Abstract
Microalgae has proven potential for producing products that are accepted as an alternate energy source. An attempt is made to further improve the efficiency of pyrolysis in terms of product yields and characteristics by adding cotton gin trash and cattle manure as a mixed feedstock (cobiomass). A statistically significant number of treatments were made by mixing different amounts of cotton gin trash and cattle manure with microalgae (Nannochloropsis oculata). These treatments were pyrolyzed at different temperatures (400 to 600 °C ) and product yields and characteristics were analyzed. The pyrolysis of cobiomass resulted in higher yield for bio-oil and char as compared to microalgae alone. An operating temperature of 500 °C was found to be the best suitable for high bio-oil yield. The high heating values (hhv) of bio-oil were observed to be maximum at 500 °C and for syngas and char, the heating value slightly increased with further increase in temperature. Comparatively, the bio-oil (30 MJ/kg) had higher heating values than char (17 MJ/kg) and syngas (13 MJ/kg). The combustible material decreased whereas fixed carbon and ash content increased in char with an increase in temperature. The bio-oil produced from cobiomass had abundant aliphatics and aromatics with low nitrogen content making it a better alternative fuel than bio-oil produced by microalgae alone. The mixing of different biomass helped improving not just the quantity but also the quality of products.
Keywords:
microalgae; cotton gin trash; pyrolysis; hydrocarbon; alternate energy; bio-oil; cobiomass 1. Introduction
Pyrolysis is a technique that successfully converts organic wastes into valuable products (biofuels) by a thermo-chemical process at controlled conditions. Pyrolysis is a promising method of converting inexpensive carbon-rich materials into valuable and useful products. The overall conversion takes place in the absence of oxygen with different operating conditions, like temperature, feedstock type, and reaction time [1]. During the process, volatile materials contribute to a gas phase in which the low molecular weight fractions form gaseous products (syngas) and large molecular weight fractions condense and constitute the liquid phase (bio-oil). The syngas and bio-oil along with char, as a residue, are potential hydrocarbons produced through pyrolysis of biomass.
Bio-oil, produced via pyrolysis, is a valuable product derived mostly from lignocellulosic biomass. Chemically, the bio-oil is composed of oxygenated organic compounds that mainly include alcohols, aldehydes, carbohydrates, organic acids, and phenols. These components suggest the application of bio-oil in different fields. The relative amount of these components mainly depends on the chemical species present in the biomass (feedstock) [2]. Biomass with low energy calorific values results in low-quality bio-oil, which may need further treatment [3]. After secondary processing, biomass produced bio-oil has a significant amount of petroleum range hydrocarbons [4]. Similarly, the quality of syngas is usually low but it contains alkanes and alkenes that are combustible and hence can be used as an alternative fuel. Usually, the presence of carbon dioxide, tar, and water vapors decrease the quality of syngas usage in combustion engines [5]. The char produced by pyrolysis of biomass is used for energy purposes, soil conditioning, and water treatment. The distribution of these three products and characteristics is dependent on pyrolysis temperature and feedstock [6,7].
Among various biomass, microalgae has proven to be a reliable raw material with high bio-oil production rates [4]. As a single biomass, a variety of microalgae have been pyrolyzed for hydrocarbon production, including Botryococcus braunii [8], Nannochloropsis Species [9], Chlorella Species, and Spirulina Species [10]. All these studies have reported high efficiency of fuel production with microalgae as a feedstock. The only problem in using microalgae as a biomass is that microalgae has a high amount of proteins resulting in a high nitrogen content in the products resulting in low-quality products. Mixing microalgae with other biomass offers a better chance of augmenting the quantity of bio-oil production and reducing the nitrogen content in the products [11].
Agriculture waste mostly contains a huge volume of biomass that requires further treatment to avoid environmental degradation due to direct disposal. Cow manure is waste that ends up in agriculture fields and causes serious environmental impacts [12]. Successful pyrolysis of cow manure for char and gas production is reported by many studies [13,14,15]. Similarly, cotton gin trash with high ligno-cellulosic material is an inexpensive and readily available carbon source [16]. Cotton gin trash and cow manure are among the readily available wastes in the agricultural state of Texas, USA.
Pyrolysing of one biomass is neither sustainable nor suitable for getting the desired quality of products. Few recent studies investigated the pyrolysis of multiple feedstocks (co-biomass) and successfully improved the quantity and quality of products [11,17]. It was hypothesized that a promising quantity and quality of hydrocarbons can be produced by pyrolyzing cobiomass. We report the quantity and quality of products (char, bio-oil, and syngas) from pyrolysis of cobiomass of microalgae (Nannochloropsis oculata), cotton gin trash, and cow manure. The study aimed to investigate the effect of temperature on product distribution and characteristics of these products.
2. Materials and Methods
2.1. Preparation of Cobiomass
Cotton gin trash and fresh cattle manure were taken from Varisco-court Cotton Gin Co. at Bryan, Texas, USA. The microalgae, Nannochloropsis oculata, was taken from Texas Agri-Life Research Algae Pond, Pecos, Texas. The microalgae was de-watered before use. The three biomasses were dried in an oven at 105 °C for 24 h to reduce the moisture content to the < 10% of dried mass (by weight). The Wiley mill was used to pulverize the feedstocks to an average particle size of 2 mm.
2.2. Experimental Setup
A pressure batch reactor (4580 HP/HT, Parr Instrument Company, Moline, IL, USA) was used to pyrolyze the biomass. Details of the experimental setup and operational parameter can be accessed from Maguyon and Capareda [1]. The temperature incremented at 5 °C/min for warming up and cooling down of the reactor. Sampling bags (0.5 L Tedlar) with a combination valve were used during the pyrolysis to collect the gas samples while the zip lock plastic bags were used for the solid products.
