Isomerization of n-C5/C6 Bioparaffins to Gasoline Components with High Octane Number
Abstract
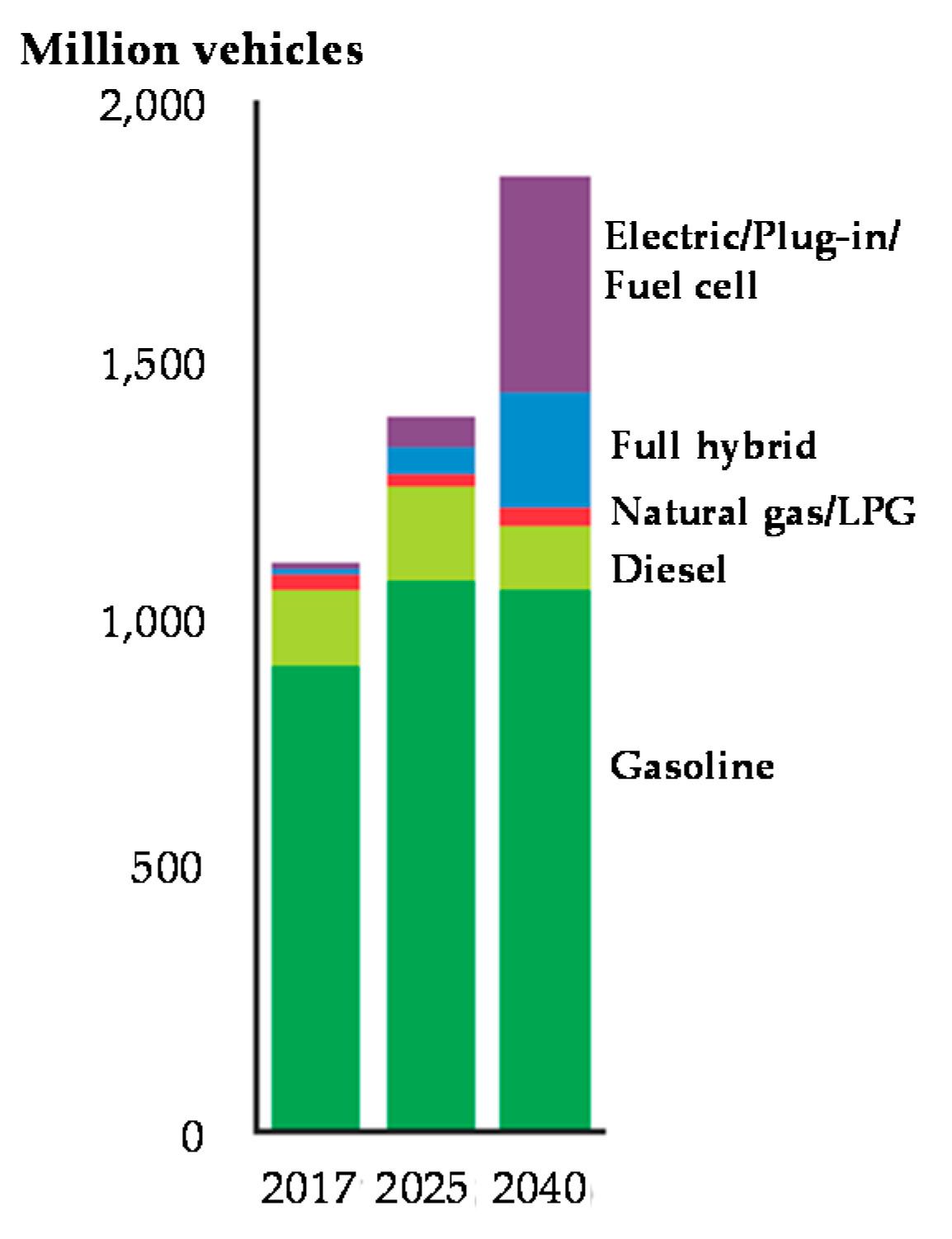
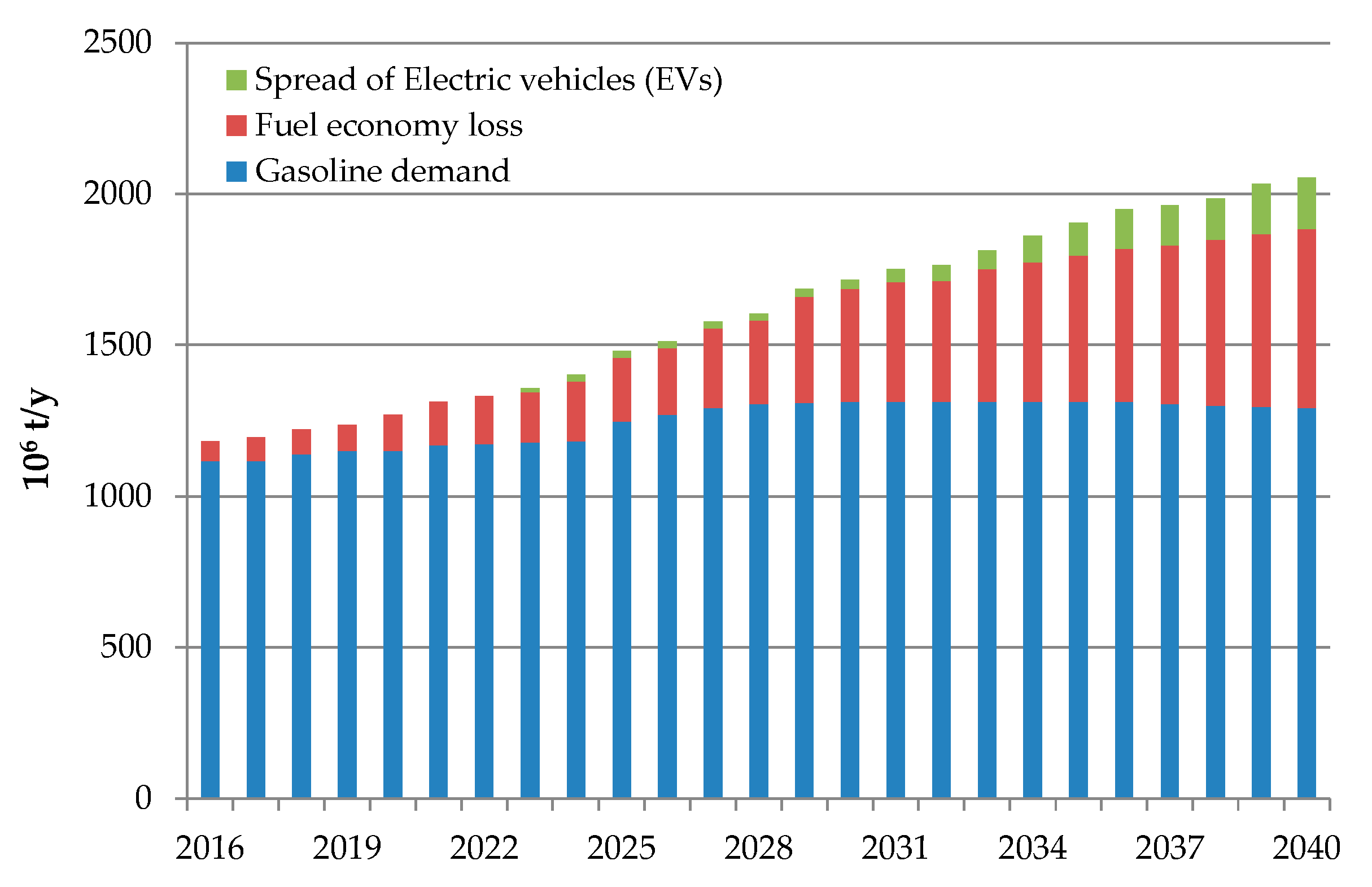
1. Introduction
- From waste or biomass-derived syngas by Fischer–Tropsch (F–T) synthesis via direct synthesis, which results in the so-called C5-C7 fraction, F–T light gasoline [11], or, eventually, via indirect route by F–T synthesis combined with the hydrocracking of heavy F–T wax [12]. The oligomerization and hydrogenation of light olefins (C2=–C3=) obtained from F–T synthesis can also yield light hydrocarbons [13].
- From the oligomerization of fuel gas rich in light olefins such as ethylene, propylene, or butylene obtained from bio-oil or other bio-originated feedstock, e.g., rice straw biomass [14]. The obtained iso-olefins need to be hydrogenated to iso-alkanes.
- Another possibility is the oligomerization and subsequent hydrogenation of light gasoline fractions having a high olefin content obtained from thermal/catalytic cracking of waste polyolefins with different structures, such as polyethylene or polypropylene [15].
- Gasoline-range hydrocarbons can be produced from sorbitol, a sugar-based compound from lignocellulosic biomass, via hydrodeoxygenation over Zr-phosphate-supported Pd-bimetallic (Pd+ Pt/Ru/Ni/W etc.) catalysts [16]. The product mixture contains a large number of individual components with very different structures, e.g., corrosive acids and compounds in the aqueous phase. The treating and separation of the products could require complex and expensive solutions.
