Developing Process Designs for Biorefineries—Definitions, Categories, and Unit Operations
Abstract
1. Introduction
Context of a Biorefinery
2. Feedstock
- (a)
- Agriculture (dedicated crops and residues);
- (b)
- Forestry;
- (c)
- Industries (process residues and leftovers);
- (d)
- Households (municipal solid waste and wastewaters);
- (e)
- Aquaculture (algae and seaweeds).
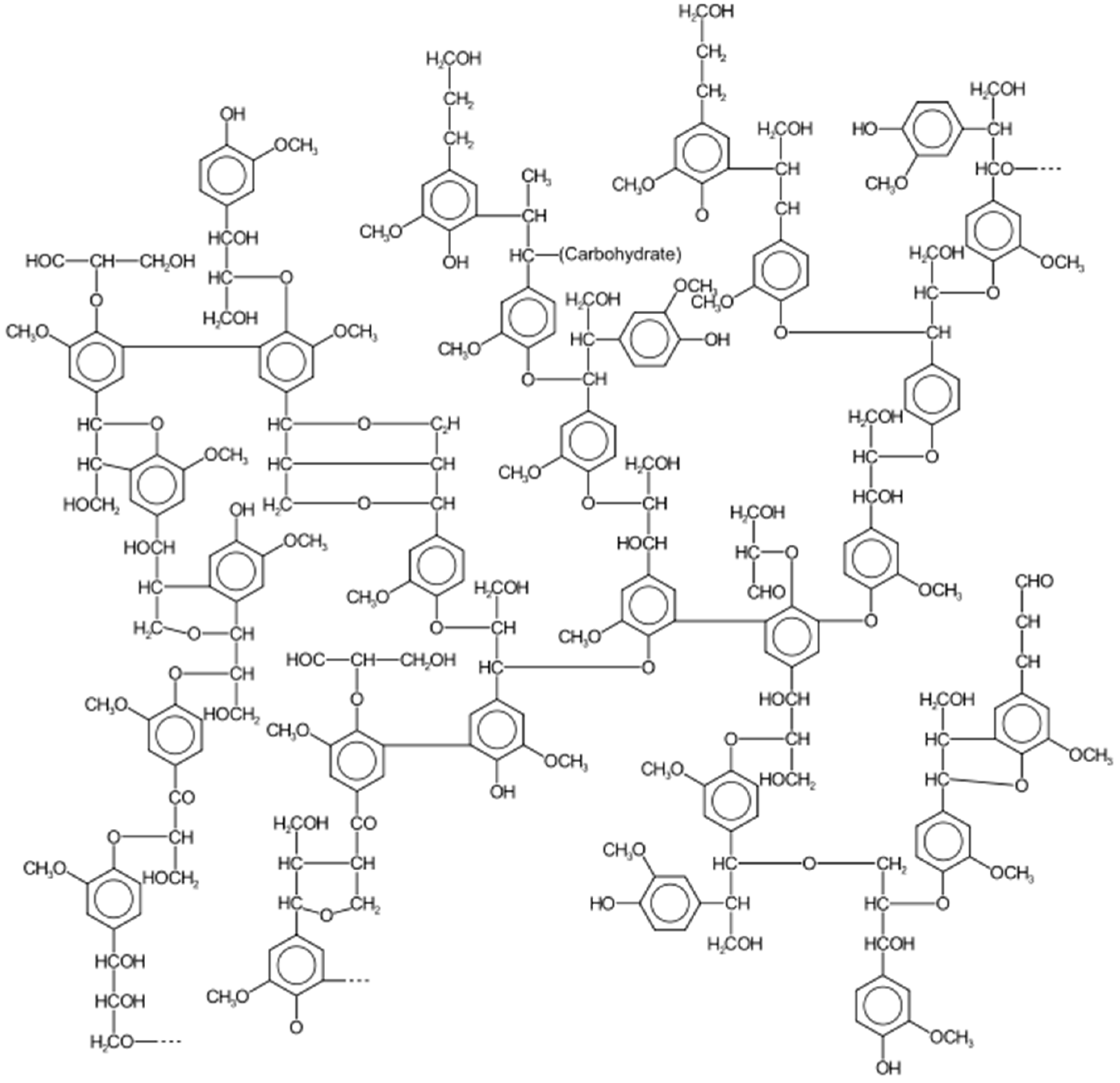
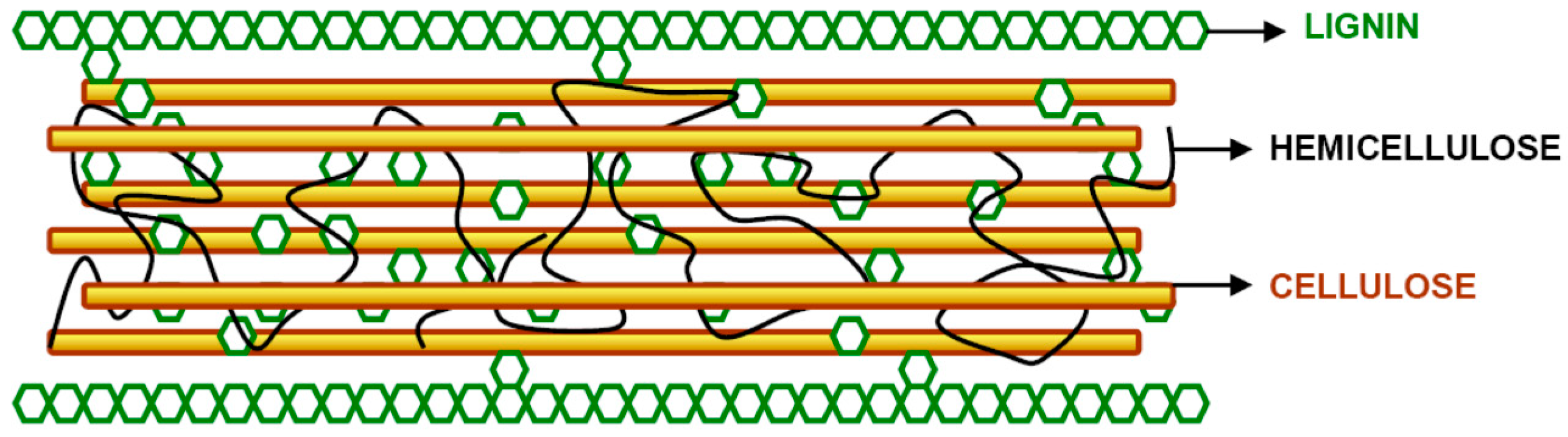
2.1. Carbohydrates and Lignin
- (1)
- P-coumaryl alcohol;
- (2)
- Coniferyl alcohol;
- (3)
- Sinapyl alcohol.