2.3. Experimental Design
The Design Expert software (Stat Ease Inc., Minneapolis, MN, USA) was used to determine the experimental runs and biomass combinations (treatments). A three-level one factorial complete randomized experimental design was prepared. This design helped in reducing the number of runs from 30 to a total of 19 runs. Table 1 shows the treatments suggested by the software. Even with different proportions of feedstocks, all treatments had the same high heating value and available carbon content (HHV = 6.2 ± 0.01 MJ/kg and total carbon = 119 ± 2 g).

Table 1.
Feedstocks and operating temperatures used as the treatments.
2.4. Chemical Analysis
A gas meter (METRIS 250, Itron, Owenton, KY, USA) was used to measure gas volume and an analytical balance (Mettler Toledo, Model XP105DR, Switzerland) was used to measure the mass of solids. The char, bio-oil, and syngas were measured for high heating values (HHV) using PARR isoperibol bomb calorimeter (Model 6200, Parr Instrument Company, Moline, IL). The ultimate analysis of char and bio-oil was done with an elemental analyzer (Vario MICRO Elemental Analyzer, Elementar Analysemsysteme GmbH, Germany). The proximate analysis was done for char according to ASTM (D3175 and E1755). The components of bio-oil were separated with gas chromatography coupled with the mass spectrometer (GC-MS, Shimadzu QP2010 Plus, Germany) for the chemical composition of bio-oil. Dichloromethane (10% vol) was used as a solvent. The separation was done in the DB-5 Mass Spectrometer column. The organic functional groups were determined with a Fourier-Transformed Infrared Spectrophotometer (FT-IR, Shimadzu, IR Affinity-1 with a MIRacle universal sampling accessory, Kyoto Prefecture, Japan). The chemical shifts were observed within the absorbance range of 4000 to 700 cm−1. The resolution was set at 4 cm−1.
2.5. Statistical Analysis
The calculations and statistics were done with XLSTAT (2018.1.49205) and the Data Analysis tool pack of Microsoft Excel (2010).
3. Results
3.1. Characterization of Three Biomass