- Alkanes can be obtained from bioethanol via dehydration, oligomerization, and hydrogenation of olefinic double bounds [19].
- N-alkanes can be manufactured through simple sugars from lignocellulose applying a very complex and expensive production process: acid-catalyzed dehydration, Aldol condensation (base catalyst), hydrogenation on metal catalyst, dehydration, and hydrogenation (acid and metal catalysts). Such products have very a low octane number, which should be increased by the catalytic process [20].
- Paraffins can be obtained from long-chain fatty aldehydes by oxidative deformylation [21].
- Liquid alkanes (≤ C13) can be produced from CO2 and H2O on Co/TiO2 catalyst by the Solar Photothermochemical Alkane Reverse Combustion (SPARC) method [22].
- Alkanes can be obtained from C5-C6 sugars through hydroxymethylfurfural, levulinic acid, and gamma-valerolactone.
2. Experimental Part
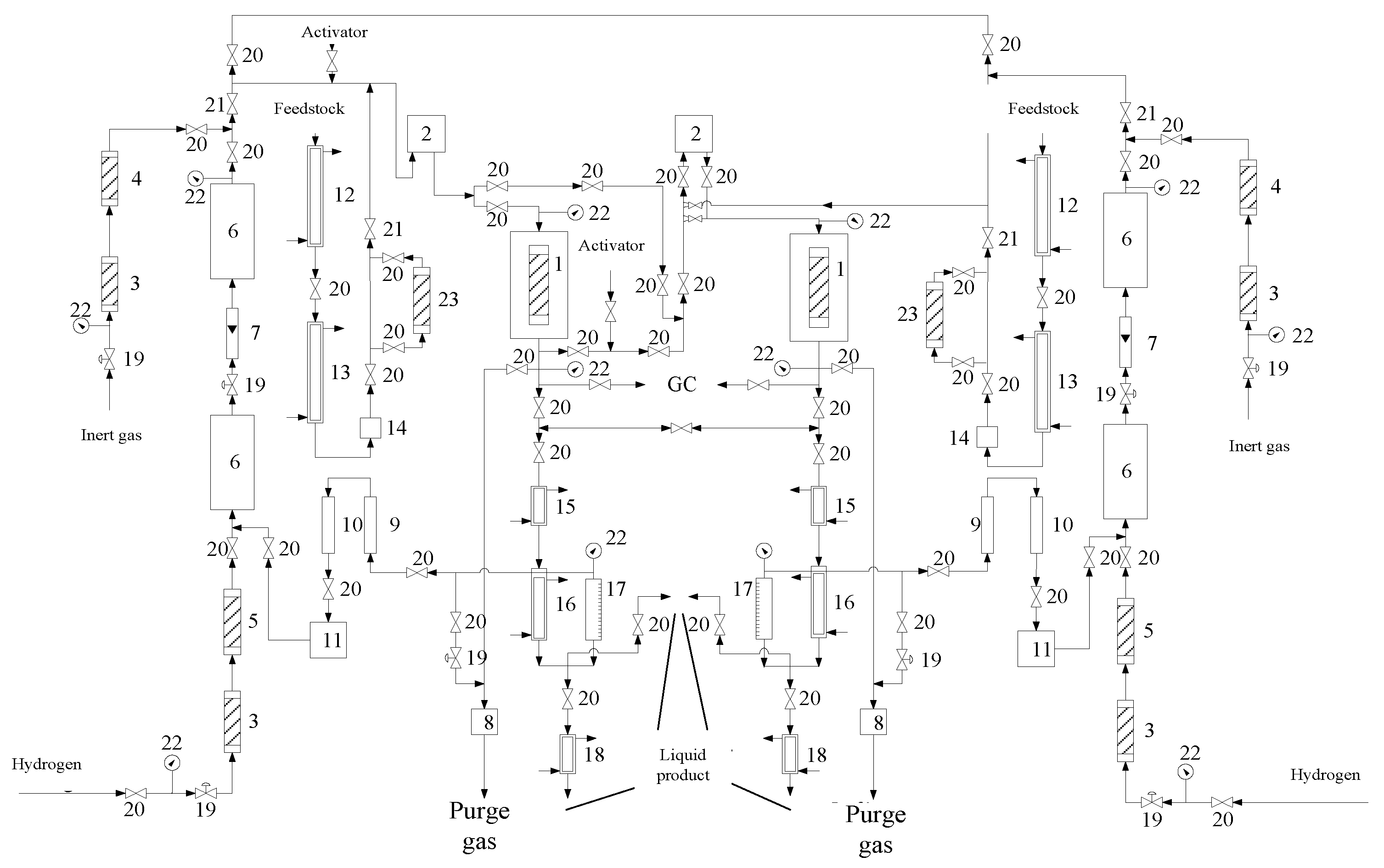
2.1. Apparatus
2.2. Materials and Methods
2.2.1. Feedstock
2.2.2. Catalysts and Adsorbents
2.2.3. Process Parameters
2.2.4. Analytical and Calculation Methods
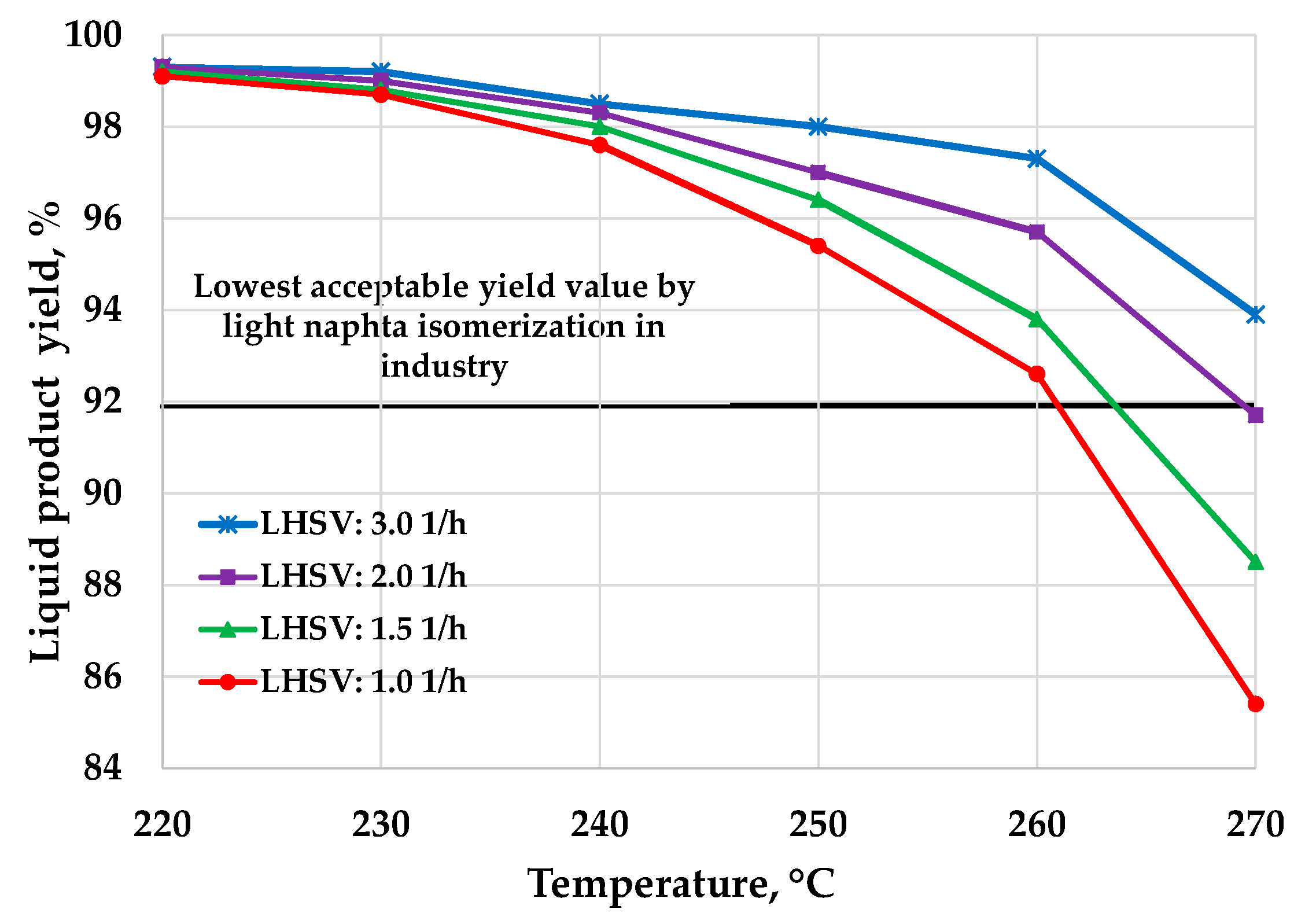
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- BP Statistical Review of World Energy. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.pdf (accessed on 9 March 2020).
- ExxonMobil, Outlook for Energy 2019: A Perspective to 2040. Available online: https://corporate.exxonmobil.com/-/media/Global/Files/outlook-for-energy/2019-Outlook-for-Energy_v4.pdf (accessed on 5 March 2020).
