A Criterion of Heat Transfer Deterioration for Supercritical Organic Fluids Flowing Upward and Its Heat Transfer Correlation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Analysis
2.2. Data Reduction
3. Results and Discussion
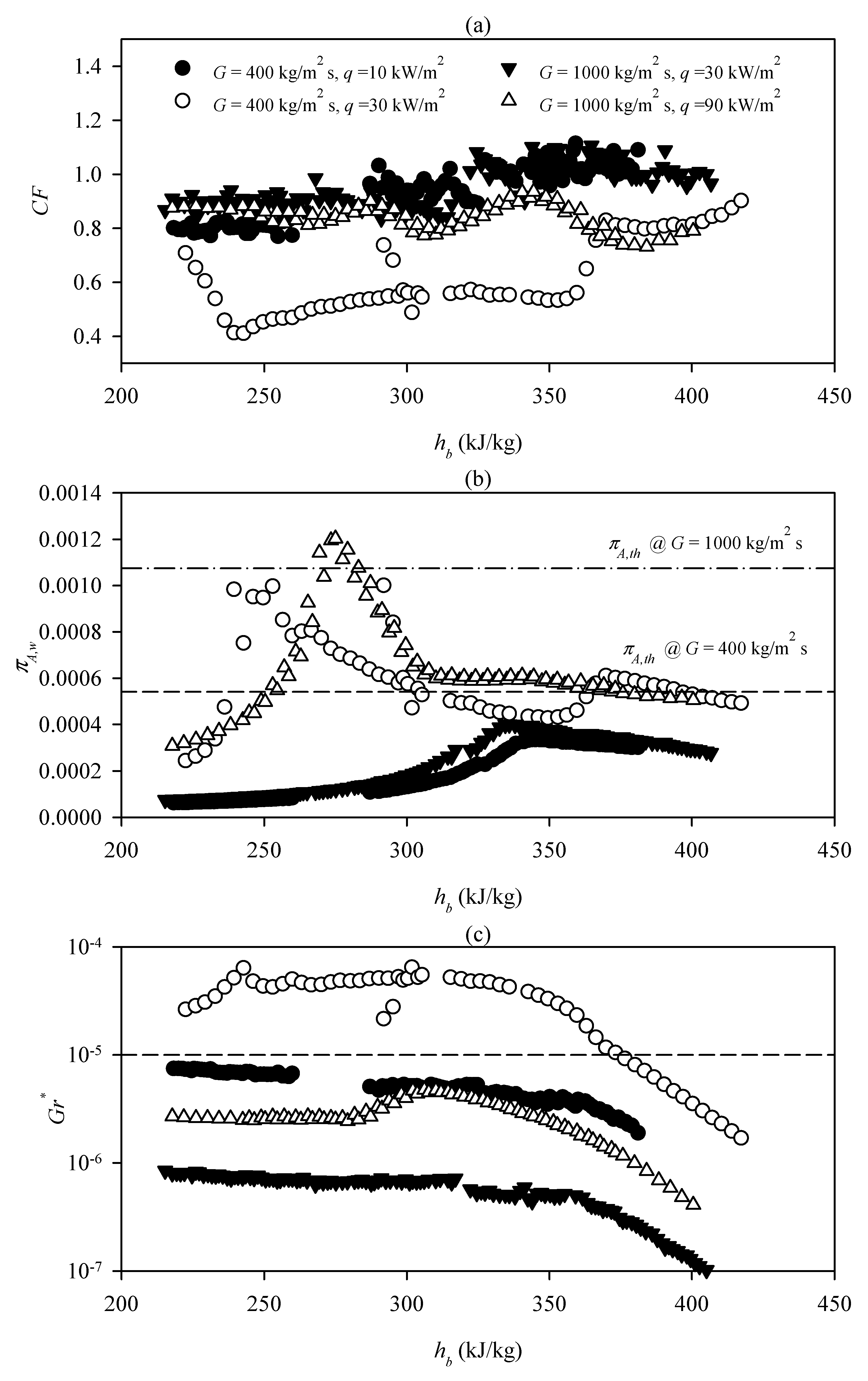
3.1. The Criterion of the Lowest Heat Flux for Heat Transfer Deterioration
3.2. The onset of Laminarization and HTD
3.3. The Correlations of the Heat Transfer
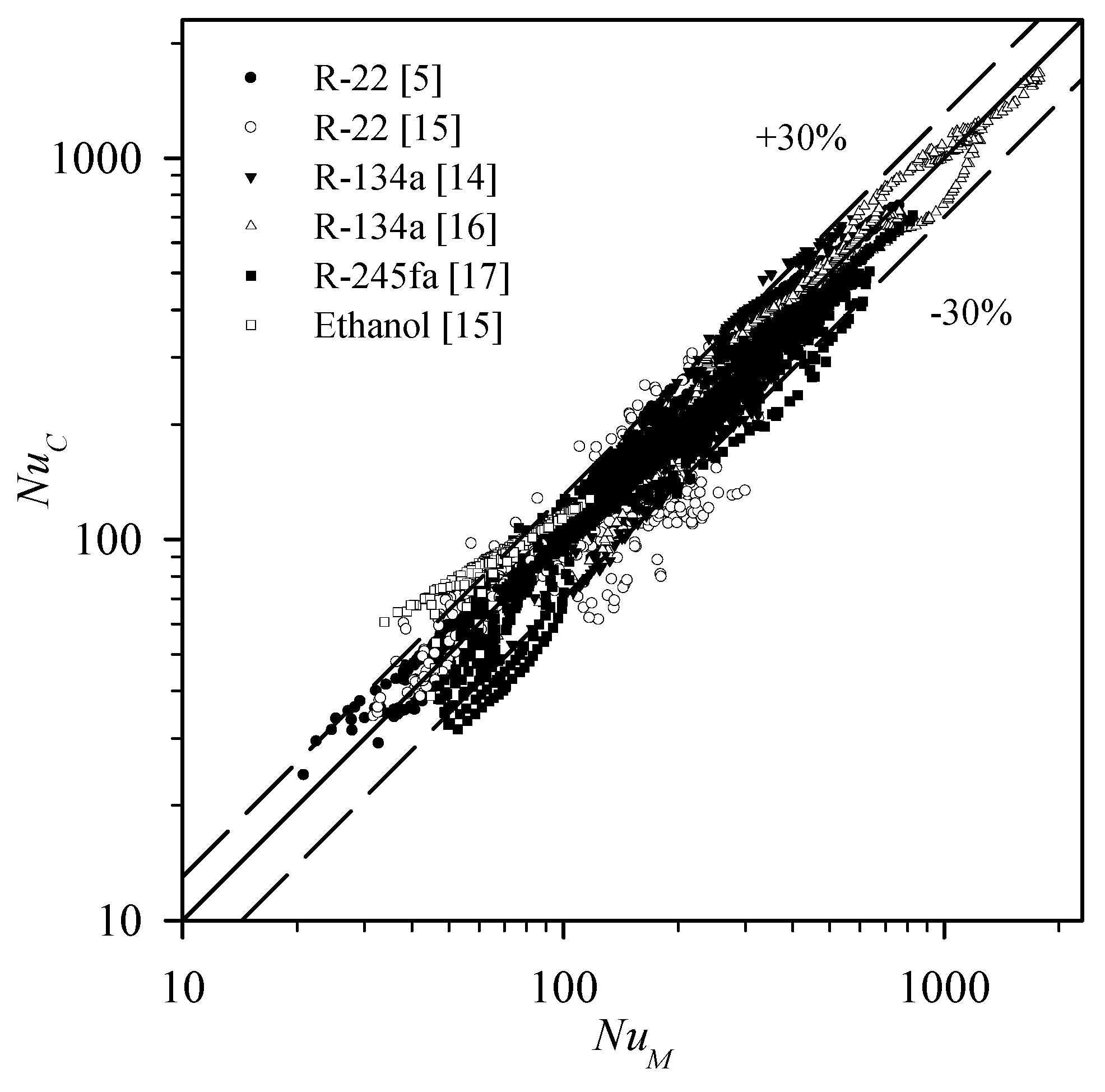
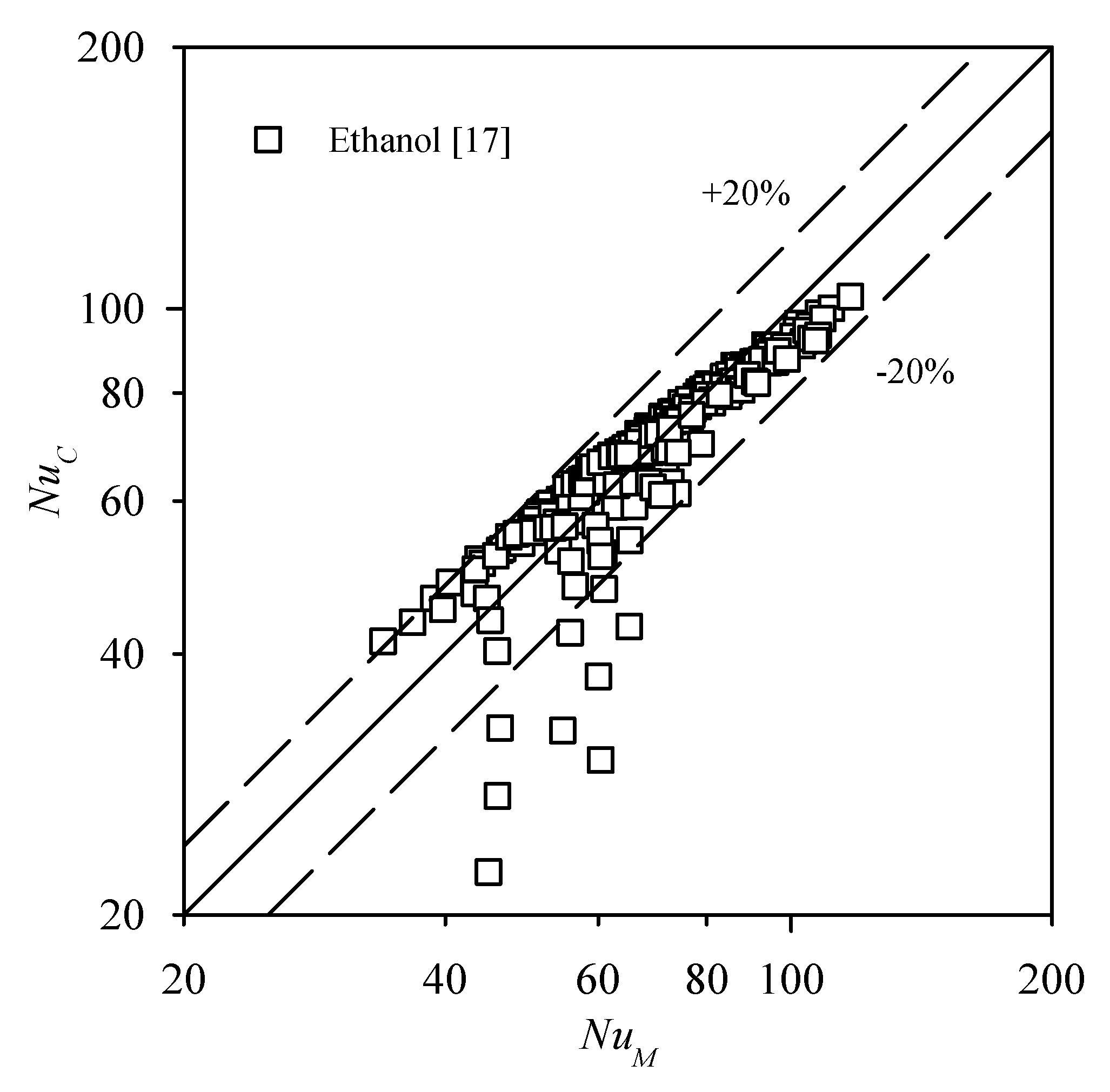
3.3.1. The Comparison Between the Experimental Data and the Existing Correlations
3.3.2. The Modified Correlation and Corresponding Correction Factor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Area, m2 |
| AD | Average deviation |
| C | Correlation constant |
| CF | Correction factor |
| Cp | Specific heat capacity, kJ/kg∙K |
| Integrated average specific heat capacity, kJ/kg∙K | |
| D | Diameter, m |
| E | Eckert number defined by the correlation of Yamagata et al. [8] |
| F | Correction factor defined by the correlation of Zhang et al. [16] |
| G | Mass flux, kg/m2∙s |
| g | Gravity acceleration, 9.81 m/s2 |
| Gr | Grashof number |
| Grashof number estimated by integrated average density | |
| Gr* | Buoyancy criterion defined by the correlation of Watts and Chou [18] |
| h | Enthalpy, kJ/kg |
| k | Thermal conductivity, W/m∙K |
| Kv | Acceleration factor proposed by McEligot et al. [9] |
| N | Number of data |
| min | Minimum operator |
| n | Exponent of dimensionless factor |
| Nu | Nusselt number |
| P | Pressure, MPa |
| Pr | Prandtl number |
| Integrated average Prandtl number | |
| q | Heat flux, kW/m2 |
| Re | Reynolds number |
| SD | Standard deviation |
| T | Temperature, K |
| u | Velocity, m/s |
| x | Unit length of flow direction, m |
| Greek letter | |
| Thermal expansion coefficient, 1/K | |
| Dynamics viscosity, Pa∙s | |
| Kinematic viscosity, m2/s | |