2.2. Triglycerides
2.3. Mixed Organic Fractions
2.4. Proteins
3. Processes
3.1. Thermochemical Processes
3.2. Low-Temperature Processes
3.3. Biochemical Processes
3.4. Mechanical Processes
3.5. Chemical Processes
4. Products
- (a)
- Gaseous biofuels (biogas, syngas, hydrogen, bio-methane);
- (b)
- Solid biofuels (pellets, lignin, charcoal);
- (c)
- Liquid biofuels for transportation (bioethanol, biodiesel, FT-fuels, bio-oil).
- Chemicals (fine chemicals, building blocks, bulk chemicals);
- Organic acids (succinic, lactic, levulinic and other sugar derivatives);
- Polymers and resins (starch-based plastics, phenol resins, furan resins);
- Biomaterials (wood panels, pulp, paper, cellulose);
- Food and animal feed (protein rich);
- Fertilizers;
- Biopolymers (example: polylactic acid).
5. Types of Biorefineries
- Raw material input (i.e., Green Biorefinery, Crop Biorefinery, Lignocellulosic Feedstock Biorefinery, Marine Biorefinery);
- Type of technology (i.e., Two Platform Concept, Thermochemical Biorefinery);
- Status-of-technology (Conventional and Advanced Biorefineries, 1st and 2nd Generation Biorefineries);
- Main or intermediate product produced (Syngas Platform, Sugar Platform, Lignin Platform).
- (a)
- A broad definition encompassing the biorefinery area which is more accessible for different stakeholders;
- (b)
- Improved overall understanding of the advantages of biorefinery processing (multi-step and integrated process) over single-product processes;
- (c)
- Market implementation and acceptance of these concepts into the global community of scientists.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamm, B.; Kamm, M. Biorefineries—Multi Product Processes. In White Biotechnology; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Pham, V.; El-Halwagi, M. Process synthesis and optimization of biorefinery configurations. AIChE J. 2012, 58, 1212–1221. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Lab.: Golden, CO, USA, 2011.
- Lino, F.A.M.; Ismail, K.A.R. Energy and environmental potential of solid waste in Brazil. Energy Policy 2011, 39, 3496–3502. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Bothast, R.J.; Schlicher, M.A. Biotechnological processes for conversion of corn into ethanol. Appl. Microbiol. Biotechnol. 2005, 67, 19–25. [Google Scholar] [CrossRef]
- Brown, D.; Li, Y. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour. Technol. 2013, 127, 275–280. [Google Scholar] [CrossRef]
- Tao, L.; Schell, D.; Davis, R.; Tan, E.; Elander, R.; Bratis, A. NREL 2012 Achievement of Ethanol Cost Targets: Biochemical Ethanol Fermentation Via Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Lab.: Golden, CO, USA, 2014.
- Tula, A.K.; Eden, M.R.; Gani, R. Process synthesis, design and analysis using a process-group contribution method. Comput. Chem. Eng. 2015, 81, 245–259. [Google Scholar] [CrossRef]
- Ashraf, M.T.; Torres, A.I.; Cybulska, I.; Fang, C.; Thomsen, M.H.; Schmidt, J.E.; Stephanopoulos, G. Optimization of Lignocellulosic Waste Biorefinery using Multi-Actor Multi-Objective Mathematical Framework. In Proceedings of the 26th European Symposium on Computer Aided Process Engineering; Elsevier: Portorož, Slovenia, 2016. [Google Scholar]
- Moncada, J.; Matallana, L.G.; Cardona, C.A. Selection of process pathways for biorefinery design using optimization tools: A colombian case for conversion of sugarcane bagasse to ethanol, poly-3-hydroxybutyrate (PHB), and energy. Ind. Eng. Chem. Res. 2013, 52, 4132–4145. [Google Scholar] [CrossRef]
- Sammons, N.E.; Yuan, W.; Eden, M.R.; Aksoy, B.; Cullinan, H.T. Optimal biorefinery product allocation by combining process and economic modeling. Chem. Eng. Res. Des. 2008, 86, 800–808. [Google Scholar] [CrossRef]
- Lynd, L.R.; Wyman, C.; Laser, M.; Johnson, D.; Landucci, R. Strategic Biorefinery Analysis: Analysis of Biorefineries; National Renewable Energy Lab.: Golden, CO, USA, 2005.
- Tay, D.; Ng, D. Fuzzy optimization approach for the synthesis of a sustainable integrated biorefinery. Ind. Eng. Chem. Res. 2011, 50, 1652–1665. [Google Scholar] [CrossRef]
- Stuart, P.R.; El-Halwagi, M.M. Integrated Biorefineries: Design, Analysis, and Optimization; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Kelloway, A.; Daoutidis, P. Process synthesis of biorefineries: Optimization of biomass conversion to fuels and chemicals. Ind. Eng. Chem. 2013, 53, 5261–5273. [Google Scholar] [CrossRef]
- Raman, J.K.; Gnansounou, E. Furfural production from empty fruit bunch—A biorefinery approach. Ind. Crops Prod. 2015, 69, 371–377. [Google Scholar] [CrossRef]
- Cheali, P.; Posada, J.A.; Gernaey, K.V.; Sin, G. Upgrading of lignocellulosic biorefinery to value- added chemicals: Sustainability and economics of bioethanol-derivatives. Biomass Bioenergy 2015, 75, 282–300. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004.
- Holladay, J.J.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value Added Chemicals from Biomass-Volume II, Results of Screening for Potential Candidates from Biorefinery Lignin; Pacific Northwest National Lab.: Richland, NJ, USA, 2007.
