Abstract
Fresh pig urine is unsuitable for microalgae cultivation due to its high concentrations of NH4+-N, high pH and insufficient magnesium. In this study, fresh pig urine was pretreated by dilution, pH adjustment, and magnesium addition in order to polish wastewater and produce microalgae biomass. Chlorella vulgaris was cultured in an in-house-designed light-receiving-plate (LRP)-enhanced raceway pond to treat the pretreated pig urine in both batch and continuous mode under outdoor conditions. NH4+-N and TP in wastewater were detected, and the growth of C. vulgaris was evaluated by chlorophyll fluorescence activity as well as biomass production. Results indicated that an 8-fold dilution, pH adjusted to 6.0 and MgSO4·7H2O dosage of 0.1 mg·L−1 would be optimal for the pig urine pretreatment. C. vulgaris could stably accumulate biomass in the LRP-enhanced raceway pond when cultured by both BG11 medium and the pretreated pig urine. About 1.72 g·m−2·day−1 of microalgal biomass could be produced and 98.20% of NH4+-N and 68.48% of TP could be removed during batch treatment. Hydraulic retention time of 7-9d would be optimal for both efficient nutrient removal and microalgal biomass production during continuous treatment.
1. Introduction
Intensive pig farming produces a large amount of piggery waste (urine and manure). The National Bureau of Statistics of the People’s Republic of China has reported that more than 11 million tons of piggery wastewater were directly discharged into the environment in 2014 [1]. Piggery waste has become a major source of pollution in rural areas of China. It releases greenhouse gases and ammonia, and causes eutrophication, algal blooms, as well as soil acidification [2]. The treatment of piggery waste is a continuing environmental issue in china.
Anaerobic digestion (AD) has been widely acknowledged as an effective method to process piggery waste, around the world [3]. AD converts complex organic matter in pig manure into biogas. However, the high content of nitrogen (urea, uric acid, and ammonium) in pig urine always leads to high ammonium levels during anaerobic digestion. This causes the inhibition of the growth rates of methanogens, and thereby reduces the yield of methane. Thus, liquid and solid separation of piggery waste is needed to alleviate the ammonium inhibition prior to the anaerobic digestion process. The separated pig urine is rich in pollutants such as nitrogen (N), phosphorus (P), and potassium (K) which are suitable nutrients for algal growth. Microalgae can effectively utilize N and P in aquatic environment as well as light energy and CO2 for photosynthesis to produce biomass [1]. The generated biomass can also be utilized for the production of a variety of high-value products such as food, bioenergy, pharmaceuticals and nutritional products [4,5]. Furthermore, some companies have used microalgae biomass in functional food and feed industry and obtained some economic benefits [6]. Furthermore, compared to the effluent from anaerobic digestion, the separated pig urine is more suitable as a medium to culture microalgae due to: (1) less contamination by heavy metals and microorganisms, (2) less content of organic acids (fulvic acid, humic acid, etc.), which easily cause flocculation of microalgae, and (3) low turbidity and good light transmission. Thus, using pig urine as culture medium for algal cultivation is a promising method for wastewater treatment, pollutants recovery and algal biomass accumulation [7].
However, pig urine is not frequently used as medium to culture microalgae due to its inhibitive effect on the microalgae metabolism. The high concentration of NH4+-N (≥ 200 mg·L−1) in fresh pig urine is toxic to the growth of microalgae due to the formation of free ammonia [8]. This could affect the photosynthetic processes in chloroplasts, electron transport chain, non-photochemical quenching, as well as the dark respiration [9], which can decrease the chlorophyll-A content and cause cell lysis of the microalgae [2,3]. In addition, high pH (commonly higher than 8.5) in pig urine can also induce the flocculation of microalgal cells [10] and decrease its population growth [11]. Moreover, magnesium is an important growth factor for green microalgae, since it is the central atom of chlorophyll [12], but it is insufficient in pig urine. Insufficient magnesium can commonly lead to the decreased chlorophyll content and enzymes activities in microalgal cells [13]. Therefore, these issues need to be sufficiently addressed to improve the successful cultivation of microalgae in pig urine.
An economical microalgae treatment system is another important factor affecting the efficiency of pig urine processing. A raceway pond is believed to be the most convenient and cost-effective facility to treat large amounts of wastewater by microalgae. It has the advantages of low capital and operation costs, ease of scale up, and the ability to handle high treatment volumes [14]. However, the self-shadowing effect of the suspended cells leads to limited light penetration, inefficient photosynthesis, and insufficient nutrient removal in the system [15]. This further leads to low dry mass content, which increases the harvest cost of the biomass.
In this study, the pig urine was pretreated by a simple and efficient method to reduce its toxicity to algal cells. Meanwhile, a light-receiving-plate (LRP)-enhanced raceway pond was designed for pig urine treatment to enhance the photosynthesis of algal for the promoted nutrient removal and the improved biomass output. The thin LRP had a short light pathway and could largely increase the irradiated area of the whole system. The microalgae involved was Chlorella vulgaris due to its widely reported robust growth property, high lipid/carbohydrate accumulation potential and large N and P removal capacity from various wastewaters [14,16]. The specific objectives were: (1) to optimize the culture conditions of C. vulgaris in pig urine and to explore a simple and efficient method for the pretreatment of pig urine; (2) to establish an efficient pig urine purification system for studying the growth characteristics of microalgae and the nutrient removal efficiency from the wastewater; (3) to explore the effect of hydraulic retention time on the continuous pig urine treatment and algal biomass production under outdoor conditions in the LRP-enhanced raceway pond. It was hoped that the results of this study would provide a scientific basis for large-scale outdoor treatment of pig urine.
2. Materials and Methods
2.1. Pig Urine Collection and Analysis
Pig urine was directly collected from a local piggery farm (Fengcheng city, Jiangxi Province, China) during the excretion of pigs. Samples were collected from pigs of different ages (2 to 8 months), and from a mixture of six different pigsties. The collected pig urine was stored in darkness at 4 °C and the supernatant was directly taken for the experiment after precipitation for 24 hours. The characteristics of the sample were analyzed, as shown in Table 1.

Table 1.
Characteristics of the collected pig urine.
2.2. Microalgae Strain and Subculture
The Chlorella vulgaris FACHB-31, purchased from the Institute of Hydrobiology, Chinese Academy of Sciences, PR China, was used for this study. The strain was preserved in 250 mL autoclaved (121 °C, 30 min) BG11 medium [17] at a temperature of 26 ± 1 °C, and light intensity of 2500 Lux. Prior to inoculation into wastewaters, microalgae cells were recovered from the culture media through centrifugation at 5000 rpm for 15 min at room temperature and washed with distilled water three times to remove the residual nutrients.
2.3. Optimization of C. vulgaris Growth Conditions in Pig Urine
A single-factor experiment was conducted to evaluate the effects of pig urine dilution rate, pH value, light intensity, and MgSO4·7H2O dosage on microalgal growth. The effect of pig urine dilution rate was studied by culturing C. vulgaris in 2, 4 and 8 fold diluted pig urine under light intensity of 2500 Lux. The effect of pig urine pH value was studied by culturing C. vulgaris, after the pig urine was diluted 8 to 1, with different pH values (4, 5, 6 and 7) under light intensity of 2500 Lux. The effect of light intensity was studied by culturing C. vulgaris in pig urine, diluted 8 to 1, with pH adjusted to 6, under light intensities of 500 Lux, 1500 Lux, 2500 Lux and 3000 Lux. The effect of MgSO4·7H2O dosage was studied by culturing C. vulgaris pig urine, diluted 8 to 1, pH adjusted to 6, with different MgSO4·7H2O dosages (0.0 g·L−1, 0.1 g·L−1 and 1.0 g·L−1) under light intensity of 2500 Lux. At least three replicate experiments were conducted for each treatment. Pig urine was diluted using deionized water. The pH value of the pig urine was adjusted by adding 1 mol·L−1 HCl or NaOH. Fresh C. vulgaris slurry, harvested from Section 2.2, was inoculated into 300 mL of pretreated pig urine in a 500 mL Erlenmeyer flask, and the OD680 (optical density at 680 nm) values of the medium were controlled between 0.20 and 0.40. All the cultures were maintained at 26 ± 1 °C for 15 days. The contents were shaken by hand twice a day and their OD680 values were also daily measured. The water evaporation loss was balanced daily by adding deionized water.