The initial characteristics of feedstocks are shown in Table 2. The table compares the initial biomass characteristics of the three components (cotton gin trash, cattle manure, and microalgae) with wood as the reference biomass. The high heating values of cotton gin trash and microalgae were higher than wood due to high lignin content [18]. The volatile combustible material in microalgae was higher than wood, which makes it a suitable candidate for syngas production at high temperatures [1]. The blending of cotton gin trash with microalgae may have decreased gaseous escape of hydrocarbons as it has high amounts of fixed carbon. As compared to wood, the feedstocks had high ash content, which shows a significant proportion of the inert material (nutrients and metals). The ultimate analysis in Table 2 shows that carbon and oxygen of wood were higher than all the feedstocks, which play a vital role in hydrocarbon production. The feedstocks have higher nitrogen showing more protein than wood, which may have potential benefits in stabilizing nitrogenous waste but lower the quality of bio-oil. Overall, the properties of three biomass were suitable for attempting pyrolysis to produce hydrocarbons. It is also important to note that the total amount of carbon in each treatment was fixed to be 120 ± 2 g with 6.2 ± 0.01 MJ/kg of high heating value. In our previous work, we have simulated these feedstocks to produce high energy products [19]. Production of bio-oil, syngas, and char was modeled to have higher heating values than single biomass. The overall energy balance suggested that using cobiomass has the potential for an improved quantity as well as the quality of the product.

Table 2.
Characteristics of feedstocks in comparison with wood as a perfect material for pyrolysis.
3.2. Products Distribution
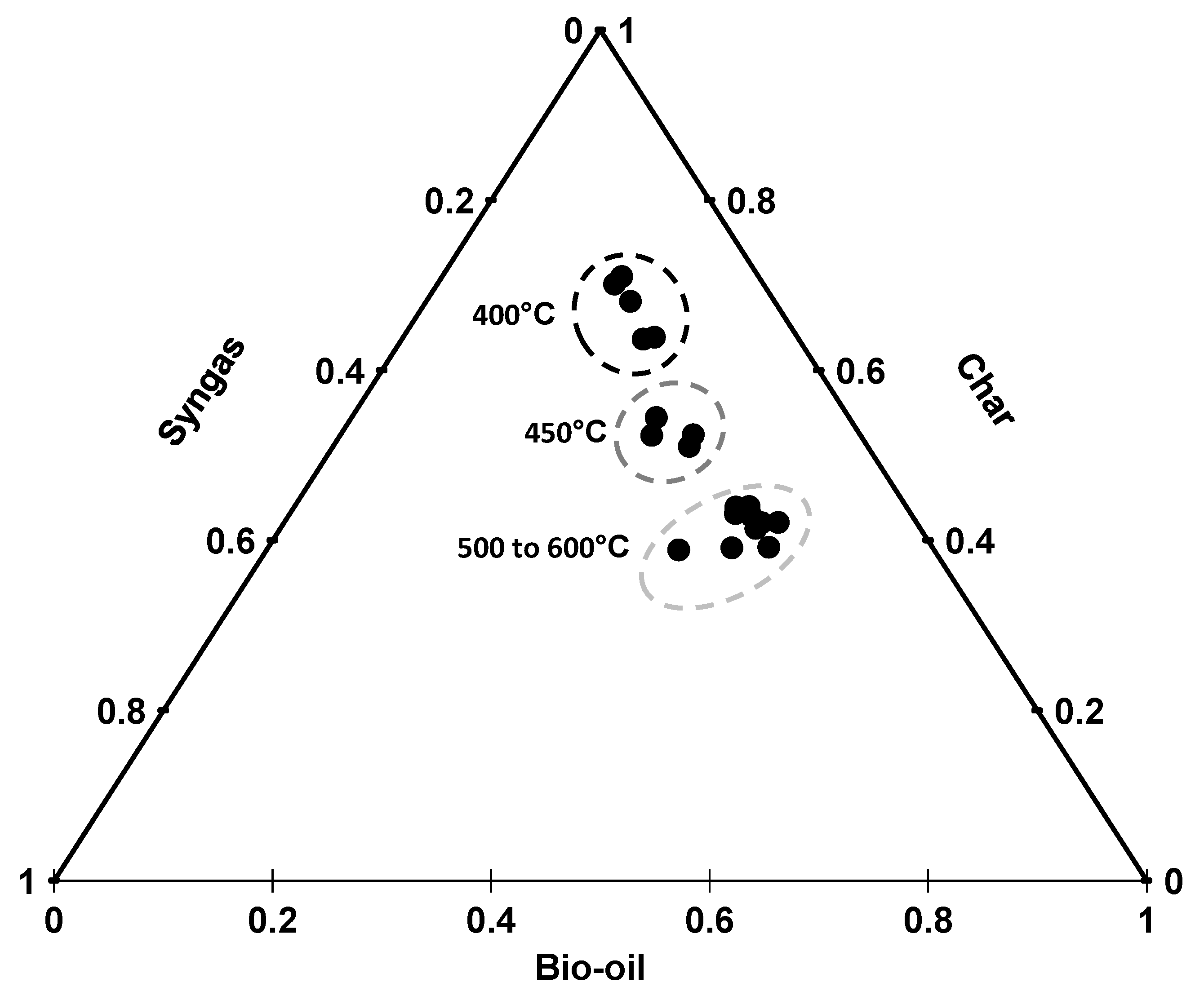
The ternary diagram (Figure 1) shows product distribution at different temperatures. The figure shows that temperature significantly affects the product distribution. Char yield showed a mirror image of bio-oil yield. Bio-oil yield increased with an increase of temperature from 400 to 500 °C and started decreasing with a further increase in temperature. The continuous increase until 500 °C is due to the decomposition of lignin, proteins, lipids, hemicellulose, and cellulose present in the feedstock [21]. Maguyon and Capareda [1] also observed a drop in the bio-oil production at higher temperatures and attributed the decline in bio-oil production to the secondary cracking of oil vapors to form gasses.
