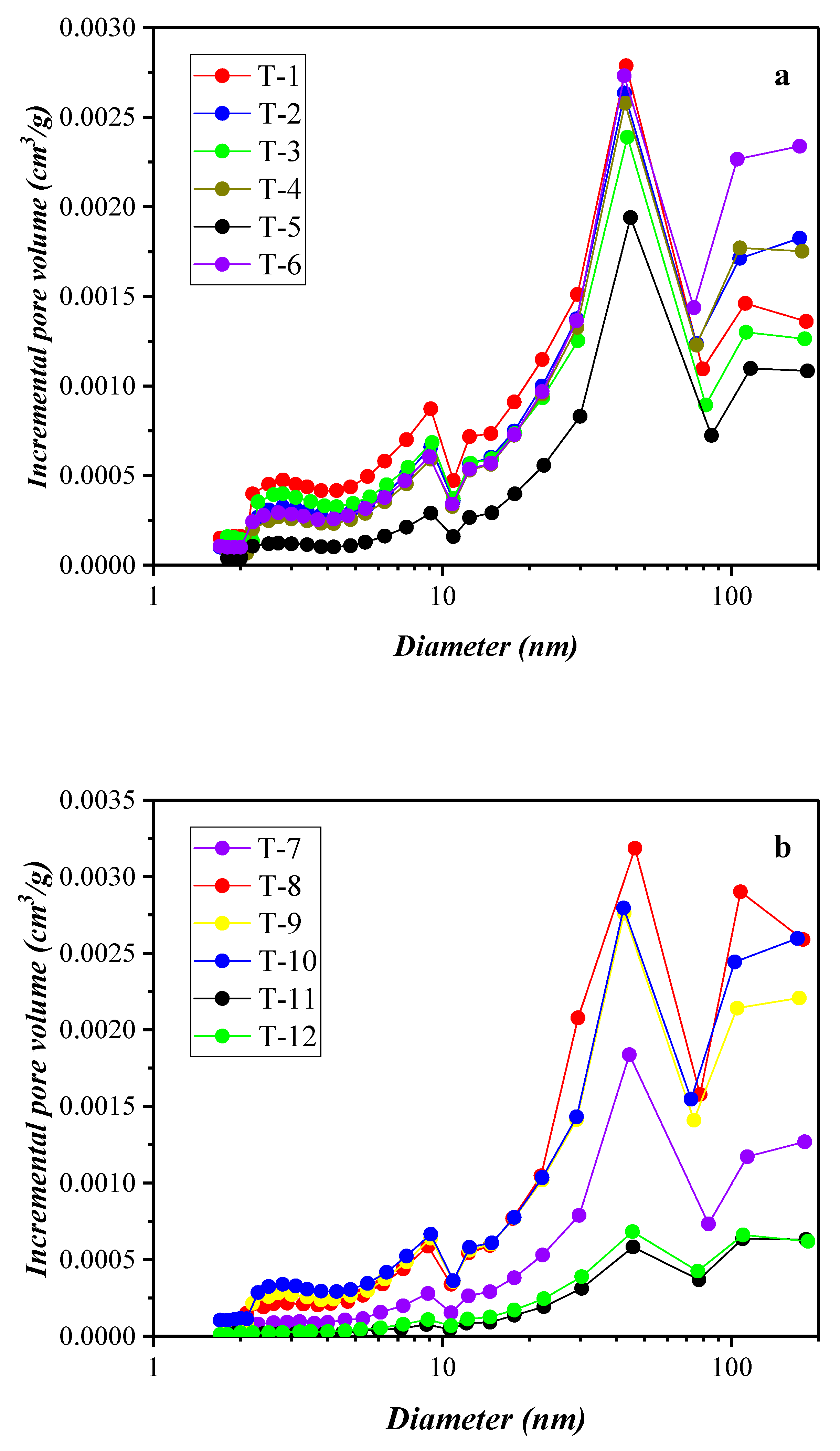
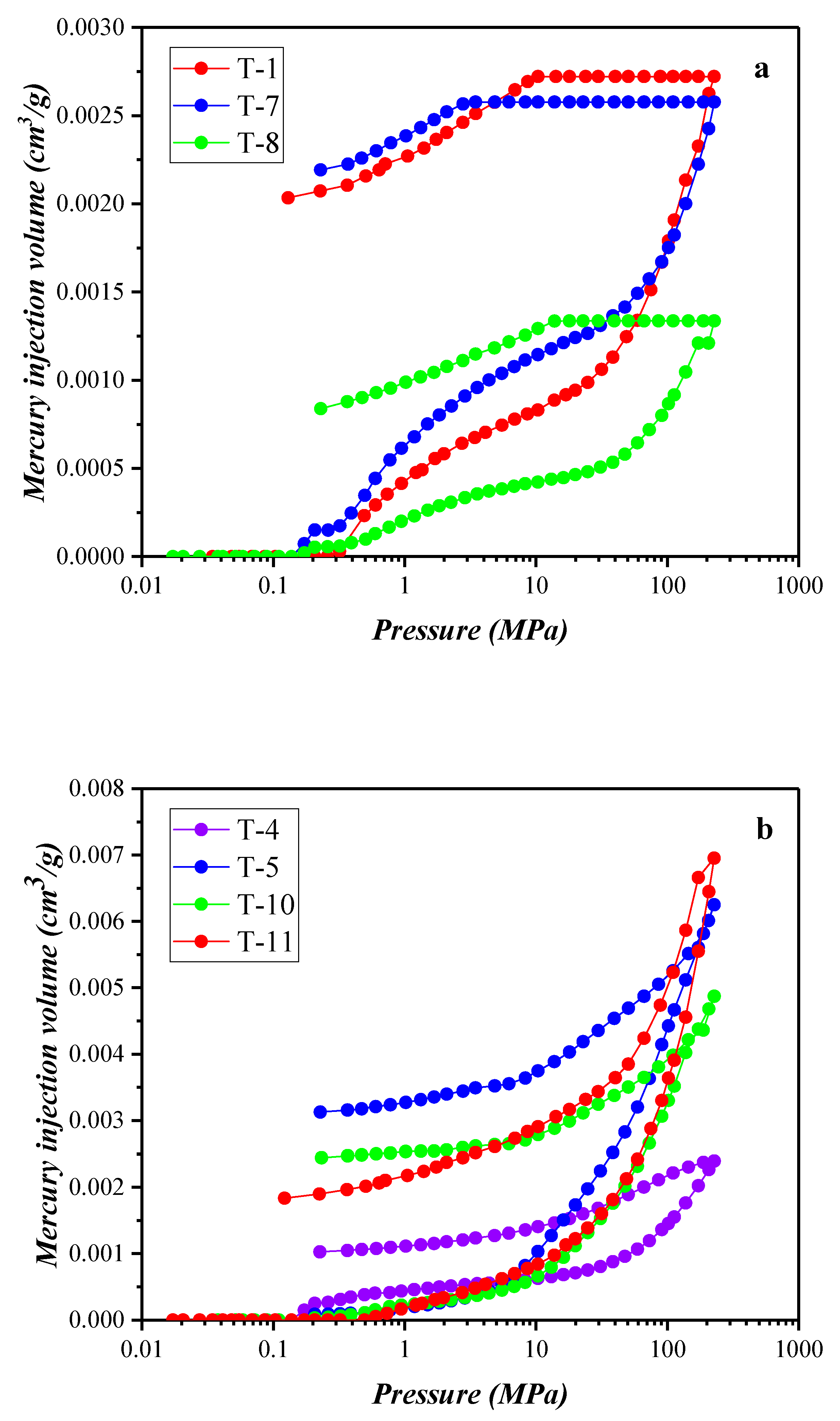
3.3. Results of the High-Pressure Mercury Injection Experiment
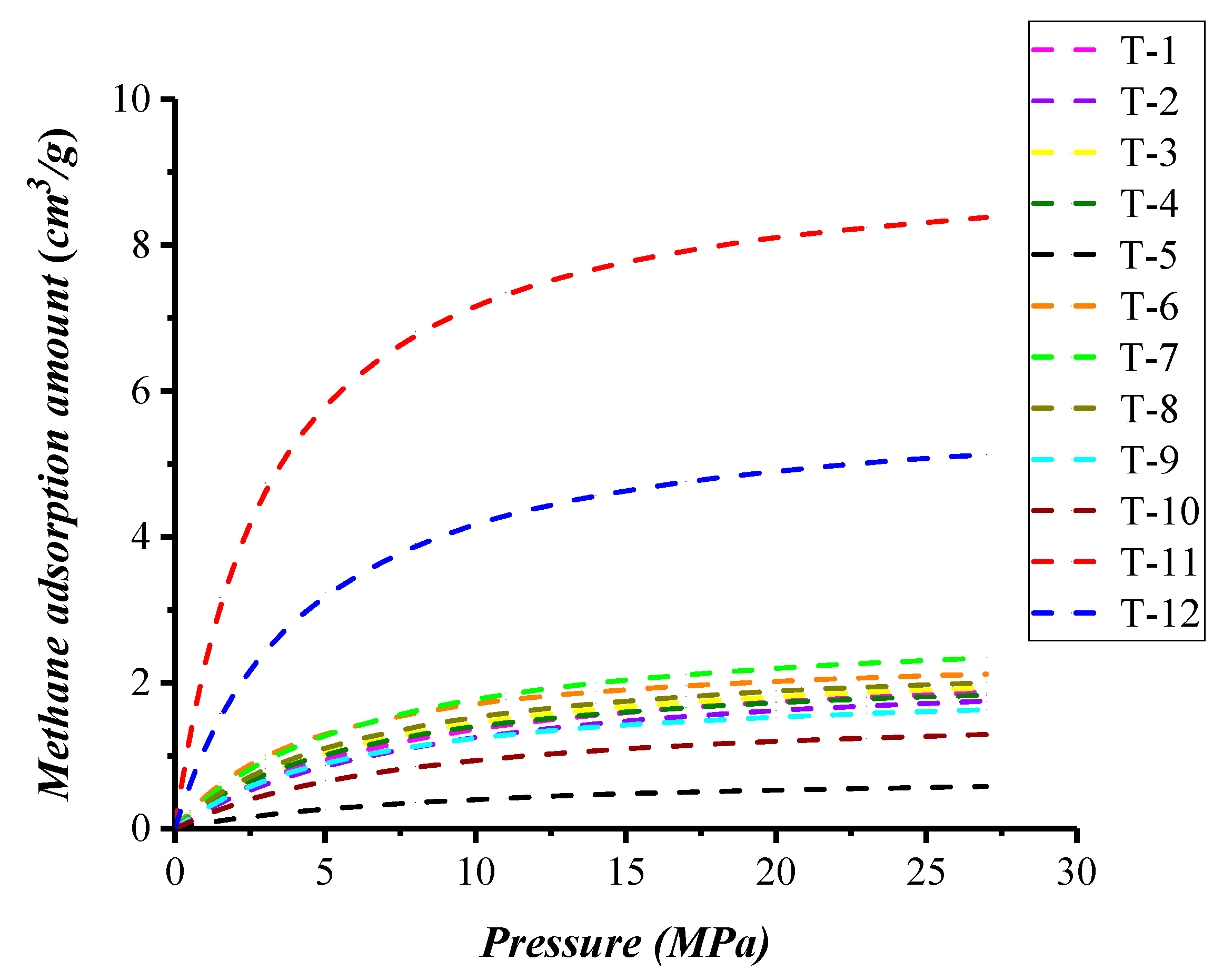
3.4. Methane Adsorption Experimental Data
4. Discussion
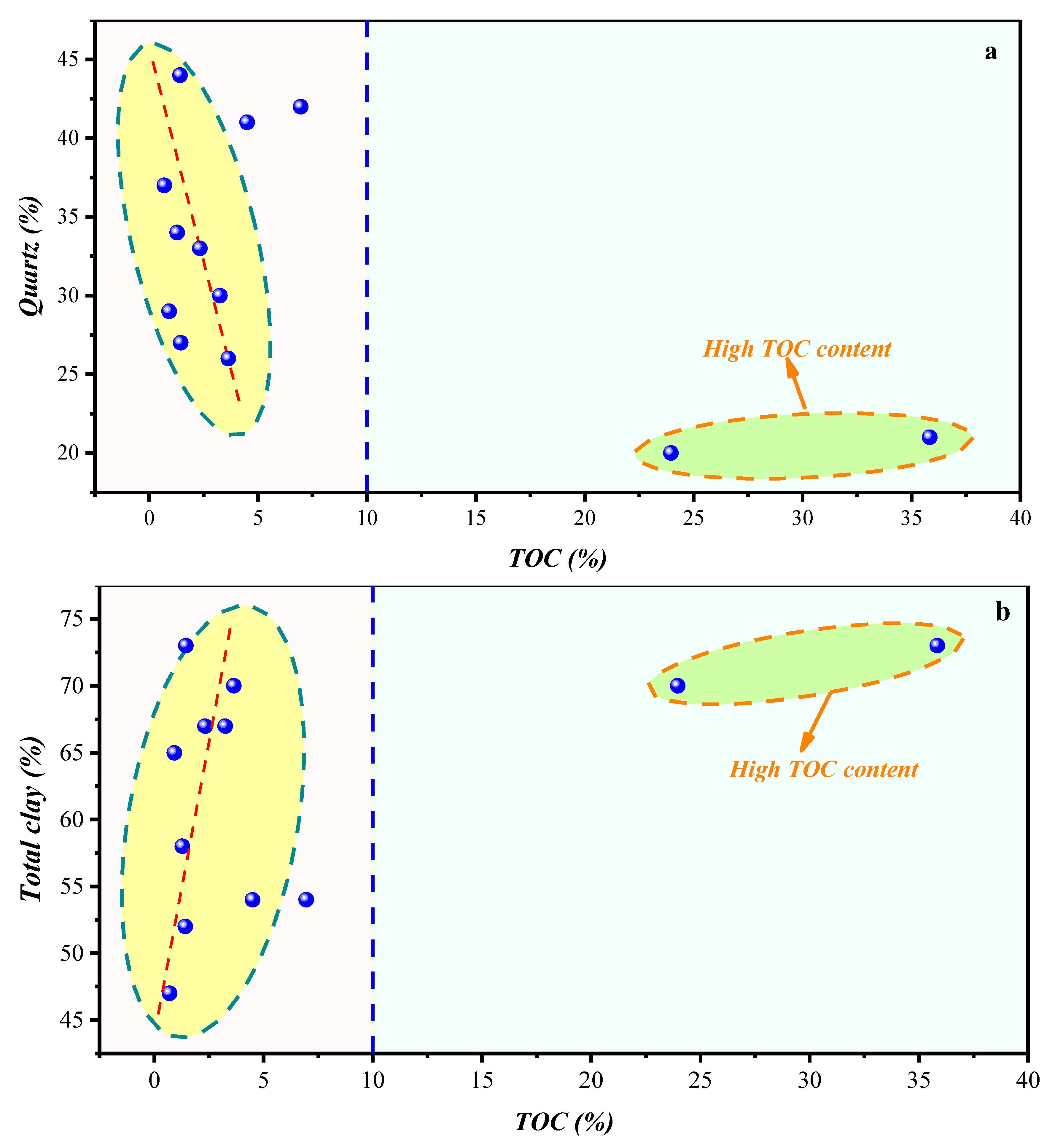
4.1. Composition of the Shale Samples with Type III Kerogen Formed in the Marine-Terrigenous Environment
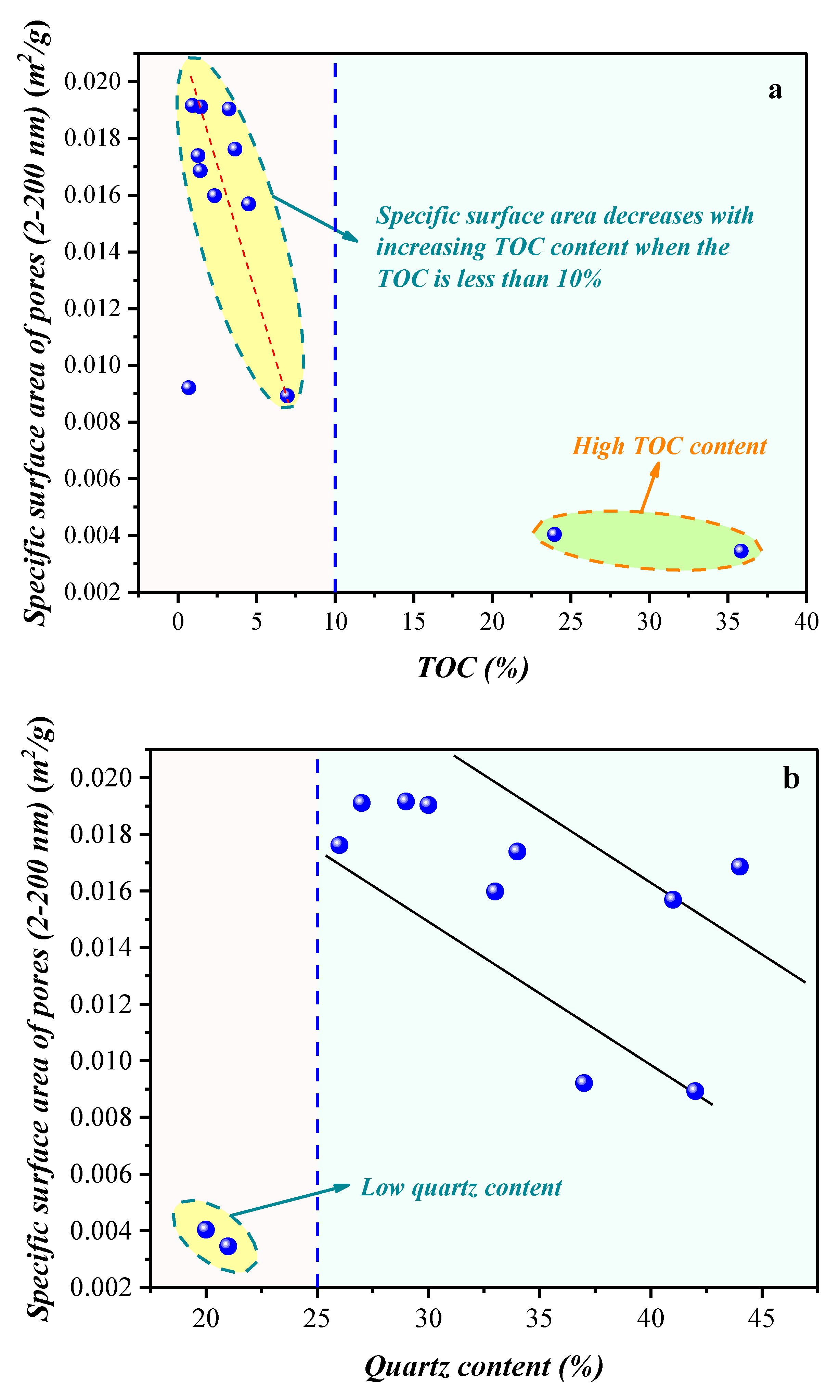
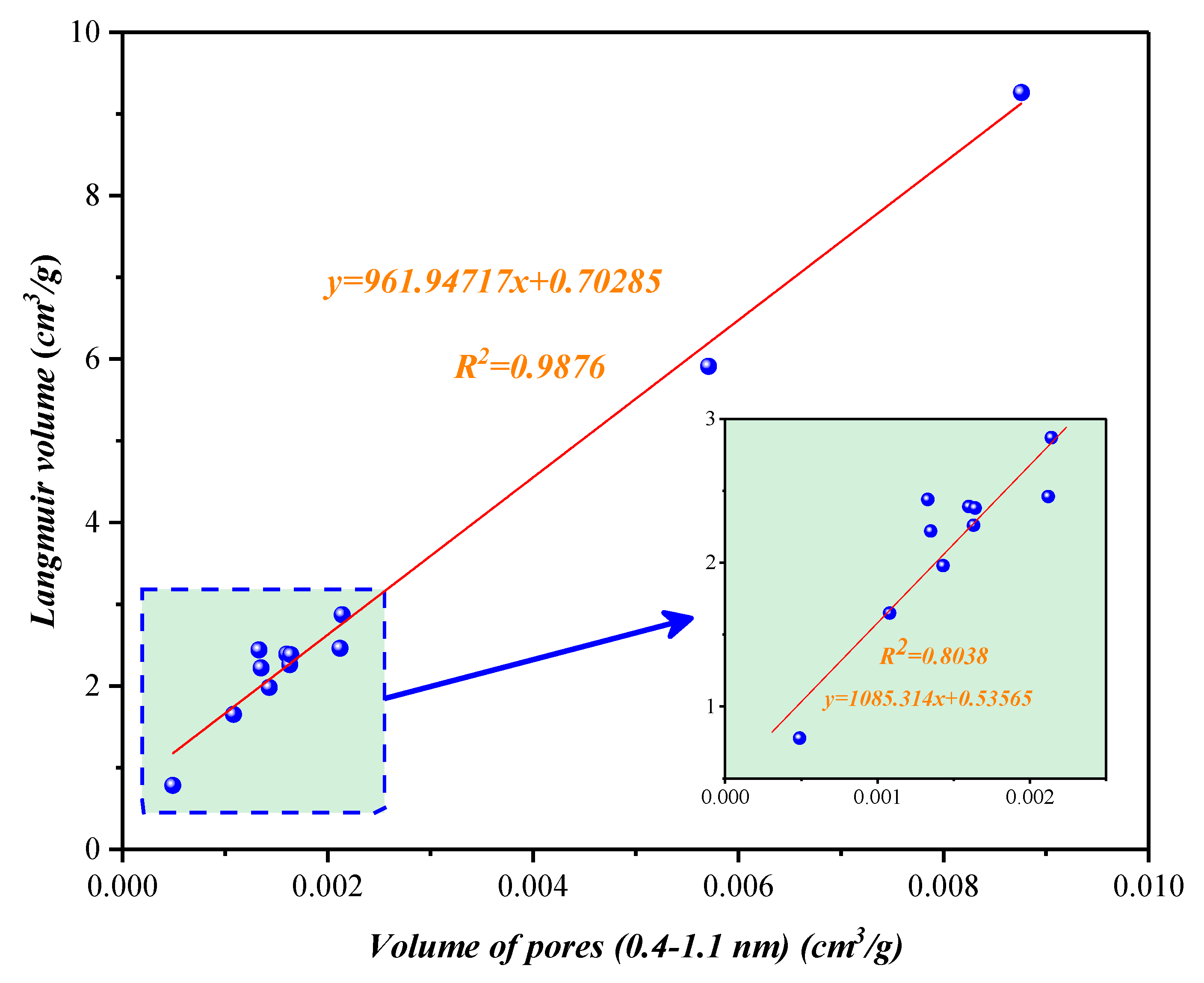
4.1.1. Compositional Influence on the Micropores (0.4–1.1 nm) Obtained by the Low-Temperature CO2 Adsorption Experiment
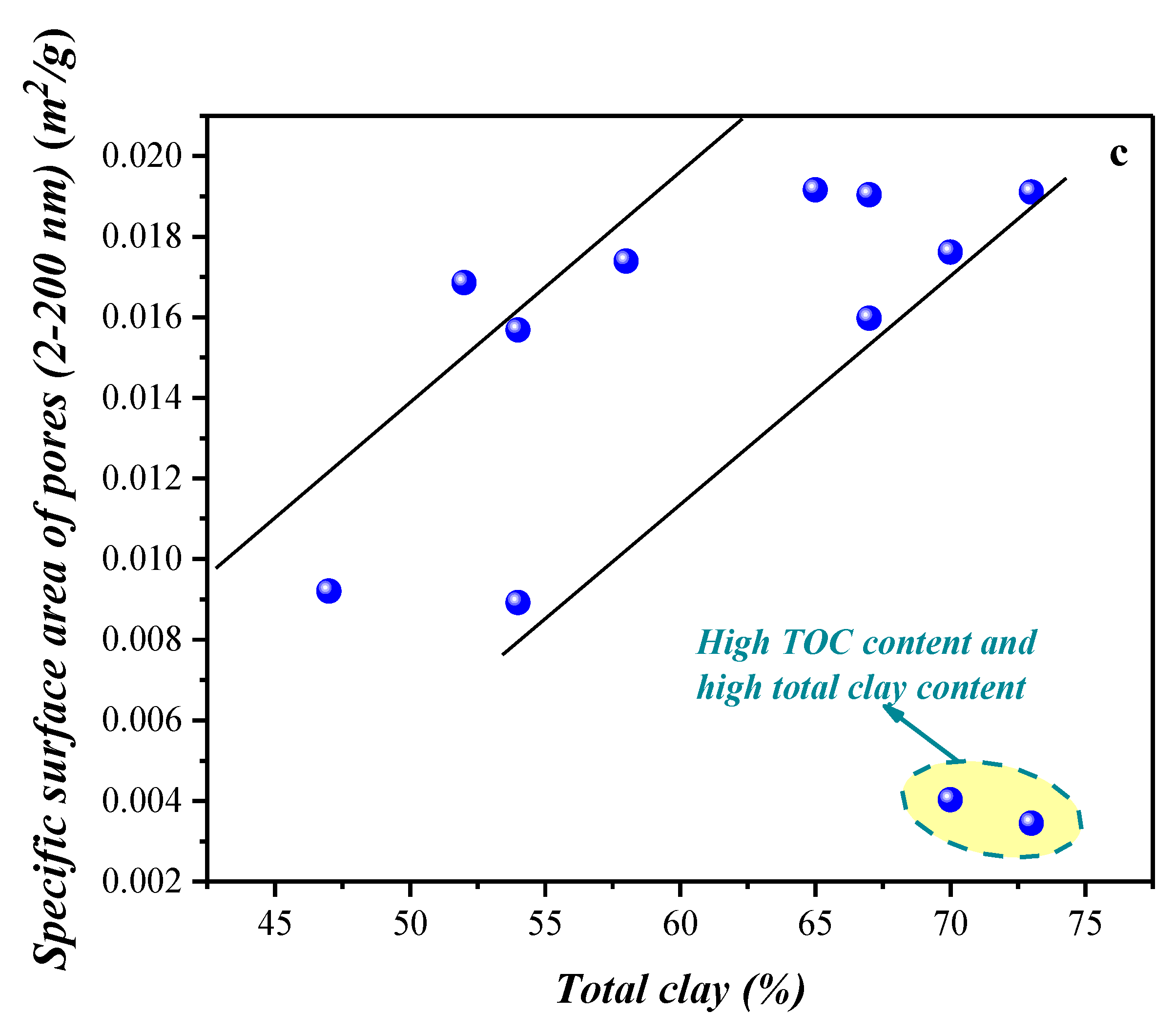
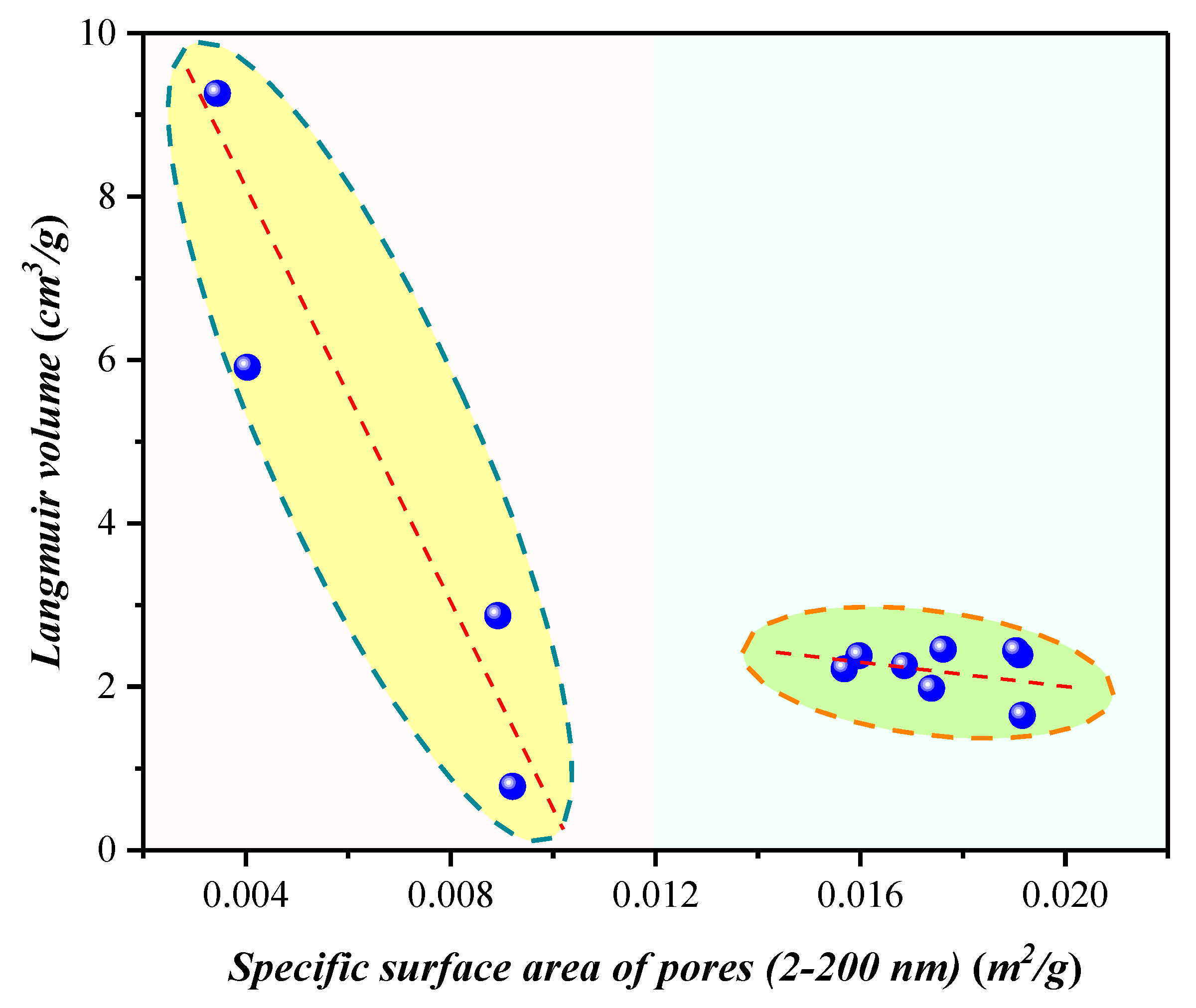
4.1.2. Compositional Influence on the Mesopores (2–50 nm) and Macropores (50–200 nm) Obtained by the Low-Temperature N2 Adsorption Experiment
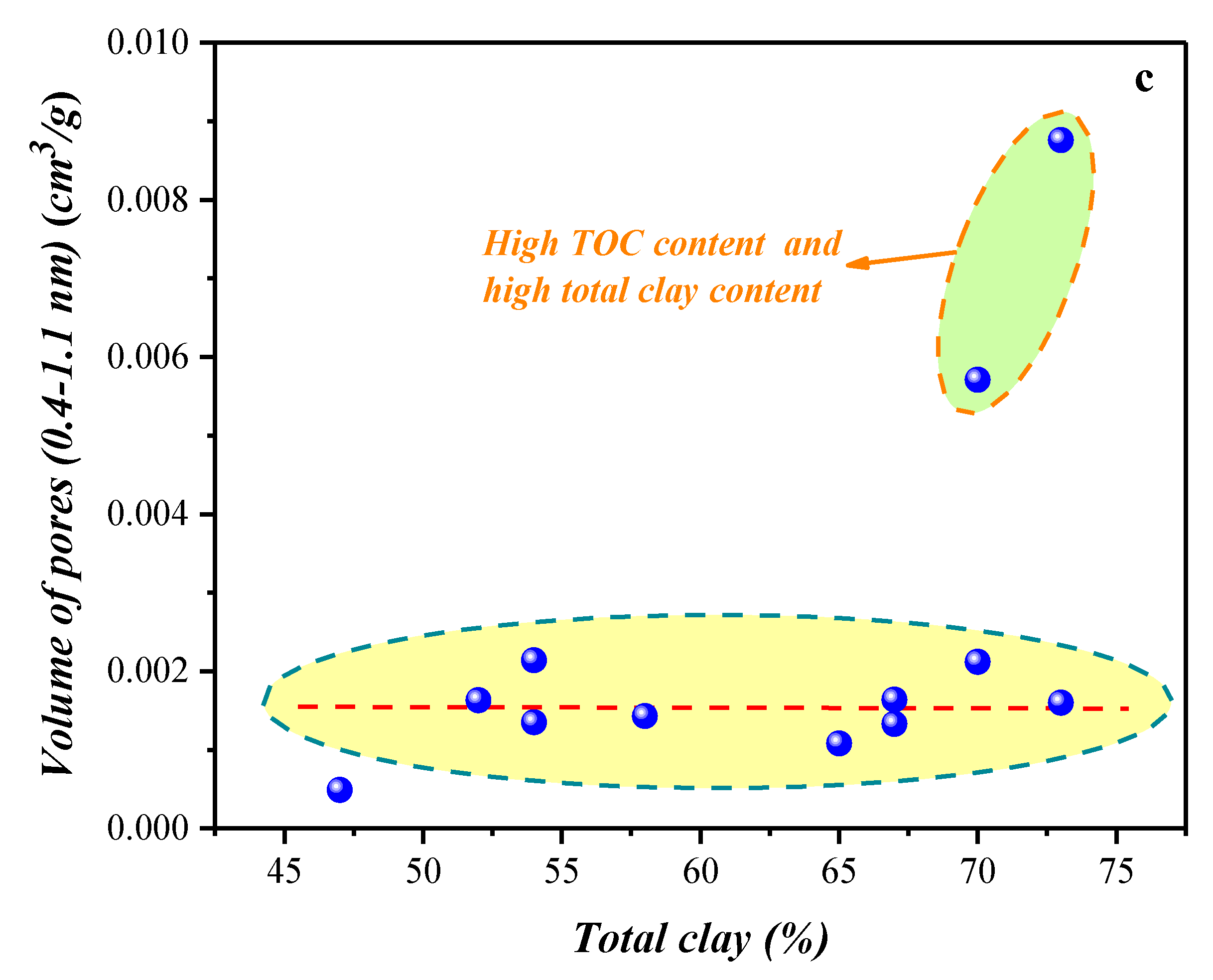
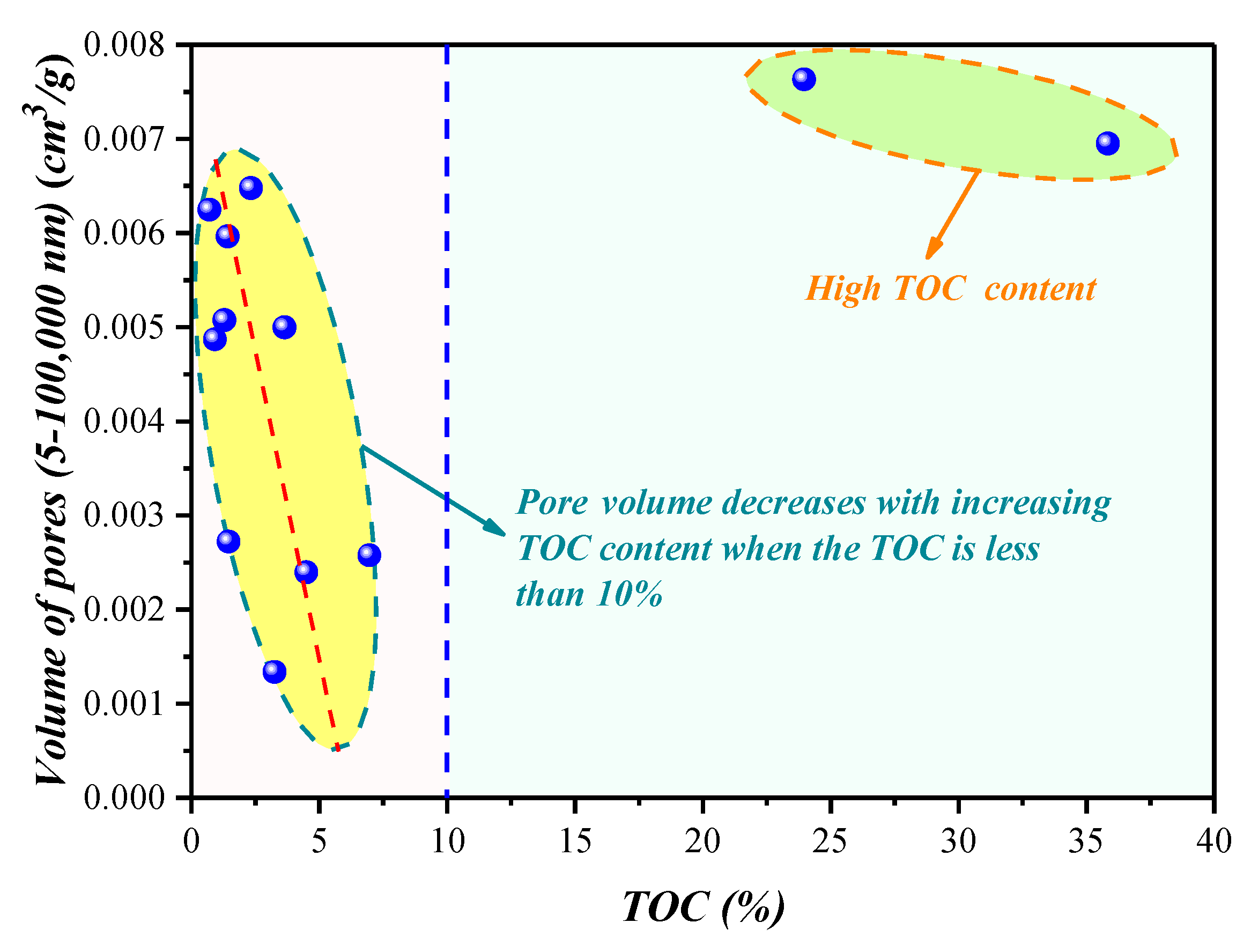
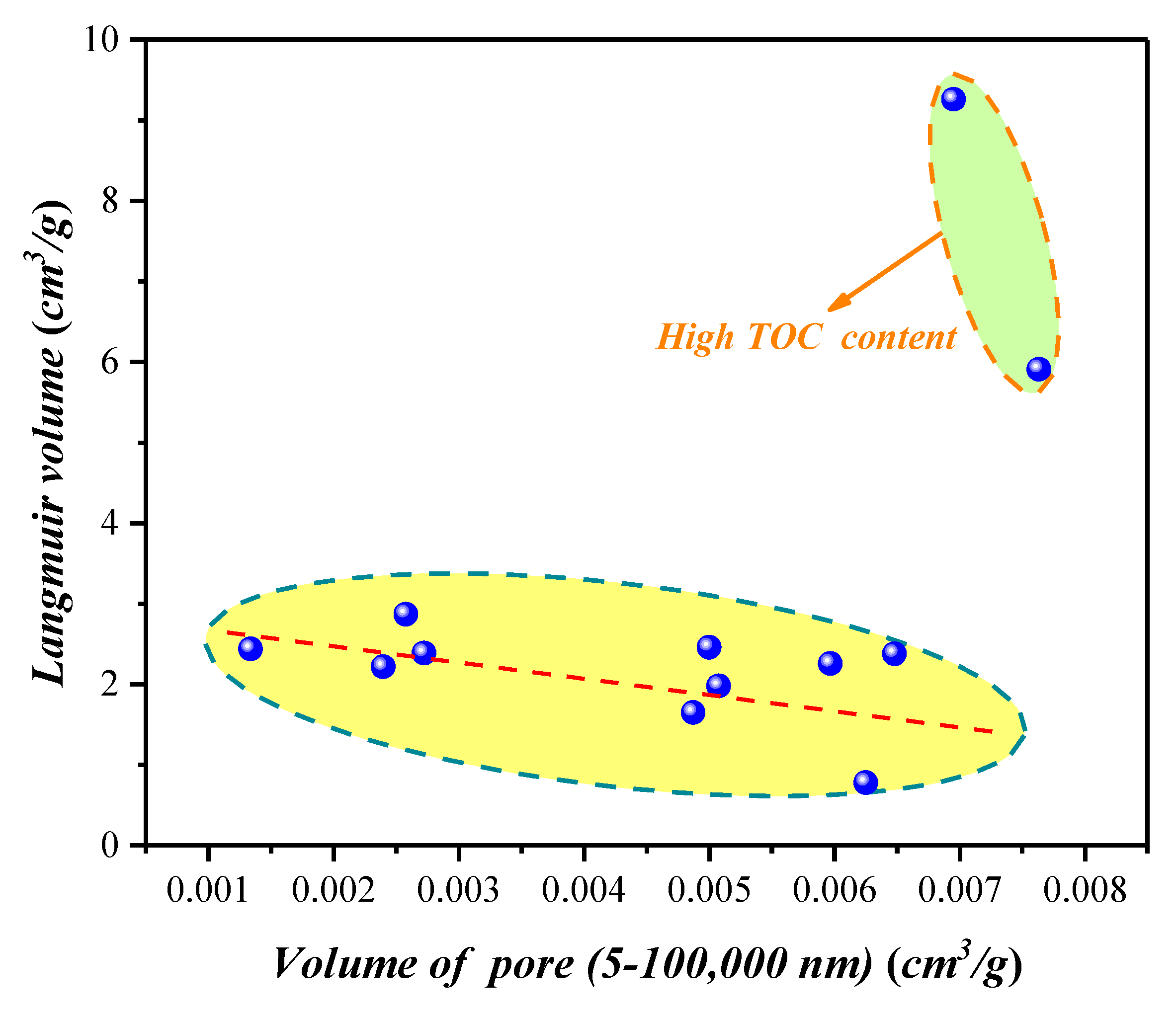
4.1.3. Compositional Influence on the Mesopores (5–50 nm) and Macropores (50–100,000 nm) Obtained by the HPMI Experiment
4.2. Compositional Effects on Methane Adsorption Capacity in Shale with Type III Kerogen
4.3. Nanopore Effect on Methane Adsorption Capacity in Shale with Type III Kerogen
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rexer, T.F.T.; Benham, M.J.; Aplin, A.C.; Thomas, K.M. Methane Adsorption on Shale under Simulated Geological Temperature and Pressure Conditions. Energy Fuel 2013, 27, 3099–3109. [Google Scholar] [CrossRef]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar]
- Li, Q.; Li, P.; Pang, W.; Bi, Q.; Du, Z.; Li, X.; Lu, D. A Novel Analytical Method to Calculate the Amounts of Free and Adsorbed Gas in Shale Gas Production. Math. Probl. Eng. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Chen, G.; Lu, S.; Zhang, J.; Xue, Q.; Han, T.; Xue, H.; Tian, S.; Li, J.; Xu, C.; Pervukhina, M. Keys to linking GCMC simulations and shale gas adsorption experiments. Fuel 2017, 199, 14–21. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, J.; Lu, S.; Pervukhina, M.; Liu, K.; Xue, Q.; Tian, H.; Tian, S.; Li, J.; Clennell, M.B.; et al. Adsorption Behavior of Hydrocarbon on Illite. Energy Fuel 2016, 30, 9114–9121. [Google Scholar] [CrossRef]