2.4. Design of the Algal Cultivation System
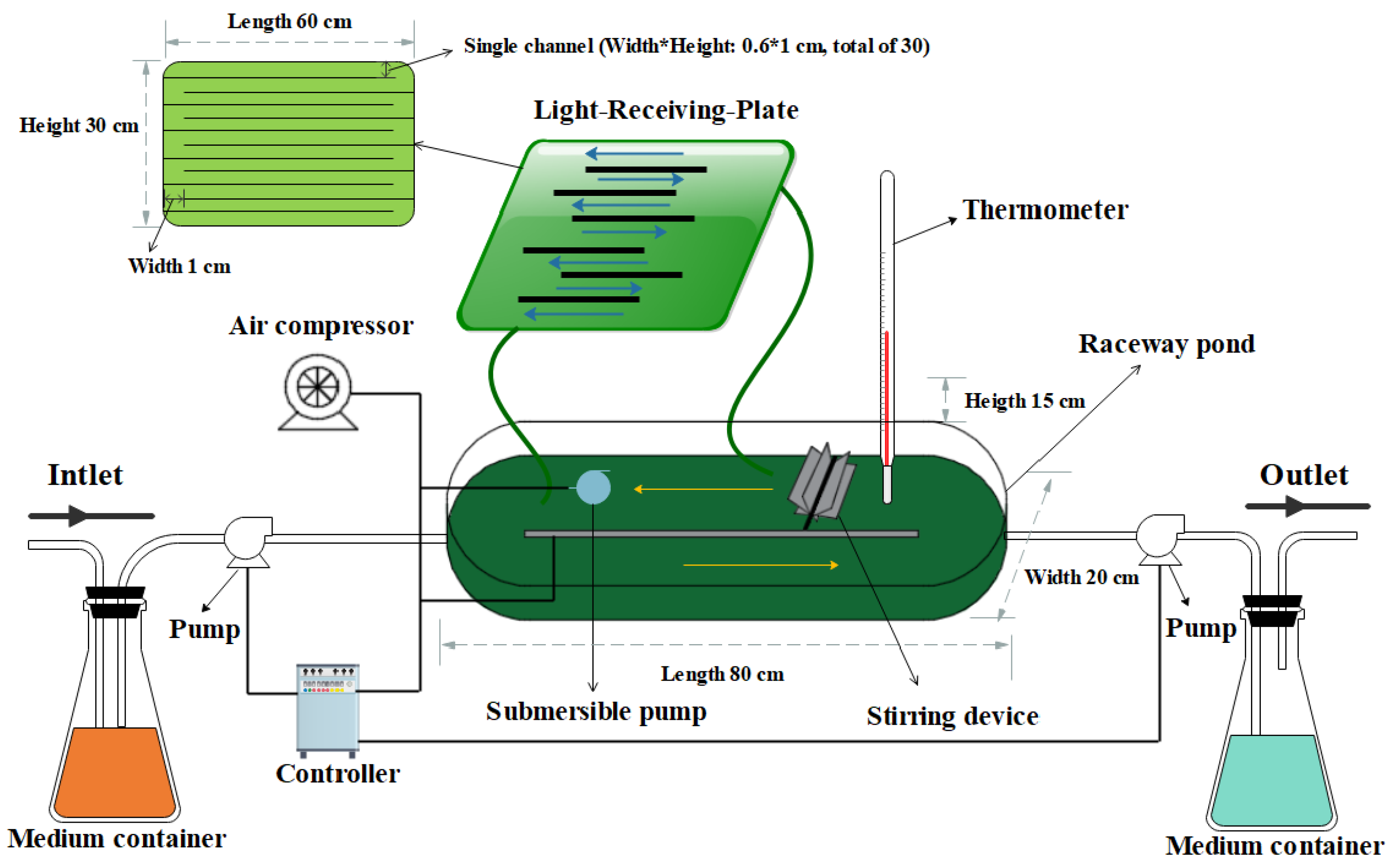
The design of the LRP-enhanced raceway pond is shown in Figure 1. In general, the system consisted of two parts, a traditional raceway pond and an LRP. The raceway pond (length*width*height: 80*20*15 cm) was made of polymethyl methacrylate (PMMA) and its work volume was 8 L. A cover plate was utilized to enclose the raceway pond to avoid contamination and evaporation. An air compressor and a stirring device were set inside the raceway pond to aerate and stir the algal culture. The temperature of the medium was monitored by a thermometer. The LRP (length*width*height: 60*0.6*30 cm) was a flat panel photobioreactor which was made of polycarbonate for increasing the light-receiving area of the whole system. It had thirty internal passages (length*width*height: 60*0.6*1 cm) and the microalgae culture inside the raceway pond could be circulated in it, to receive light by the submersible pump. In addition, pig urine was pumped into the system from a container for nutrients supplement by a peristaltic pump and the medium flow rate was controlled by a controller. The medium was simultaneously pumped out of the system at the same flow rate by another peristaltic pump. The water evaporation loss was daily balanced by deionized water. After each batch experiment, the whole system was washed twice by deionized water and the 10 g·L−1 NaClO was used to clean the system twice for disinfection. Afterwards, sterilized cold water was applied to clean up the residual NaClO [17]. The outdoor experiments were conducted during July 10, 2017- August 20, 2017 at 115°94′ E, 28°69′ N.
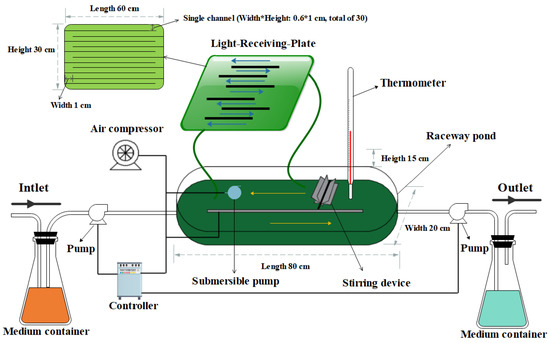
Figure 1.
Schematic diagram of the light-receiving-plate (LRP)-enhanced raceway pond.
2.5. C. vulgaris Cultivation and Pig Urine Treatment in LRP-enhanced Raceway Pond
2.5.1. Cultivation of C. vulgaris in LRP-Enhanced Raceway Pond
8 Liters of autoclaved BG11 medium were added into the raceway pond and the system was inoculated with C. vulgaris slurry (Section 2.2). Then, the air compressor, the stirrer, and the submersible pump were turned on to uniformly disperse the algal cells into the medium, and the OD680 value of the medium was controlled at 0.15. The system was run continuously for 10 days under outdoor conditions. The aeration rate was set at 0.7 L·min−1; the flow rate of the algae culture inside the internal passages of the LRP was controlled as 0.2 L·min−1 (5.56 cm·s−1). The stirring speed of the stirrer was set at 30 rpm. During the process, all of the on-off switches for the air compressor, the stirrer, and the submersible pump, were controlled by a controller, in order to balance the energy consumption and photosynthetic efficiency of the system (Figure 1). The system was controlled to run for an hour and powered off for an hour with a total of 6 cycles during the daylight hours (07:00-18:00). The temperature and light intensity were measured every 2 hours during daylight (07:00-18:00). In total, 20 mL of algal culture was sampled daily for further analysis at 18:00.
2.5.2. Pig Urine Treatment by LRP-Enhanced Raceway Pond in Batch Mode
During this experiment, the autoclaved BG11 medium (8 L) was first introduced into the raceway pond, and then the system was run under the same conditions of Section 2.5.1. After the OD680 value of the algal culture reached about 1.2, half of the medium was replaced with the pretreated pig urine. Pig urine was first diluted 4 to 1 with deionized water, and then treated by the addition of MgSO4·7H2O (0.1 g·L−1) and the adjustment of pH to 6.0. Finally, the dilution rate of the pig urine was adjusted to 8 to 1, after 1:1 mixed with algal culture. The treatment of the pig urine by C. vulgaris was conducted under the same conditions of Section 2.5.1 for 5 days and 20 mL algal culture was sampled at 08:00 and 20:00 every day for further analysis.