- Be Future Forward, Honeywell UOP. Available online: https://www.digitalrefining.com/article_1002373.pdf (accessed on 6 January 2020).
- Srivastava, S.P.; Hancsók, J. Fuels and Fuel-Additives; John Wiley & Sons: Hoboken, NJ, USA, 2014; p. 374. [Google Scholar]
- European Parliament, 2018, Directive of the European Parliament and of the Council cast, 2016/0382 (COD). Available online: http://data.consilium.europa.eu/doc/document/PE-48-2018-INIT/en/pdf (accessed on 5 February 2020).
- Mansouri, A.; Rihani, R.; Laoufi, A.N.; Özkan, M. Production of bioethanol from a mixture of agricultural feedstocks: Biofuels characterization. FUEL 2016, 185, 612–621. [Google Scholar] [CrossRef]
- Abdellatif, F.H.H.; Babin, J.; Arnal-Herault, C.; Nouvel, C.; Six, J.-L.; Jonquieres, A. Bio-based membranes for ethyl-tert-butyl-ether (ETBE) bio-fuel purification by pervaporation. J. Membr. Sci. 2017, 524, 449–459. [Google Scholar] [CrossRef]
- Manzetti, S.; Andersen, O. A review of emission products from bioethanol and its blends with gasoline. Background for new guidelines for emission control. FUEL 2015, 140, 293–301. [Google Scholar] [CrossRef]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef] [PubMed]
- Padella, M.; O’Connell, A.; Prussi, M. What is still Limiting the Deployment of Cellulosic Ethanol? Analysis of the Current Status of the Sector. Appl. Sci. 2019, 9, 4523. [Google Scholar] [CrossRef]
- Sauciuc, A.; Abosteif, Z.; Weber, G.; Potetz, A.; Rauch, R.; Hofbauer, H.; Schaub, G.; Dumitrescu, L. Influence of operating conditions on the performance of biomass-based Fischer-Tropsch synthesis. Biomass. Convers. Bior. 2012, 2, 253–263. [Google Scholar] [CrossRef]
- Ershov, M.; Potanin, D.; Gueseva, A.; Abdellatief, T.M.M.; Kapustin, V. Novel strategy to develop the technology of high-ocatane alternative fuel based on low-octane gasoline Fischer-Tropsch process. FUEL 2020, 261, 116330. [Google Scholar] [CrossRef]
- Atashi, H.; Dinarvandi, K. Determination of selectivity equations heavy and light product of petroleum on iron based catalysts in Fischer-Tropsch synthesis. Petrol. Sci. Technol. 2019, 37, 2035–2042. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Q.; Ding, M.; Wang, C.; Li, Y.; Zhang, Q.; Ma, L. Bio-gasoline production by coupling of biomass catalytic pyrolysis and oligomerization. Energy Proced. 2017, 105, 858–863. [Google Scholar] [CrossRef]
