1. Introduction
Our world is highly reliant on a single source of energy, fossil fuel. Cheapness, cost effectiveness, and wide accessibility are some of the recognizable advantages of fossil fuel. These advantages come at a steep price, however, as the excessive exploitation of fossil fuel has released enormous amounts of greenhouse gases into our atmosphere, leading to global warming. As of 2016, CO
2 emissions had reached 32.31 GtCO
2, almost 2 times greater than the output of the early industrial revolution [
1]. In addition, fossil fuel is not a renewable resource, making the need for alternative sources even more urgent.
Whereas the global energy supply is still dominated by fossil fuel, the proportion of renewables has been on the rise, reaching 10.2% of the primary energy supply in the OECD (Organisation for Economic Co-operation and Development) countries by 2017 [
1]. This uptrend is projected to continue as numerous other countries race to increase their utilization of renewables. For instance, South Korea released a plan to its renewables proportion from 6% to 20% by 2030. Following South Korea’s step is Indonesia, which plans to increase its renewable proportion from 6.2% (2016) to 23% by 2025.
Biodiesel is a product of a transesterification reaction in which triglycerides found in vegetable oils or animal fats combine with alcohol in the presence of a catalyst to produce fatty acid methyl esters (FAME) and glycerol. Biodiesel is considered superior to fossil fuel in terms of biodegradability, renewability, toxicity, ecofriendliness [
2], and better physicochemical properties except for those such as freezing point and oxidation stability [
3,
4]. Biodiesel has similar characteristics to petroleum-based diesel, facilitating its incorporation as a mixture or direct utilization with existing technologies without complex modification [
5].
Transesterification is widely adapted for biodiesel production because of its relatively mild operating conditions. As it is reversible, excess amounts of reactants can be used to promote the forward reaction and increase product yields thereby. An alkaline catalyst is commonly used in transesterification reaction as it has a faster reaction rate relative to an acid catalyst [
6]. However, the transesterification system is highly sensitive to high free fatty acid (FFA) content. High FFA content in feedstock can promote the formation of soap through a saponification reaction, leading to decreased yields and difficulties in the separation and purification of biodiesel. Thus, in systems where high FFA contents are present, an additional pretreatment step to reduce acid value (FFA) level is needed.
Pretreatment of FFA can be achieved using an esterification reaction, in which the FFA is reacted with alcohol in the presence of an acid catalyst to produce FAME and water. Sulfuric acid is commonly used in esterification reactions, given its advantage of high FFA conversion yield under moderate conditions [
7]. Unfortunately, sulfuric acid can cause corrosion and environmental problems, making it relatively difficult to utilize on large scales. For these reasons, a heterogeneous catalyst is preferred as it is can be easily recovered and recycled and with less waste production.
The feedstock for biodiesel production usually comes from vegetable oils as they are easier to source on large scales [
8]. Palm oil is one of the most extensively utilized vegetable oils in biodiesel production, due to its cheap price, high kernel-oil yield (±25%) [
9], and long productivity (±23 years) [
10]. Excessive utilization of palm oil, however, has led to massive deforestation, especially through direct burning, to make room for palm tree plantations. Hence, while biodiesel is considered to be a greener alternative to petroleum fuel, the overall greenhouse gas reduction credit from the use of palm oil is considered to be insignificant due to the enormous amounts of greenhouse gases emitted during the deforestation process. Palm oil also is an edible oil, which makes for competition with the food industry. Thus, researchers have shifted their attention towards other, non-edible feedstock alternatives, such as
Jatropha curcas and
Pongamia pinnata [
11].
Reutealis trisperma is another oil-producing crop that has the potential to be commercialized as a biodiesel production feedstock. This species is native to the Philippines and is cultivated throughout the South East Asia region. Compared to palm trees,
R.trisperma has a much higher oil kernel yield (±50%) [
12] and longer productivity (±70 years), making it an especially promising feedstock candidate. It can grow on marginal land, which could help both the restoration of marginal lands and the prevention of deforestation. Furthermore
R.trisperma oil is known to contain a toxin called eleostearic acid, making it non-edible [
13]. All these factors make
R.trisperma a very promising alternative indeed for commercialization as biodiesel production feedstock.