2.5.3. Pig Urine Treatment by LRP-Enhanced Raceway Pond in Continuous Mode
To evaluate the effect of hydraulic retention time on the pig urine, treatment efficiency in the LRP-enhanced raceway pond, the pretreated pig urine (8 to 1 dilution, 0.1 g·L−1 MgSO4·7H2O, pH=6.0) was continuously pumped into the system with different flow rates. First, algal biomass in the system was accumulated using the same method of Section 2.5.2. After the OD680 value of the algal culture reached about 0.75, the pretreated pig urine was continuously pumped into the raceway pond and the medium with algal cells was also continuously pumped out of the system at a same flow rate as the influent. This was the initiation of a continuous process. Then the continuous treatment experiment was started, and the system was run for 20 days under the same conditions of Section 2.5.1. The inflow rate as well as the outflow rate of pig urine was adjusted every 5 days to generate four different hydraulic retention times (HRT), e.g., 9-days, 7-days, 5-days and 3-days, respectively. The corresponding medium inflow rates were 0.89 L·day−1, 1.14 L·day−1, 1.60 L·day−1, 2.67 L·day−1, respectively. Furthermore, the corresponding rates of urine exchange were 11.1%, 14.3%, 20%, 33.3%, respectively. In total, 20 mL of algal culture was daily sampled for further analysis at 18:00.
2.6. Analytical Method
2.6.1. Microalgal Growth Analysis
The optical density of the samples at wavelength of 680 nm were determined daily by using a spectrophotometer (Metash, UV-9000, China); the microalgal biomass density (mg·L−1) was calculated based on a calibration curve as follows:
To plot the calibration curve (Equation (1)), C. vulgaris was first cultured in pretreated pig urine (the same in Section 2.5.3) under the same conditions as Section 2.2. After the OD680 of the culture reached about 1.64, the suspended cells were harvested and rinsed following the same method in Section 2.2. Then the algal slurry was extensively diluted using BG11 medium to obtain samples with an absorbance (OD680) in the range of 0.2–1.5. After this, 50 mL of each diluted sample were harvested by filtering using the pre-weighed 0.45 µm GF/C filter membrane (Whatman, Little Chalfont, England), and their cell dry mass contents (mg·L−1) were determined by oven drying at 105 °C for 12 h. Finally, the sample cell dry mass content was plotted against the value of OD680.
During the pig urine treatment in batch mode, the microalgal biomass production and productivity were calculated using Equations (2) and (3):
where D0 and D5 are the algal biomass density at day 0 and day 5; 8 (L) and 0.16 (m2) are the volume of the medium in the raceway pond and the footprint area of the raceway pond, respectively; 5 is the duration time of the treatment.
During the pig urine treatment in continuous mode, the microalgal biomass production and productivity during each HRT were calculated using Equations (4) and (5):
where is the function of microalgal biomass density (A) vs culture time (t); i is the day that a new HRT was applied; µ is the flow rate under each HRT; 5 is the duration time of each HRT.
2.6.2. Wastewater Nutrient Analysis
The samples for nutrient analysis were first centrifuged at 5000× g for 10 min. Then, the supernatants were collected and filtered using 0.45 μm membrane filters (Millipore Corporation, USA). After which, the filtrates were properly diluted and the Chemical Oxygen Demand (COD), Ammonium (NH4+-N), Total Nitrogen (TN) and Total Phosphorus (TP) were analyzed by a Hach DR 5000 Spectrophotometer following the method reported by Zheng et al. [2]. The Total Organic Carbon (TOC) and Inorganic Carbon (IC) were determined by a multi N/C3100 Analyzer (Analytik Jena AG, Jena, Germany). The concentrations of the metal ions (Zn2+, Cu2+, Cd2+, K+, and Mg2+) were measured using a C200 Multi-parameter Ion Specific Meter (Hanna Instruments, Italy) following the method reported by Cao et al. [18]. The nutrient removal efficiency and removal rate during pig urine treatment in batch mode were calculated according to Equations (6) and (7):
where (mg·L−1) and (mg·L−1) are the nutrient concentrations at day 0 and day 5, respectively. For the nutrient removal efficiency and removal rate during pig urine treatment in continuous mode, results were calculated following Equations (8) and (9):
where is the function of nutrient concentration (C) vs culture time (t).
2.6.3. Chlorophyll Fluorescence Analysis
The chlorophyll fluorescence parameters of C. vulgaris were measured using Water-PAM (WALZ, Effeltrich, Germany). The samples were first diluted by deionized water to obtain the same biomass concentration (OD680) before measuring the light response curves of photosynthesis. Then each sample of 3 mL microalgae suspension was placed in darkness for 15 min. After which, the values of maximum photochemical efficiency (Fv/Fm) of PS II and the actual conversion efficiency of light energy (ΦPSII) were recorded, details are reported elsewhere [2].
2.7. Statistical Analysis
All of the analytical experiments were conducted in triplicate, and the results are presented as mean values ± standard deviation. Analysis of variance (ANOVA) was carried out wherever applicable.
3. Results and Discussion
3.1. Optimization of C. vulgaris Growth Conditions in Pig Urine
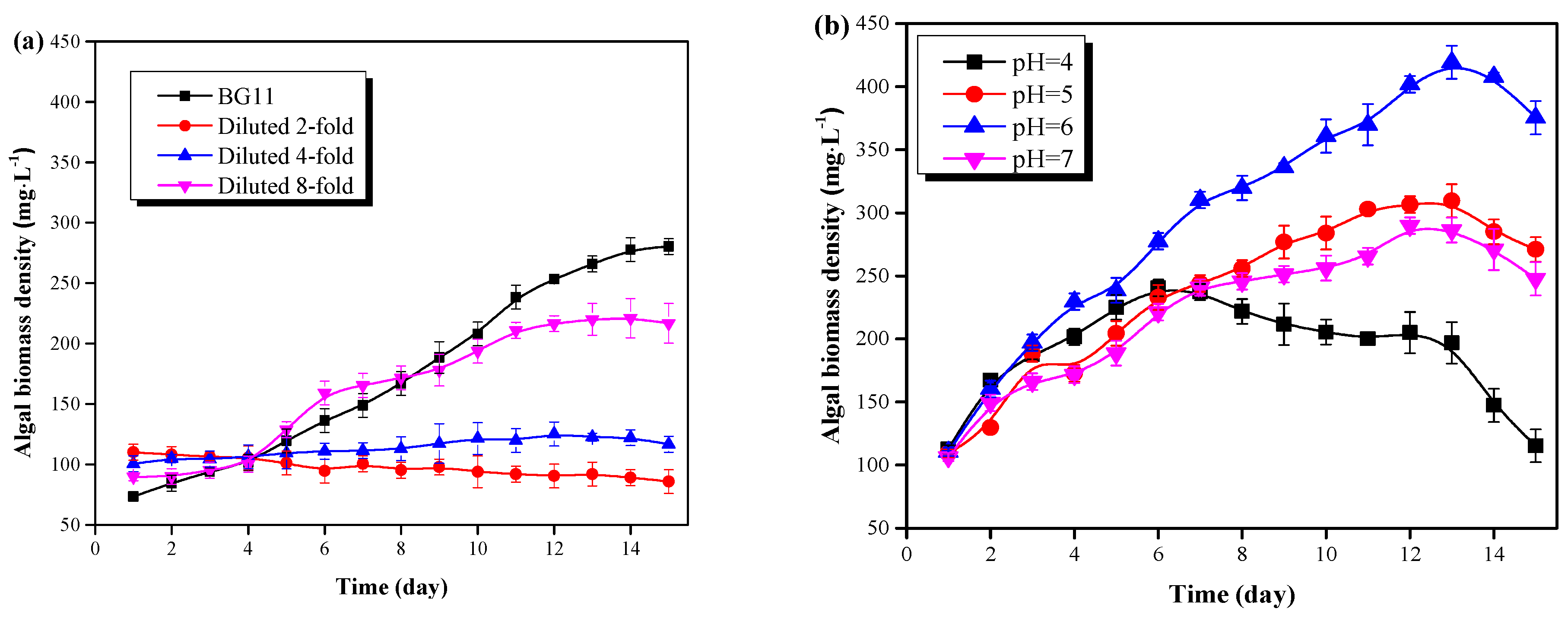
The characterization of pig urine is listed in Table 1. The C. vulgaris growth could be inhibited by the high concentration of NH4+-N (1155.5 mg·L−1) in unpretreated pig urine due to the uncoupling effect of free ammonia on the photosynthetic processes in chloroplasts [7]. Meanwhile, the K (1150.9 mg·L−1) was sufficient but the Mg (0.62 mg·L−1) was insufficient for microalgal growth when compared to BG11 medium. Furthermore, the pH value of 9.2 also showed strong alkalinity in unpretreated pig urine. Therefore, the pig urine needed to be pretreated for the optimized cultivation of microalgae. In this study, pig urine was diluted, and its pH as well as the Mg content were both adjusted. The effects of dilution rate, pH value, light intensity, and Mg dosage on the growth of C. vulgaris were studied. Results are illustrated in Figure 2.
