Abstract
In this review, we present a comprehensive revisit of past research and advances developed on the stay-green (SG) paradigm. The study aims to provide an application-focused review of the SG phenotypes as crop residuals for bioenergy. Little is known about the SG trait as a germplasm enhancer resource for energy storage as a system for alternative energy. Initially described as a single locus recessive trait, SG was shortly after reported as a quantitative trait governed by complex physiological and metabolic networks including chlorophyll efficiency, nitrogen contents, nutrient remobilization and source-sink balance. Together with the fact that phenotyping efforts have improved rapidly in the last decade, new approaches based on sensing technologies have had an impact in SG identification. Since SG is linked to delayed senescence, we present a review of the term senescence applied to crop residuals and bioenergy. Firstly, we discuss the idiosyncrasy of senescence. Secondly, we present biological processes that determine the fate of senescence. Thirdly, we present the genetics underlying SG for crop-trait improvement in different crops. Further, this review explores the potential uses of senescence for bioenergy crops. Finally, we discuss how high-throughput phenotyping methods assist new technologies such as genomic selection in a cost-efficient manner.
1. Introduction
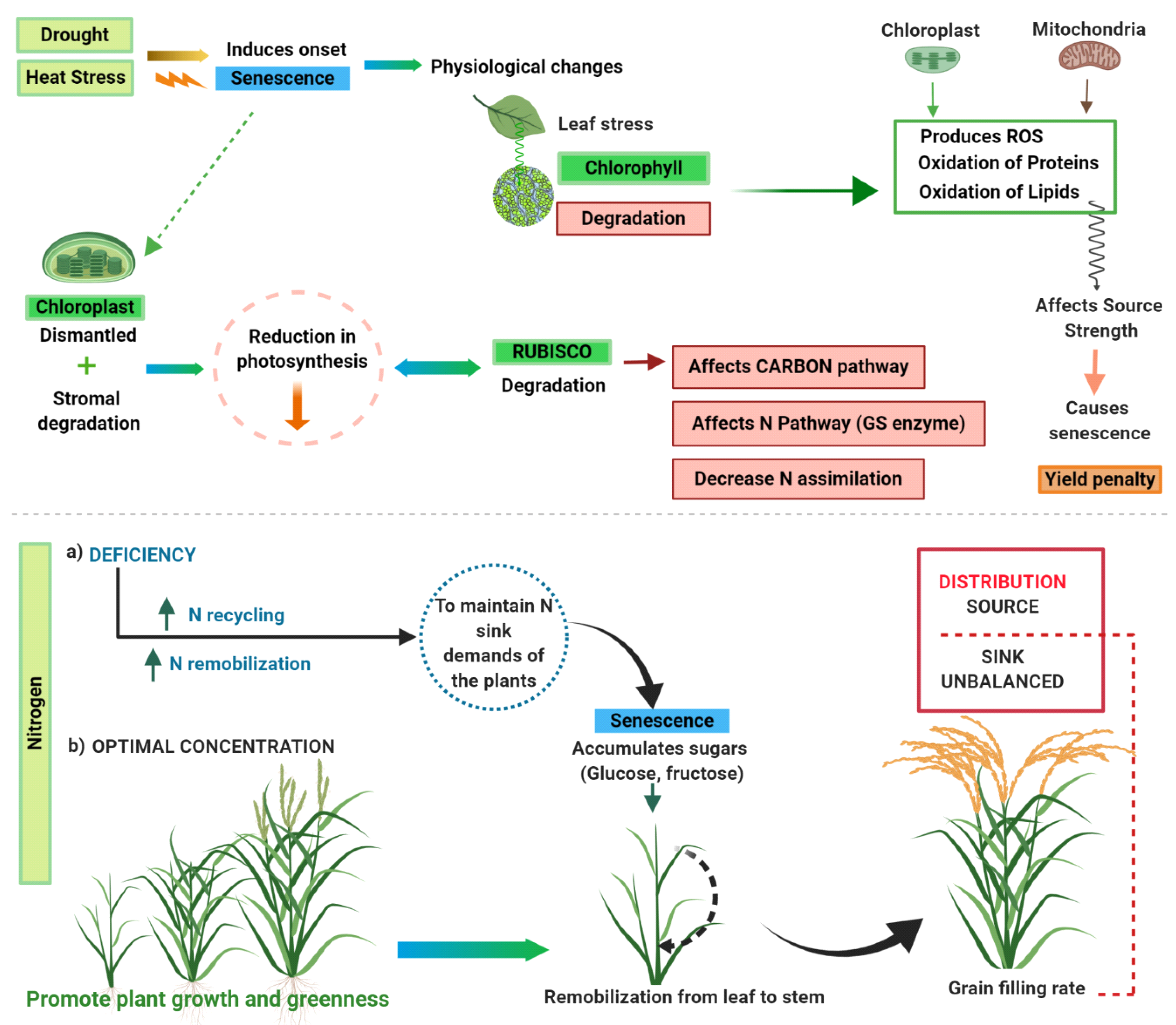
The genotypes with the Stay green (SG) characteristic maintain greenness during the final stage of leaf development due to coordinated genetic mechanisms that regulate the transition from nutrient assimilation to nutrient remobilization [1,2] and the biochemical processes of chlorophyll breakdown during leaf senescence [3] (Figure 1). Senescence is a general term that can be applied to cells (programmed cell death), organs or the whole plant [4]. An example of the whole plant senescence is the gradual deterioration of cell functioning due to aging of annual crops in which it is coordinated with the seed production. Similarly, in perennial crops as miscanthus (Miscanthus spp.) the whole aerial part ages, although subterranean organs remain alive for the next season. In this review, we will focus on the whole plant senescence of herbaceous crops and its relevance for the production of bioenergy. Whole plant senescence involves changes in nutrient absorption, nutrient remobilization [1,5], the dismantling of the photosynthesis apparatus [3] and many other catabolic reactions such as the transport of molecules to sinks and so forth. (Figure 1). Thousands of genes, integrated in many interacting metabolic paths and controlled by transcription factors, internal and external signals, participate in the process of senescence. The senescence networks are age-dependent with a “never senescence” phase, followed by a “senescence competence” phase and finally an “always senescence” phase [6,7]. The progression of ageing depends on age related changes including