- Fehér, C.; Tomasek, S.; Hancsók, J.; Skoda-Földes, R. Oligomerization of light olefins in the presence of a supported Brønsted acidic ionic liquid catalyst. Appl. Catal. B-Environ. 2018, 239, 52–60. [Google Scholar] [CrossRef]
- Kwon, E.E.; Kim, Y.T.; Kim, H.J.; Lin, K.Y.L.; Kim, K.H.; Lee, J.; Huber, G.W. Production of high-octane gasoline via hydrodeoxygenation of sorbitol over palladium-based bimetallic catalysts. J. Environ. Manage. 2018, 227, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Herz, G.; Reichelt, E.; Jahn, M. Design and evaluation of a Power-to-Liquid process for the production of valuable hydrocarbons from CO2 and H2O. DGMK Tag. 2017, 2, 181–186. [Google Scholar]
- Mesfun, S.; Sanchez, D.L.; Leduc, S.; Wetterlund, E.; Lundgren, J.; Biberacher, M.; Kraxner, F. Power-to-gas and power-to-liquid for managing renewable electricity intermittency in the Alpine Region. Renew Energy 2017, 107, 361–372. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Zamani, A.; Taherzadeh, M.J. Bioethylene Production from Ethanol: A Review and Techno-economical Evaluation. Chem. Bio. Eng. Rev. 2017, 4, 75–91. [Google Scholar] [CrossRef]
- Huber, G.W.; Chedda, J.N.; Barre, C.J.; Dumestic, J.A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Shokri, D.; Que, L. Conversion of Aldehyde to Alkane by a Peroxoiron(III) Complex: A Functional Model for the Cyanobacterial Aldehyde-Deformylating Oxygenase. J. Am. Chem. Soc. 2015, 137, 7686–7691. [Google Scholar] [CrossRef]
- Chanmanee, W.; Islam, M.F.; Dennis, B.H.; MacDonell, F.M. Solar photothermochemical alkane reverse combustion. PNAS 2016, 113, 2579–2584. [Google Scholar] [CrossRef]
- Bezergianni, S.; Kalogianni, A. Hydrocracking of used cooking oil for biofuels production. Bioresour. Technol. 2009, 100, 3927–3932. [Google Scholar] [CrossRef]
- Rosson, E.; Sgarbossa, P.; Pedrielli, F.; Mozzon, M.; Bertani, R. Bioliquids from raw waste animal fats: An alternative renewable energy source. Biomass Conv. Bioref. 2020. [Google Scholar] [CrossRef]