- Moncada, J.; Tamayo, J.A.; Cardona, C.A. Integrating first, second, and third generation biorefineries: Incorporating microalgae into the sugarcane biorefinery. Chem. Eng. Sci. 2014, 118, 126–140. [Google Scholar] [CrossRef]
- Moncada, J.; El-Halwagi, M.M.; Cardona, C.A. Techno-economic analysis for a sugarcane biorefinery: Colombian case. Bioresour. Technol. 2013, 135, 533–543. [Google Scholar] [CrossRef]
- Kumar, M.; Goyal, Y.; Sarkar, A.; Gayen, K. Comparative economic assessment of ABE fermentation based on cellulosic and non-cellulosic feedstocks. Appl. Energy 2012, 93, 193–204. [Google Scholar] [CrossRef]
- Quintero, J.A.; Moncada, J.; Cardona, C.A. Techno-economic analysis of bioethanol production from lignocellulosic residues in Colombia: A process simulation approach. Bioresour. Technol. 2013, 139, 300–301. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Simmons, B.A.; Blanch, H.W. Techno-economic analysis of a lignocellulosic ethanol biorefinery with ionic liquid pre-treatment. Biofuels Bioprod. Biorefining 2011, 5, 562–569. [Google Scholar] [CrossRef]
- Axelsson, L.; Franzén, M.; Ostwald, M.; Berndes, G.; Lakshmi, G.; Ravindranath, N.H. Perspective: Jatropha cultivation in southern India: Assessing farmers’ experiences. Biofuels Bioprod. Biorefining 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Baliban, R.C.; Elia, J.A.; Floudas, C.A. Biomass and natural gas to liquid transportation fuels: Process synthesis, global optimization, and topology analysis. Ind. Eng. Chem. Res. 2013, 52, 3381–3406. [Google Scholar] [CrossRef]
- IEA. IEA Bioenergy Task 42 on Biorefineries: Co-Production of Fuels, Chemicals, Power and Materials from Biomass; IEA bioenergy task: Paris, France, 2010. [Google Scholar]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, P.; Su, Y. An overview of biofuels policies and industrialization in the major biofuel producing countries. Renew. Sustain. Energy Rev. 2015, 50, 991–1003. [Google Scholar] [CrossRef]
- Kircher, M. Sustainability of biofuels and renewable chemicals production from biomass. Curr. Opin. Chem. Biol. 2015, 26, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Biddy, M.J.; Scarlata, C.; Kinchin, C. Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential; National Renewable Energy Lab.: Golden, CO, USA, 2016.
- Preisig, H.A.; Wittgens, B. Thinking towards synergistic green refineries. Energy Procedia 2012, 20, 59–67. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Jacobsen, N. Industrial symbiosis in Kalundborg, Denmark: A quantitative assessment of economic and environmental aspects. J. Ind. Ecol. 2006, 10, 239–255. [Google Scholar] [CrossRef]
- Sims, R.E.; Mabee, W.; Saddler, J.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef]
- Stephen, J.; Sokhansanj, S.; Bi, X. The impact of agricultural residue yield range on the delivered cost to a biorefinery in the Peace River region of Alberta, Canada. Biosyst. Eng. 2010, 105, 298–305. [Google Scholar] [CrossRef]
- Hess, J.; Wright, C.; Kenney, K. Cellulosic biomass feedstocks and logistics for ethanol production. Biofuels Bioprod. Biorefining 2007, 1, 181–190. [Google Scholar] [CrossRef]
- Caputo, A.C.; Palumbo, M.; Pelagagge, P.M.; Scacchia, F. Economics of biomass energy utilization in combustion and gasification plants: Effects of logistic variables. Biomass Bioenergy 2005, 28, 35–51. [Google Scholar] [CrossRef]
- Galbe, M.; Sassner, P.; Wingren, A.; Zacchi, G. Process engineering economics of bioethanol production. In Biofuels; Olsson, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Damartzis, T.; Zabaniotou, A. Thermochemical conversion of biomass to second generation biofuels through integrated process design—A review. Renew. Sustain. Energy Rev. 2011, 15, 366–378. [Google Scholar] [CrossRef]
- Bulushev, D.; Ross, J. Catalysis for conversion of biomass to fuels via pyrolysis and gasification: A review. Catal. Today 2011, 171, 1–13. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Double, J.M. Production costs of liquid fuels from biomass. Fuel 1991, 70, 1209–1224. [Google Scholar] [CrossRef]
- Demirbas, A. Biorefinery technologies for biomass upgrading. Energy Sources Part A Recover. Util. Environ. Eff. 2010, 32, 1547–1558. [Google Scholar] [CrossRef]
- Centi, G.; Lanzafame, P.; Perathoner, S. Analysis of the alternative routes in the catalytic transformation of lignocellulosic materials. Catal. Today 2011, 167, 14–30. [Google Scholar] [CrossRef]
- Cherubini, F.; Strømman, A.H. Principles of biorefining. In Biofuels: Alternative Feedstocks and Conversion Processes; Pandey, A., Larroche, C., Ricke, S.C., Dussap, C.-G., Gnansounou, E., Dussap, C.-G., Eds.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Kokossis, A.C.; Yang, A. On the use of systems technologies and a systematic approach for the synthesis and the design of future biorefineries. Comput. Chem. Eng. 2010, 34, 1397–1405. [Google Scholar] [CrossRef]
- Tsai, W.; Lin, C.; Yeh, C. An analysis of biodiesel fuel from waste edible oil in Taiwan. Renew. Sustain. Energy Rev. 2007, 11, 838–857. [Google Scholar] [CrossRef]
- Lebo, S.E., Jr.; Gargulak, J.D.; McNally, T.J. Lignin. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley Online Library: Hoboken, NJ, USA, 2000. [Google Scholar]
- Wardrop, A.B. The structure of the cell wall in lignifield collenchyma of Eryngium sp.(Umbelliferae). Aust. J. Bot. 1969, 17, 229–240. [Google Scholar] [CrossRef]
- Glazer, A.N.; Nikaido, H. Microbial Biotechnology: Fundamentals of Applied Microbiology; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Koutinas, A.A.; Wang, R.; Campbell, G.M.; Webb, C. A Whole Crop Biorefinery System: A Closed System for the Manufacture of Non-food Products from Cereals. In Biorefineries-Industrial Processes and Products; Wiley-VCH Verlag GmbH: Weinheim, Germany, 16 December 2005. [Google Scholar]
- Deswarte, F.E.I.; Clark, J.H.; Wilson, A.J.; Hardy, J.J.E.; Marriott, R.; Chahal, S.P.; Jackson, C.; Heslop, G.; Birkett, M.; Bruce, T.J.; et al. Toward an integrated straw-based biorefinery. Biofuels Bioprod. Biorefining 2007, 1, 245–254. [Google Scholar] [CrossRef]