levels of some hormones as ethylene, abscisic, jasmonic and salicylic acids and brassinosteroids [6,7]. The internal changes associated to age and the external factors such as light, temperature, water and nutrients availability or biotic interactions determine the timing and rate of the senescence. Plant senescence is often described as a paradox because this “survival” strategy encourages the death of the organism; Schippers et al. [6] described this paradox as living to die and dying to live. However, this sacrifice is not in vain since the plant aims to secure a greater good to undergo successful reproduction before death [8]. Leaf senescence has also a central function against abiotic stresses providing substantial tolerance to plants particularly exposed to drought stress [9] by reprogramming expression of genes controlling steps towards the death of the leaf [10]. Instead of senescence itself, several studies have examined a related trait named stay-green (SG) which is a “general term given to a variant in which senescence is delayed compared with a standard reference genotype” [11]. This classical definition of SG illustrates the relativity of the term as SG depends on the standard genotype which is used as reference. The SG trait might be ambiguous if we do not define the standard genotype but also if we do not define the moment and environment of measurement. A SG genotype could have a delayed senescence in the absence of stresses that is a longer cycle in a specific environment. Alternatively, a SG genotype could have a standard senescence under optimum conditions but higher stability against abiotic or biotic stresses that allows the genotype to remain greener than susceptible genotypes under stress conditions. For example, on the commercial description of many maize hybrids the level of SG is shown (acceptable, good, very good, etc.) and it is not clear if the SG is due to a longer cycle or to a late resistance to diseases and plagues. While the association between agronomic traits, such as yield [12,13] and abiotic stresses [14,15] and SG has been widely reported in food or feed crops, the association with bioenergy crops has been less studied. In this review, we address the role of senescence or SG as a highly genetic controlled trait that helps plant to respond to biotic and environmental stresses and discuss the extent to which bioenergy crops can benefit from this paradigmatic trait. First, we focus on internal factors looking at the biological processes that determine the fate of senescence. Next, we discuss trait association analysis and adaptive advantage of senescence for plant survival in different crops. Then, we explore the relationship and potential use on bioenergy crops. Finally, we discuss new high-throughput phenotyping methods to make the measurement of SG more efficient.
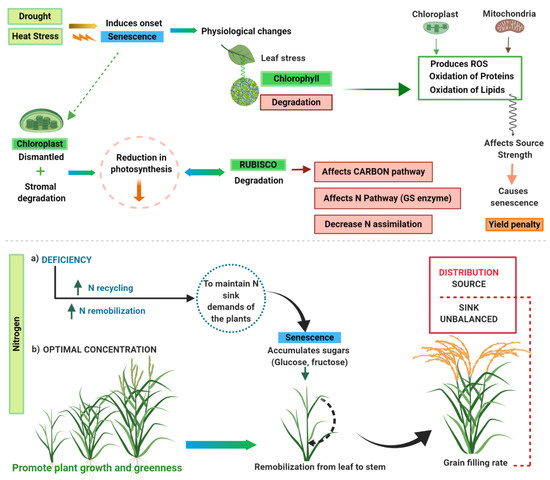
Figure 1.
Schematic diagram showing physiological changes caused by external factors that leads to senescence in plants.