Several studies have been conducted regarding the production of biodiesel from
R.trisperma oil. This oil has a relatively high acid value, averaging 30 mg KOH/g. Thus, an esterification reaction is deemed necessary to maximize the biodiesel production from this oil. A two-step esterification/transesterification reaction of
R.trisperma oil produced a 95.15% biodiesel yield [
14]. One study found that the highest FAME yield achieved was 61.1% [
13]. Another study incorporated an infrared-assisted technology for biodiesel production from
R.trisperma oil and successfully achieved a 97.78% methyl ester yield [
15]. Several gaps have been detected based on these studies. First, all of these studies performed the esterification reaction with sulfuric acid, which is preferentially avoided for larger-scale production. Second, there is limited data available on the kinetics of
R.trisperma oil esterification, which is considered important for scaling up the production process. Hence, to fill that data lacuna, the present study aimed to investigate and optimize the esterification reaction of
R.trisperma oil with a heterogeneous ion exchange resin Lewatit K2640. A kinetics study of the esterification reaction was performed as well.
4. Conclusions
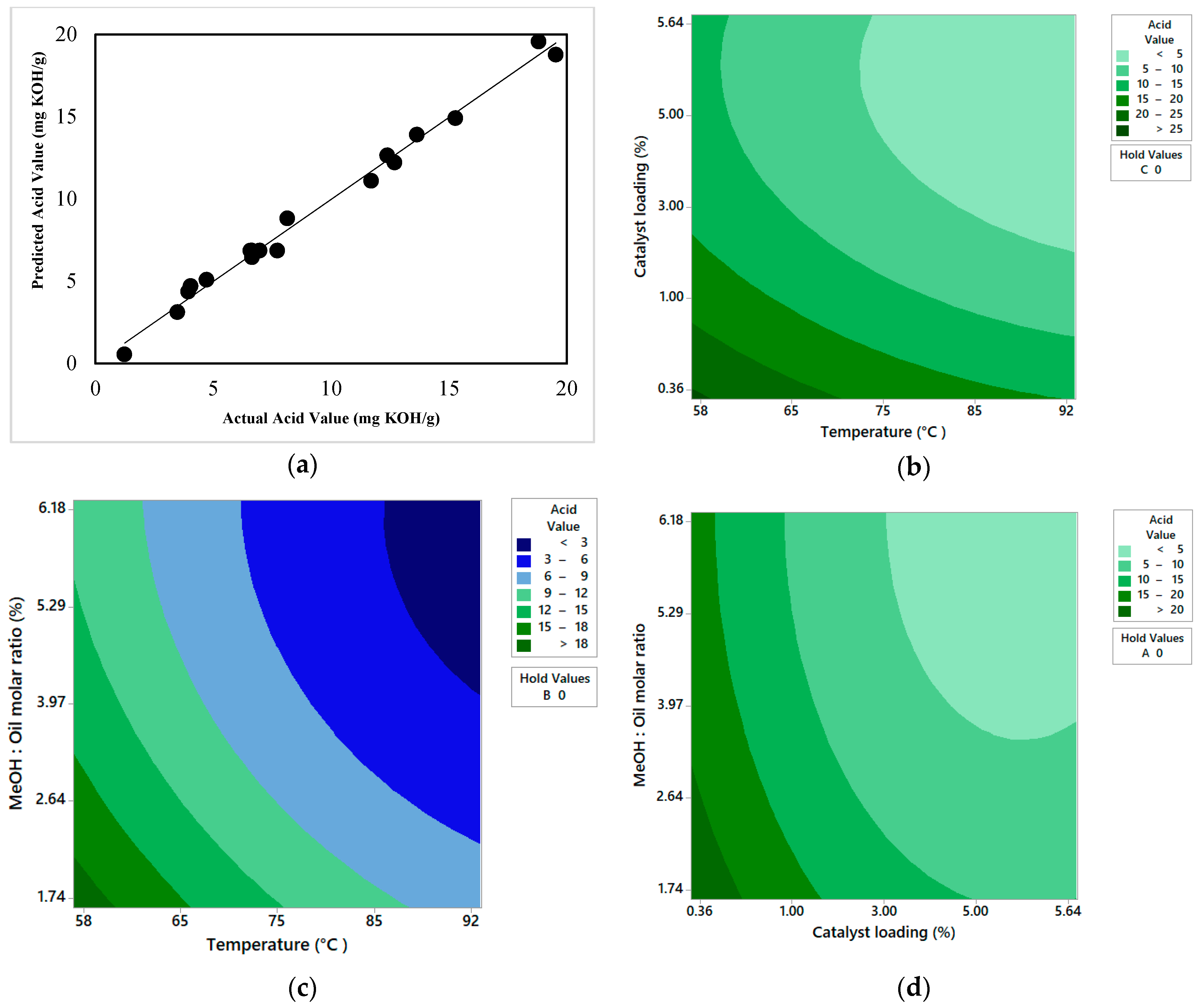
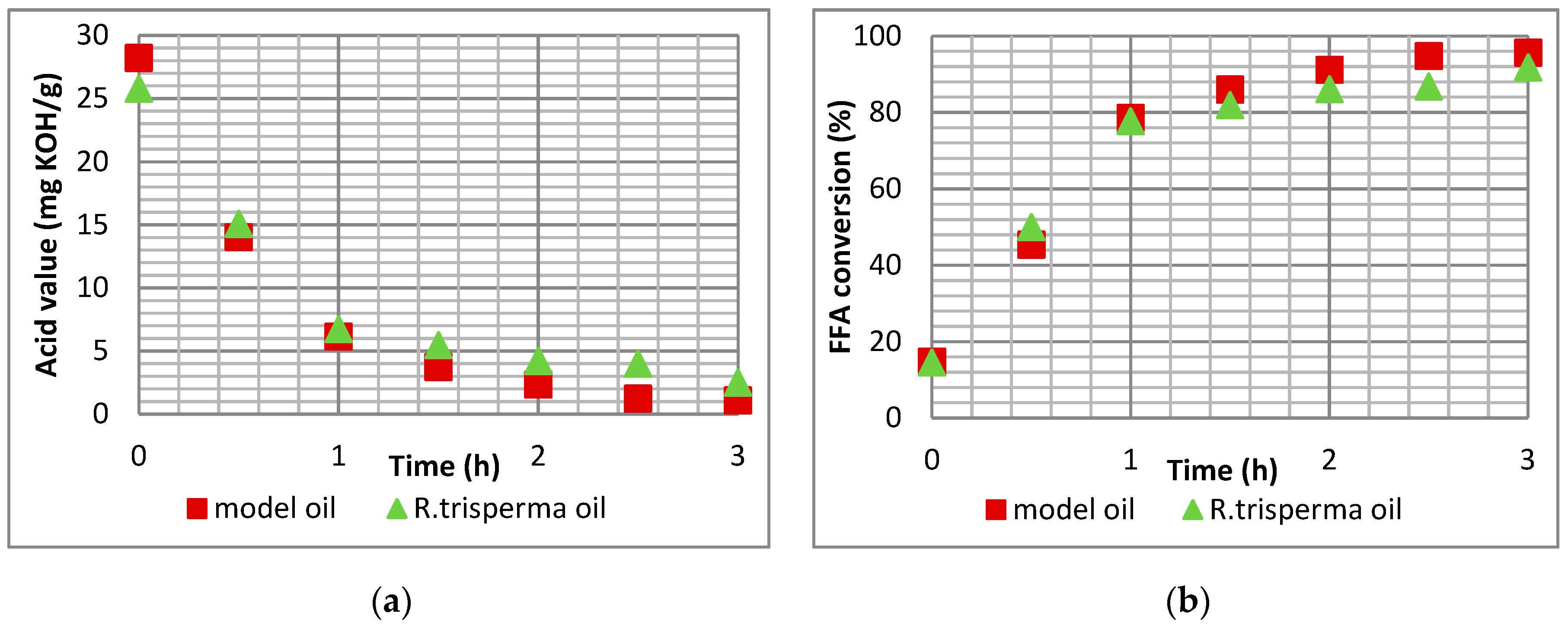
The esterification of R.trisperma oil was evaluated and optimized statistically using a response surface methodology via a central composite design with a model oil for optimization of esterification reaction conditions. It was revealed that all of the individual factors (temperature, catalyst loading, and methanol-to-oil molar ratio) observed in this study had a significant impact on the acid value of the esterified oil, catalyst loading having the most significant impact, followed by temperature and methanol-to-oil molar ratio, respectively. The two-way interaction of the individual factors did not have any impact on the acid value of the esterified oil. The developed model could represent the experimental data well with high reliability. Based on the optimization process, the optimal conditions for the esterification reaction of R.trisperma oil were 92 °C temperature, 5.34 wt% catalyst loading, and 5.82:1 methanol-to-oil molar ratio, with a final acid value of 2.49 mg KOH/g and an achieved FFA conversion of 91.75%.