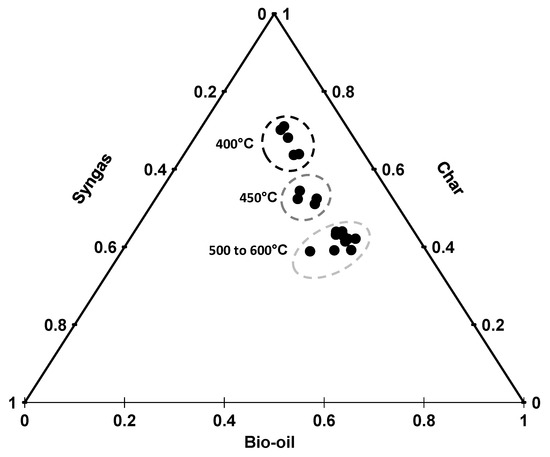
Figure 1.
Ternary diagram showing unit product yields with respect to various temperatures.
The char yield kept on decreasing till 500 °C and stabilized at higher temperatures. The trend of both char and bio-oil production indicates that the process of decomposition has completed at 500 °C [22]. The amount of syngas did not change significantly with the change in temperature. The ternary diagram (Figure 1) suggests that the product distribution varies at low temperature but the change in temperature does not change the product distribution significantly at higher temperatures (>500 °C ). The average losses were less than 20% for all the treatments. These losses were higher at low temperatures and as the temperature increased the losses were reduced to a minimum of 4.4%.
Maguyon and Capareda [1] used microalgae (Nannochloropsis oculata) as a single feedstock for hydrocarbon production through pyrolysis and reported similar trends for product yields with respect to temperature but the amount of product yields for our cobiomass is higher than their findings. Mixing of cotton gin trash and cattle manure with microalgae has significantly increased the quantity of bio-oil and char but overall gas production was reduced. The conversion of cobiomass suggests that combining two or more biomass is a reliable option for bio-oil and char production.
3.3. Products Characteristics
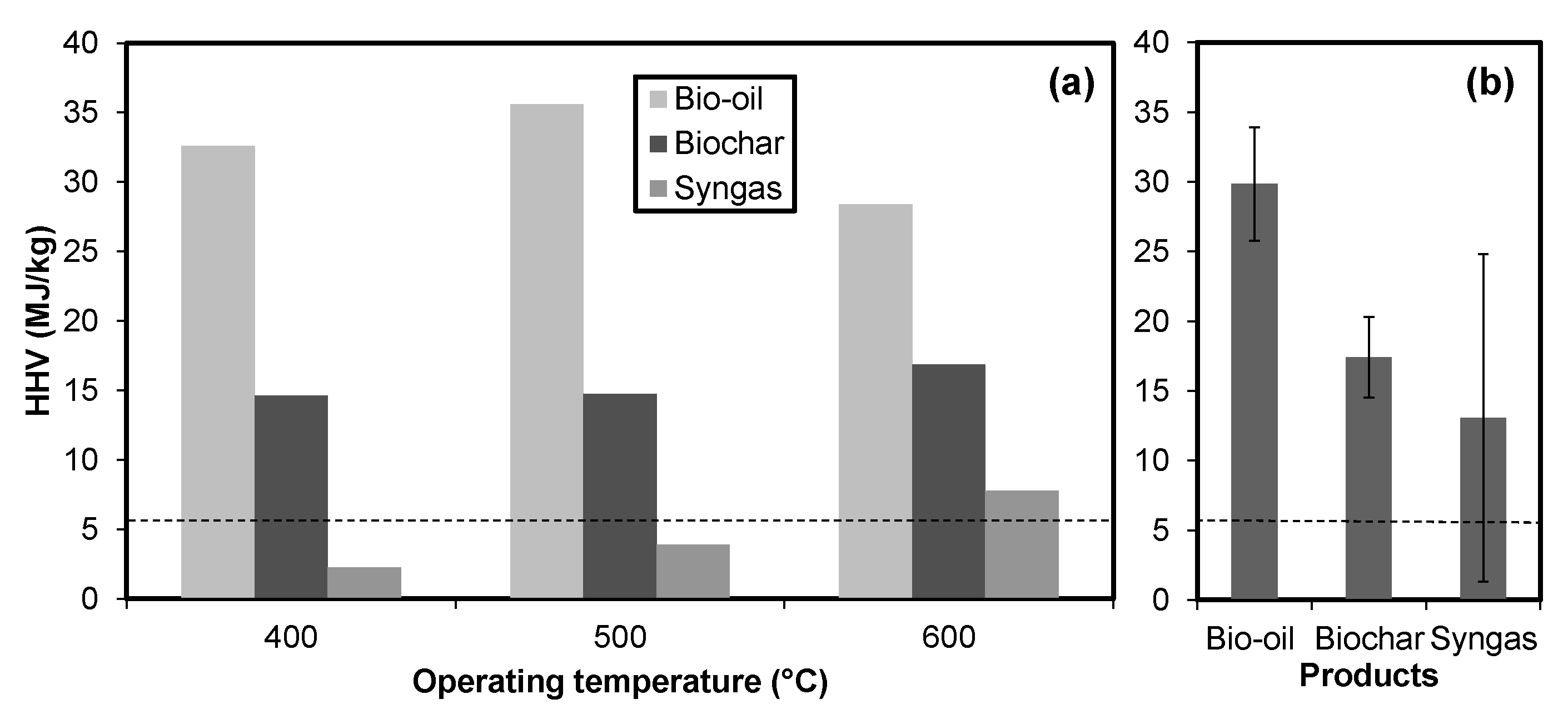
Pyrolysis successfully increased the high heating values of cobiomass. The heating values of syngas was as high as 29 MJ/kg at low temperatures (400 °C), which dropped to 1 MJ/kg at high temperatures (Figure 2a). The results are entirely the opposite of Maguyon and Capareda [1]. Mixing of cobiomass must have resulted in fixing more hydrocarbons resulting in low combustible compounds. Further, the chemical analysis of gas samples could explain the reason for lowering high heating values with an increase in temperature. The bio-oil high heating value was maximum at 500 °C, which is the most suitable temperature for high bio-oil production, especially when microalgae is used as a feedstock [1]. Overall, the bio-oil high heating values were significantly higher than other products with low standard deviation (4.1, see Figure 2b).