2. Biological Processes of SG and Relationship with Chlorophyll
Although the genetic and physiological routes to SG phenotype are quite diverse among species [11], it is directly related to chlorophyll content of leaves since loss of chlorophyll leads to visible symptoms of senescence (Figure 1 and Figure 2). Therefore, two possible routes to SG phenotype are: (a) impaired or delayed catabolism of chlorophyll and (b) continued biosynthesis of chlorophyll in excess of its catabolism [16]. Among two, the first route has been explored to a great extent and mechanisms/genes responsible for SG phenotype have been identified in several crops. The chlorophyll degradation is basically a multistep process that at first involves conversion of chlorophyll b to chlorophyll a catalyzed by enzymes chlorophyll b reductase (CBR) and 7-hydroxymethyl chlorophyll a reductase (HCAR). This is followed by removal of Mg to form pheophytin by metal-chelating substance and removal of phytol tail catalyzed by pheophytinase (PPH) enzyme to form pheophorbide a. Further, action of pheide O oxygenase and chlorophyll catabolite reductase convert pheophorbide a to a chlorophyll catabolite which is then transported to vacuoles [3]. SG phenotype is either cosmetic (non-functional) or functional in nature. A cosmetic phenotype is the one in which chlorophyll pigments are retained but plant loses its capacity to photosynthesize. This happens because during the first steps of chlorophyll degradation, ring is not broken, and green color is preserved but the chlorophyll becomes non-functional. The cosmetic SG is due to a loss of function mutation in the genes for example, HCAR, PPH and so forth, that breaks the ring. On the contrary, a functional phenotype is the one in which plant continues to photosynthesize and entire senescence process is delayed or slowed down [17]. Therefore, plant breeders mostly rely on functional SG for enhancing yields or for imparting stress tolerance in plants. However, to assess whether the SG is functional or not it is important to measure the CO2 interchange intensity which is slow and laborious and is particularly limiting when many genotypes are evaluated as is common in crop breeding. For that reason, the visual SG has been improved in crop breeding, for example, in private maize [18,19] but it is unknown if it is cosmetic or functional.

Figure 2.
Comparison of different delay of senescence phenotypes of maize planted in a nursery (Baden-Württemberg, Germany). Photo courtesy of Hans Seifert.
Delayed leaf senescence in SG phenotype can enhance crop yields by remobilizing nutrients from source to sink under various stresses and nutrient limited conditions. Transgenic approaches to achieve SG phenotype include enhancing endogenous cytokinin level, reducing abscisic acid (ABA) production, increasing chlorophyll biosynthesis and impairing its degradation. For example, over-expression of cytokinin biosynthetic pathway gene Isopentenyl transferase (IPT) under the control of senescence-associated promoter delayed leaf senescence in maize [20]. Over-expression of IPT gene in wheat (Triticum aestivum L.) plants resulted in delayed leaf senescence by retaining chlorophyll for longer period and resulted in markedly increased grain yields under water deficit conditions [21]. Similarly, expression of ADP-glucose pyrophosphorylase large subunit of maize in wheat seeds stimulated photosynthesis and carbon metabolism and resulting in increased seed number and total plant biomass [22]. An activation tagged line of rice (Oryza sativa L.) for OsMYB102 transcription factor expressed under CaMV35S promoter resulted in delayed leaf senescence by down-regulating ABA accumulation and signaling whereas opposite results were observed by its down-regulation [23]. In another study, a NAC TF named OsNAC2 was identified as a positive regulator of leaf senescence and development of its null mutants exhibited a stay-green phenotype [24].
The chlorophyll degradation pathways are now well understood, and the corresponding genes/enzymes have been identified. Kusaba et al. [25] identified a non-yellow coloring (nyc1) mutant in rice that give it a SG phenotype by impairing chlorophyll degradation during senescence. NYC1 gene encodes a short-chain dehydrogenase/reductase (SDR) with proposed chlorophyll b reductase activity for conversion of Chl b to Chl a, which is then degraded through Chl a degradation pathway [26]. STAYGREEN (SGR) initially identified from a forage crop meadow fescue (Festuca pratensis Huds) is also a positive regulator of chlorophyll degradation, although its catalytic role is not defined yet [27]. Several loss of function mutants (sgr) of SGR gene have been reported from plants like Arabidopsis thaliana L. Heynh. [28], rice [29] and tomato (Solanum lycopersicum L.) [30] and so forth. In addition to yield enhancements, SG phenotypes are of interest for enhancing biomass especially in bioenergy crops like maize (Zea mays L.), sugarcane (Saccharum officinarum L.), sweet sorghum (Sorghum bicolor L.), switchgrass (Panicum virgatum L.) and cassava (Manihot esculenta Cranzt). Genetic manipulation by downregulation of an NAC-domain containing transcription factor nac7 in maize enhanced biomass and N content in vegetative tissues by delaying senescence [31]. Rice /wheat husk and straw also have potential to be utilized as a bioenergy resource, which otherwise is burnt in the fields especially in developing countries. Therefore, a better understanding of mechanisms/process involved in SG phenotype in these crops will help us exploit these resources for production of bioenergy.
Drought (heat stress) diminishes photosynthesis and triggers leaf senescence during the stimulation of complex physiological changes that take place in the chloroplast and mitochondria (Figure 1). Metabolic changes such oxidation of proteins, production of reactive oxygen species (ROS) and lipids may impact source strength. ROS may affect leaf senescence causing a yield penalty. Due to those abiotic stresses leaf is susceptible to chloroplast dismantle and rubisco degradation to balance N demand of sink organs under faster senescence decay. Meanwhile, carbon and nitrogen cycles as sources of energy begin to remobilize by accumulating sugars (glucose and fructose) in the leaves to maintain nitrogen demands of the sink. On the other hand, lack of nitrogen triggers leaf senescence and promotes N recycling and remobilization. Optimal N concentrations stimulate foliage greenness and growth which in turn remobilize N that otherwise would require degradation of chloroplast protein to form molecules of N available. Deficient conditions trigger senescence remobilization of C and N from “green” tissues to fasten grain-filling. These physiological changes alter C and N metabolism by impairing translocation mechanisms leading to a source-sink unbalanced distribution. Physiological changes were further described in Jagadish et al. [32].