| Density, kg/m3 | |
| Integrated average density, kg/m3 | |
| Acceleration parameter proposed by Cheng et al. [10]. | |
| Fanning fiction factor | |
| Subscript | |
| b | Bulk condition |
| base | Baseline |
| C | Calculated value |
| i | Index |
| M | Measured value |
| pc | Pseudo-critical condition |
| th | Threshold |
| w | Wall condition |
| Abbreviations | |
| HTC | Heat transfer coefficient |
| HTD | Heat transfer deterioration |
| LHF | Limit heat flux |
| ORC | Organic Rankine cycle |
References
- Hung, T.-C.; Shai, T.; Wang, S.K. A review of organic Rankine cycles (ORCs) for the recovery of low-grade waste heat. Energy 1997, 22, 661–667. [Google Scholar] [CrossRef]
- Schuster, A.; Karellas, S.; Aumann, R. Efficiency optimization potential in supercritical Organic Rankine Cycles. Energy 2010, 35, 1033–1039. [Google Scholar] [CrossRef]
- Karellas, S.; Schuster, A.; Leontaritis, A.-D. Influence of supercritical ORC parameters on plate heat exchanger design. Appl. Therm. Eng. 2012, 33–34, 70–76. [Google Scholar] [CrossRef]
- Chen, H.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. A supercritical Rankine cycle using zeotropic mixture working fluids for the conversion of low-grade heat into power. Energy 2011, 36, 549–555. [Google Scholar] [CrossRef]
- Yamashita, T.; Yoshida, S.; Mori, H.; Morooka, S.; Komita, H.; Nishida, K. Heat transfer study under supercritical pressure conditions. In Proceedings of the GENES4/ANP2003, Kyoto, Japan, 15–19 September 2003. [Google Scholar]
- Petukhov, B. Heat transfer and friction in turbulent pipe flow with variable physical properties. In Advances in Heat Transfer; Elsevier: Amsterdam, The Netherlands, 1970; Volume 6, pp. 503–564. [Google Scholar]
- Pioro, I.L.; Duffey, R.B. Experimental heat transfer in supercritical water flowing inside channels (survey). Nucl. Eng. Des. 2005, 235, 2407–2430. [Google Scholar] [CrossRef]