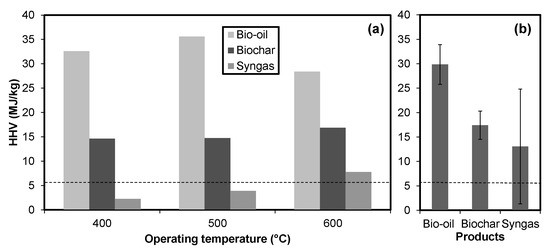
Figure 2.
High heating values (HHV) for products of pyrolysis. The plot shows HHV values of syngas, char, and bio-oil with the same feedstock (CGT:CM:MA = 3.5:1.5:5) at different temperatures (a) and average HHV of products from all treatments (b). The broken line shows the initial HHV of treatments (6.2 ± 0.01).
The high heating value of char kept on increasing with increasing temperature. This is due to the increase in carbon content at increasing temperatures [23]. Char had an average high heating value of 17 MJ/kg with a standard deviation of 2.9 (Figure 2b). The detailed chemical properties of products are discussed as follows.
3.3.1. Bio-Oil
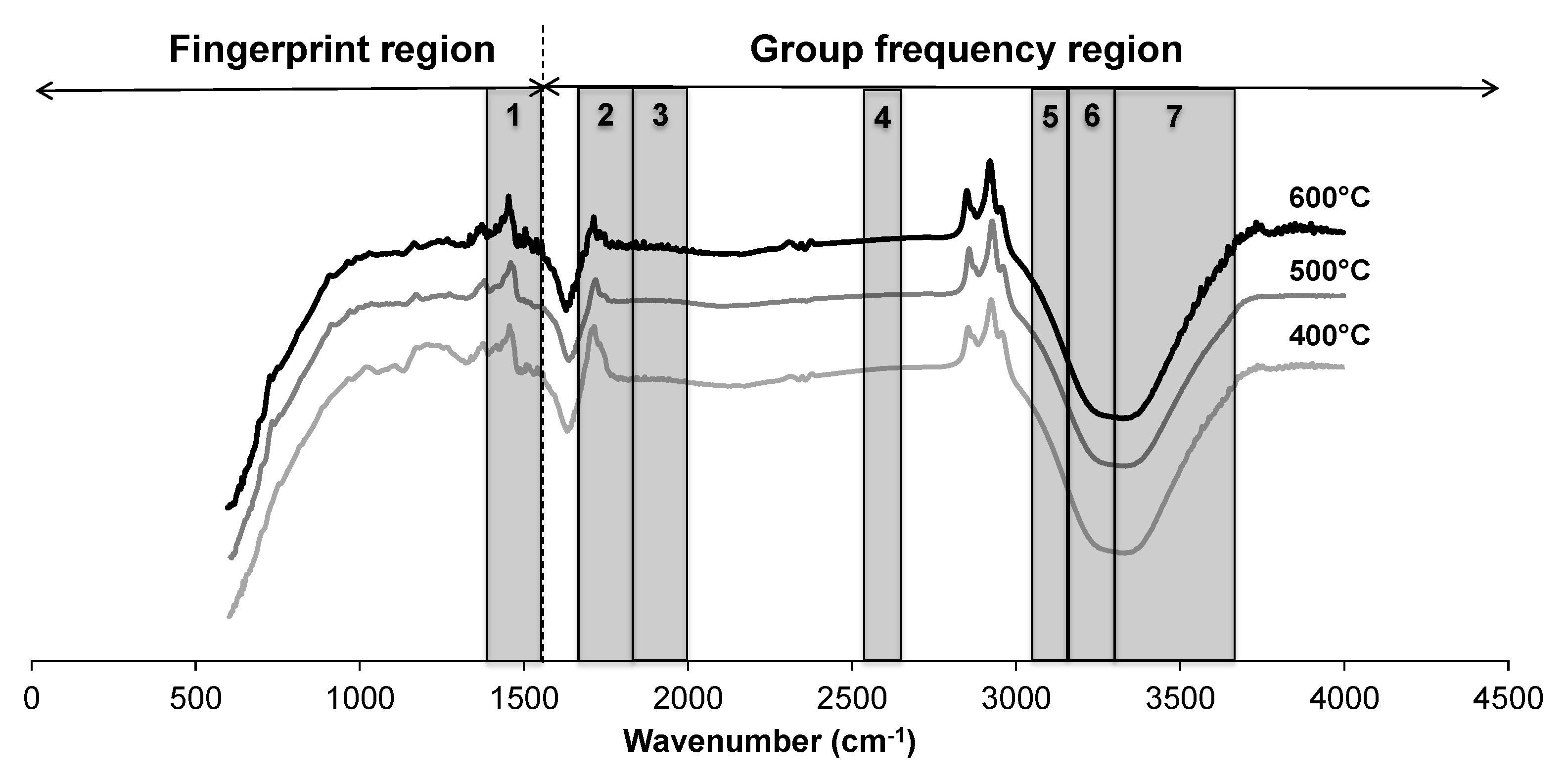
Figure 3 shows the FTIR spectra of three bio-oils at the same feedstocks (CGT:CM:MA = 3.5:1.5:5) but different temperatures (400, 500, and 600 °C). The relative chemical structure of bio-oil did not change significantly, but the peaks of bio-oil produced at 600 °C are well pronounced than the bio-oil produced at lower temperatures. The spectrum shows several absorption bands that can be attributed to many functional groups. Major stretches of silicates, polysaccharides, fatty acids, aldehydes, amides, alkanes, alkenes, aromatics, aliphatics, alcohols, phenols, carboxyl groups, and water are highlighted on spectra. A wide variety of chemical groups are present in the bio-oil showing the complexity of bio-oil composition.
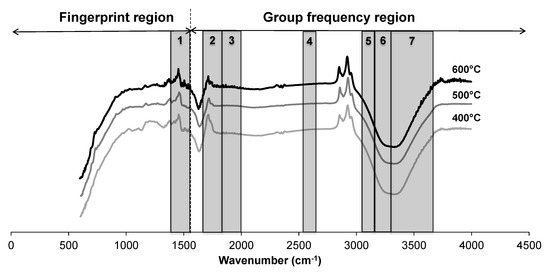
Figure 3.
FTIR spectra of bio-oil produced at 400, 500, and 600 °C. The spectral ranges of various compounds are highlighted as (1) silicates and polysacchrides, (2) CH2 and CH3 compound, (3) fatty acids, aldehydes, amides, alkenes, and aromatics, (4) C-N groups, (5) aliphatics, (6) aromatics, and (7) water, alcohols, phenols, and carboxylic acids.
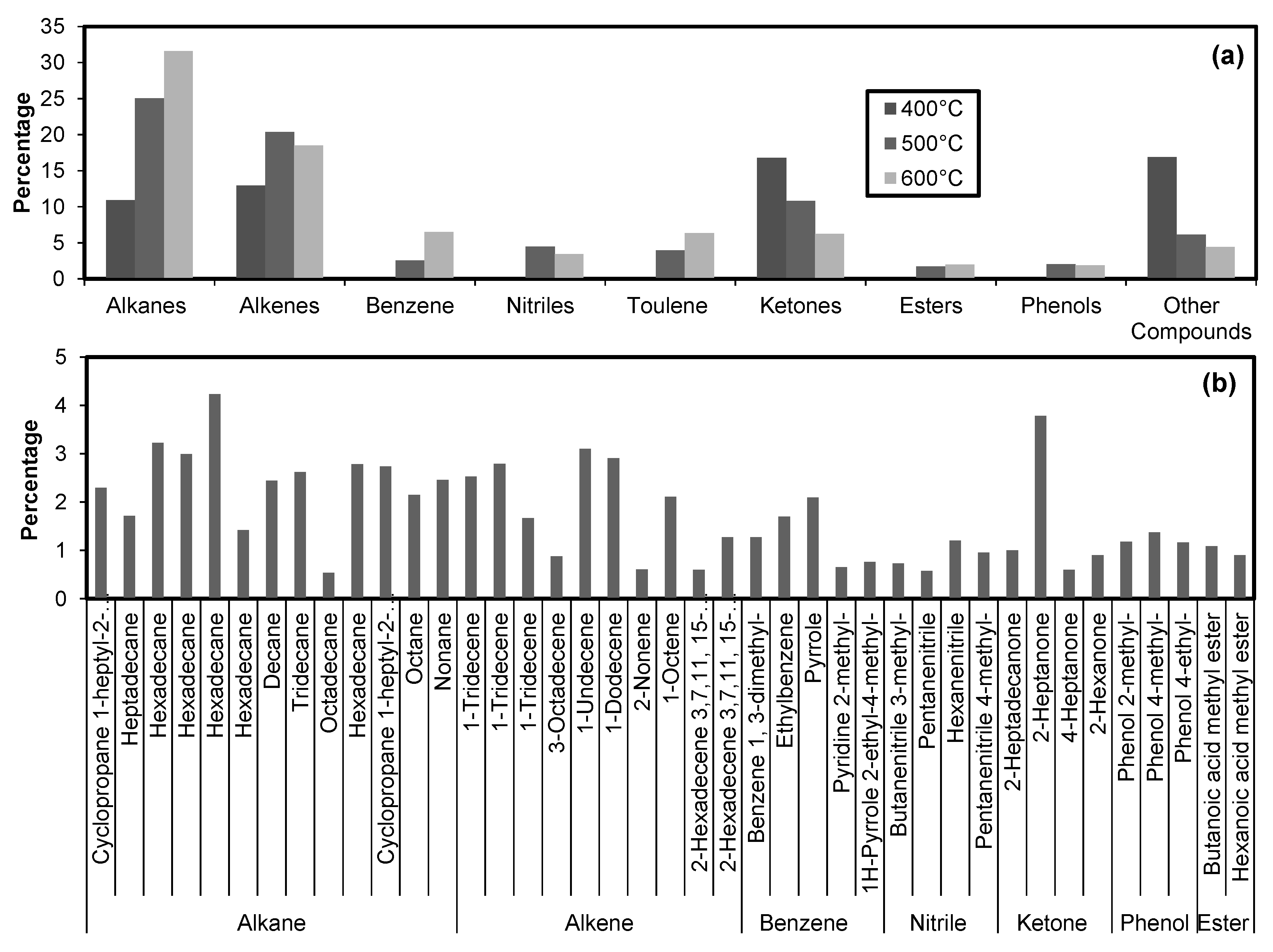
The GC–MS analysis for molecular characterization of bio-oil produced by the same treatments is shown in Figure 4. The abundant compounds found in bio-oils are alkanes, alkenes, and ketones (Figure 4a). At 400 °C, benzene, nitriles, toluene, esters, and phenols were not found. The relative abundance of compounds in bio-oil at 600 °C remained alkanes > alkenes > ketones > whereas at 400 °C the relative abundance of compounds was found