- Chalmers, G.R.; Bustin, R.M.; Power, I.M. Characterization of gas shale pore systems by porosimetry, pycnometry, surface area, and field emission scanning electron microscopy/transmission electron microscopy image analyses: Examples from the Barnett, Woodford, Haynesville, Marcellus, and Doig units. AAPG Bull. 2012, 96, 1099–1119. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, Genesis, and Distribution of Nanometer-Scale Pores in Siliceous Mudstones of the Mississippian Barnett Shale. J. Sediment Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Hu, H.; Hao, F.; Lin, J.; Lu, Y.; Ma, Y.; Li, Q. Organic matter-hosted pore system in the Wufeng-Longmaxi (O3w-S1l) shale, Jiaoshiba area, Eastern Sichuan Basin, China. Int. J. Coal Geol. 2017, 173, 40–50. [Google Scholar] [CrossRef]
- Guo, C.; Xu, J.; Wu, K.; Wei, M.; Liu, S. Study on gas flow through nano pores of shale gas reservoirs. Fuel 2015, 2015, 107–117. [Google Scholar] [CrossRef]
- Song, W.; Yao, J.; Ma, J.; Li, A.; Li, Y.; Sun, H.; Zhang, L. Grand canonical Monte Carlo simulations of pore structure influence on methane adsorption in micro-porous carbons with applications to coal and shale systems. Fuel 2018, 215, 196–203. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Chen, S.; Li, W. Characteristics of the Nanoscale Pore Structure in Northwestern Hunan Shale Gas Reservoirs Using Field Emission Scanning Electron Microscopy, High-Pressure Mercury Intrusion, and Gas Adsorption. Energy Fuel 2014, 28, 945–955. [Google Scholar] [CrossRef]
- Li, J.; Yan, X.; Wang, W.; Zhang, Y.; Yin, J.; Lu, S.; Chen, F.; Meng, Y.; Zhang, X.; Chen, X.; et al. Key factors controlling the gas adsorption capacity of shale: A study based on parallel experiments. Appl. Geochem. 2015, 2015, 88–96. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Liu, S.; Zhang, R. Pore characterization and its impact on methane adsorption capacity for organic-rich marine shales. Fuel 2016, 2016, 227–237. [Google Scholar] [CrossRef]
- Ji, W.; Song, Y.; Rui, Z.; Meng, M.; Huang, H. Pore characterization of isolated organic matter from high matured gas shale reservoir. Int. J. Coal Geol. 2017, 174, 31–40. [Google Scholar] [CrossRef]
- Robert, G.; Loucks, R.M.R.S.; Hammes, A.U. Spectrum of pore types and networks in mudrocks and a descriptive classification for matrix-related mudrock pores. AAPG Bull. 2012, 96, 1071–1098. [Google Scholar]