- Wenzl, H. The Chemical Technology of Wood; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Teixeira, J.A. Lignocellulose as raw material in fermentation processes. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Formatex Research Center: Badajoz, Spain, 2010. [Google Scholar]
- Pan, X.; Gilkes, N.; Kadla, J.; Pye, K.; Saka, S.; Gregg, D.; Ehara, K.; Xie, D.; Lam, D.; Saddler, J. Bioconversion of Hybrid Poplar to Ethanol and Co-Products Using an Organosolv Fractionation Process: Optimization of Process Yields. Biotechnol. Bioeng. 2006, 94, 851–861. [Google Scholar] [CrossRef]
- Achten, W.M.; Mathijs, E.; Verchot, L.; Singh, V.P.; Aerts, R.; Muys, B. Jatropha biodiesel fueling sustainability? Biofuels Bioprod. Biorefining 2007, 1, 283–291. [Google Scholar] [CrossRef]
- Cybulska, I.; Chaturvedi, T.; Brudecki, G.P.; Kádár, Z.; Meyer, A.S.; Baldwin, R.M.; Thomsen, M.H. Bioresource Technology Chemical characterization and hydrothermal pretreatment of Salicornia bigelovii straw for enhanced enzymatic hydrolysis and bioethanol potential. Bioresour. Technol. 2014, 153, 165–172. [Google Scholar] [CrossRef]
- Nwobi, A. Techno-economic Evaluation of Biofuel Production from Organic Fraction of Municipal Solid Waste (OFMSW). Master’s Thesis, Masdar Institute of Science and Technology, Abu Dhabi, UAE, 2013. [Google Scholar]
- Mulder, W. Proteins in Biomass Streams; Food & Biobased Research: Wageningen, The Netherlands, 2010. [Google Scholar]
- Soh, L.; Montazeri, M.; Haznedaroglu, B.Z.; Kelly, C.; Peccia, J.; Eckelman, M.J.; Zimmerman, J.B. Evaluating microalgal integrated biorefinery schemes: Empirical controlled growth studies and life cycle assessment. Bioresour. Technol. 2014, 151, 19–27. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Alexandri, M.; Strati, A.; Gardeli, C.; Papanikolaou, S.; Komaitis, M.; Kookos, I.K.; Koutinas, A.A. Integrated sunflower-based biorefinery for the production of antioxidants, protein isolate and poly (3-hydroxybutyrate). Ind. Crops Prod. 2015, 71, 106–113. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of Vegetable Oils with Ethanol and Characterization of the Key Fuel Properties of Ethyl Esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Du, Z.; Mohr, M.; Ma, X.; Cheng, Y.; Lin, X.; Liu, Y.; Zhou, W.; Chen, P.; Ruan, R. Hydrothermal pretreatment of microalgae for production of pyrolytic bio-oil with a low nitrogen content. Bioresour. Technol. 2012, 120, 13–18. [Google Scholar] [CrossRef]
- Helle, S.; Bennett, N.; Lau, K.; Matsui, J.; Duff, S. A kinetic model for production of glucose by hydrolysis of levoglucosan and cellobiosan from pyrolysis oil. Carbohydr. Res. 2007, 342, 2365–2370. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.N.; Sanchez, A.; Rocha, G.J.M.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef]
- Taherzadeh, M.M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Hosseini, S.; Shah, N. Multiscale modelling of hydrothermal biomass pretreatment for chip size optimization. Bioresour. Technol. 2009, 100, 2621–2628. [Google Scholar] [CrossRef]
- Heitz, M.; Vincent, D.; Chornet, E.; Overend, R.P.; Sastre, H. Solvent Effects on Liquefaction: Solubilization Profiles of a Tropical Prototype Wood, Eucalyptus, in the Presence of Simple Alcohols, Ethylene Glycol, Water and Phenols. In Research in Thermochemical Biomass Conversion; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzym. Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Thygesen, A.; Thomsen, A.B. Identification and characterization of fermentation inhibitors formed during hydrothermal treatment and following SSF of wheat straw. Appl. Microbiol. Biotechnol. 2009, 83, 447–455. [Google Scholar] [CrossRef]
- Garrote, G.; Dominguez, H.; Parajo, J. Hydrothermal processing of lignocellulosic materials. Holz als Roh und Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Argyros, D.A.; Tripathi, S.A.; Barrett, T.F.; Rogers, S.R.; Feinberg, L.F.; Olson, D.G.; Foden, J.M.; Miller, B.B.; Lynd, L.R.; Hogsett, D.A.; et al. High ethanol titers from cellulose by using metabolically engineered thermophilic, anaerobic microbes. Appl. Environ. Microbiol. 2011, 77, 8288–8294. [Google Scholar] [CrossRef]
- Montgomery, L.F.R.; Bochmann, G. Pretreatment of Feedstock for Enhanced Biogas Production; IEA Bioenergy: Cork, Ireland, 2015. [Google Scholar]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Fitzgerald, G.C.; Themelis, N.J. Technical and Economic Analysis of Pre-Shredding Municipal Solid Wastes Prior to Disposal. Master’s Thesis, Columbia University, New York, NY, USA, 2009. [Google Scholar]
- Conde-Mejia, C.; Jimenez-Gutierrez, A. A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Process Saf. Environ. Prot. 2012, 90, 189–202. [Google Scholar] [CrossRef]
- Butler, N. How Petrochemicals are Fuelling Oil Demand. Available online: https://www.ft.com/content/5ae88252-cb9b-11e8-b276-b9069bde0956 (accessed on 20 March 2020).
- IEA. The Future of Petrochemicals; IEA: Paris, France, 2018. [Google Scholar]
- Team, C.W.; Pachauri, R.K.; Meyer, L.A. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Kromus, S.; Wachter, B.; Koschuh, W.; Mandl, M.; Krotscheck, C.; Narodoslawsky, M. The green biorefinery Austria-development of an integrated system for green biomass utilization. Chem. Biochem. Eng. Q. 2004, 18, 7–12. [Google Scholar]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Naik, S.N.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Van Ree, R.; Annevelink, B. Status Report Biorefinery 2007; Food & Biobased Research: Wageningen, The Netherlands, 2007. [Google Scholar]
- Kamm, B.; Gruber, P.; Kamm, M. Biorefineries–Industrial Processes and Products, 1st ed.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Chandel, A.K.; Chandrasekhar, G.; Silva, M.B.; Silvério da Silva, S. The realm of cellulases in biorefinery development. Crit. Rev. Biotechnol. 2012, 32, 187–202. [Google Scholar] [CrossRef]