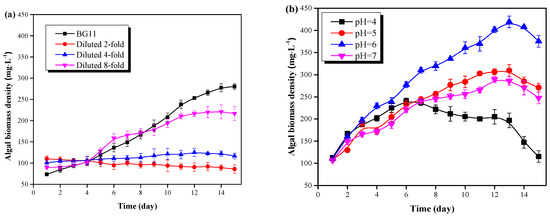
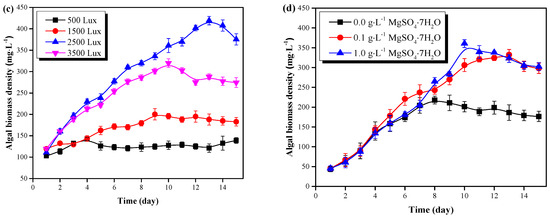
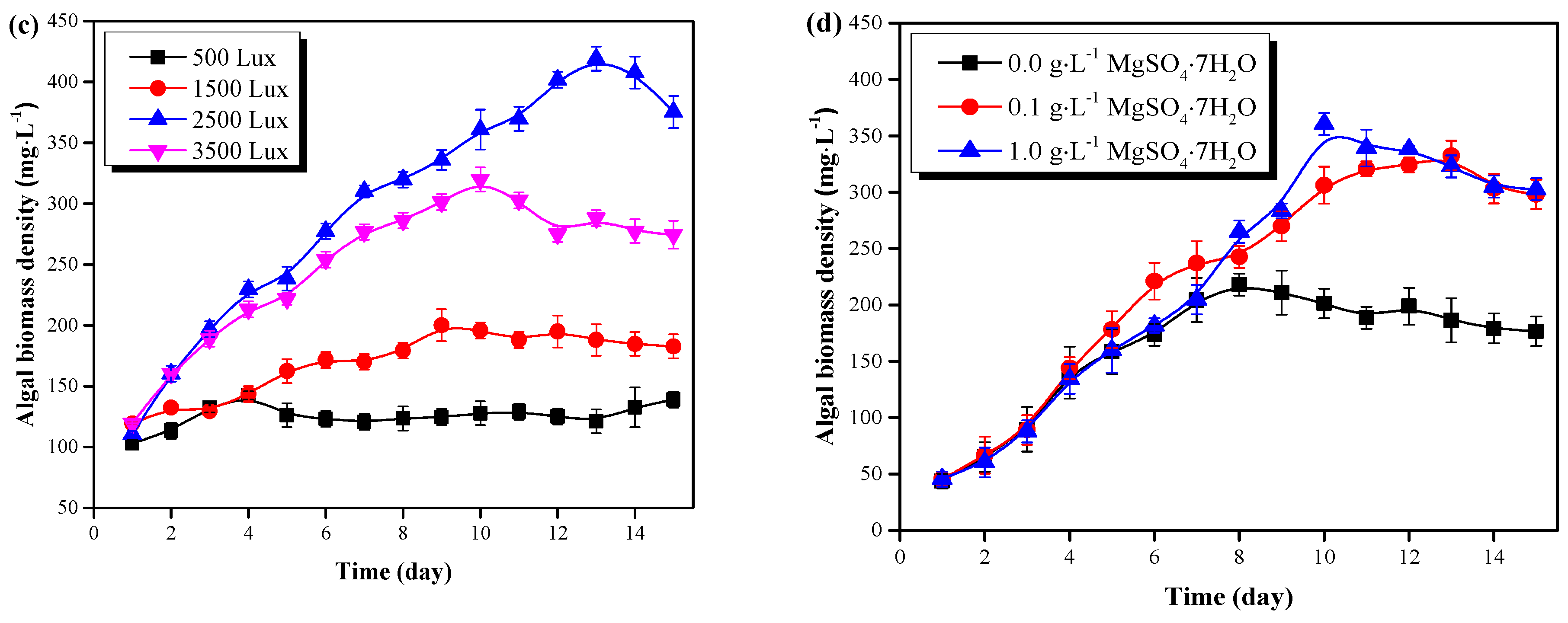
Figure 2.
C. vulgaris growth results in the pretreated pig urine. (a) The effect of pig urine dilution rate on the microalgal growth. (b) The effect of pig urine pH value on the microalgal growth. (c) The effect of light intensity on the microalgal growth. (d) The effect of pig urine MgSO4·7H2O dosage on the microalgal growth.
As can be seen from Figure 2a, C. vulgaris in 8:1 diluted pig urine witnessed a rapid growth after 4 days of lag phase, and the biomass density reached to 220.08 mg·L−1 at day 13. C. vulgaris grew more slowly in 4:1 diluted pig urine, and only a 10% increase of the biomass was obtained after 15 days cultivation. For the 2:1 diluted pig urine, C. vulgaris failed to grow, and plenty of cells were dead and formed as sedimentation at the bottom of the flask. Possibly the high concentration of NH4+-N and the salts contribute to the growth inhibition or even the death of C. vulgaris in pig urine with low dilution rate. In this study, the NH4+-N concentration of the 2- and 4- diluted pig urine reached 577.5 mg·L−1 and 288.75 mg·L−1, respectively. The high concentration of NH4+-N was detrimental to algal cells due to the free ammonia [8]. In addition, high levels of salts (Table 1) could also affect the nutrient assimilation and metabolism of microalgae, and even threaten its growth, due to high osmotic pressure [3]. Thus, the pig urine needed to be diluted; a dilution rate of 8:1 was found to be optimal, according to this study. This was in consistent with the findings of Deng et al [19]. They found that C. vulgaris grew well by properly diluted wastewater.
The effect of pH on the growth of C. vulgaris in 8-fold diluted pig urine is shown in Figure 2b. C. vulgaris could grow well in a pH range of 5.0-7.0 without a lag phase. The optimal pH value of pig urine for C. vulgaris growth was found to be 6.0, with the largest amount of biomass (419.15 mg·L−1) being accumulated at this pH value, after 13 days cultivation. Such density was significantly (P < 0.05) larger than that of pH of 5.0 (309.46 mg·L−1) and 7.0 (286.22 mg·L−1) as well as the values in Figure 2a (≤ 221 mg·L−1). This signified that acidification of the pig urine enhanced the growth of microalgae, which was in consistent with the findings of Qiu et al. [11]. They found that biomass productivity increased with decreasing pH. This was mainly because the acidic condition could: (1) inhibit the formation of free ammonia, (2) promote the absorption and utilization of macromolecular nutrients by C. vulgaris via acidolysis, and (3) cause metabolic disturbance of other microbial cells [2,18]. However, C. vulgaris could not grow well at a pH of 4.0, under which the biomass density increased only for 6 days and then sharply decreased to 115.30 mg·L−1 at day 15. Lower pH (4.0) could have a negative effect on cell division and enzyme activity, and thus inhibit cell growth [7].
The growth results of C. vulgaris in pretreated pig urine (8-fold dilution, pH=6.0) under different light intensity, is shown in Figure 2c. Results demonstrate that light intensity of 2500 Lux to 3500 Lux would be optimal for C. vulgaris growth in pretreated pig urine. C. vulgaris could grow well under light intensity of 1500 Lux, 2500 Lux and 3500 Lux, and increased light intensity could enhance its growth. In contrast, C. vulgaris could hardly accumulate biomass under light intensity of 500 Lux. This possibly indicates that the light intensity of 500 Lux would be the light compensation point of C. vulgaris, where the microalgae photosynthetic accumulation rate equilibrates with the respiration rate [20]. Proper light supplement is important for the efficient accumulation of microalgal biomass; too high or too low light intensity would inhibit the C. vulgaris growth due to light inhibition or light starvation [21].