- Zhang, J.; Li, X.; Xie, Z.; Li, J.; Zhang, X.; Sun, K.; Wang, F. Characterization of microscopic pore types and structures in marine shale: Examples from the Upper Permian Dalong formation, Northern Sichuan Basin, South China. J. Nat. Gas Sci. Eng. 2018, 59, 326–342. [Google Scholar] [CrossRef]
- Zhu, H.; Ju, Y.; Qi, Y.; Huang, C.; Zhang, L. Impact of tectonism on pore type and pore structure evolution in organic-rich shale: Implications for gas storage and migration pathways in naturally deformed rocks. Fuel 2018, 228, 272–289. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Liu, H. Fractal characteristics of shales from a shale gas reservoir in the Sichuan Basin, China. Fuel 2014, 115, 378–384. [Google Scholar] [CrossRef]
- Zhou, S.; Ning, Y.; Wang, H.; Liu, H.; Xue, H. Investigation of methane adsorption mechanism on Longmaxi shale by combining the micropore filling and monolayer coverage theories. Adv. Geo-Energy Res. 2018, 2, 269–281. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Z.; Liu, K.; Gao, F. Quantitative characterization of micropore structure for organic-rich Lower Silurian shale in the Upper Yangtze Platform, South China: Implications for shale gas adsorption capacity. Adv. Geo-Energy Res. 2017, 1, 112–123. [Google Scholar] [CrossRef]
- Ji, W.; Song, Y.; Jiang, Z.; Chen, L.; Li, Z.; Yang, X.; Meng, M. Estimation of marine shale methane adsorption capacity based on experimental investigations of Lower Silurian Longmaxi formation in the Upper Yangtze Platform, south China. Mar. Petrol. Geol. 2015, 2015, 94–106. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, X.; Liang, L.; Zeng, Q. Methane Adsorption on Carbon Models of the Organic Matter of Organic-Rich Shales. Energy Fuel 2017, 31, 1489–1501. [Google Scholar] [CrossRef]
- Li, P.; Jiang, Z.; Zheng, M.; Bi, H.; Chen, L. Estimation of shale gas adsorption capacity of the Longmaxi Formation in the Upper Yangtze Platform, China. J. Nat. Gas Sci. Eng. 2016, 34, 1034–1043. [Google Scholar] [CrossRef]