- Psycha, M.; Pyrgakis, K.; Kokossis, A.C. Integrated Designs of Microalgae Biorefineries Using a Fixed Selection of Halophytic Algae. Energy 2014, 50, 275–301. [Google Scholar]
- Gebreslassie, B.; Waymire, R.; You, F. Sustainable design and synthesis of algae based biorefinery for simultaneous hydrocarbon biofuel production and carbon sequestration. AIChE J. 2013, 59, 1599–1621. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Dale, B. ’Greening’the chemical industry: Research and development priorities for biobased industrial products. J. Chem. Technol. Biotechnol. 2003, 70, 1093–1103. [Google Scholar] [CrossRef]
- Sues, A.; Juraščík, M.; Ptasinski, K. Exergetic evaluation of 5 biowastes-to-biofuels routes via gasification. Energy 2010, 35, 996–1007. [Google Scholar] [CrossRef]
- O’Keeffe, S.; Schulte, R.P.O.; Sanders, J.P.M.; Struik, P.C. II. Economic assessment for first generation green biorefinery (GBR): Scenarios for an Irish GBR blueprint. Biomass Bioenergy 2012, 41, 1–13. [Google Scholar]
- Santamaría-Fernández, M.; Molinuevo-Salces, B.; Lübeck, M.; Uellendahl, H. Biogas potential of green biomass after protein extraction in an organic biorefinery concept for feed, fuel and fertilizer production. Renew. Energy 2017, 129, 769–775. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Bech, D.; Kiel, P. Manufacturing of stabilised Brown juice for L-lysine production from university lab scale over pilot scale to industrial production. Chem. Biochem. Eng. Q. 2004, 18, 37–46. [Google Scholar]
- Arora, A.; Banerjee, J.; Vijayaraghavan, R.; Macfarlane, D.; Patti, A.F. Industrial Crops & Products Process design and techno-economic analysis of an integrated mango processing waste biore fi nery. Ind. Crop. Prod. 2018, 116, 24–34. [Google Scholar]
- Rajendran, K.; Kankanala, H.R.; Martinsson, R.; Taherzadeh, M.J. Uncertainty over techno-economic potentials of biogas from municipal solid waste (MSW): A case study on an industrial process. Appl. Energy 2014, 125, 84–92. [Google Scholar] [CrossRef]
- Niziolek, A.M.; Onel, O.; Floudas, C.A. Municipal solid waste to liquid transportation fuels, olefins, and aromatics: Process synthesis and deterministic global optimization. Comput. Chem. Eng. 2017, 102, 169–187. [Google Scholar] [CrossRef]
- Montagnana, M.; Leme, V.; Henrique, M.; Eduardo, E.; Lora, S.; José, O.; Marciano, B. Resources, Conservation and Recycling Techno-economic analysis and environmental impact assessment of energy recovery from Municipal Solid Waste ( MSW ) in Brazil. Resour. Conserv. Recycl. 2014, 87, 8–20. [Google Scholar]
- Han, W.; Fang, J.; Liu, Z.; Tang, J. Techno-economic evaluation of a combined bioprocess for fermentative hydrogen production from food waste. Bioresour. Technol. 2015, 202, 107–112. [Google Scholar] [CrossRef]
- Demichelis, F.; Fiore, S.; Pleissner, D.; Venus, J. Technical and economic assessment of food waste valorization through a biore fi nery chain. Renew. Sustain. Energy Rev. 2018, 94, 38–48. [Google Scholar] [CrossRef]
- Shahzad, K.; Narodoslawsky, M.; Sagir, M.; Ali, N.; Ali, S.; Rashid, M.I.; Ismail, I.M.I.; Koller, M. Techno-economic feasibility of waste biorefinery: Using slaughtering waste streams as starting material for biopolyester production. Waste Manag. 2017, 67, 73–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- Audsley, E.; Sells, J.E. Determining the Profitability of a Wholecrop Biorefinery. In Cereals; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Wooley, R.; Ruth, M.; Sheehan, J.; Ibsen, K.; Majdeski, H.; Galvez, A. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis Current and Futuristic Scenarios; National Renewable Energy Lab.: Golden, CO, USA, 1999.
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of acetone butanol (AB) from liquefied corn starch, a commercial substrate, using Clostridium beijerinckii coupled with product recovery by gas stripping. J. Ind. Microbiol. Biotechnol. 2007, 34, 771–777. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Cotta, M.A.; Singh, V. An economic evaluation of biological conversion of wheat straw to butanol: A biofuel. Energy Convers. Manag. 2013, 65, 456–462. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Baroia, G.N.; Gavalab, H.N.; Westermanna, P.; Skiadas, I.V. Fermentative production of butyric acid from wheat straw: Economic evaluation. Ind. Crop. Prod. 2017, 104, 68–80. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, Y.; Kapu, N.S.; Beatson, R. Evaluation of an organosolv-based biorefinery process to fractionate wheat straw into ethanol and co-products. Ind. Crops Prod. 2018, 121, 294–302. [Google Scholar] [CrossRef]
- Cherubini, F.; Jungmeier, G. LCA of a biorefinery concept producing bioethanol, bioenergy, and chemicals from switchgrass. Int. J. Life Cycle Assess. 2010, 15, 53–66. [Google Scholar] [CrossRef]
- Villegas, J.D.; Gnansounou, E. Techno-economic and environmental evaluation of lignocellulosic biochemical refineries: Need for a modular platform for integrated assessment (MPIA). J. Sci. Ind. Res. 2008, 67, 927–940. [Google Scholar]
- Mariano, A.P.; Dias, M.O.S.; Junqueira, T.L.; Cunha, M.P.; Bonomi, A.; Filho, R.M. Butanol production in a first-generation Brazilian sugarcane biorefinery: Technical aspects and economics of greenfield projects. Bioresour. Technol. 2013, 135, 316–323. [Google Scholar] [CrossRef]
- Buruiana, C.T.; Vizireanu, C.; Garrote, G.; Parajó, J.C. Optimization of corn stover biorefinery for coproduction of oligomers and second generation bioethanol using non-isothermal autohydrolysis. Ind. Crops Prod. 2014, 54, 32–39. [Google Scholar] [CrossRef]
- Luo, L.; Voet EVan, D.e.r.; Huppes, G.; van der Voet, E.; Huppes, G. Biorefining of lignocellulosic feedstock—Technical, economic and environmental considerations. Bioresour. Technol. 2010, 101, 5023–5032. [Google Scholar] [CrossRef]
- Kazi, F.K.; Fortman, J.; Anex, R.; Kothandaraman, G.; Hsu, D.; Aden, A.; Dutta, A. Techno-Economic Analysis of Biochemical Scenarios for Production of Cellulosic Ethanol; National Renewable Energy Lab.: Golden, CO, USA, 2010.