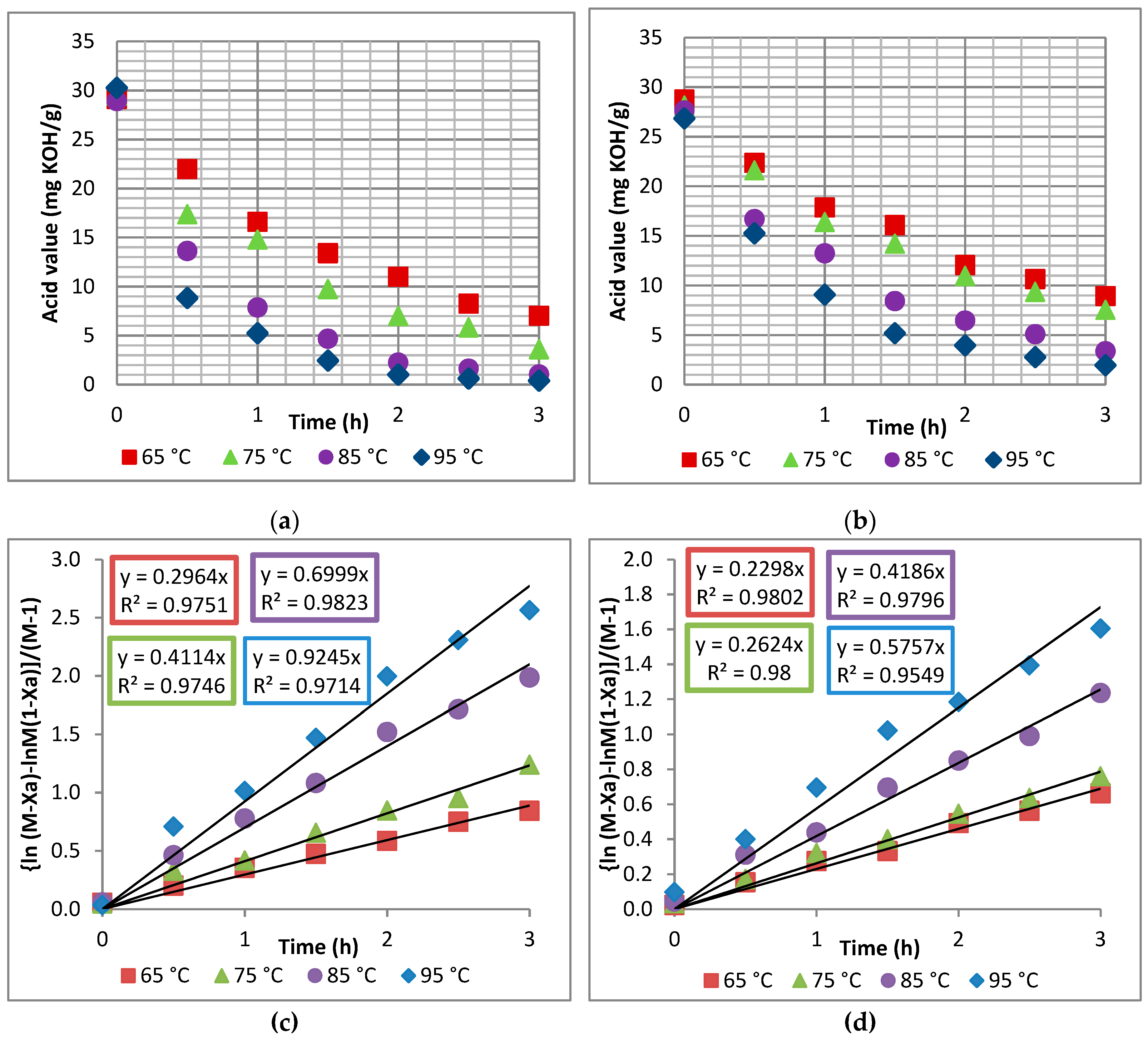
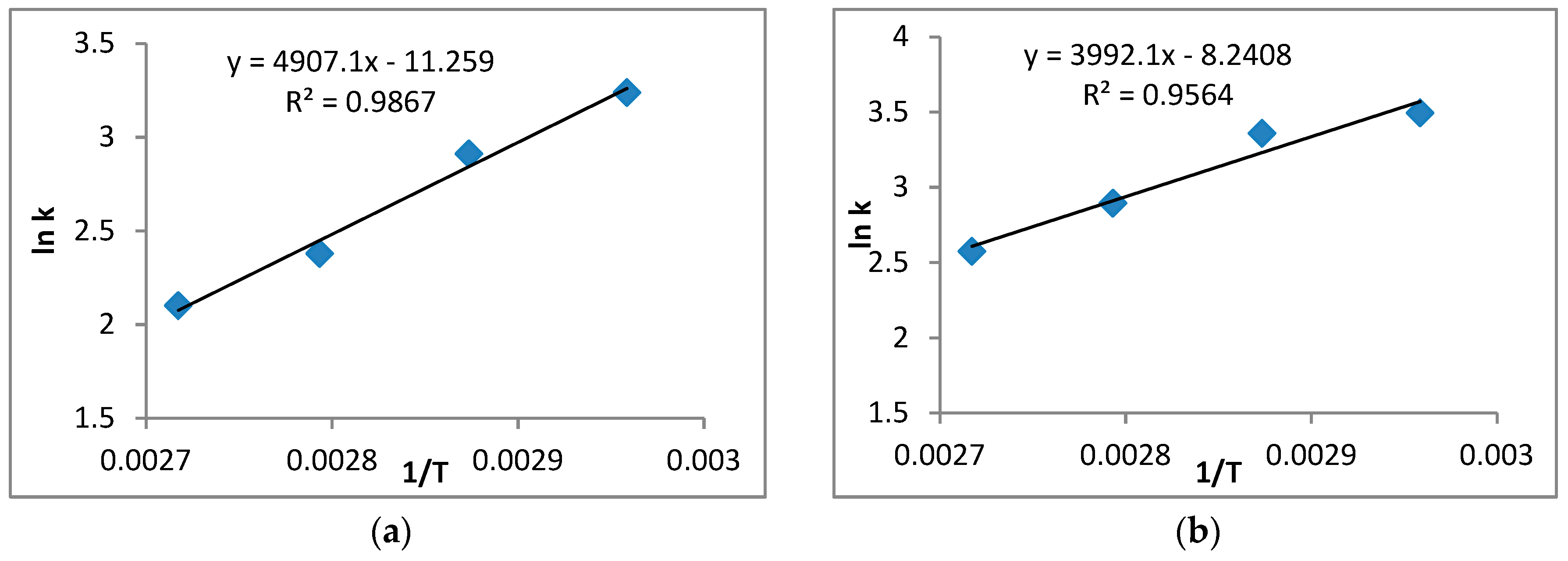
The kinetics of R.trisperma were investigated under the assumption of pseudo-homogeneous second-order kinetics. It was discovered that the experimental data could fit the developed reaction rate equation well and with high reliability, indicating that the kinetics of R.trisperma oil esterification followed a pseudo-homogeneous second-order reaction under the specified conditions. The activation energy required for the esterification of R.trisperma oil was 33.2 kJ/mol.