- Cao, T.; Song, Z.; Wang, S.; Cao, X.; Li, Y.; Xia, J. Characterizing the pore structure in the Silurian and Permian shales of the Sichuan Basin, China. Mar. Petrol. Geol. 2015, 61, 140–150. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chen, D.X.; Qiu, N.S.; Wang, Y.; Fu, J.; Huyan, Y.; Jia, J.K.; Wu, H. Reservoir characteristics and methane adsorption capacity of the Upper Triassic continental shale in Western Sichuan Depression, China. Aust. J. Earth Sci. 2017, 64, 807–823. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.S.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Marc Bustin, R. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Petrol. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Shabani, M.; Moallemi, S.A.; Krooss, B.M.; Amann-Hildenbrand, A.; Zamani-Pozveh, Z.; Ghalavand, H.; Littke, R. Methane sorption and storage characteristics of organic-rich carbonaceous rocks, Lurestan province, southwest Iran. Int. J. Coal Geol. 2018, 2018, 51–64. [Google Scholar] [CrossRef]
- Tan, J.; Weniger, P.; Krooss, B.; Merkel, A.; Horsfield, B.; Zhang, J.; Boreham, C.J.; Graas, G.V.; Tocher, B.A. Shale gas potential of the major marine shale formations in the Upper Yangtze Platform, South China, Part II: Methane sorption capacity. Fuel 2014, 129, 204–218. [Google Scholar] [CrossRef]
- Hu, H.; Hao, F.; Guo, X.; Dai, F.; Lu, Y.; Ma, Y. Investigation of methane sorption of overmature Wufeng-Longmaxi shale in the Jiaoshiba area, Eastern Sichuan Basin, China. Mar. Petrol. Geol. 2018, 91, 251–261. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, T.; Milliken, K.L.; Qu, J.; Zhang, X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Gasparik, M.; Ghanizadeh, A.; Bertier, P.; Gensterblum, Y.; Bouw, S.; Krooss, B.M. High-Pressure Methane Sorption Isotherms of Black Shales from The Netherlands. Energy Fuel 2012, 26, 4995–5004. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, X.; Li, Y.; Wu, K.; Shi, J.; Yang, L.; Feng, D.; Zhang, T.; Yu, P. Water distribution characteristic and effect on methane adsorption capacity in shale clay. Int. J. Coal Geol. 2016, 2016, 135–154. [Google Scholar] [CrossRef]
- Yang, R.; He, S.; Hu, Q.; Hu, D.; Zhang, S.; Yi, J. Pore characterization and methane sorption capacity of over-mature organic-rich Wufeng and Longmaxi shales in the southeast Sichuan Basin, China. Mar. Petrol. Geol. 2016, 77, 247–261. [Google Scholar] [CrossRef]