- Giarola, S.; Romain, C.; Williams, C.K.; Hallett, J.P.; Shah, N. Techno-economic assessment of the production of phthalic anhydride from corn stover. Chem. Eng. Res. Des. 2015, 7, 181–194. [Google Scholar] [CrossRef]
- Meyer, P.A.; Tews, I.J.; Magnuson, J.K.; Karagiosis, S.A.; Jones, S.B. Techno-economic analysis of corn stover fungal fermentation to ethanol. Appl. Energy 2013, 111, 657–668. [Google Scholar] [CrossRef]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Hsu, D.D. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 2013, 89, 463–469. [Google Scholar]
- Chen, S.; Xu, Z.; Li, X.; Yu, J.; Cai, M.; Jin, M. Integrated bioethanol production from mixtures of corn and corn stover. Bioresour. Technol. 2018, 258, 18–25. [Google Scholar] [CrossRef]
- Tyner, W.E. The US ethanol and biofuels boom: Its origins, current status, and future prospects. Bioscience 2008, 58, 646–653. [Google Scholar] [CrossRef]
- Ekman, A.; Campos, M.; Lindahl, S.; Co, M.; Borjesson, P.; Karlson, N.E.; Turner, C. Bioresource utilisation by sustainable technologies in new value-added biorefinery concepts - two case studies from food and forest industry. J. Clean. 2013, 57, 46–58. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, A.; Sultana, A. Biofuels and biochemicals production from forest biomass in Western Canada. Energy 2011, 36, 6251–6262. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, A. Large-scale biohydrogen production from bio-oil. Bioresour. Technol. 2010, 101, 7350–7361. [Google Scholar] [CrossRef]
- Shabangu, S.; Woolf, D.; Fisher, E.M.; Angenent, L.T.; Lehmann, J. Techno-economic assessment of biomass slow pyrolysis into different biochar and methanol concepts. Fuel 2014, 117, 742–748. [Google Scholar] [CrossRef]
- Trippe, F.; Fröhling, M.; Schultmann, F.; Stahl, R.; Henrich, E.; Dalai, A. Comprehensive techno-economic assessment of dimethyl ether (DME) synthesis and Fischer-Tropsch synthesis as alternative process steps within biomass-to-liquid production. Fuel Process. Technol. 2013, 106, 577–586. [Google Scholar] [CrossRef]
- Tan, E.C.D.; Talmadge, M.; Dutta, A.; Hensley, J.; Schaidle, J.; Biddy, M.; Humbird, D.; Snowden-Swan, L.J.; Ross, J.; Sexton, D.; et al. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons via Indirect Liquefaction. Thermochemical Research Pathway to High-Octane Gasoline Blendstock Through Methanol/Dimethyl Ether Intermediates; National Renewable Energy Lab.: Golden, CO, USA, 2015.
- Dutta, A.; Sahir, A.; Tan, E.; Humbird, D.; Snowden-swan, L.J.; Meyer, P.; Ross, J.; Sexton, D.; Yap, R.; Lukas, J.; et al. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels Thermochemical Research Pathways with In Situ and Ex Situ Upgrading of Fast Pyrolysis Vapors; National Renewable Energy Lab.: Golden, CO, USA; Pacific NorthwestNational Lab: Richland, NJ, USA, 2015.
- Haro, P.; Trippe, F.; Stahl, R.; Henrich, E. Bio-syngas to gasoline and olefins via DME—A comprehensive techno-economic assessment. Appl. Energy 2013, 108, 54–65. [Google Scholar] [CrossRef]
- Jones, S.; Meyer, P.; Snowden-Swan, L.; Asanga, P.; Eric, T.; Abhijit, D.; Jacob, J.; Cafferty, K. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels Fast Pyrolysis and Hydrotreating Bio-Oil Pathway; National Renewable Energy Lab.: Golden, CO, USA; Pacific Northwest National Lab.: Richland, NJ, USA; Idaho National Lab.: Idaho Falls, ID, USA, 2013.
- Sanchez, A.; Valdez-Vazquez, I.; Soto, A.; Sanchez, S.; Tavarez, D. Biomass and Bioenergy Lignocellulosic n-butanol co-production in an advanced biore fi nery using mixed cultures. Biomass Bioenergy 2017, 102, 1–12. [Google Scholar] [CrossRef]
- Jang, M.O.; Choi, G. Techno-economic analysis of butanol production from lignocellulosic biomass by concentrated acid pretreatment and hydrolysis plus continuous fermentation. Biochem. Eng. J. 2018, 134, 30–43. [Google Scholar] [CrossRef]
- Wang, W. Techno-economic analysis of a bio-refinery process for producing Hydro-processed Renewable Jet fuel from Jatropha. Renew. Energy 2016, 95, 63–73. [Google Scholar] [CrossRef]
- Zaafouri, K.; Ziadi, M.; Ben Farah, R.; Farid, M.; Hamdi, M.; Regaya, I. Potential of Tunisian Alfa (Stipa tenassicima) fibers for energy recovery to 2G bioethanol: Study of pretreatment, enzymatic saccharification and fermentation. Biomass Bioenergy 2016, 94, 66–77. [Google Scholar] [CrossRef]
- Tirpanalan, Ö.; Reisinger, M.; Smerilli, M.; Huber, F.; Neureiter, M.; Kneifel, W.; Novalin, S. Wheat bran biorefinery—An insight into the process chain for the production of lactic acid. Bioresour. Technol. 2015, 180, 242–249. [Google Scholar] [CrossRef]
- Ahmad, M.; Mohd, K.; Arif, H.; Noriznan, M.; Salihon, J.; Shirai, Y.; Ali, M. Case study for a palm biomass biore fi nery utilizing renewable non-food sugars from oil palm frond for the production of poly (3-hydroxybutyrate).Bioplastic. J. Clean. Prod. 2015, 87, 284–290. [Google Scholar]
- He, M.X.; Wang, J.L.; Qin, H.; Shui, Z.X.; Zhu, Q.L.; Wu, B.; Tan, F.R.; Pan, K.; Hu, Q.C.; Dai, L.C.; et al. Bamboo: A new source of carbohydrate for biorefinery. Carbohydr. Polym. 2014, 111, 645–654. [Google Scholar] [CrossRef]
- Dassanayake, G.D.M.; Kumar, A. Techno-economic assessment of triticale straw for power generation. Appl. Energy 2012, 98, 236–245. [Google Scholar] [CrossRef]
- Zhu, L. Biorefinery as a promising approach to promote microalgae industry: An innovative framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384. [Google Scholar] [CrossRef]
- Davis, R.; Kinchin, C.; Markham, J.; Tan, E.; Laurens, L.; Sexton, D.; Knorr, D.; Schoen, P.; Lukas, J. Process Design and Economics for the Conversion of Algal Biomass to Biofuels: Algal Biomass Fractionation to Lipid- and Carbohydrate-Derived Fuel Products; National Renewable Energy Lab.: Golden, CO, USA, 2014.