- Yamagata, K.; Nishikawa, K.; Hasegawa, S.; Fujii, T.; Yoshida, S. Forced convective heat transfer to supercritical water flowing in tubes. Int. J. Heat Mass Transf. 1972, 15, 2575–2593. [Google Scholar] [CrossRef]
- McEligot, D.; Coon, C.; Perkins, H. Relaminarization in tubes. Int. J. Heat Mass Transf. 1970, 13, 431–433. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Y.H.; Huang, S.F. A simplified method for heat transfer prediction of supercritical fluids in circular tubes. Ann. Nucl. Energy 2009, 36, 1120–1128. [Google Scholar] [CrossRef]
- Jackson, J.D. Fluid flow and convective heat transfer to fluids at supercritical pressure. Nucl. Eng. Des. 2013, 264, 24–40. [Google Scholar] [CrossRef]
- Pioro, I.L.; Khartabil, H.F.; Duffey, R.B. Heat transfer to supercritical fluids flowing in channels—Empirical correlations (survey). Nucl. Eng. Des. 2004, 230, 69–91. [Google Scholar] [CrossRef]
- Yoo, J.Y. The turbulent flows of supercritical fluids with heat transfer. Annu. Rev. Fluid Mech. 2013, 45, 495–525. [Google Scholar] [CrossRef]
- Kang, K.-H.; Chang, S.-H. Experimental study on the heat transfer characteristics during the pressure transients under supercritical pressures. Int. J. Heat Mass Transf. 2009, 52, 4946–4955. [Google Scholar] [CrossRef]
- Jiang, P.-X.; Zhao, C.-R.; Liu, B. Flow and heat transfer characteristics of r22 and ethanol at supercritical pressures. J. Supercrit. Fluids 2012, 70, 75–89. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, H.; Cheng, X.; Xiong, Z. Experimental study on heat transfer of supercritical Freon flowing upward in a circular tube. Nucl. Eng. Des. 2014, 280, 305–315. [Google Scholar] [CrossRef]
- He, J.; Dang, C.; Hihara, E. Experimental investigation of heat transfer to supercritical R245fa flowing vertically upward in a circular tube. Int. J. Heat Mass Transf. 2018, 127, 286–295. [Google Scholar] [CrossRef]