- Caineng, Z.; Dazhong, D.; Shejiao, W.; Denghua, L.; Keming, C. Geological characteristics and resource potential of shale gas in China. ScienceDirect 2010, 37, 641–653. [Google Scholar]
- Yu, W.; Sepehrnoori, K.; Patzek, T.W. Modeling Gas Adsorption in Marcellus Shale with Langmuir and BET Isotherms. Isotherms SPE J. 2016, 21, 589–600. [Google Scholar] [CrossRef]
- Peischl, J.; Ryerson, T.B.; Aikin, K.C.; de Gouw, J.A.; Gilman, J.B.; Holloway, J.S.; Lerner, B.M.; Nadkarni, R.; Neuman, J.A.; Nowak, J.B.; et al. Quantifying atmospheric methane emissions from the Haynesville, Fayetteville, and northeastern Marcellus shale gas production regions. J. Geophys. Res. Atmos. 2015, 120, 2119–2139. [Google Scholar] [CrossRef]
- Lewan, M.D.; Pawlewicz, M.J. Reevaluation of thermal maturity and stages of petroleum formation of the Mississippian Barnett Shale, Fort Worth Basin, Texas. AAPG Bull. 2017, 101, 1945–1970. [Google Scholar] [CrossRef]
- WANG, H.; WANG, Y.; LI, X.; CHEN, B.; WU, W.; DONG, D.; ZHANG, J.; HAN, J.; MA, J.; DAI, B.; et al. Lower limit of thermal maturity for the carbonization of organic matter in marine shale and its exploration risk. Pet. Explor. Dev. Online 2018, 45, 402–411. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Liu, L.; Chen, L.; Chen, J. Evolution characteristics and model of nanopore structure and adsorption capacity in organic-rich shale during artificial thermal maturation: A pyrolysis study of the Mesoproterozoic Xiamaling marine shale with type II kerogen from Zhangjiakou, Hebei, China. Energy Explor. Exploit. 2018, 37, 493–518. [Google Scholar] [CrossRef]
- Petersen, H.I.; Hertle, M.; Sulsbrück, H. Upper Jurassic–lowermost Cretaceous marine shale source rocks (Farsund Formation), North Sea: Kerogen composition and quality and the adverse effect of oil-based mud contamination on organic geochemical analyses. Int. J. Coal Geol. 2017, 173, 26–39. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Zhao, H.; Li, M. Molecular simulation of adsorption and thermodynamic properties on type II kerogen: Influence of maturity and moisture content. Fuel 2017, 190, 198–207. [Google Scholar] [CrossRef]
- Gasparik, M.; Bertier, P.; Gensterblum, Y.; Ghanizadeh, A.; Krooss, B.M.; Littke, R. Geological controls on the methane storage capacity in organic-rich shales. Int. J. Coal Geol. 2014, 123, 34–51. [Google Scholar] [CrossRef]