3. Genetics of SG and Associated Traits
SG trait is highly genetically regulated and dependent on expression of specific genes [14,33]. Senescence in maize was firstly described as a single dominant locus [34] where the delay in senescence was dominant over senescence satisfying goodness of fit (3:1) ratio. However, Gentinetta et al. [34] also observed that visually scored foliar phenotypes showed “unexpected symptoms of senescence” in the F1 and BC1 crosses. Segregating families produced from crosses of stay-green phenotypes revealed that SG trait has a polygenic inheritance [35,36]. Finding marker-trait associations with the SG and developing consistent molecular markers allows the marker assisted characterization for evaluating senescence before any physiological and visible changes. Linkage mapping analysis has been used to associate SG with molecular variants. In maize, quantitative trait locus (QTL) studies found associated markers across all chromosomal regions (Table 1). Beavis et al. [35] using a biparental population Mo17 × B73 detected 3 QTLs on chromosomes 2, 6 and 9 with a percentage of phenotypic variance explained ranging from 10% to 25% and a high heritability at 0.73.Guei and Wasson [37] quantified the genetic variability for SG in two elite tropical maize populations (dent × flint/dent) reporting that additive effects were greater than dominance effects and suggesting that greener plants at maturity may increase yield. Câmara [38] used maize populations from Brazil and reported 33 QTLs of minor effect averaging 5% of the phenotypic variance explained; although, 65% of the QTLs where localized on chromosomes 1, 2 and 5. Zheng et al. [39] using a biparental population of a stay-green inbred Q319 and an inbred line Mo17 reported 6 QTLs on chromosomes 1, 2, 5 and 8 with relatively small percentage of variation explained ranged at 5.40 to 10.67%. They also reported transgressive segregation for SG and a high correlation between SG and grain yield.Wang et al. [40] measured SG related trait at different stages, 30 days after flowering, grain-ripening stage and considering the whole growing period, reporting 4, 6 and 5 QTLs, respectively. The phenotypic variance explained was relatively low from 2.4% to 11.7% and additive genetic effects were larger than non-additive, although both significant at 0.05 level. Belicuas et al. [41] using tropical lines and a visual scoring scale reported 17 QTLs localized on chromosomes 1, 2, 3, 4, 6 and 9. Most of the QTLs detected showed small additive effects averaging 7.7% of the genotypic variance. However, a QTL on chromosome 1 had a large effect with over 38% of the genetic variance suggesting that this region might be important for SG. Furthermore, linkage mapping analysis also studied SG with photosynthesis efficiency and chlorophyll rates. Almeida et al. [42] detected 8 QTLs associated with senescence across chromosomes 1, 3, 4, 5, 8 and 10, with a sizeable percentage of phenotypic variance explained between 13% and 21%. Almeida et al. [42] reported that SG was negatively correlated with grain yield at −0.71 on average. In this study, they also detected a QTL associated with chlorophyll only under water stressed conditions using a SPAD meter. Kante et al. [43] using two European F2 population of flint by dent origin detected 3 QTLs associated with SG on chromosomes 1, 5 and 10. Kante et al. [43] also found the same region on chromosome 1 associated with chlorophyll levels suggesting that SG may be due to chlorophyll synthesis. Yang et al. [36] reported a broad sense heritability for SG measured as leaf stay-green area index at 0.78. They detected 8 QTLs across seven chromosomes (1, 3, 4, 5, 6, 8 and 10) explaining a percentage of phenotypic variation ranging from 4.8% and 13.5%. SG was highly significantly correlated with relative chlorophyll contents in the leaf at 0.73. Zhang et al. [31] detected a major QTL on chromosome 3 associated with visually scored SG in an Illinois maize population. This QTL mapped to a NAC-domain transcription factor named “NAC7”. Validation analysis utilizingribonucleic acid interference (RNAi) showed that NAC7 negatively regulates biomass production and delayed senescence. In summary, quantitative genetics research on maize have shown marker-trait associations suggesting that genomic regions like chromosome 1 may be of importance for SG. Additives effects were shown generally larger than non-additive effects, although dominance effects have been reported as significant. The later would allow exploiting hybrid vigor performance.

Table 1.
Genetics of stay-green: trait scoring method, number of quantitative trait loci (QTLs), germplasm used and reference for maize and rice crops.