- Watts, M.J.; Chou, C.T. Mixed convection heat transfer to supercritical pressure water. In Proceedings of the 7th International Heat Transfer Conference, Munchen, Germany, 6–10 September 1982. [Google Scholar]
- Jackson, J.D. Consideration of the heat transfer properties of supercritical pressure water in connection with the cooling of advanced nuclear reactors. In Proceedings of the 13th Pacific Basin Nuclear Conference, Shenzhen, China, 21–25 October 2002. [Google Scholar]
- Jackson, J.D.; Fewster, J. Forced Convection Data for Supercritical Pressure Fluids; Report No. HTFS 21540; Simon Engineering Laboratory, University of Manchester: Manchester, UK, 1975. [Google Scholar]
- Dittus, F.; Boelter, L. Heat transfer in automobile radiators of the tubular type. Int. Commun. Heat Mass Transf. 1985, 12, 3–22. [Google Scholar] [CrossRef]
- Duffey, R.B.; Pioro, I.L. Experimental heat transfer of supercritical carbon dioxide flowing inside channels (survey). Nucl. Eng. Des. 2005, 235, 913–924. [Google Scholar] [CrossRef]
- Huang, D.; Wu, Z.; Sunden, B.; Li, W. A brief review on convection heat transfer of fluids at supercritical pressures in tubes and the recent progress. Appl. Energy 2016, 162, 494–505. [Google Scholar] [CrossRef]
- Kim, J.K.; Jeon, H.K.; Yoo, J.Y.; Lee, J.S. Experimental study on heat transfer characteristics of turbulent supercritical flow in vertical circular/non-circular tubes. In Proceedings of the 11th International Topical Meeting on Nuclear Reactor Thermal Hydraulics (NURETH 11), Avignon, France, 2–6 October 2005; p. 85. [Google Scholar]
- Yin, F.; Chen, T.-K.; Li, H.-X. An Investigation on Heat Transfer to Supercritical Water in Inclined Upward Smooth Tubes. Heat Transf. Eng. 2007, 27, 44–52. [Google Scholar] [CrossRef]
- Styrikovich, M.; Margulova, T.K.; Miropol’Skii, Z. Problems in the development of designs of supercritical boilers. Therm. Eng. 1967, 32, 4–7. [Google Scholar]