- Guo, H.; Jia, W.; Peng, P.; Lei, Y.; Luo, X.; Cheng, M.; Wang, X.; Zhang, L.; Jiang, C. The composition and its impact on the methane sorption of lacustrine shales from the Upper Triassic Yanchang Formation, Ordos Basin, China. Mar. Petrol. Geol. 2014, 57, 509–520. [Google Scholar] [CrossRef]
- Chalmers, G.R.L.; Bustin, R.M. Lower Cretaceous gas shales in northeastern British Columbia, Part I: Geological controls on methane sorption capacity. Bull. Can. Pet. Geol. 2008, 56, 1–21. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Car, R.; Parrinello, M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Tang, X.; Ripepi, N.; Stadie, N.P.; Yu, L.; Hall, M.R. A dual-site Langmuir equation for accurate estimation of high pressure deep shale gas resources. Fuel 2016, 185, 10–17. [Google Scholar] [CrossRef]
- Hou, X.; Liu, S.; Zhu, Y.; Yang, Y. Experimental and theoretical investigation on sorption kinetics and hysteresis of nitrogen, methane, and carbon dioxide in coals. Fuel 2020, 268, 117349. [Google Scholar] [CrossRef]
- Tang, X.; Jiang, Z.; Li, Z.; Gao, Z.; Bai, Y.; Zhao, S.; Feng, J. The effect of the variation in material composition on the heterogeneous pore structure of high-maturity shale of the Silurian Longmaxi formation in the southeastern Sichuan Basin, China. J. Nat. Gas Sci. Eng. 2015, 23, 464–473. [Google Scholar] [CrossRef]
- Kuila, U.; McCarty, D.K.; Derkowski, A.; Fischer, T.B.; Topór, T.; Prasad, M. Nano-scale texture and porosity of organic matter and clay minerals in organic-rich mudrocks. Fuel 2014, 135, 359–373. [Google Scholar] [CrossRef]
- Schmitt, M.; Fernandes, C.P.; Neto, J.A.B.D.; Wolf, F.G.; Santos, V.S.S.D. Characterization of pore systems in seal rocks using Nitrogen Gas Adsorption combined with Mercury Injection Capillary Pressure techniques. Mar. Petrol. Geol. 2013, 39, 138–149. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, T.; Sun, Y.; Milliken, K.L.; Sun, D. Geochemical evidence of organic matter source input and depositional environments in the lower and upper Eagle Ford Formation, south Texas. Org. Geochem. 2016, 98, 66–81. [Google Scholar] [CrossRef]