- Jung, K.A.; Lim, S.R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Song, W.; Xia, A.; Zhou, J.; Cen, K. Cogeneration of hydrogen and methane from Arthrospira maxima biomass with bacteria domestication and enzymatic hydrolysis. Int. J. Hydrogen Energy 2011, 36, 1474–1481. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, R.; Xu, X.; Fan, X.; Luo, S. Hydrogen and methane production from lipid-extracted microalgal biomass residues. Int. J. Hydrogen Energy 2011, 36, 3465–3470. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Lin, R.; Lu, H.; Zhou, J.; Cen, K. Comparison in dark hydrogen fermentation followed by photo hydrogen fermentation and methanogenesis between protein and carbohydrate compositions in Nannochloropsis oceanica biomass. Bioresour. Technol. 2013, 138, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.; Bhatia, S. Catalytic processes towards the production of biofuels in a palm oil and oil palm biomass-based biorefinery. Bioresour. Technol. 2008, 99, 7911–7922. [Google Scholar] [CrossRef] [PubMed]
- Laser, M.; Jin, H.; Jayawardhana, K.; Dale, B.E.; Lynd, L.R. Projected mature technology scenarios for conversion of cellulosic biomass to ethanol with coproduction thermochemical fuels, power, and/or animal feed protein. Biofuels Bioprod. Biorefining 2009, 3, 231–246. [Google Scholar] [CrossRef]
- Pinatti, D.G.; Conte, R.A.; Soares, A.G.; Pereira, M.L.G.; Romao, E.L.; Ferreira, J.C.; Oliveira, I.; Marton, L.F.M. Biomass refinery as a renewable complement to the petroleum refinery. Int. J. Chem. React. Eng. 2010, 8, A94. [Google Scholar] [CrossRef]
- Bao, B.; Ng, D.K.S.; Tay, D.H.S.; Jiménez-Gutiérrez, A.; El-Halwagi, M.M. A shortcut method for the preliminary synthesis of process-technology pathways: An optimization approach and application for the conceptual design of integrated biorefineries. Comput. Chem. Eng. 2011, 35, 1374–1383. [Google Scholar] [CrossRef]
- Sharma, P.; Sarker, B.R.; Romagnoli, J.A. A decision support tool for strategic planning of sustainable biorefineries. Comput. Chem. Eng. 2011, 35, 1767–1781. [Google Scholar] [CrossRef]
| EXCEL | EXTEND | SuperPRO | ASPEN | gPROMS | |
|---|---|---|---|---|---|
| Mass balance | ☑ | - | ☑ | ☑ | - |
| Mass and heat balance | ☑ | - | ☑ | ☑ | - |
| Batch—Discrete | - | ☑ | ☑ | ☑ | - |
| Continuous | - | - | ☑ | ☑ | ☑ |
| Dynamic | - | - | ☑ | ☑ |
| Type of Biorefinery | Feedstock | Processing Techniques | Products | References |
|---|---|---|---|---|
| Green Biorefineries (GB) | wet biomass: green grasses and green crops, such as lucerne and clover | pretreatment, pressing, fractionation, separation, digestion | Lactic acid, amino acids, ethanol, proteins, biogas, dyes, pigments. | [29,83,84] |
| Whole Crop Biorefineries (WCB) | whole crop (including straw) cereals such as rye, wheat, and maize | dry or wet milling, biochemical conversion | Syngas, sorbitol, glucose amine, ethanol, biogas, ethylene glycol, propylene glycol, glycerin | [84,85,86,87] |
| Lignocellulosic Feedstock Biorefineries (LB) | lignocellulosic-rich biomass: e.g., straw, chaff, reed, miscanthus, wood | pretreatment, chemical and enzymatic hydrolysis, fermentation, separation | Energy, syngas, methanol, levulinic acid, ethanol, acetic acid, lactic acid, furfural, 5-hydroxymethyl-furfural (HMF) | [3,45,69,88] |
| Marine Biorefineries (MB) | aquatic biomass: microalgae and macroalgae (seaweed) | cell disruption, product extraction and separation | Protein for fish farming, dietary or health food, lipids, especially high-value fatty acids linoleic acid and g-linolenic acid. | [89,90,91] |
| Feedstock | Processes * | Products | Modelled | References |
|---|---|---|---|---|
| Grass and organic waste fractions | 1,8,9,10 | Syn Gas | Yes | [93] |
| 1,8,9,15 | Methanol | Yes | ||
| 1,8,9,15 | Fischer–Tropsch Fuels | Yes | ||
| 1,8,9,11 | Hydrogen | Yes | ||
| Agricultural waste/Green waste | 1,8,9,10 | Syn Gas | Yes | |
| 1,8,9,15 | Methanol | Yes | ||
| 1,8,9,15 | Fischer–Tropsch Fuels | Yes | ||
| 1,8,9,11 | Hydrogen | Yes | ||
| Grass and silage | 4,7,14 | Lactic Acid, proteins, methane | No | [94] |
| Silage | No | |||
| Red clover (Trifolium pratense) | 1,12,14 | Methane, by-products (Press Cake and Brown Juice) | No | [95] |
| Clover grass (Trifolium pretense and Lolium multiflorum) | ||||
| Alfalfa (Medicago sativa) | ||||
| Oilseed radish (Raphanus sativus var. Oleiferus) | ||||
| Grass Juice | 1,2,3,5,6,11,12 | Lactic Acid and protein feed | Yes | [96] |
| Mango Waste | 2,5,13 | Pectin, oil seeds, polyphenols, and cattle feed | No | [97] |
| Feedstock | Processes ** | Products | Modelled | References |
|---|---|---|---|---|
| Sludge and manure | 1,4,5,6 | Syn Gas | Yes | [93] |
| 1,4,5,15 | Methanol | Yes | ||
| 1,4,5,15 | Fischer-Tropsch Fuels | Yes | ||
| 1,4,5,7 | Hydrogen | Yes | ||
| Municipal Solid Waste | 1,4,5,6 | Syn Gas | Yes | |
| 1,4,5,15 | Methanol | Yes | ||
| 1,4,5,15 | Fischer–Tropsch Fuels | Yes | ||
| 1,4,5,7 | Hydrogen | Yes | ||
| 3,7,14 | Biogas | Yes | [98] | |
| 1,3,5,12,13,15 | Fischer–Tropsch Fuels, olefins, and aromatics | Yes | [99] | |
| 1,14 | Energy | No | [100] | |
| Food Waste | 2,3,11,13,14 | Biogas and hydrogen | Yes | [101] |
| 2,7,9,10,11,13,14 | Lactic acid and biogas | Yes | [102] | |
| Slaughter Waste | 2,11,13,14 | Polyhydroxyalkanoate (PHA) | No | [103] |