- Mokry, S.; Pioro, I.; Farah, A.; King, K.; Gupta, S.; Peiman, W.; Kirillov, P. Development of supercritical water heat-transfer correlation for vertical bare tubes. Nucl. Eng. Des. 2011, 241, 1126–1136. [Google Scholar] [CrossRef]
- Lemmon, E.; Huber, M.; McLinden, M. Reference fluid thermodynamic and transport properties–REFPROP Version 8.0. In NIST Stand. Ref. Database; 2007; 23. Available online: https://www.nist.gov/publications/nist-standard-reference-database-23-reference-fluid-thermodynamic-and-transport-0?pub_id=50520 (accessed on 2 April 2007).
- Krasnoshchekov, E.; Protopopov, V.; Van, F.; Kuraeva, I. Experimental investigation of heat transfer for carbon dioxide in the supercritical region. In Proceedings of the 2nd All-Soviet Union Conference on Heat and Mass Transfer, Minsk, Belarus, 5–9 May 1964; pp. 26–35. [Google Scholar]
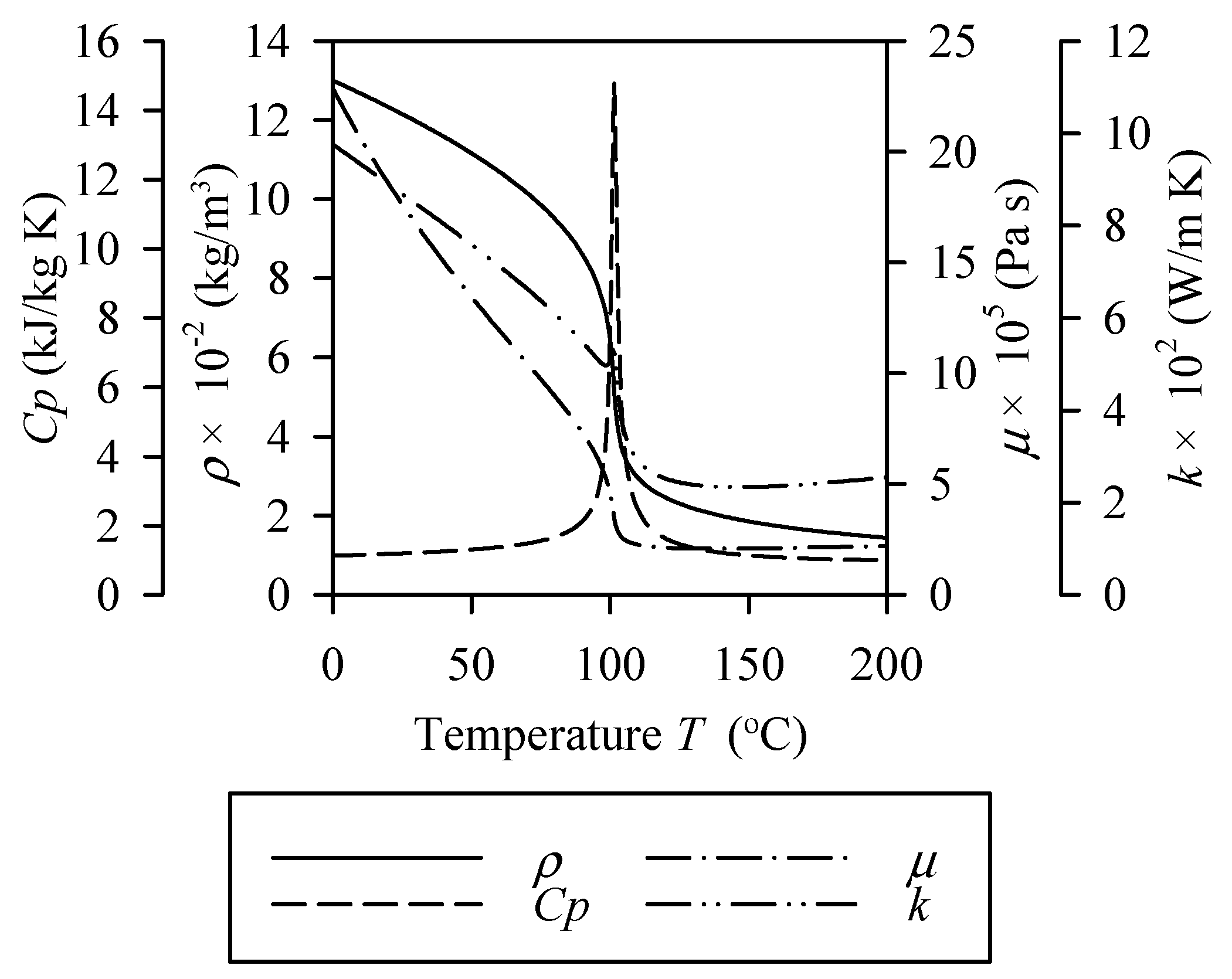

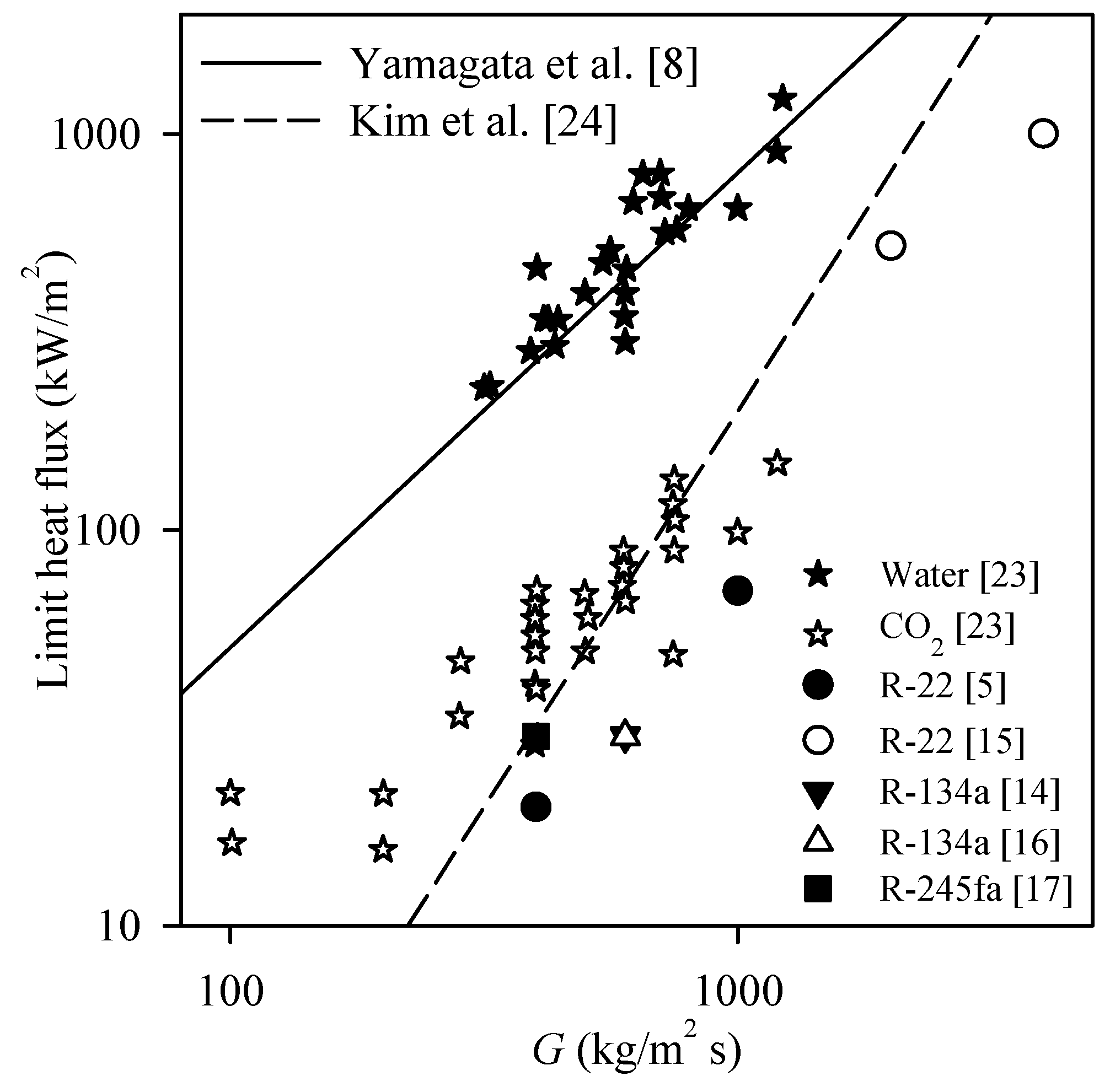

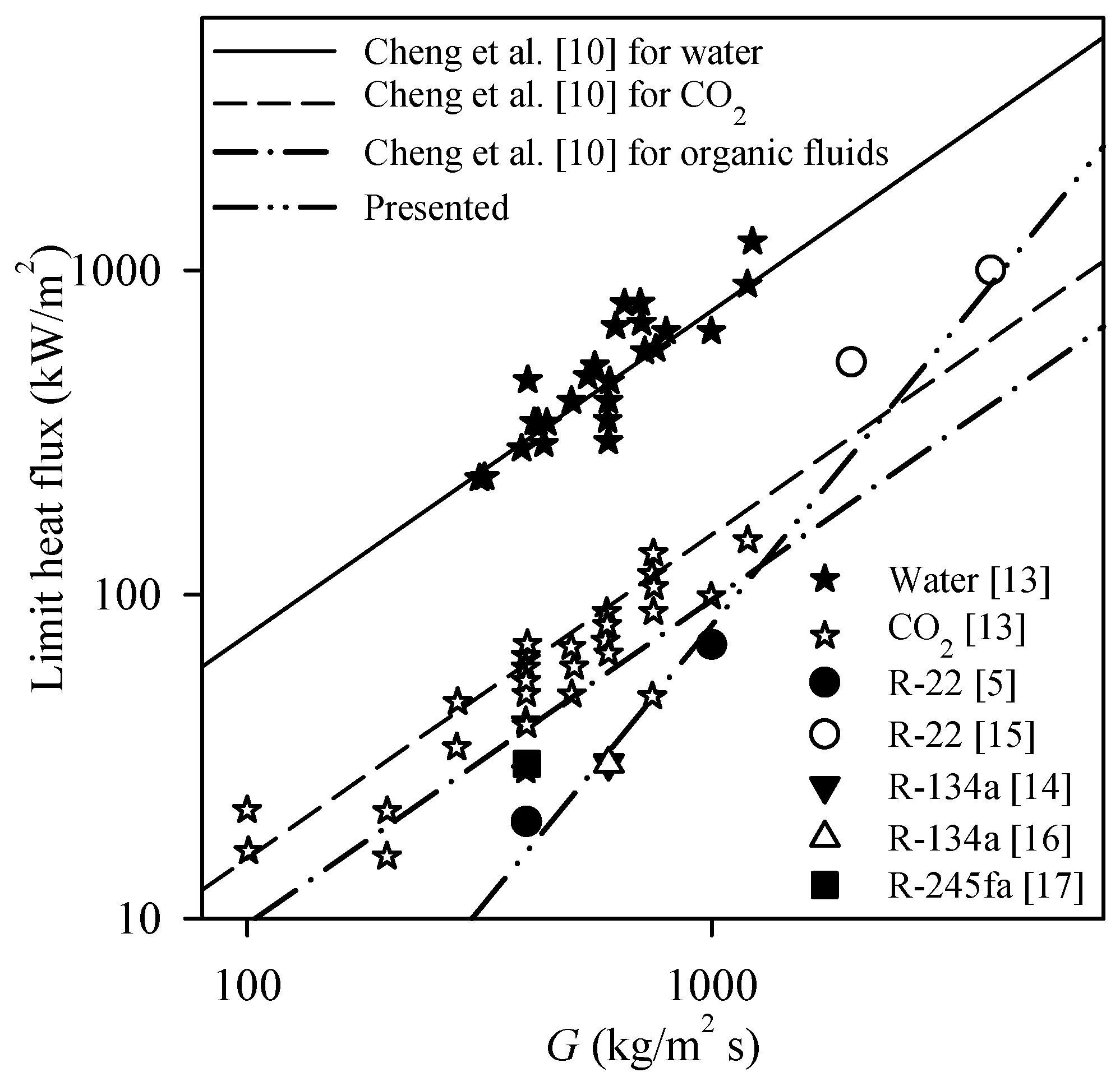


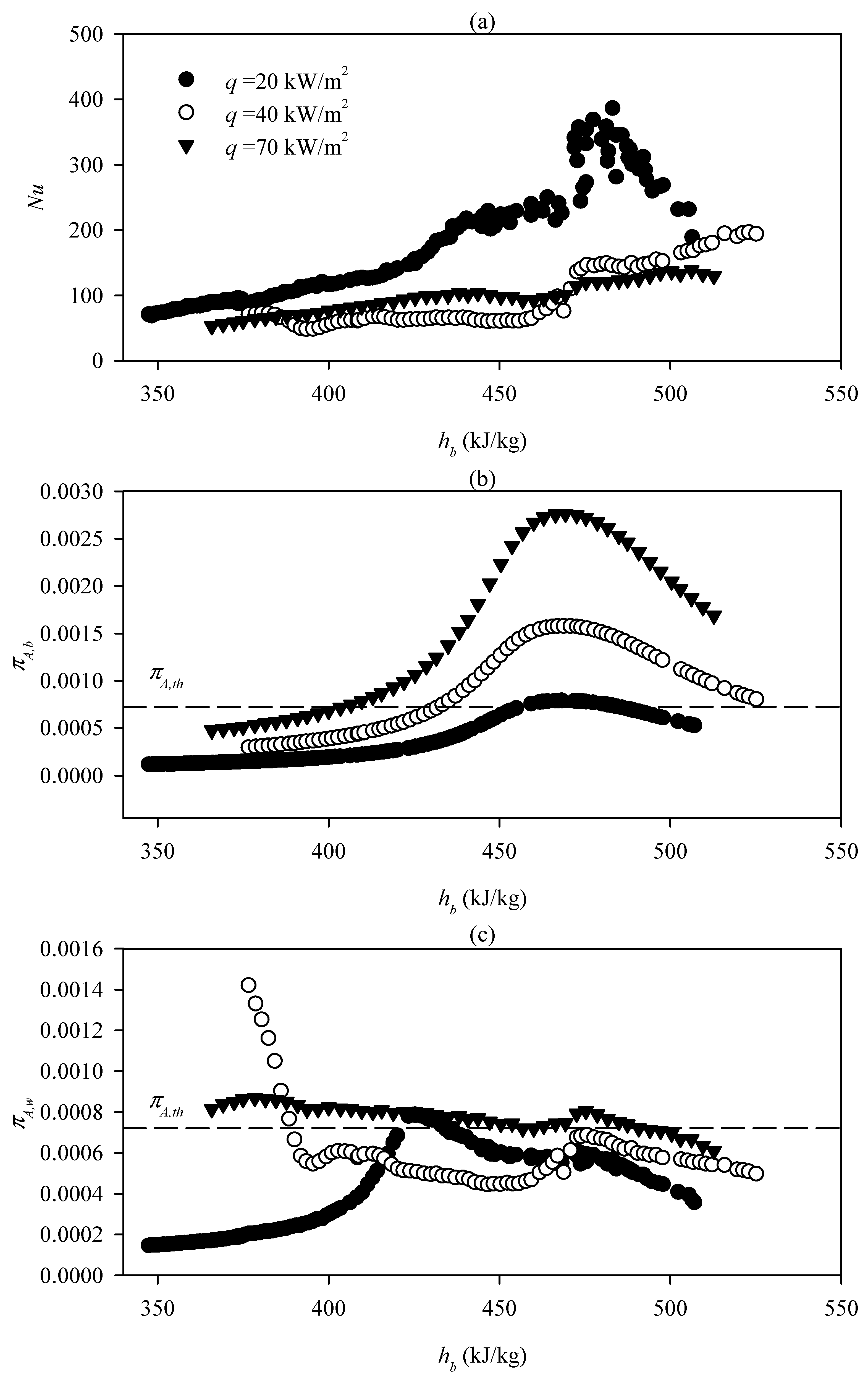

| Reference | Organic Fluid | Investigation of Limit Heat Flux | Recommended Correlation | New Correlation | Ability to Predict others’ Data |
|---|---|---|---|---|---|
| Yamashita et al. [5] | R-22 | Yes | Watts and Chou [18] | No | No |
| Kang and Chang [14] | R-134a | No | No | Yes | Yes |
| Jiang et al. [15] | Ethanol | Yes | No | No | No |
| Jiang et al. [15] | R-22 | Yes | No | No | No |
| Zhang et al. [16] | R-134a | Yes | Jackson [19] | Yes | No |
| He et al. [17] | R-245fa | Yes | Yamagata et al. [8], Jackson [19] | No | No |
| References | Criterion | For | Water | Carbon Dioxide | Organic Fluids | |
|---|---|---|---|---|---|---|
| Yin et al. [25] | Water | AD SD | −0.535 0.198 | 1.138 0.181 | 1.293 0.448 | |
| Yamagata et al. [8] | Water | AD SD | −0.119 0.216 | 1.358 0.180 | 1.551 0.243 | |
| Styrikovich et al. [26] | Water | AD SD | −0.323 0.208 | 1.279 0.159 | 1.407 0.394 | |