- Bechtel, A.; Oberauer, K.; Kostić, A.; Gratzer, R.; Milisavljević, V.; Aleksić, N.; Stojanović, K.; Groß, D.; Sachsenhofer, R.F. Depositional environment and hydrocarbon source potential of the Lower Miocene oil shale deposit in the Aleksinac Basin (Serbia). Org. Geochem. 2018, 115, 93–112. [Google Scholar] [CrossRef]
- Gao, G.; Titi, A.; Yang, S.; Tang, Y.; Kong, Y.; He, W. Geochemistry and depositional environment of fresh lacustrine source rock: A case study from the Triassic Baijiantan Formation shales in Junggar Basin, northwest China. Org. Geochem. 2017, 113, 75–89. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, J.; Liang, L. Investigation of pore structure and fractal characteristics of organic-rich Yanchang formation shale in central China by nitrogen adsorption/desorption analysis. J. Nat. Gas Sci. Eng. 2015, 22, 62–72. [Google Scholar] [CrossRef]
- Tian, H.; Pan, L.; Xiao, X.; Wilkins, R.W.T.; Meng, Z.; Huang, B. A preliminary study on the pore characterization of Lower Silurian black shales in the Chuandong Thrust Fold Belt, southwestern China using low pressure N2 adsorption and FE-SEM methods. Mar. Petrol. Geol. 2013, 48, 8–19. [Google Scholar] [CrossRef]
- Chalmers, G.R.L.; Ross, D.J.K.; Bustin, R.M. Geological controls on matrix permeability of Devonian Gas Shales in the Horn River and Liard basins, northeastern British Columbia, Canada. Int. J. Coal Geol. 2012, 103, 120–131. [Google Scholar] [CrossRef]
- Hou, X.; Liu, S.; Zhu, Y.; Yang, Y. Evaluation of gas contents for a multi-seam deep coalbed methane reservoir and their geological controls: In situ direct method versus indirect method. Fuel 2020, 265, 116917. [Google Scholar] [CrossRef]
- Rani, S.; Padmanabhan, E.; Prusty, B.K. Review of gas adsorption in shales for enhanced methane recovery and CO2 storage. J. Petrol. Sci. Eng. 2019, 175, 634–643. [Google Scholar] [CrossRef]
- Ma, Y.; Zhong, N.; Li, D.; Pan, Z.; Cheng, L.; Liu, K. Organic matter/clay mineral intergranular pores in the Lower Cambrian Lujiaping Shale in the north-eastern part of the upper Yangtze area, China: A possible microscopic mechanism for gas preservation. Int. J. Coal Geol. 2015, 137, 38–54. [Google Scholar] [CrossRef]
- Rahman, H.M.; Kennedy, M.; Löhr, S.; Dewhurst, D.N.; Sherwood, N.; Yang, S.; Horsfield, B. The influence of shale depositional fabric on the kinetics of hydrocarbon generation through control of mineral surface contact area on clay catalysis. Geochim. Cosmochim. AC 2018, 220, 429–448. [Google Scholar] [CrossRef]