Although the onset of senescence develops because of leaf aging, other environmental conditions can trigger this condition (Figure 1). SG genetic basis have been studied in other crops and associated with other agronomic traits. Similarly as in maize, the delay of senescence as mentioned was firstly reported as a qualitative trait in other crops, for example, Festuca pratensis [44] or rice [45] but rapidly described as controlled by more than one locus in soybean (Glycine max (L.) Merr.) [46], wheat [47], sorghum [48,49] and rice [50]. In rice, Jiang et al. [50] identified 46 QTLs associated with delay of senescence looking at chlorophyll content and retention degree of greenness. This study showed high phenotypic correlations between different stay-green traits and yield performance, suggesting that high correlations were explained by the co-localization of the QTLs for these traits likely due to linkage or pleiotropic effects. Furthermore, Yoo et al. [51] identified three QTLs with large additive effects, 5.0 and 5.6, in rice located on chromosomes 7 and 9, respectively. The QTL on chromosome 9 was named as “SNU-SG1” after the SG donor parent and contributed to grain yield by improving the source strength (i.e., higher chlorophyll amounts and photosynthetic rates). Furthermore, Yamamoto et al. [52] in a high yielder rice biparental population also identified 6 QTLs for leaf chlorophyll amounts and 5 QTLs for rate of nitrogen transport, however did not show changes in yield (Table 1). Furthermore, delay of senescence could enhance drought adaptation in rice [53] as well as in other grasses like sorghum and maize [48,49,54]. The effect of SG on yield performance [39,55] and grain protein relies on the nitrogen accessibility during specific phenology stages of the plant [56] and this aspect has raised concerns for breeding purposes orientated to bioenergy and biomass production.
4. The Senescence or SG in the Breeding of Bioenergy Crops
The knowledge of the genes and pathways involved in senescence can be used to modify crop characteristics in order to optimize the plant tissues for energy exploitation. However, only a small portion of networks and interactions between genes, transcription factors, hormones and signals relevant to the senescence process has been identified [57,58]. Actually, the networks involved in senescence are not specific, for example, some of them overlap with those involved in plant defense. Therefore, biotic stresses accelerate senescence and, several genes upregulated during developmental senescence are involved in plant senescence [58,59]. This introduces further complexities into the study of the mechanisms involved in senescence. The elucidation of those complex networks will be facilitated by global analysis using mathematical tools of system biology [60].
The knowledge of the genes and metabolic paths can facilitate bioenergy crop breeding, harnessing the natural variation or generating new variation by genome editing [61] or transgenic approaches. Gregersen et al. [62] showed that the effectivity of the transgenic approach to increase the biosynthesis of the hormone cytokinin varied among species and that some unexpected pleiotropic effects have occurred when introducing the IPT gene. This highlights that the knowledge acquired about genes and metabolic pathways should be completed with information of allelic variation, genotype × environment interaction and pleiotropy to be of practical use in breeding. In addition, it is crucial to know the magnitude of the gene effects and their relevance in the whole process of senescence. However, given the complexity of networks involved, it is not surprising that the traits related with senescence, as onset, rate of progression or amount of remobilization during the process, are highly quantitative as several QTL experiments, that we have previously commented, have showed. For those types of traits, the breeding based on individual genes, except for those genes of large effects, could not be the most effective [63] and alternatively methodologies as genomic selection should be used [64].
Senescence influences bioenergy production by its effect on residual moisture and yield and on remobilization of nutrients that, in turn affects grain and residual composition. The typical cycle of a crop, for example maize, can be divided into a vegetative phase from emergence to flowering and a grain filling phase until the grain is physiological mature. The senescence overlaps with grain filling. Finally, there is a phase of dry down in which the mature grain only loses moisture. In maize, we found that the functional SG is positively correlated with the duration of grain filling and N uptake after flowering and negatively correlated with the remobilization of C and N to the grain (unpublished data). Actually, the higher N uptake after flowering in late senescence genotypes is supported by other studies [65]. More time for active photosynthesis allows more time for the uptake of nutrients but less time to recycle the nutrients to the grain and the remobilization is less efficient. This tradeoff between uptake and remobilization is what some authors have named the “dilemma of senescence” [66,67]. However, Chen et al. [68] found that new maize hybrids not only take up more N but also remobilize more N due to a higher proportion of dry matter in leaves than in stalks at silking, which benefits both uptake and remobilization. In the new hybrids, the remobilization could also be favored by an increased efficiency of transferring source from cob and husk to grain [69]. Thus, the selection applied in private maize breeding could have overcome the senescence dilemma.
Regardless of the selection methodology, the improvement of the characteristics of senescence genotypes to obtain more valuable feedstocks for bioenergy and potentially more genetic gain depends on several factors:
- The specific characteristics of the crop, including if the crop is annual or perennial
- The part of the plant used for obtaining energy: the whole plant or only the residuals. For example, in cereals the grain can be used for animal feeding and the remaining parts (leaves, stalks and cobs), that are traditional seen as residuals, can be used for energy.
Maize is one of the most favorable crops for development of dual-purpose varieties (grain for feeding and residual for energy) because it produces a large amount of residuals. A late senescence supposes higher yield of residuals and grain but also higher moisture. The higher moisture of the grain is detrimental because it may cause problems during transport and storage. The higher moisture of grain can be due to a longer period of grain filling which is associated to delayed senescence but also to higher moisture of husks that could hinder the dry down of the grain. There is not direct information, as far as we know, about the relationship between the senescence of husks and the dry down of grain. However, much of the modern maize hybrids present a delayed senescence in all leaves, except the husks which dry faster that the remaining leaves. We found genetic variation for the synchrony of senescence of the husks and the remaining leaves: in some maize genotypes both husks and the remaining leaves age at the same rate but in some genotypes the husks dry faster than the remaining leaves (unpublished data). Interestingly, Arriola et al. [70] defined SG in maize as an asynchronous dry down of ear and stover, with the ear maturing faster than the stover. Also, an improvement of the dry down rate could allow the cultivation of genotypes with delayed senescence.