to be ketones > alkenes > alkanes. The aliphatic and aromatic compounds are relatively higher in bio-oil produced at high temperatures (> 400 °C). The amount of nitrogen-containing compounds (mainly nitriles) significantly reduced in bio-oil by cobiomass as compared to microalgae alone [1]. It is important to mention here that, on average, nitrogen-containing compounds were found in small amounts in bio-oil produced at 500 °C among all the treatments. The relative abundance of these compounds in descending order were nitriles (1% to 3%), pyridines (0.5% to 1.04%), and indoles (0% to 0.5%). It was observed that with the increase in temperature the diversity in nitrogen-containing compound increases in bio-oil. The bio-oil produced by co-biomass has a better candidacy for environment-friendly alternate fuel as compared to the bio-oil produced by microalgae alone.
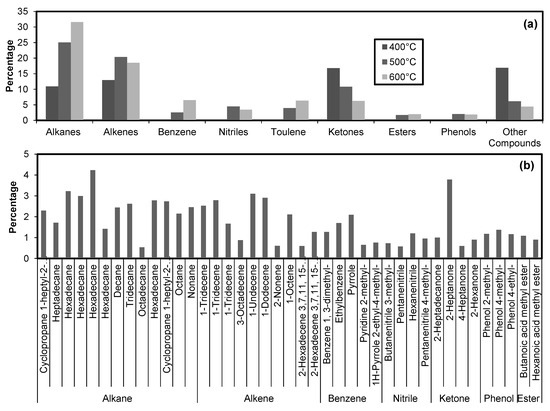
Figure 4.
Chemical composition of bio-oil obtained at 400, 500, and 600 °C (a) with further elaboration of these groups at 600 °C (b).
Figure 4b shows the further breakup of broader groups present in bio-oil produced at 600 °C. Hexadecane, 1-tridecane, pyrole, hexa-nitrile, 2-heptanone, phenol 4-methyl, and butanoic acid methyl ester were the most abundant compounds found among the alkanes, alkenes, benzene, nitrile, ketones, phenols, and esters, respectively.
3.3.2. Char
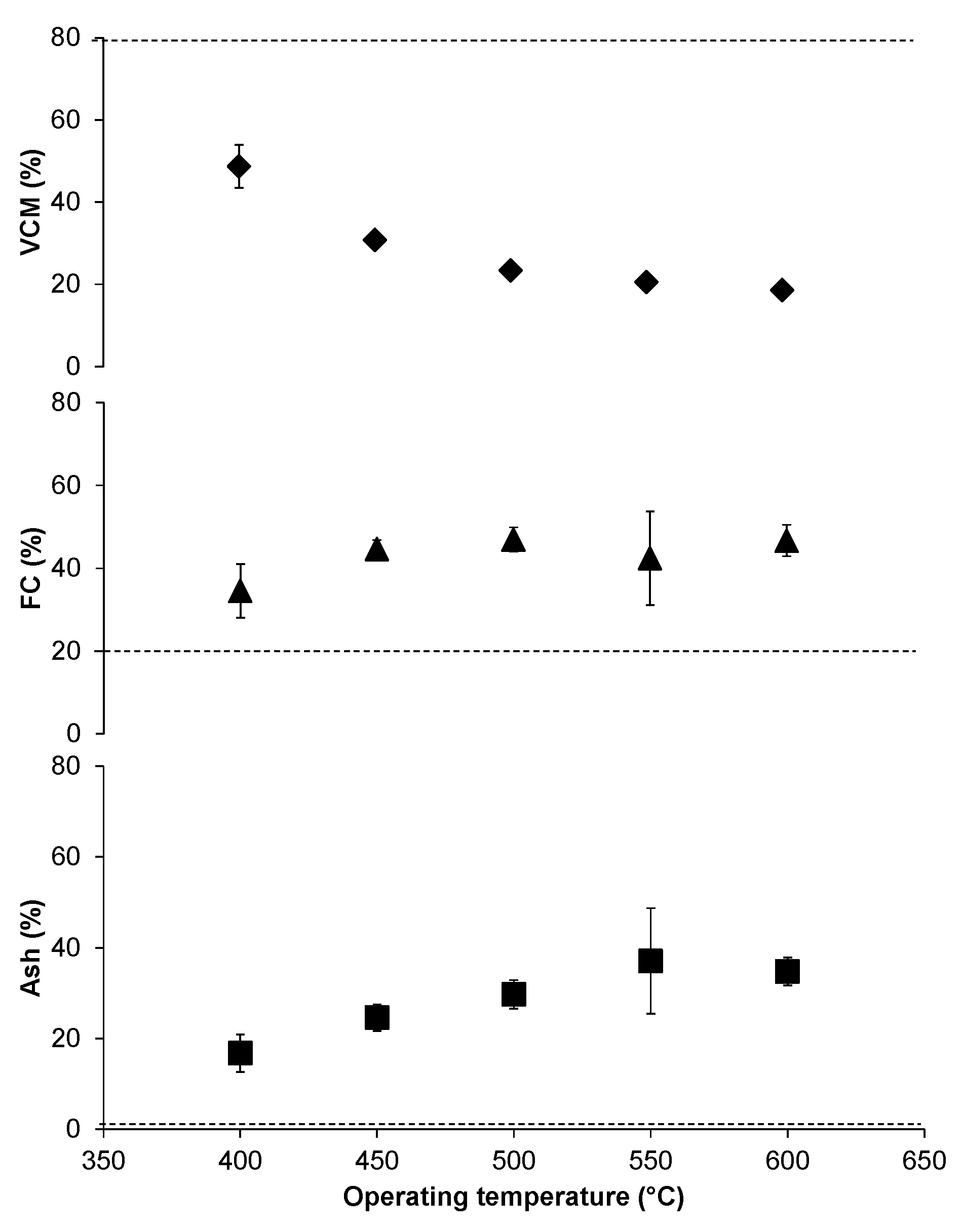
The proximate analysis of char shows that there is a significant effect of temperature on ash, fixed carbon, and volatile combustible material (Figure 5). The volatile combustible material in char decreased with an increase in temperature, as the volatiles are converted to the gas phase of products leaving behind fixed carbon and ash content. In comparison with the wood, our char had almost a half volatile combustible material and double the amount of fixed carbon. Figure 5 shows that the ash content is thirty times higher than wood making it more suitable for agricultural applications rather than as an alternative source of fuel. The mass balance of carbon transformation from feedstock to fixed carbon in char is almost 45% having a maximum of 68% at low temperature and a minimum value of 30% at high temperatures. The char can be used for sequestering carbon to avoid environmental degradation.