- Rahman, H.M.; Kennedy, M.; Löhr, S.; Dewhurst, D.N. Clay-organic association as a control on hydrocarbon generation in shale. Org. Geochem. 2017, 105, 42–55. [Google Scholar] [CrossRef]
- Zhu, X.; Cai, J.; Wang, G.; Song, M. Role of organo-clay composites in hydrocarbon generation of shale. Int. J. Coal Geol. 2018, 192, 83–90. [Google Scholar] [CrossRef]






| Sample ID | TOC (wt %) | Mineral Components (wt %) | Clay Components (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | Plagioclase | Dolomite | Siderite | Pyrite | Total Clay | Illite | Kaolinite | Mixed-Layer Illite/Smectite | ||
| T-1 | 1.45 | 27 | 0 | 0 | 0 | 0 | 73 | 24 | 62 | 14 |
| T-2 | 1.42 | 44 | 3 | 0 | 1 | 0 | 52 | 26 | 51 | 23 |
| T-3 | 2.33 | 33 | 0 | 0 | 0 | 0 | 67 | 21 | 58 | 21 |
| T-4 | 4.50 | 41 | 0 | 0 | 5 | 0 | 54 | 37 | 43 | 20 |
| T-5 | 0.70 | 37 | 12 | 0 | 4 | 0 | 47 | 34 | 35 | 31 |
| T-6 | 3.64 | 26 | 4 | 0 | 0 | 0 | 70 | 26 | 41 | 33 |
| T-7 | 6.96 | 42 | 4 | 0 | 0 | 0 | 54 | 36 | 31 | 33 |
| T-8 | 3.25 | 30 | 3 | 0 | 0 | 0 | 67 | 25 | 41 | 34 |
| T-9 | 1.29 | 34 | 5 | 0 | 3 | 0 | 58 | 35 | 38 | 27 |
| T-10 | 0.92 | 29 | 3 | 0 | 3 | 0 | 65 | 51 | 44 | 5 |
| T-11 | 35.84 | 21 | 0 | 3 | 0 | 3 | 73 | 8 | 92 | 0 |
| T-12 | 23.96 | 20 | 0 | 0 | 0 | 10 | 70 | 44 | 47 | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Zhu, Y.; Liu, Y.; Wang, Y.; Zhang, H.; Yu, W. Nanostructure Effect on Methane Adsorption Capacity of Shale with Type III Kerogen. Energies 2020, 13, 1690. https://doi.org/10.3390/en13071690
Han Y, Zhu Y, Liu Y, Wang Y, Zhang H, Yu W. Nanostructure Effect on Methane Adsorption Capacity of Shale with Type III Kerogen. Energies. 2020; 13(7):1690. https://doi.org/10.3390/en13071690
Chicago/Turabian StyleHan, Yong, Yanming Zhu, Yu Liu, Yang Wang, Han Zhang, and Wenlong Yu. 2020. "Nanostructure Effect on Methane Adsorption Capacity of Shale with Type III Kerogen" Energies 13, no. 7: 1690. https://doi.org/10.3390/en13071690
APA StyleHan, Y., Zhu, Y., Liu, Y., Wang, Y., Zhang, H., & Yu, W. (2020). Nanostructure Effect on Methane Adsorption Capacity of Shale with Type III Kerogen. Energies, 13(7), 1690. https://doi.org/10.3390/en13071690