The optimum moisture of the residuals depends of the final use; for example, for biogas should be high while for combustion should be low. Biogas maize is generally ensiled which is the best-known preservation technique for biomass with high water content [71]. For ensiling, the optimum range of moisture should be about 60–70%: lower values will produce leachate and higher values predispose to seepages and clostridial fermentation [70]. Then, from the point of view of moisture, the late senescence could be a good option for dual purpose varieties of maize in which residuals are used for producing biogas and the grain is used for feeding animals with the restriction that the grain moisture should be maintained low. This could be a good option, for example, for the humid Atlantic regions of Europe. However, for dry regions as Mediterranean Europe, the residuals are probably collected too dry to be ensiled and a best choice is combustion to produce heat. In this case, the senescence should be adapted to reduce not only the moisture of the grain but also of the residuals. In the case of combustion, the residuals harvested with high water content are more expensive to store, transport and drying [72,73].
Regarding the effect of senescence on the quality of the residuals, the few results published so far showed a positive effect of a late senescence on digestibility [74,75] which is also favorable for biogas or ethanol production. However, this probably means a lower content of lignin and a reduction of the heat value of the feedstock which is the less valuable for combustion. In the case of residuals for combustion, an incomplete remobilization of N from residuals to grain could be particularly problematic because during the combustion the N leads to pollutant emissions of nitrogen oxides [76]. In addition to crop residuals, perennial grasses such as miscanthus has been proposed as a valuable option for bioenergy because they can grow with little or no fertilization and also on poor lands not adequate for crops [77]. In perennial grasses, the nutrients such as N, are remobilized during senescence from leaves and stems to underground organs and are used for re-growth in the following season [78]. Similar to maize, miscanthus show a strong correlation between senescence and moisture at harvest which determine the energetic use of the biomass [79]. In the case of biogas, Mangold et al. [71] have found that the SG genotype Sin55 of Miscanthus sinensis Anderson has better digestibility than a no SG genotype of Miscanthus xgiganteus Greef and Deuter ex Hodkinson andRenvoize (Mxg). However, Sin55, because of its late senescence that produces an incomplete remobilization of nutrients, had higher potassium than Mg and this element has an alkaline effect and producing poorer acidification and reduced silage quality [80]. In the case of combustion, the genotypes of miscanthus with late senescence are not adequate for this use because they have higher moisture at harvest and higher concentration of detrimental elements as N, P K and Cl [81].
One of the biofuels with highest potential to be used as an alternative to fossil fuels is biodiesel because is similar to petroleum diesel and could be used in conventional machines without modifications [82]. Oil crops, mainly palm (Elaeis spp.), rapeseed (Brassica napus L.) and soybeen (Gycine max (L.) Merr.), are widely used to obtain biodiesel [83]. Changing the lipid content and composition by phenotypic selection or transgenics can be useful for improving the biodiesel production in soybean [84,85] and rapeseed [86]. In these species, the timing or rate of senescence influences N uptake and remobilization [87,88,89] but the effect of senescence in lipid content and composition have not been reported. The changes in lipid composition during senescence is complex, for example, the function of most of the enzymes that degrade the lipids of the chloroplasts remains to be elucidated [90]. Also, although 68 putative lipases up-regulated at senescence has been annotated in Arabidopsis, the specific function of most of those genes remains elusive [91]. The relationship of senescence and agronomic traits in oil crops is similar to other crops, increasing both the yield but also the moisture. In this aspect, complications in the harvesting operations and a reduction in the quality of the grain due to a delayed senescence have being described in soybean [92].
5. Field High-Throughput Phenotyping for SG
Plant phenotyping (the quantitative description of physiological, biochemical and anatomical properties) is crucial to evaluate the effect of genetic attributes and their interaction with environmental issues [93]. It is especially interesting on critical traits, such as senescence (SG), the yield potential or tolerance to abiotic (concerning non-living factors such drought or nutrient deficiency) or biotic (including living factors such as weeds, insects, parasites, …) stresses [93,94]. Depending on the purpose of the phenotyping and the heritability of the trait, plant phenotyping may be implemented under either controlled conditions or field environments, yet the latter has proven to be more effective and easiest to extrapolate to the farmer conditions as well as more adequate in representing genotype by environment interaction [95]. In a direct or indirect way reliable high-throughput precision phenotyping (HTP) has a critical role to play in all the aspects of a complete and modern plant-breeding process [96,97] by:(i) improving selection intensity by increasing the size of the breeding program; (ii) enhancing the repeatability of the breeding program; (iii) guaranteeing and assessing adequate genetic variation; (iv) speeding up the breeding cycles and their evaluation; (v) enhancing decision support approaches.