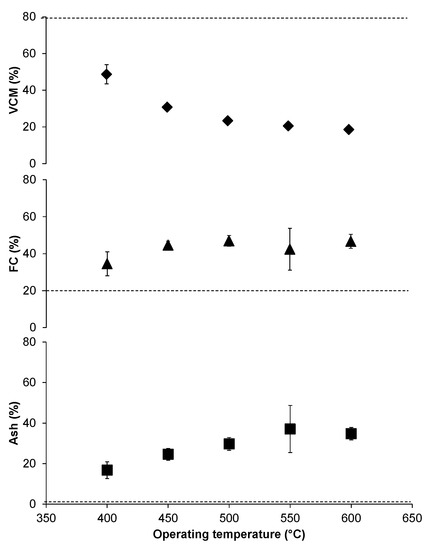
Figure 5.
Proximate analysis of char. The percentages of volatile combustible material (VCM), fixed carbon (FC), and ash at different temperatures. Note that the broken line shows the specific properties of wood.
3.3.3. Syngas
The temperature and feedstock affected the relative amounts and trends of syngas components (Table 3). The table shows that carbon dioxide was maximum at low temperature (400 °C) and the hydrocarbons () were maximum at 500 °C. This confirms that the high heating value of syngas were higher at 500 °C in Figure 2. The table also compares the components of syngas with the relative amount of cow manure in treatments at 500 °C. Production of methane (CH4) was higher in syngas where cow manure was lowest showing that cow manure alone did not contribute towards hydrocarbon presence in syngas. The mass balance of carbon showed that methane had almost 6% of the total carbon available in the feedstock.

Table 3.
Composition and high heating values of syngas at different temperatures.
4. Conclusions
The amount of bio-oil and char increased by mixing cow manure and cotton gin trash with microalgae. Specifically for bio-oil, a pyrolysis temperature of 500 °C is suitable for maximum production. The product distribution does not change significantly at temperature higher than 500 °C. Further increase in pyrolysis temperature is only recommended if specific chemical combinations are required in products. The high heating value of bio-oil (30 MJ/kg) was at a maximum at 500 °C whereas for syngas (13 MJ/kg) and char (17 MJ/kg) the heating value slightly increased with further increase in temperature. Aliphatics (25 to 50%) and aromatics (5 to 15%) were abundant in bio-oil with low nitrogen content (<5%) making it a better alternative fuel than the bio-oil produced by microalgae alone (5%). The nitrogen-containing compounds were found at a minimum in the bio-oil produced at 500 °C. The relative abundance of these compounds in descending order that remained were nitriles (1% to 3%), pyridines (0.5% to 1.04%), and indoles (0 to 0.5%). The combustible material decreased (from 50% to 20%) whereas fixed carbon and ash content increased (from 30% to 42% and from 18% to 38%, respectively) in char with increase in temperature.