| Waste Oil | 1,8,13 | Fatty Acid Methyl Esters (FAME) and glycerol | Yes | [104,105] |
| Feedstock | Processes *** | Products | Modelled | References |
|---|---|---|---|---|
| Yellow Poplar | 3,11,15 | Ethanol, Biogas | Yes | [107] |
| Saccharified Liquefied Cornstarch (SLCS) with moisture content of approximately 60% | 6,12,15 | Ethanol and methane | Yes | [108] |
| Wheat Straw | 1,4,5,16 | Butanol | Yes | [109] |
| 1,6,7,12,16 | Bioethanol, hydrogen, and biogas | No | [110] | |
| 1,6,10,12,15 | Butyric Acid | Yes | [111] | |
| 1,6,12,16 | Ethanol | Yes | [112] | |
| 1,10,12,16 | Biomethane, ethanol, electricity and phenols | No | [113] | |
| Sugarcane | 2,8,14 | Ethanol, electricity, gypsum, fertilizers, | No | [22] |
| 1,4,5,12,16 | Electricity, ethanol, animal feed | Yes | [114] | |
| 5,12,13,16 | Ethanol, sugar, power, and n-butanol, acetone– butanol–ethanol | Yes | [115] |
| Feedstock | Processes † | Products | Modelled | References |
|---|---|---|---|---|
| Corn Stover | 1,18 | Ethanol | No | [116] |
| 1,7,19,25 | Ethanol, succinic acid, acetic acid, electricity | Yes | [117] | |
| 1,5,14,19,25 | Biomethane, ethanol, electricity and phenols | No | [113] | |
| 1,17,19,20,22,25 | Gypsum, methane and ethanol | Yes | [118] | |
| 3,17 | Phthalic Anhydride | Yes | [119] | |
| 1,17,19,23,25 | Ethanol | Yes | [120] | |
| 10,21,23 | Naphtha and diesel range fuels | Yes | [121] | |
| 1,6,7,17,19,25 | Bioethanol | Yes | [122] | |
| Corn | 3,18,25 | Ethanol | Yes | [123] |
| Yellow onion (allium cepa) | 2,24 | Quercetin, biogas | No | [124] |
| Birch Forest (betula spp.) | 15,16 | Electricity, betulin | No | |
| Wood and forest waste | 1,9,11,12 | Syn Gas | Yes | [93] |
| 1,9,11,25 | Methanol | Yes | ||
| 1,9,11,25 | Fischer–Tropsch Fuels | Yes | ||
| 1,9,11,13 | Hydrogen | Yes | ||
| Forest Residue | 9,21,23,25 | Methanol, Dimethyl Ether, ammonia, Fischer Tropsch Fuels | No | [125] |
| Forest Residue and straw | 9,23 | Biohydrogen | Yes | [126] |
| Pine | 9,11,21,23 | Methanol | No | [127] |
| Lignocellulose | 10,21,23,25 | Gasoline, diesel, Fischer Tropsch fuel, and biochar | Yes | [128] |
| 6,9,23,25 | Gasoline and Methanol intermediates | Yes | [129] | |
| 10,11,13,21,23,25 | Gasoline, diesel, and bio-oil | Yes | [130] | |
| 10,21,23 | Ethylene and propylene | Yes | [131] | |
| 6,10,23,25 | Gasoline and diesel | Yes | [132] | |
| 1,19,23,24 | Acetone, butanol, ethanol, biogas, and hydrogen | Yes | [133] | |
| 1,5,6,13,19,23 | Butanol | Yes | [134] | |
| Salicornia bigelovii | 1,2,3,4,5,7,18,24,25 | Ethanol, and biogas | No | [59] |
| Dried Oil Palm | 1,18 | Furfural, ethanol and lignin | No | [17] |
| Jatropha curcas | 6,10,20,23,25 | Jet Fuel (light gases, naphtha, jet fuel, and diesel) | Yes | [135] |
| Switch Grass | 1,5,14,19,24 | Bioethanol, biomethane, electricity, phenols | No | [113] |
| Tunisian Alfa (Stipa tenassicima) | 1,7,17,19,23,25 | Bioethanol | No | [136] |
| Sunflower seed | 7,8,19 | Poly(3-hydroxybutyrate) PHB, levulinic acid, protein isolate and antioxidants | No | [64] |
| Wheat Barn | 1,16,19 | Lactic Acid, lignin fraction | No | [137] |
| Palm oil fronds | 6,17,25 | Bioplastic, poly(3-hydroxybutyrate) PHB | Yes | [138] |
| Bamboo | 5,7,19 | Xylitol, Lactic Acid, succinic acid, biomethane, ethanol | No | [139] |
| Triticale straw | 14 | Electricity | No | [140] |
| Feedstock | Processes ‡ | Products | Modelled | References |
|---|---|---|---|---|
| Microalgae | 3,6,10,11,15 | High value products, biodiesel, bioethanol and biogas | No | [141] |
| Microalgae (Chlorella strain) | 1,5,13,14,15,16 | Naphtha, biogas, renewable diesel blendstock, AD digestate | Yes | [142] |
| Brown macroalgae (Laminaria japonica) | 4,7,15 | Hydrogen and methane | No | [143] |
| Microalgae A. maxima | 7,15 | Hydrogen and methane | No | [144] |
| Lipid extracted microalgae (Scenedesmus spp.) | 7,15 | No | [145] | |
| Microalga Chlorella (Pyrenoidosa spp.) | 7,8,9,15 | No | [146] | |
| Microalgae (Nannochloropsis oceanica spp.) | 2,7,8,9,15 | No | ||
| Neochloris oleoabundans | 6,11,12 | Lipid, protein and starch | No | [62] |
| Chlorella sorokiniana | No | |||
| Tetraselmis suecica | No | |||
| Nannochloropis oculata | No | |||
| Microalgae | 6,11,16 | Bioethanol, heat and power, biodiesel (from microalgae oil), and glycerol | No | [21] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaturvedi, T.; Torres, A.I.; Stephanopoulos, G.; Thomsen, M.H.; Schmidt, J.E. Developing Process Designs for Biorefineries—Definitions, Categories, and Unit Operations. Energies 2020, 13, 1493. https://doi.org/10.3390/en13061493
Chaturvedi T, Torres AI, Stephanopoulos G, Thomsen MH, Schmidt JE. Developing Process Designs for Biorefineries—Definitions, Categories, and Unit Operations. Energies. 2020; 13(6):1493. https://doi.org/10.3390/en13061493
Chicago/Turabian StyleChaturvedi, Tanmay, Ana I. Torres, George Stephanopoulos, Mette Hedegaard Thomsen, and Jens Ejbye Schmidt. 2020. "Developing Process Designs for Biorefineries—Definitions, Categories, and Unit Operations" Energies 13, no. 6: 1493. https://doi.org/10.3390/en13061493
APA StyleChaturvedi, T., Torres, A. I., Stephanopoulos, G., Thomsen, M. H., & Schmidt, J. E. (2020). Developing Process Designs for Biorefineries—Definitions, Categories, and Unit Operations. Energies, 13(6), 1493. https://doi.org/10.3390/en13061493