| Kim et al. [24] | CO2 | AD SD | −1.470 0.189 | −0.299 0.581 | 0.584 0.387 | |
| Mokry et al. [27] | Water | AD SD | −0.232 0.211 | 1.219 0.417 | 1.471 0.324 |
| References | Fluid | N | L (m) | D (mm) | P (MPa) | G (kg/m2∙s) | q (kW/m2) | Tb (°C) | Tw (°C) |
|---|---|---|---|---|---|---|---|---|---|
| Yamashita et al. [5] | R-22 | 927 | 2 | 4.4 | 5.5 | 400–2000 | 10–170 | 12–120 | 26–154 |
| Kang and Chang [14] | R-134a | 560 | 2 | 9.4 | 4.1–4.5 | 600–2000 | 10–160 | 49–113 | 59–188 |
| Jiang et al. [15] | Ethanol | 262 | 0.152 | 1.004 | 5.5–10 | 2000–4000 | 110–1800 | 23–110 | 46–326 |
| Jiang et al. [15] | R-22 | 345 | 0.152 | 1.004 | 5.5–10 | 2000–4000 | 110–1800 | 22–183 | 41–384 |
| Zhang et al. [16] | R-134a | 530 | 2.3 | 7.6 | 4.3–4.7 | 600–2500 | 20–180 | 74–107 | 86–182 |
| He et al. [17] | R-245fa | 1636 | 1.04 | 4 | 4–5 | 400–800 | 15–100 | 100–195 | 121–234 |
| Fluid | P (MPa) | |
|---|---|---|
| Water | 22.6 | 1.79 |
| CO2 | 7.5 | 8.80 |
| R-22 | 5.5 | 13.43 |
| R-134a | 4.3 | 15.27 |
| R-245fa | 4 | 15.77 |
| 4.5 | 13.23 |
| Author | Criterion | For | Water | Carbon Dioxide | Organic Fluids | |
|---|---|---|---|---|---|---|
| Cheng et al. [10] | Water | AD SD | −0.069 0.213 | 0.199 0.271 | 0.111 0.650 | |
| This study | Organic | AD SD | - - | - - | −0.232 0.336 |
| Authors | Correlation | Fluid |
|---|---|---|
| Dittus-Boelter [21] | Water | |
| Krasnoshchekov et al. [29] | Water, Carbon dioxide | |
| Yamagata et al. [8] | Water | |
| Jackson and Fewster [20] | Water | |
| Watts and Chou [18] | Water | |
| Jackson [19] | Water | |
| Kang and Chang [14] | R-134a | |
| Zhang et al. [16] | R-134a |
| Correlation | R-22 | R-22 | R-134a | R-134a | R-245fa | Ethanol | Total | |
|---|---|---|---|---|---|---|---|---|
| Yamashita et al. [5] | Jiang et al. [15] | Kang and Chang [14] | Zhang et al. [16] | He et al. [17] | Jiang et al. [15] | |||