Nowadays experts have drawn the attention to the need for new reliable, automatic, multifunctional and high-throughput phenotyping (HTP) platforms [94] (Table 2).Field-based plant phenotyping has become a bottleneck in crop breeding while advances in sensors development and information and communication technologies are envisaged as a de facto, affordable and non-destructive solution with great potential [96]. These technologies have addressed certain shortcomings of conventional plant phenotyping and substantially contribute to improving both capture and processing of large amounts of data for a great number of plant traits [96]. Furthermore, remotely and proximal sensed precision phenotyping fosters genetic gain. The potential of remote sensing to capture simultaneous and exhaustive data at the field trial level (with multiple measures at plant level), plays an interesting role in the estimation of heritability, being a turning point in relation to conventional techniques and thus contributing to genetic gain, as it decreases the noise generated by the effect of spatial heterogeneity inherent in the field trial (Table 3).

Table 2.
Currently used sensors onboard high-throughput precision phenotyping (HTP) platforms, advantages and limitations for potential uses on the stay green (SG) phenotyping.

Table 3.
Heritability measured with currently used remote sensing (RS) methods: ground-based, aerial platforms and sensor deployed across senescence and biomass related traits.
5.1. Current Platforms and Sensors in Field-High-Throughput Phenotyping
At the present time most of the high-throughput phenotyping (HTP) approaches are based on remote and proximal sensing technologies deployed on phenotyping platforms. Generally speaking, those platforms can be classified into ground-based or remote HTP platforms which can be equipped with multiple sensors enabled to capture and deliver manifold measures on key plant traits at successive growth stages in a rapid, precise and accurate way and both at leaf and canopy level (Table 2 and Table 3). The range of ground-based HTP devices embraces from handheld sensors (from spectroradiometers to smartphones, among a large series) to ground-wheeled sensors (phenomobiles). Interestingly, smartphones are evolving into a low-cost alternative in phenotyping, as they (i) can manage spectral images (carrying RGB and even thermal cameras); (ii) can manage a certain amount of data; (iii) have georeferencing functions; (iv) have connectivity via wi-fi and mobile data networks [98]. Ground-based platforms (proximal remote sensing) enable acquisition of highly accurate data, yet they show two limitations related to time and space since the working procedure is time-consuming and constrained to restricted operating areas. Alternatively, the use of HTP remote platforms (both satellite and airborne platforms) has been increasing significantly over the last decade thanks to their flexibility, effectivity and ability to collect crucial and simultaneous information with daily (or less) patterns. However, some constraints arise regarding remote sensing based on satellite imaging especially related to the significant cost and the lack of optimal spatial and temporal resolutions in the context of precision plant phenotyping. On the other hand, manned airborne platforms have limited use in breeding research due to high operating costs and operational complexity [94]. On its behalf, unmanned airborne systems (UAS) have proven that they can effectively supply optimal spectral, spatial and temporal resolutions as well as low-cost approaches [99]. The key advantages in the use of UAS have to do with the fact that they contribute to a quick (near real-time) and non-destructive acquisition of the phenotypic parameters of the crop under natural conditions. One of the most valuable assets of UAS is the capacity to measure with several sensors at the same time coupled with its flexibility and adaptability to endless spatial and temporal solutions. Some limitations in the use of UAS for field phenotyping are related to three main facts [94,98]:(i) the lack of integration of both UAS-flight and sensors acquisition control systems; (ii) current strict airspace regulations; (iii) remotely sensed data acquired through the UAS require considerable post-processing.
Regarding sensors technologies on board the UAS, the main restrictions have to do with UAS limited payload capacity. Accordingly, sensors on board UAS must consider light weight and small size parallel to high precision and low power consumption [94]. In light of the current state of the art, the most frequently deployed sensors onboard these platforms at this time [94,100] are mentioned in Table 2. It is also worth highlighting the advantage that represents the combined use of the sensors and platforms series described above, which provides countless (more or less) complex solutions with a wide range of potentialities to characterize crucial functional plant traits like senescence.
A selection of recent studies of accuracy parameters compiling previously described methods of sensing technologies is described in Table 3. The research shows that the combination of high-throughput phenotyping technologies (platforms + sensors + methods) currently available can be combined into a multitude of optimal approaches and provides insights on how approaches can be applied to address different targets in crop breeding programs. Approaches for a wide range of plant traits (morphological, physiological, phenological or ecological) are already been developed, evaluated and improved. We have used the heritability parameter as an indicator to compare across studies showing that both ground and aerial platforms combined with appropriate sensors and models have the potential to significantly contribute to the efficiency of breeding programs by increasing the accuracy of selection and subsequently the genetic gain [103]. However, in a broad sense we can assume that ground level platforms are mostly used as experimental solutions, almost always custom-designed and they are not considered to be the most suitable for implementing globally generalizable systems. While the best potentialities of the aerial platforms (with enhancements in the different resolutions while advancing in their miniaturization and costs) turns them into the most generalizable and popular alternatives [98] in crop breeding programs to increase both selection intensity (for example, by increasing population size) and selection accuracy (since improving the scale and the cost efficiency of phenotyping can allow enhanced selection intensity).
5.2. Current Models in Field-High-Throughput Phenotyping
By using different methodological models, field HTP based on the combination of previously described sensors and platforms shows optimal potential to adequately characterize both morphological (plant height, LAI, crop canopy cover, …) and physiological crop traits (chlorophyll content, biomass, pigment content, photosynthesis rate, …). In addition, it makes it possible to monitor nutrients levels (nitrogen, protein contents, …) or abiotic/biotic stress indicators as stomatal conductance, leaf water potential, canopy temperature or senescence rate (Stay-Green) [94]. Especially relevant is the fact that this characterization can be conducted in real-time and non-destructive way and at different growth stages of the crop cycle, for example at critical points previously established, due to the affordability of the technology (Table 3). Principally ground-based and UAS platforms can deliver data in near real-time of the entire field trial several times per day or along the whole growing cycle with massive amounts of data being generated for analysis and storage [93].