- Baladincz, P.; Hancsók, J. Fuel from waste animal fats. Chem. Eng. J. 2015, 282, 152–160. [Google Scholar] [CrossRef]
- Jeffery, G.H.; Bassett, J.; Mendham, J.; Denney, R.C. Vogel’s Textbook of Quantitative Chemical Analysis, 5th ed.; Longman Scientific & Technical: New York, NY, USA, 1989; pp. 355–356. [Google Scholar]
- Hancsók, J.; Holló, A.; Debreczeni, É.; Perger, J.; Kalló, D. Benzene saturating isomerization. Stud. Surf. Sci. Catal. 1999, 125, 417–424. [Google Scholar]
- Holló, A.; HancsóK, J.; Kalló, D. Kinetics of hydroisomerization of C5-C7 alkanes and their mixtures over platinum containing mordenite. Appl. Catal. A-Gen. 2002, 229, 93–102. [Google Scholar]


| Characteristics | Feedstock | |
|---|---|---|
| A | B | |
| Composition, % | ||
| C1-C4 | 0.2 | 0.2 |
| i-C5 | 3.9 | 4.0 |
| n-C5 | 48.6 | 48.1 |
| 2,2-dimethylbutane (2,2-DMB) | <0.01 | <0.01 |
| 2,3-dimethylbutane (2,3-DMB) | 0.2 | 0.3 |
| 2-methylpentane (2-MP) | 4.1 | 3.9 |
| 3-methylpentane (3-MP) | 2.7 | 2.6 |
| n-C6 | 36.8 | 37.3 |
| methyl-cyclopentane | 0.3 | 0.2 |
| cyclohexane | 1.0 | 1.1 |
| benzene | 1.3 | 1.4 |
| ΣC7 | 0.9 | 0.9 |
| Oxygen-containing compounds, mg/kg | n.d. | 41 |
| Sulfur content, mg/kg | <1.0 | 2.5 |
| Water content, mg/kg | <1.0 | 14 |
| C5 paraffin hydrocarbons in feedstock, % | 52.5 | 52.1 |
| Research octane number (RON) | 48.4 | 48.6 |
| Motor octane number (MON) | 47.3 | 47.5 |
| Sensibility (RON-MON) | 1.1 | 1.1 |
| Paraffin hydrocarbons, % | 98.7 | 98.6 |
| Total liquid i-, c-paraffin content, % | 12.2 | 12.1 |
| Properties | Pt/Al2O3/Cl | Pt/H-Mordenite/Al2O3 |
|---|---|---|
| Pt-content, % | 0.28 | 0.38 |
| Pt-dispersion, % | 84 | 89 |
| Specific surface area (BET), m2/g | 426 | 448 |
| Si/Al molar ratio | - | 19.5 |
| Chlorine content, % (Reactor I/Reactor II) | 3.1/7.6 | - |
| Length/diameter of catalyst extrudates (average, mm) | 3.9/1.8 | 4.8/2.4 |
| Process Parameters | Pt/Al2O3/Cl | Pt/H-Mordenite/Al2O3 |
|---|---|---|
| Temperature, °C | 115–145 | 220–270 |
| LHSV 1, h−1 | 1.0–3.0 2 | 1.0–3.0 |
| Pressure, bar | 30 | 30 |
| H2/feedstock molar ratio | 0.15:1.0 | 1.0:1.0 |
| Liquid Hourly Space Velocity 1, h−1. | Yield of Liquid Products, % | |||
|---|---|---|---|---|
| 115 °C | 125 °C | 135 °C | 145 °C | |
| 1.0 | 98.6 | 98.1 | 97.6 | 97.2 |