| Dittus-Boelter [21] | N AD SD | 927 0.252 0.342 | 345 0.298 0.556 | 560 0.358 0.382 | 530 0.048 0.390 | 1636 −0.164 0.366 | 262 −0.071 0.227 | 4260 0.065 0.433 |
| Krasnoshchekov et al. [29] | N AD SD | 927 0.165 0.297 | 345 −0.062 0.586 | 560 0.125 0.340 | 530 0.093 0.323 | 1636 −0.226 0.278 | 262 0.044 0.216 | 4260 −0.025 0.369 |
| Yamagata et al. [8] | N AD SD | 927 0.439 0.175 | 345 0.252 0.462 | 560 0.505 0.150 | 530 0.367 0.099 | 1636 0.061 0.287 | 262 0.780 0.171 | 4260 0.300 0.329 |
| Jackson and Fewster [20] | N AD SD | 927 0.207 0.184 | 345 0.050 0.467 | 560 0.226 0.157 | 530 0.063 0.112 | 1636 −0.194 0.241 | 262 0.143 0.202 | 4260 0.021 0.294 |
| Watts and Chou [18] | N AD SD | 927 0.165 0.169 | 345 −0.026 0.477 | 560 0.162 0.158 | 530 0.012 0.105 | 1636 −0.228 0.232 | 262 0.232 0.195 | 4260 −0.017 0.291 |
| Jackson [19] | N AD SD | 927 0.209 0.188 | 345 0.045 0.468 | 560 0.237 0.183 | 530 0.047 0.142 | 1636 −0.195 0.246 | 262 0.130 0.200 | 4260 0.019 0.301 |
| Kang and Chang [14] | N AD SD | 927 0.068 0.272 | 345 0.014 0.468 | 560 0.117 0.172 | 530 −0.040 0.174 | 1636 −0.311 0.310 | 262 0.114 0.225 | 4260 −0.086 0.339 |
| Zhang et al. [16] | N AD SD | 927 0.319 0.246 | 345 -0.554 1.201 | 560 0.261 0.380 | 530 0.080 0.195 | 1636 −0.372 0.532 | 262 0.027 0.226 | 4260 −0.072 0.608 |
| Correlation | R-22 | R-134a | R-245fa | Ethanol | Total | |
|---|---|---|---|---|---|---|
| Dittus-Boelter [21] | N ±20% ±30% | 1272 42 55 | 1090 48 61 | 1636 25 52 | 262 55 87 | 4260 37 57 |
| Krasnoshchekov et al. [29] | N ±20% ±30% | 1272 30 55 | 1090 32 48 | 1636 50 64 | 262 70 82 | 4260 40 58 |
| Yamagata et al. [8] | N ±20% ±30% | 1272 11 20 | 1090 0 7 | 1636 47 67 | 262 0 0 | 4260 22 34 |
| Jackson and Fewster [20] | N ±20% ±30% | 1272 43 69 | 1090 66 76 | 1636 48 68 | 262 62 72 | 4260 51 70 |
| Watts and Chou [18] | N ±20% ±30% | 1272 52 72 | 1090 76 86 | 1636 47 69 | 262 47 59 | 4260 55 73 |