The growth of C. vulgaris under different MgSO4·7H2O dosage rates (0.0 mg·L−1, 0.1 mg·L−1 and 1.0 mg·L−1) in the pretreated pig urine (8-fold dilution, pH=6.0) is shown in Figure 2d. In the first 7 days of cultivation, C. vulgaris witnessed a rapid growth with no significant difference between the treatments. After day 7, C. vulgaris continued with its increase in the treatments with the addition of MgSO4·7H2O (0.1 g·L−1 and 1.0 g·L−1). However, the microalgae without MgSO4·7H2O addition adhered to the wall of the flasks, and the cells gradually turned yellow. By the end of the experiment, the biomass densities of C. vulgaris were similar (298.00 mg·L−1 and 302.59 mg·L−1) in the pretreated pig urine with the addition of MgSO4·7H2O (0.1 g·L−1 and 1.0 g·L−1), which were significantly higher than the treatment without MgSO4·7H2O addition (P < 0.05). The supplement of Mg in the Mg deficient pig urine (0.62 mg·L−1) enhanced the growth of C. vulgaris; these results agree with the findings of Tuantet et al. [22]. Mg is essential for microalgal photosynthesis since it is the fundamental element for chlorophyll synthesis [12]. However, Mg in pig urine in this study was insufficient to sustain satisfactory phototrophic growth. Excessive concentration of Mg might cause flocculation of C. vulgaris and high cost [10], but a MgSO4·7H2O dosage of 0.1 g·L−1 was found to be optimal for C. vulgaris growth in pretreated pig urine.
3.2. Growth of C. vulgaris in the Reactor
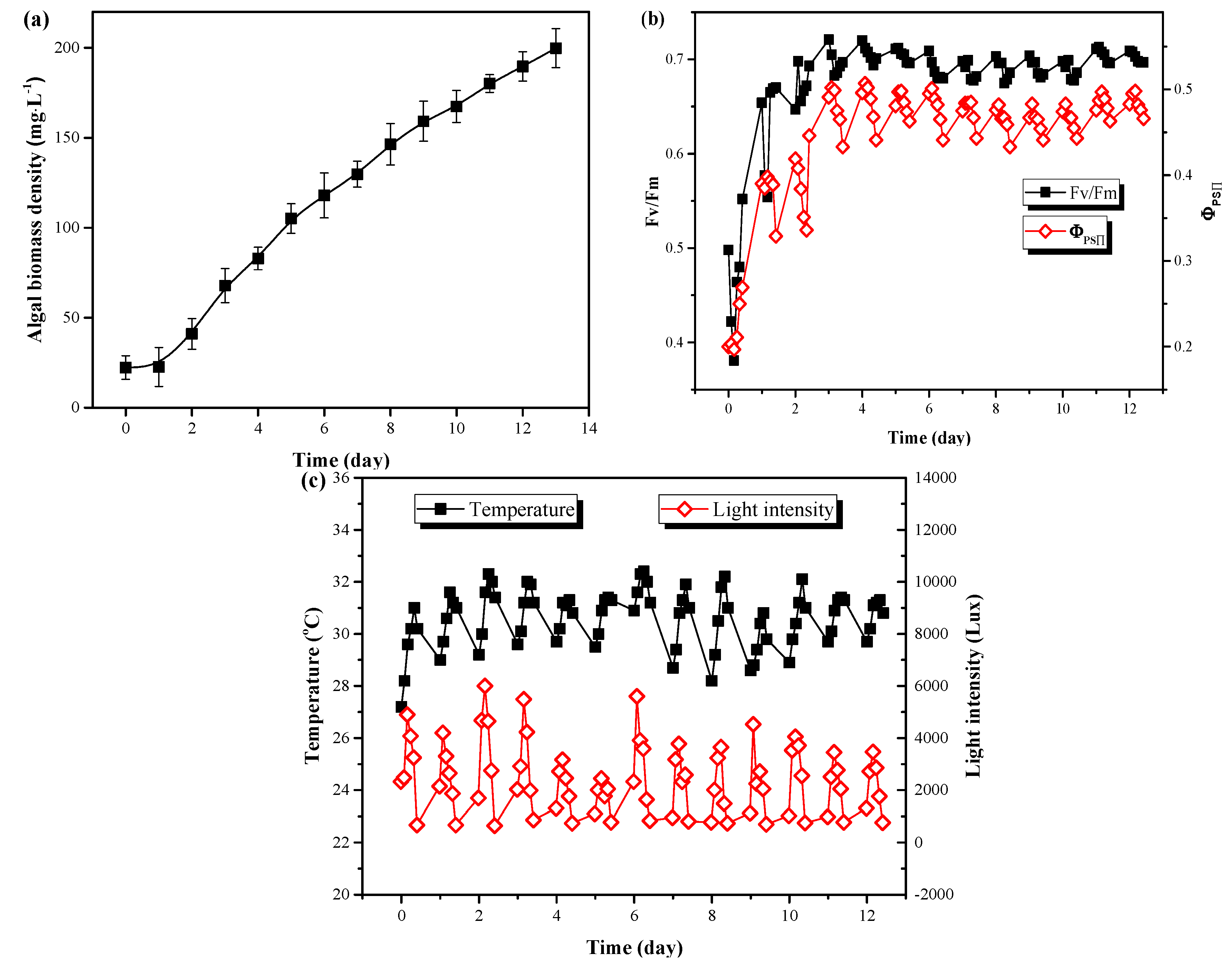
To explore the growth performance of C. vulgaris in LRP-enhanced raceway pond under outdoor conditions, the whole system was put outdoors, and sunlight was applied for the microalgae growth with no temperature control. As shown in Figure 3a, C. vulgaris could grow rapidly in LRP-enhanced raceway pond under outdoor conditions with only a short lag phase (1 day). The biomass density continuously increased and the maximum value of OD680 reached 199.78 mg·L−1 at day 10 and the algal biomass productivity was 0.89 g·m−2·day−1. Fv/Fm and ΦPSⅡ are the maximum quantum yield of PSΠ and the actual quantum yield of PSII, respectively. They are important parameters of microalgal photosynthesis and are commonly used to evaluate photosynthetic performance under stress [23]. It can be seen from Figure 3b that both Fv/Fm and ΦPSⅡ were first decreased and then gradually increased toward stability. After two days of cultivation, the values of Fv/Fm and ΦPSⅡ reached 0.70 and 0.50, respectively, which were significantly higher than the initial values (P < 0.05). The adaption (lag phase, Figure 3a) of C. vulgaris from indoor to outdoor conditions might contribute to the decrease of both Fv/Fm and ΦPSⅡ on day 1. After adaption, the growth of C. vulgaris moved into the exponential phase due to the sufficient light and nutrients supplement and the suitable temperature. The values of Fv/Fm and ΦPSⅡ only varied with the temperature and light intensity. During the experiment, the temperature and light intensity ranged from 27 °C to 33 °C and 700 Lux to 5000 Lux during the day, respectively. Their mean values were 30 °C and 2444 Lux (Figure 3c), respectively, which were suitable conditions for the enhanced growth of C. vulgaris [14]. Moreover, efficient photosynthesis in this system would be another reason for the rapid growth of C. vulgaris. By enhancing the raceway pond with LRP, algal cells could be alternately pumped into the plate to receive additional light for photosynthesis. Thus, the LRP increased the total irradiated area of the whole system, leading more cells to be illuminated.
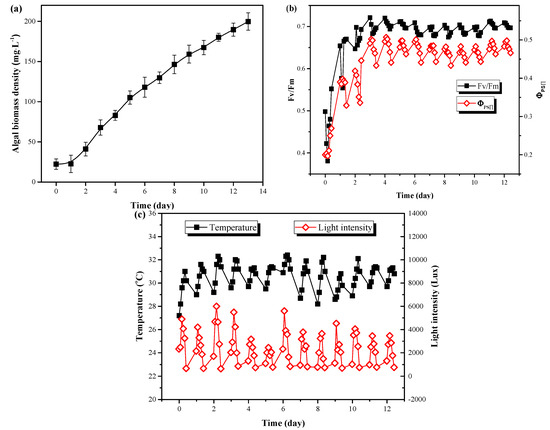
Figure 3.
The changes of microalgal biomass density (a) and chlorophyll fluorescence parameters (b) as well as environmental parameters (c) during outdoor cultivation of C. vulgaris in LRP-enhanced raceway pond.