Author Contributions
Conceptualization, S.C.C., M.U.H. and A.B.; data curation, M.U.H. and H.I; investigation, M.U.H., M.Z. and M.A; methodology, S.C.C.; supervision, S.C.C.; validation, B.F.F., M.A., and A.W.; visualization, A.W., A.B., and M.Z.; writing—original draft, M.U.H., M.Z., S.C.C., H.I., M.A., B.F.F., A.B., and A.W.; writing—review and editing, M.U.H., M.Z., S.C.C., H.I., M.A., B.F.F., A.B., and A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This article was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah. The author, therefore, acknowledges with thanks DSR for technical and financial support.
Acknowledgments
The authors are grateful to the Higher Education Commission (HEC) of Pakistan, Bio-Energy Testing and Analysis Laboratory (BETA Lab), Biological and Agricultural Engineering Department, Texas A&M University, and National University of Sciences and Technology for supporting the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maguyon, M.C.; Capareda, S.C. Evaluating the effects of temperature on pressurized pyrolysis of Nannochloropsis oculata based on products yields and characteristics. Energy Convers. Manag. 2013, 76, 764–773. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel 2014, 128, 162–169. [Google Scholar] [CrossRef]
- Isa, Y.M.; Ganda, E.T. Bio-oil as a potential source of petroleum range fuels. Renew. Sustain. Energy Rev. 2018, 81, 69–75. [Google Scholar]
- Aneke, M.; Wang, M. Thermodynamic Comparison of alternative Biomass Gasification Techniques for producing Syngas for Gas Turbine Application. Energy Procedia 2017, 142, 829–834. [Google Scholar] [CrossRef]
- Mafu, L.D.; Hein, W.J.P. Neomagus and Raymond C. Everson and Christien A. Strydom and Marion Carrier and Gregory N. Okolo and John R. Bunt, Chemical and structural characterization of char development during lignocellulosic biomass pyrolysis. Bioresour. Technol. 2017, 243, 941–948. [Google Scholar] [CrossRef]
- Gomez-Casanovas, N.; DeLucia, N.J.; Hudiburg, T.W.; Bernacchi, C.J.; DeLucia, E.H. Conversion of grazed pastures to energy cane as a biofuel feedstock alters the emission of GHGs from soils in Southeastern United States. Biomass Bioenerg. 2018, 108, 312–322. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Naqvi, M.; Noor, T.; Hussain, A.; Iqbal, N.; Uemura, Y.; Nishiyama, N. Catalytic Pyrolysis of Botryococcus Braunii (microalgae) Over Layered and Delaminated Zeolites For Aromatic Hydrocarbon Production. Energy Procedia 2017, 142, 381–385. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, L.; Yang, X. Pyrolysis characteristics and pathways of protein, lipid and carbohydrate isolated from microalgae Nannochloropsis sp. Bioresour. Technol. 2017, 229, 119–125. [Google Scholar] [CrossRef]
- Huang, F.; Tahmasebi, A.; Maliutina, K.; Yu, J. Formation of nitrogen-containing compounds during microwave pyrolysis of microalgae: Product distribution and reaction pathways. Bioresour. Technol. 2017, 245, 1067–1074. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, W.; Tian, X.; Yang, B. Synergistic effects from co-pyrolysis of low-rank coal and model components of microalgae biomass. Energy Convers. Manag. 2017, 135, 212–225. [Google Scholar] [CrossRef]
- Hanifzadeh, M.; Sarrafzadeh, M.; Nabati, Z.; Tavakoli, O.; Feyzizarnagh, H. Technical, economic and energy assessment of an alternative strategy for mass production of biomass and lipid from microalgae. J. Environ. Chem. Eng. 2018, 6, 866–873. [Google Scholar] [CrossRef]
- Yue, Y.; Singh, H.; Singh, B.; Mani, S. Torrefaction of sorghum biomass to improve fuel properties. Bioresour. Technol. 2017, 232, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; He, T.; Cao, H.; Yuan, Q. Cattle manure pyrolysis process: Kinetic and thermodynamic analysis with isoconversional methods. Renew. Energy 2017, 107, 489–496. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, D.; Li, X.Q.; Yuan, Q.; Cao, H. Influence of moisture content on cattle manure char properties and its potential for hydrogen rich gas production. J. Aanal. Appl. Pyrol. 2018, 130, 224–232. [Google Scholar] [CrossRef]
- Ranjithkumar, M.; Ravikumar, R.; Sankar, M.K.; Kumar, M.N.; Thanabal, V. An Effective Conversion of Cotton Waste Biomass to Ethanol: A Critical Review on Pretreatment Processes. Waste Biomass Valori. 2017, 8, 57–68. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Ristovski, Z.; Milic, A.; Gu, Y.; Islam, M.S.; Wang, S.; Hao, J.; Zhang, H.; He, C. A review of biomass burning: Emissions and impacts on air quality, health and climate in China. Sci. Total Environ. 2017, 579, 1000–1034. [Google Scholar] [CrossRef]
- White, R.H. Effect of lignin content and extractives on the higher heating value of wood. Wood Fiber Sci. 1987, 19, 446–452. [Google Scholar]
- Hanif, M.U.; Capareda, S.C.; Iqbal, H.; Arazo, R.O.; Baig, M.A. Effects of Pyrolysis Temperature on Product Yields and Energy Recovery from Co-Feeding of Cotton Gin Trash, Cow Manure, and Microalgae: A Simulation Study. PLoS ONE 2016, 11, 1–11. [Google Scholar]
- Parikh, J.; Channiwala, S.A.; Ghosal, G.K. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Bui, H.; Tran, K.; Chen, W. Pyrolysis of microalgae residues—A kinetic study. Bioresour. Technol. 2016, 199, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Hu, C.; Yang, W.; Li, Y.; Dong, L.; Zhu, L.; Tong, D.; Qing, R.; Fan, Y. The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils. Bioresour. Technol. 2010, 101, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Imam, T.; Capareda, S. Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J. Aanal. Appl. Pyrol. 2012, 93, 170–177. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).