| Jackson [19] | N ±20% ±30% | 1272 45 68 | 1090 63 73 | 1636 45 68 | 262 63 73 | 4260 50 69 |
| Kang and Chang [14] | N ±20% ±30% | 1272 63 74 | 1090 78 89 | 1636 18 43 | 262 63 77 | 4260 50 66 |
| Zhang et al. [16] | N ±20% ±30% | 1272 21 38 | 1090 46 64 | 1636 42 52 | 262 74 85 | 4260 39 52 |
| Correlation | R-22 | R-22 | R-134a | R-134a | R-245fa | Ethanol | Total | |
|---|---|---|---|---|---|---|---|---|
| Yamashita et al. [5] | Jiang et al. [15] | Kang and Chang [14] | Zhang et al. [16] | He et al. [17] | Jiang et al. [15] | |||
| Present study | N AD SD | 927 0.076 0.090 | 345 −0.059 0.292 | 560 0.043 0.196 | 530 −0.013 0.124 | 1636 −0.084 0.137 | 262 0.279 0.105 | 4260 0.007 0.181 |
| Correlation | R-22 | R-134a | R-245fa | Ethanol | Total | |
|---|---|---|---|---|---|---|
| Presented study | N ±20% ±30% | 1272 82 90 | 1090 70 94 | 1636 86 95 | 262 16 42 | 4260 76 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-M.; Liaw, J.-S.; Wang, C.-C. A Criterion of Heat Transfer Deterioration for Supercritical Organic Fluids Flowing Upward and Its Heat Transfer Correlation. Energies 2020, 13, 989. https://doi.org/10.3390/en13040989
Li Y-M, Liaw J-S, Wang C-C. A Criterion of Heat Transfer Deterioration for Supercritical Organic Fluids Flowing Upward and Its Heat Transfer Correlation. Energies. 2020; 13(4):989. https://doi.org/10.3390/en13040989
Chicago/Turabian StyleLi, Yung-Ming, Jane-Sunn Liaw, and Chi-Chuan Wang. 2020. "A Criterion of Heat Transfer Deterioration for Supercritical Organic Fluids Flowing Upward and Its Heat Transfer Correlation" Energies 13, no. 4: 989. https://doi.org/10.3390/en13040989
APA StyleLi, Y.-M., Liaw, J.-S., & Wang, C.-C. (2020). A Criterion of Heat Transfer Deterioration for Supercritical Organic Fluids Flowing Upward and Its Heat Transfer Correlation. Energies, 13(4), 989. https://doi.org/10.3390/en13040989