3.3. Pig Urine Treatment by LRP-Enhanced Raceway Pond in Batch Mode
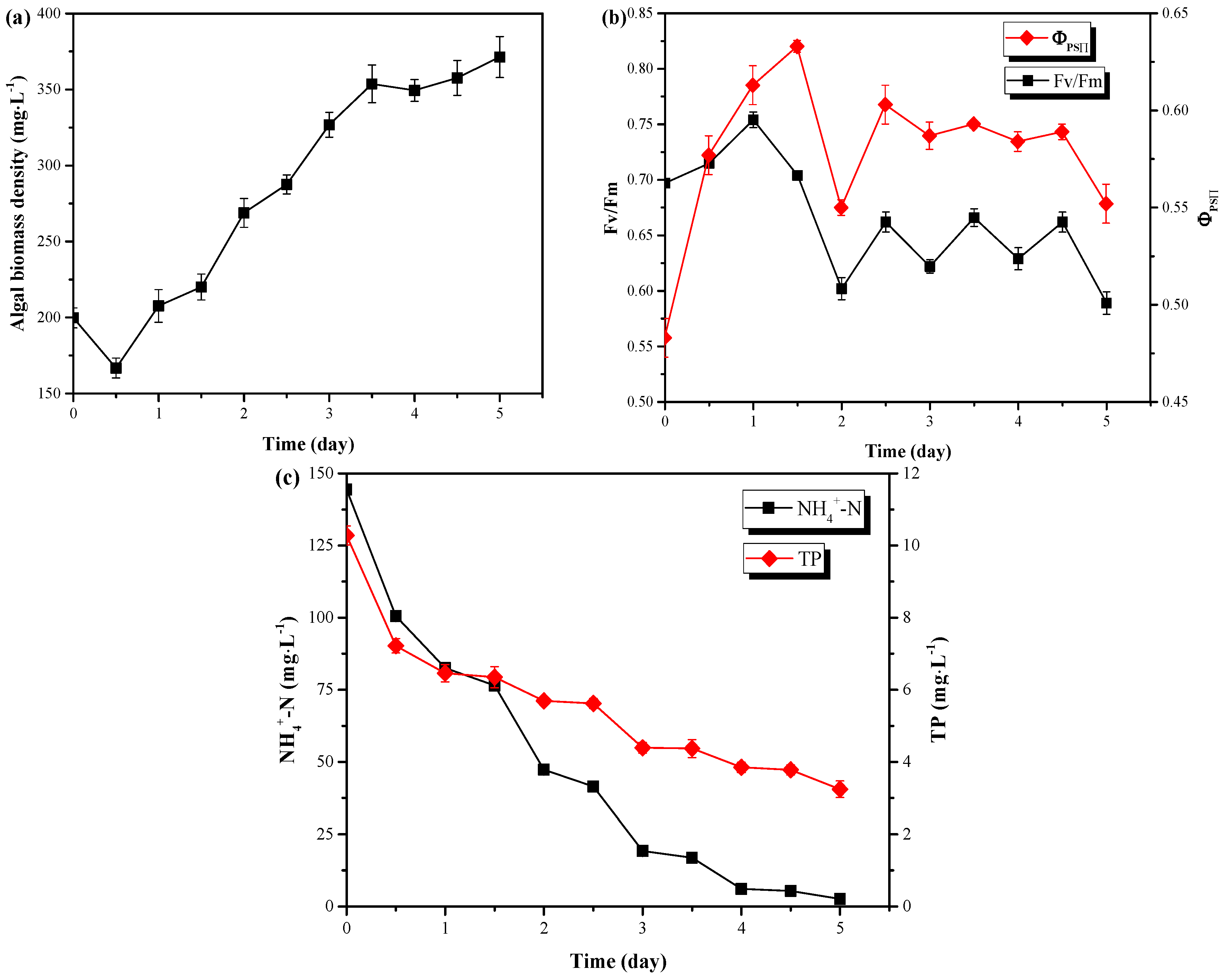
The pretreated pig urine was treated by C. vulgaris in the LRP-enhanced raceway pond under outdoor conditions (same as Section 3.2) to evaluate the wastewater treatment performance of this system. In the pretreated pig urine (8-fold of dilution, 0.1 g·L−1 MgSO4·7H2O, pH=6.0), C. vulgaris could rapidly accumulate biomass from biomass density of 199.78 mg·L−1 to 371.35 mg·L−1 in 5 days (Figure 4a), corresponding to biomass productivity of 1.72 g·m−2·day−1. Meanwhile, the biomass both increased during day and night (Figure 4a and Table 2), with the biomass accumulated during day (1.39 g·m−2·day−1) significantly larger than during night (0.33 g·m−2·day−1) (P < 0.05). Compared to the results of Section 3.2, C. vulgaris accumulated more biomass in a short time in fresh pig urine medium than in BG11 medium. This is possibly due to the following reasons: (1) the nitrogen source in the pretreated pig urine (acidized) was mainly NH4+-N which is the preferred form by C. vulgaris when compared to NO3--N in BG11, because NH4+-N is already in reduced form and it can be used directly for the synthesis of proteins with less energy consumed [14,24]; (2) The organics (TOC, 2146 mg·L−1) in pig urine might have induced the mixotrophic/heterotrophic growth of C. vulgaris during day/night [15]. Pig urine contains certain amounts of simple organics like amino acids, which could be directly utilized by C. vulgaris. This might also be the reason why C. vulgaris accumulated biomass during the night. (3) Lower inoculation (199.78 vs. 22.32) of the BG11 medium might be another reason for its lower biomass productivity since lower cell density commonly led to photo-inhibition due to the weak shadowing effect between cells [25]. (4) Bacteria might also contribute to the robust growth of C. vulgaris in the un-sterilized pig urine [26]. The changes of chlorophyll fluorescence parameters during the treatment of the pretreated pig urine are shown in Figure 4b. As can be seen from Figure 4b, Fv/Fm did not decrease with the addition of pig urine, and ΦPSII increased first and then stabilized at 0.6. Both of Fv/Fm and ΦPSII were higher in the pig urine than in the BG11 medium (Section 3.2). Thus, no negative effect of the pretreated pig urine on C. vulgaris photosynthesis existed. Furthermore, the pretreated pig urine could be used as a good medium to enhance the growth of C. vulgaris when compared to BG11 medium.
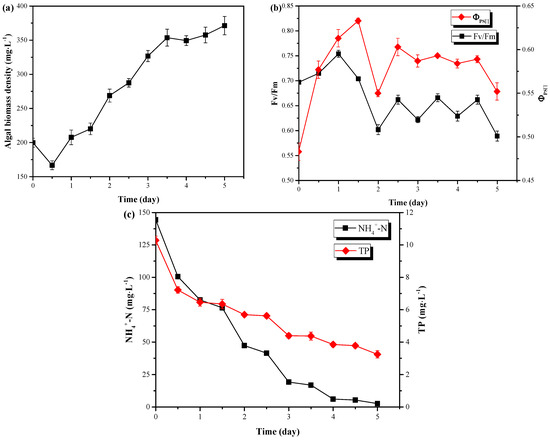
Figure 4.
The changes of C. vulgaris biomass density (a) and chlorophyll fluorescence parameters (b) as well as nutrients concentrations (c) during outdoor treatment of pretreated pig urine in LRP-enhanced raceway pond.

Table 2.
Pig urine treatment results in batch mode.
Microalgae can utilize N, P and other elements in the wastewater to form cells through photosynthesis. It has been reported that during the growth of C. vulgaris, phosphorus (P) is used to synthesize nucleic acids, phospholipids, ATP, lipids, proteins, carbohydrates, etc. [16], while nitrogen is used to synthesize various biological substances such as peptides, proteins, enzymes, chlorophyll, energy transfer molecules, genetic materials, etc. [15,27]. Figure 4c shows the changes of NH4+-N and TP in the pretreated pig urine. C. vulgaris could sharply decrease the NH4+-N and TP concentrations from 100.5 mg·L−1 to 2.6 mg·L−1 and from 7.22 mg·L−1 to 3.24 mg·L−1 within 5 days, respectively. These results effectively met the discharge standard of these pollutants for piggery wastewater (GB18596-2001, NH4+-N ≤ 80 mg·L−1, TP ≤ 8 mg·L−1), given by Ministry of environmental protection of People’s Republic of China. Furthermore, most of this removal occurred during the day, but not at night (excluding the removal in day 1) (Figure 4c and Table 2). The removal efficiencies of NH4+-N and TP by C. vulgaris were 98.20% and 68.48%, respectively. Possibly the unusable phosphorus in organic forms resulted in this low TP removal efficiency since C. vulgaris could not utilize them for growth [28]. The removal rates of NH4+-N and TP in this system was 1418.40 mg·m−2·day−1 and 70.40 mg·m−2·day−1, respectively, which were significantly higher than the results of a vertical-algae-biofilm reactor (N: 130 mg·m−2·day−1, P: 23 mg·m−2·day−1) [29] and a pilot scale revolving algae biofilm reactor (N: < 800 mg·m−2·day−1, P: 30 mg·m−2·day−1) [30]. The results from this study might be due to the efficient photosynthesis of LRP enhanced system.