| 1.33 | 98.8 | 98.3 | 97.9 | 97.4 |
| 1.66 | 99.1 | 98.6 | 98.2 | 97.7 |
| 2.0 | 99.4 | 98.9 | 98.5 | 98.0 |
| LHSV, h−1 | Yield of Gas Products, % | |||||
|---|---|---|---|---|---|---|
| 220 °C | 230 °C | 240 °C | 250 °C | 260 °C | 270 °C | |
| 1.0 | 0.9 | 1.3 | 2.4 | 4.6 | 7.4 | 14.6 |
| 1.5 | 0.8 | 1.2 | 2.0 | 3.6 | 6.2 | 11.5 |
| 2.0 | 0.7 | 1.0 | 1.7 | 3.0 | 4.3 | 8.3 |
| 3.0 | 0.7 | 0.8 | 1.5 | 2.0 | 2.7 | 6.1 |
| Characteristics | Products | |
|---|---|---|
| Pt/Al2O3/Cl | Pt/H-Mordenite | |
| Liquid yield, % (0 % recirculation) | 97.6–98.3 | 92.9–93.8 |
| ATEC, % | ||
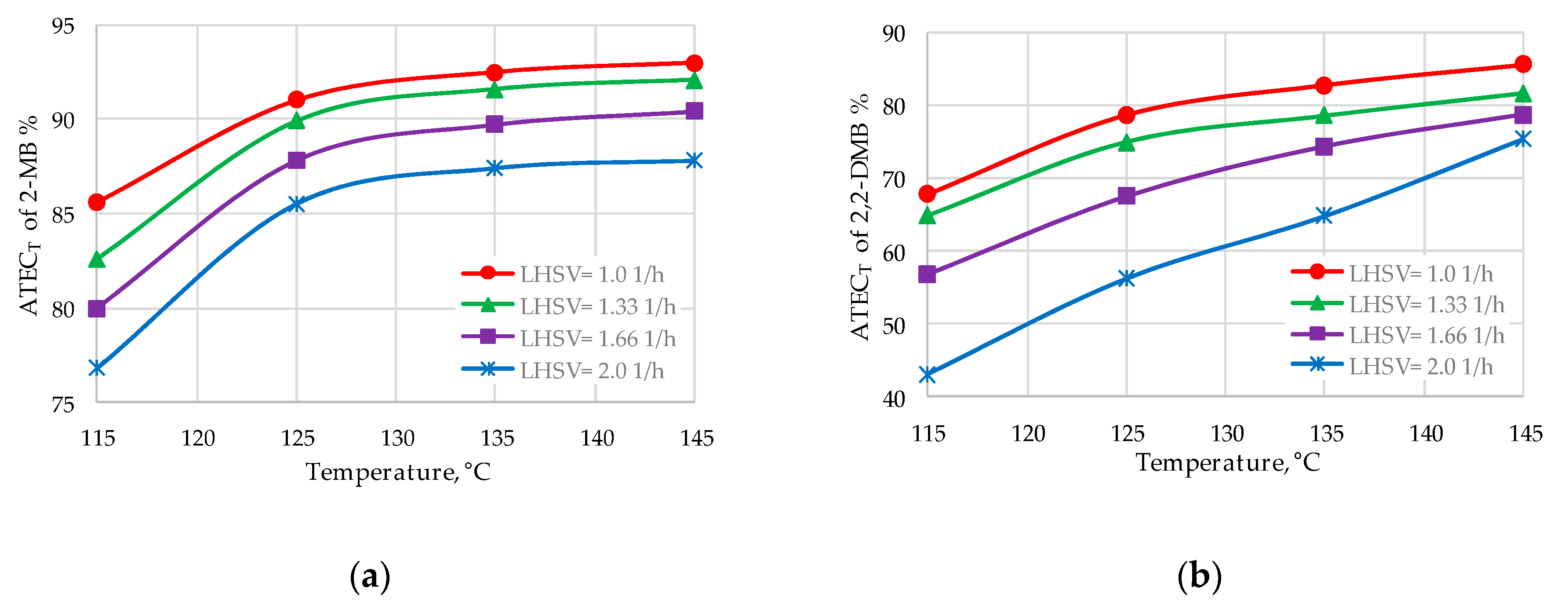
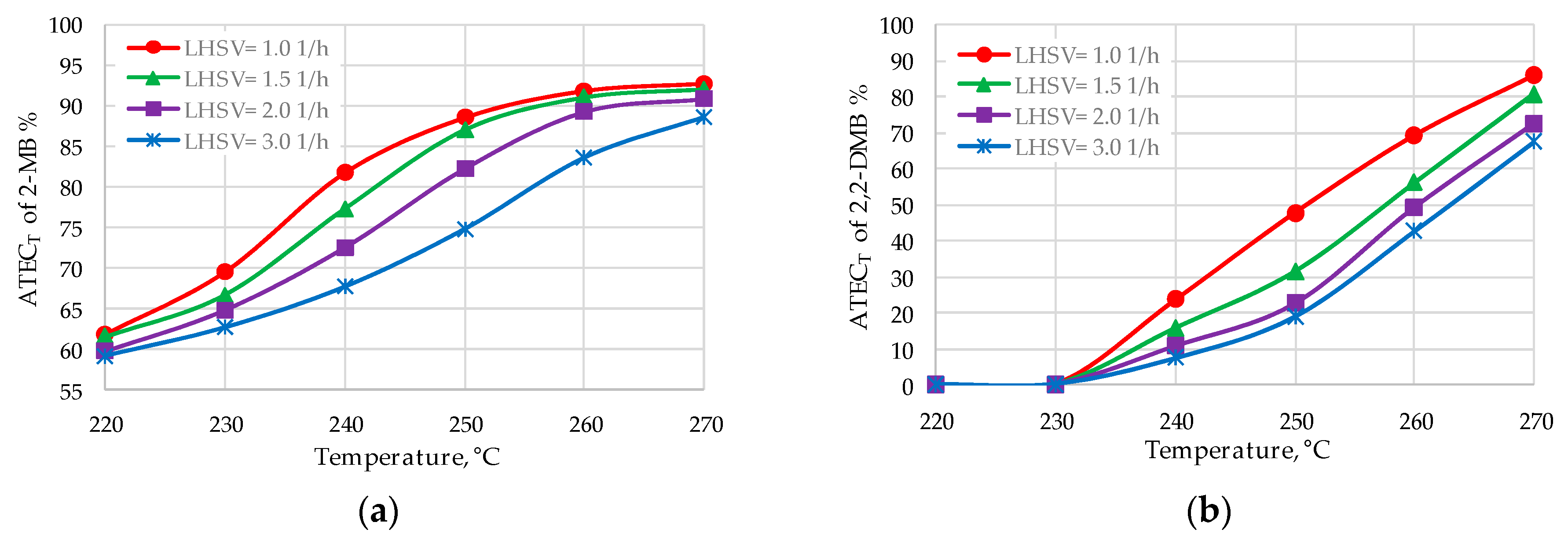
| 2-MB | 89.9–92.5 | 90.5–91.8 |
| 2,2-DMB | 75.0–82.8 | 57.6–70.4 |
| 2-MP | 89.3–97.3 | 90.5–92.7 |
| RON/MON (0 % recirculation) | 79.4–80.9/78.1–79.5 | 74.9–76.4/73.9–75.4 |
| Sensibility (RON-MON) | 1.3–1.4 | 1.1–1.0 |
| ΔRON/ΔMON (product-feedstock) | 30.8–32.3/29.5–32.0 | 24.7–28.0/24.7–28.1 |
| Liquid yield, % (95 % n-Cx recirculation) | 75.4–78.1 | 68.1–69.3 |
| RON/MON (95 % n-Cx recirculation ) | 87.1–88.8/85.3–86.7 | 84.1–85.3/83.0–84.3 |
| Sensibility (RON-MON) | 1.8–2.1 | 1.1–1.0 |
| Liquid yield, % (95 % n-Cx, 2-MP, 3-MP recirculation) | 57.6–60.4 | 52.7–54.8 |
| RON/MON (95 % n-Cx, 2-MP, 3-MP recirculation) | 91.7–92.3/90.0–90.7 | 88.7–90.0/87.5–88.9 |
| Sensibility (RON-MON) | 1.7–1.6 | 1.2–1.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hancsók, J.; Kasza, T.; Visnyei, O. Isomerization of n-C5/C6 Bioparaffins to Gasoline Components with High Octane Number. Energies 2020, 13, 1672. https://doi.org/10.3390/en13071672
Hancsók J, Kasza T, Visnyei O. Isomerization of n-C5/C6 Bioparaffins to Gasoline Components with High Octane Number. Energies. 2020; 13(7):1672. https://doi.org/10.3390/en13071672
Chicago/Turabian StyleHancsók, Jenő, Tamás Kasza, and Olivér Visnyei. 2020. "Isomerization of n-C5/C6 Bioparaffins to Gasoline Components with High Octane Number" Energies 13, no. 7: 1672. https://doi.org/10.3390/en13071672
APA StyleHancsók, J., Kasza, T., & Visnyei, O. (2020). Isomerization of n-C5/C6 Bioparaffins to Gasoline Components with High Octane Number. Energies, 13(7), 1672. https://doi.org/10.3390/en13071672