3.4. Pig Urine Treatment by LRP-Enhanced Raceway Pond in Continuous Mode
To further evaluate the applicability and feasibility of pig urine treatment by LRP-enhanced raceway pond, the pretreated pig urine (8-fold dilution, 0.1 g·L−1 MgSO4·7H2O, pH=6.0) was treated by C. vulgaris under outdoor condition (same as Section 3.2) with different hydraulic retention times (HRT: 9d, 7d, 5d, 3d). Figure 5a shows the trend of C. vulgaris biomass density with different HRT. It can be seen from the figure that the density of C. vulgaris rapidly increased with a HRT of 9 days and 7days, with the value increasing from 236.78 mg·L−1 to 262.31 mg·L−1 and from 262.31 mg·L−1 to 302.91 mg·L−1, respectively. However, when the HRT was adjusted to 5 days, the density of C. vulgaris decreased, from 302.91 mg·L−1 to 257.40 mg·L−1 after 5 days operation. As the HRT was further shortened, the density of C. vulgaris decreased sharply. When the HRT was adjusted to 3 days, the biomass density decreased from 257.40 mg·L−1 to 167.36 mg·L−1. The microalgal biomass productivity under HRT of 9d, 7d, 5d and 3d was 1.56 g·m−2·day−1, 2.39 g·m−2·day−1, 2.32 g·m−2·day−1 and 2.78 g·m−2·day−1, respectively. Clearly, biomass productivity increased with the increase of HRT. The obtained biomass productivity was slightly higher than a Twin-Layer photobioreactor (1.3 g·m−2·day−1) [31] and a revolving algae biofilm reactor (0.3 g·m−2·day−1-1.0 g·m−2·day−1) [30] but significantly lower than other systems, e.g., 6.30 g·m−2·day−1 [31] and 19.01 g·m−2·day−1-27.18 g·m−2·day−1 [14].
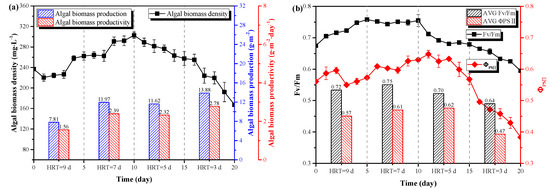
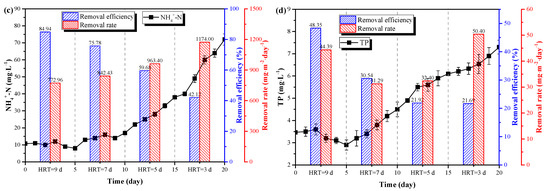
Figure 5.
The results of pretreated pig urine treatment under different HRT. (a) The variations of C. vulgaris biomass density and production. (b) the variations of C. vulgaris chlorophyll fluorescence parameters. The variations of NH4+-N (c) and TP (d) concentrations, removal efficiencies and removal rates.
Figure 5b shows the changes in chlorophyll fluorescence parameters. As the HRT shortened, the Fv/Fm and ΦPSII values gradually decreased, and the photosynthesis of C. vulgaris was inhibited. When HRT was adjusted to 9 days and 7days, the average values of Fv/Fm and ΦPSII were relatively stable at 0.72 and 0.57, respectively. This was consistent with the increase of biomass density in Figure 5a. However, with shorter HRTs of 5 days and 3 days, the Fv/Fm and ΦPSII decreased significantly and photosynthesis of C. vulgaris was inhibited even though its productivity was increased, which possibly contributed to the decrease of biomass density in Figure 5a.
Figure 5c and 5d show the changes of NH4+-N and TP in the reactor under different HRT. It can be seen from the figures that NH4+-N and TP contents in the wastewater were low when the HRT was adjusted to 9d and 7d, e.g., 8 mg·L−1 to 17 mg·L−1 and 2.9 mg·L−1 to 4.5 mg·L−1, respectively. The corresponding NH4+-N and TP removal rates were 84.94% (772.96 mg·L−1·day−1) and 48.35% (44.39 mg·L−1·day−1) for HRT=9d, and 75.78% (842.43 mg·L−1·day−1) and 30.54% (31.29 mg·L−1·day−1) for HRT=7d, respectively. When the HRT was adjusted to 5d and 3d, the contents of NH4+-N and TP in the medium increased rapidly, and the purification effect of C. vulgaris on the wastewater decreased. The corresponding NH4+-N and TP removal rates were 59.68% (963.40 mg·L−1·day−1) and 21.92% (32.40 mg·L−1·day−1) for HRT=5d, and 42.12% (1174.00 mg·L−1·day−1) and 21.69% (50.40 mg·L−1·day−1) for HRT=3d, respectively. As the HRT decreased, the nutrients removal efficiencies also decreased but the nutrient removal rates increased. However, during the four HRTs, the effluent NH4+-N and TP levels could always effectively meet the discharge standard of pollutants for piggery wastewater (GB18596-2001).
The shorter HRT (5 days and 3 days) would lead to the large dilution rate of the medium, under which the growth rate of the cells was lower than the dilution rate. Thus, the biomass density decreased. Meanwhile, the decrease of the HRT could lead to the increased nutrient loading rate of the system. Furthermore, increased nutrient loading rates could further enhance the nutrients uptake of microalgal cell [15,32], which might contribute to the increased nutrients removal rates of the system (Figure 5c,d). However, increased nutrient loading rate also increased the nutrients concentrations in the system, leading to a higher salinity as well as inhibitor (NH4+-N, NH3, etc.) contents, which further increased to inhibit effects of pig urine on C. vulgaris. This might also contribute to the decrease of both biomass density and chlorophyll fluorescence parameters of C. vulgaris when the HRT is 5 days and 3 days. In addition, decreased biomass density might also lead to the enhanced light irradiation, which might cause light inhibition and further decrease chlorophyll fluorescence parameters of C. vulgaris under HRT of 5 days and 3 days. This could be effectively confirmed by the results in Figure 2c, where the biomass density significantly decreased as the light intensity increased from 2500 Lux to 3500 Lux. Therefore, HRT for 7 days to 9 days would be optimal for efficient nutrient removal and microalgal biomass production based on the above discussion.
In this study, the LRP-enhanced raceway pond was designed and used for the treatment of pig urine for the first time, and high nutrient removal efficiency was obtained with this system under energy saving operating conditions (Section 2.5.1). Furthermore, the obtained results fully indicated the excellent scale-up potential of this technology. It is a cost-effective way by using LRP to enhance the raceway ponds for pig urine treatment and microalgal biomass production. The capital cost of the system could be largely reduced compared to other new technologies, because the current, existed commercial raceway ponds could be sufficiently upgraded and better utilized [15]. Meanwhile, the system biomass productivity could be significantly improved by increasing the pond depth. Furthermore, the treated wastewater could also be utilized for the dilution of the fresh pig urine to save freshwater resources. The produced microalgal biomass could also be utilized for the production of feed, fertilizer, or biodiesel, due to its rich contents in proteins, lipids and carbohydrates [7]. However, further studies should be conducted, regarding the pilot scale operation of the LRP-enhanced raceway pond and the corresponding life cycle assessment, chemical composition analysis of the produced microalgal biomass, and nutrient removal of other pollutants (TOC, COD, Cu2+, Zn2+, etc.) prior to the scale-up of this technology.
4. Conclusions
C. vulgaris could grow very well in pretreated fresh pig urine (at 8-fold dilution, pH=6, MgSO4·7H2O dosage of 0.1 mg·L−1). The LRP-enhanced raceway pond has been shown to support the robust growth of C. vulgaris. Furthermore, the pretreated pig urine could be efficiently polished by C. vulgaris in this system in both batch and continuous mode, under outdoor conditions. About 1.72 g·m−2·day−1 of microalgal biomass could be produced, and 98.20% of NH4+-N and 68.48% of TP could be removed during the batch mode, respectively. A hydraulic retention time of 7 days to 9 days would be optimal for efficient nutrient removal and microalgal biomass production during continuous mode.
Author Contributions
G.Z. designed the experiments, conducted the study, performed the statistics analysis and drafted the manuscript. T.Z., S.X., Z.G., Q.H., H.Y. and M.L. participated in the pig urine treatment and help to revise the manuscript. H.Z., Q.Z., X.W., Y.W., R.R. and Y.L. participated in the design of the study and helped to draft the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 21466022, 21706087, 21766019, 21878139); The “Post-doctoral Innovative Talents Support Program” (BX20190147); The Funding for postdoctoral research projects in Jiangxi Province (2019KY04); The Key Project of Jiangxi Provincial Department of Science and Technology (20161BBF60057, 20181BBF60026).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, W.; Chen, P.; Min, M.; Ma, X.; Wang, J.; Griffith, R.; Hussain, F.; Peng, P.; Xie, Q.; Li, Y.; et al. Environment-enhancing algal biofuel production using wastewaters. Renew. Sustain. Energy Rev. 2014, 36, 256–269. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, X.; Zou, G.; Zhou, T.; Liu, Y.; Ruan, R. Cultivation of Chlorella vulgaris in manure-free piggery wastewater with high-strength ammonium for nutrients removal and biomass production: Effect of ammonium concentration, carbon/nitrogen ratio and pH. Bioresour. Technol. 2019, 273, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Kusmayadi, A.; Yen, H.; Dong, C.; Lee, D. Current advances in biological swine wastewater treatment using microalgae-based processes. Bioresour. Technol. 2019, 289, 121718. [Google Scholar] [CrossRef] [PubMed]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Z.; Jin, S.; Zhu, L.; Liu, C.; Zheng, H.; Zhou, T. Lignocellulosic residue as bio-carrier for algal biofilm growth: Effects of carrier physicochemical proprieties and toxicity on algal biomass production and composition. Bioresour. Technol. 2019, 293, 122091. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs. 2019, 17, 312. [Google Scholar] [CrossRef]
- Zhu, L.; Nugroho, Y.K.; Shakeel, S.R.; Li, Z.; Martinkauppi, B.; Hiltunen, E. Using microalgae to produce liquid transportation biodiesel: What is next? Renew. Sustain. Energy Rev. 2017, 78, 391–400. [Google Scholar] [CrossRef]
- Wang, M.; Payne, K.A.; Tong, S.; Ergas, S.J. Hybrid algal photosynthesis and ion exchange (HAPIX) process for high ammonium strength wastewater treatment. Water Res. 2018, 142, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Depraetere, O.; Muylaert, K. Effect of ammonia on the photosynthetic activity of Arthrospira and Chlorella: A study on chlorophyll fluorescence and electron transport. Algal Res. 2016, 16, 449–457. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Ben, H.; Ayed, A.; Taidi, B.; Ayadi, H.; Pareau, D.; Stambouli, M. Effect of magnesium ion concentration in autotrophic cultures of Chlorella vulgaris. Algal Res. 2015, 9, 291–296. [Google Scholar]
- Jyoti, S.; Kumar, R.; Kaur, S.; Le, Y.; Buelna, G.; Verma, M.; Ricardo, C. Application of magnesium sulfate and its nanoparticles for enhanced lipid production by mixotrophic cultivation of algae using biodiesel waste. Energy 2014, 78, 16–22. [Google Scholar]
- Zhang, Q.; Yu, Z.; Zhu, L.; Ye, T.; Zuo, J.; Li, X.; Xiao, B.; Jin, S. Vertical-algal-biofilm enhanced raceway pond for cost-effective wastewater treatment and value-added products production. Water Res. 2018, 139, 144–157. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Guo, D.; Ye, T.; Xiong, M.; Zhu, L.; Liu, C.; Jin, S.; Hu, Z. Operation of a vertical algal biofilm enhanced raceway pond for nutrient removal and microalgae-based byproducts production under different wastewater loadings. Bioresour. Technol. 2018, 253, 323–332. [Google Scholar] [CrossRef]
- Salama, E.S.; Kurade, M.B.; Abou-Shanab, R.A.I.I.; El-dalatony, M.M.; Yang, I.S.; Min, B.; Jeon, B.H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, C.; Li, Y.; Yu, Z.; Chen, Z.; Ye, T.; Wang, X.; Hu, Z.; Liu, S.; Xiao, B.; et al. Cultivation of algal biofilm using different lignocellulosic materials as carriers. Biotechnol. Biofuels 2017, 10, 115. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, T.; Li, Z.; Wang, J.; Tang, J.; Ruan, R.; Liu, Y. Effect of combining adsorption-stripping treatment with acidification on the growth of Chlorella vulgaris and nutrient removal from swine wastewater. Bioresour. Technol. 2018, 263, 10–16. [Google Scholar] [CrossRef]
- Deng, X.; Gao, K.; Addy, M.; Li, D.; Xia, A.; Zhang, R.; Lu, Q.; Ma, Y.; Cheng, Y.; Chen, P.; et al. Cultivation of Chlorella vulgaris on anaerobically digested swine manure with daily recycling of the post-harvest culture broth. Bioresour. Technol. 2018, 247, 716–723. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, W.; Liao, Q.; Fu, Q.; Xia, A.; Zhu, X.; Sun, Y. Comparison of Chlorella vulgaris biomass productivity cultivated in biofilm and suspension from the aspect of light transmission and microalgae affinity to carbon dioxide. Bioresour. Technol. 2016, 222, 367–373. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Hu, Q.; Cheng, P.; Ji, B.; Liu, J.; Chen, Y.; Zhang, W.; Chen, X.; Chen, L.; et al. Attached cultivation technology of microalgae for efficient biomass feedstock production. Bioresour. Technol. 2013, 127, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Tuantet, K.; Temmink, H.; Zeeman, G.; Janssen, M.; Wijffels, R.H.; Buisman, C.J.N. Nutrient removal and microalgal biomass production on urine in a short light-path photobioreactor. Water Res. 2014, 55, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, J.; Zheng, H.; Wu, X.; Wang, Y.; Liu, M.; Xiang, S.; Cao, L.; Ruan, R.; Liu, Y. Characterization of additional zinc ions on the growth, biochemical composition and photosynthetic performance from Spirulina platensis. Bioresour. Technol. 2018, 269, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Champagne, P. Nutrient removal, microalgal biomass growth, harvesting and lipid yield in response to centrate wastewater loadings. Water Res. 2016, 88, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Liu, T.; Gao, L. Effects of initial population density (IPD) on growth and lipid composition of Nannochloropsis sp. J. Appl. Phycol. 2012, 24, 1623–1627. [Google Scholar] [CrossRef]
- Bai, X.; Lant, P.; Pratt, S. The contribution of bacteria to algal growth by carbon cycling. Biotechnol. Bioeng. 2015, 112, 688–695. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Hu, T.; Nugroho, Y.K.; Yin, Z.; Hu, D.; Chu, L.; Mo, F.; Liu, C.; Hiltunenb, E. Effects of nitrogen source heterogeneity on nutrient removal and biodiesel production of mono- and mix-cultured microalgae. Energy Convers. Manag. 2019, 201, 112–114. [Google Scholar] [CrossRef]
- Ge, S.; Qiu, S.; Tremblay, D.; Viner, K.; Champagne, P.; Jessop, P.G. Centrate wastewater treatment with Chlorella vulgaris: Simultaneous enhancement of nutrient removal, biomass and lipid production. Chem. Eng. J. 2018, 342, 310–320. [Google Scholar] [CrossRef]
- Boelee, N.C.; Janssen, M.; Temmink, H.; Shrestha, R.; Buisman, C.J.N.; Wijffels, R.H. Nutrient removal and biomass production in an outdoor pilot-scale phototrophic biofilm reactor for effluent polishing. Appl. Biochem. Biotechnol. 2014, 172, 405–422. [Google Scholar] [CrossRef]
- Zhao, X.; Kumar, K.; Gross, M.A.; Kunetz, T.E.; Wen, Z. Evaluation of revolving algae biofilm reactors for nutrients and metals removal from sludge thickening supernatant in a municipal wastewater treatment facility. Water Res. 2018, 143, 467–478. [Google Scholar] [CrossRef]
- Shi, J.; Podola, B.; Melkonian, M. Application of a prototype-scale Twin-Layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour. Technol. 2014, 154, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Yu, Z.; Zhou, T.; Gu, Z.; Huang, Q.; Xiao, B.; Zhou, W.; Ruan, R.; Liu, Y. Pine sawdust as algal biofilm biocarrier for wastewater treatment and algae-based byproducts production. J. Clean. Prod. 2020, 265, 120. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).