Specific phenotyping of SG has historically grounded on breeder’s experience but nowadays, as stated above, remote and proximal sensing technologies represent a great potential to estimate plant phenotyping senescence-parameters at the field-scale. Currently a clear target is to identify the SG phenotype besides to discriminate between cosmetic and functional Stay-Green inbred lines [114]. In a wide sense, the two main potential approaches for crop phenotyping (SG phenotyping) to process and model collected spectral datasets are related to: empirical statistic (which are site and species specific) or biophysical methods combined with machine learning (ML) (which generalize well as they are based on the modeling of the optical behavior of leaves and canopies). ML approaches give plant breeders the opportunity to discover patterns into large datasets by simultaneous analysis of combined variables. This is both crucial to analyze big data coming from the huge dimensionality of the image databases and key to model plant stress by connecting the dots between the holistic effect of genetic, agronomic, meteorological and anthropic factor on crop stress and, eventually, crop yield [93,96].
The study of the direct statistical relationship between ground-measured biochemical parameters and reflectance measures has the potential to achieve significant correlations but does not allow spatial or temporal predictive capabilities since estimations are constrained by species and canopy structure. As well, they always require field measurements corresponding to remote data collection for calibration and validation procedures [2,115]. Alternatively, biophysical methods rely on the modeling of leaf optical properties by characterizing the light interception by plant canopies (both absorption and scattering). Consequently, they are highly useful to precisely capture vegetation traits from remotely sensed data [116]. The key involved traits in those models are related to leaf biochemistry (primarily chlorophyll and moisture contents and dry matter) and canopy architecture (specifically leaf area index and leaf angle distribution). Biophysical methods, usually referred to as Radiative Transfer Models RTM, have the capacity to make use of the maximum potential of the available electromagnetic spectrum and implicitly minimize the model-calibration needs.
Empirical approaches are normally built up on the use of regression models between spectral indices and vegetation traits. Among the most widely used vegetation indices (VI) is the NDVI (Normalized Difference Vegetative Index) which is the normalized ratio between reflected light in the red and near-infrared data of the electromagnetic spectrum and is highly correlated to canopy greenness. For example, Christopher et al. [112] showed that the use of temporal NDVI measurements have great potential to identify high yielding stay-green phenotypes by consistent monitoring and analysis based on a logistic model. Building on this study, Rebetzke et al. [114] also modeled dynamic change in leaf greenness and canopy architecture (based on LIDAR measurements) to discriminate functional vs cosmetic stay-green. On the other hand, Berger et al. [115] evaluated the use of RTM biophysical models in the context of agricultural crop monitoring and specifically in maize crop. They have shown the great potential of these approaches in the retrieval of biophysical and biochemical variables from multi- and hyperspectral remotely sensed data and explicitly for chlorophyll content and Leaf Area Index.
6. Conclusions and Further Research
The timing or rate of senescence is a trait important for the adaptation of the crops to the specific environments. In that sense, it is not reasonable to define an ideotype with early or late senescence because the optimization of the senescence depends on the target environment where the crops are going to be cultivated. We have also to take into account that the environments are dynamic and change with time which raises concern about the future conditions for cultivation. In this regard, the traits related to adaptation, particularly flowering time and senescence, could have a pivotal role in hampering the expected reductions in crop yields due to climate change. A recent research conducted by Tardieu and collaborators showed that the predicted reduction in maize yield in Europe due to climate change can be reverted if the flowering date of the varieties is adapted to the new conditions [117].
In addition to the adaptation to the environment, the senescence serves also to adapt the crop to specific bioenergetics use. The better crop and use for a specific environment is not obvious and depends not only on the characteristics of the crop and environment but on social and historical components of the target region. The breeders working on varietal improvement must work in collaboration with scientists of other fields that give them precise indications of the favorable traits. For the improvement to be effective, more information about the senescence is needed, including genomic regions, genes and metabolic pathways, internal and external signals, transcription factors and so forth. Also more information about the environment and the genotype × environment interaction is important because it has never to be forgotten that the crops have to be cultivated in a particular environment. All this knowledge will allow the effective use of selection using molecular markers in genomic selection or molecular tools such as genome editing or recombination targeting. One of the main limitations for an effective breeding is the cost and labor needed for phenotyping many genotypes and for recording environmental variables. This is particularly true for a trait like senescence that needs to be recorded dynamically along the station. However, in the last decade technical advances in artificial intelligence, robotics and sensor technologies have brought about a new era in the use of spectral remote sensing tools which retrieve unprecedented temporal, high spatial and spectral resolutions [2,118]. Consequently, precision high-throughput image-based phenotyping has an enormous potential to quickly supply highly accurate measurements of plant-growth dynamics contributing to the development of predictive models on environmental responses [100]. Regardless of the algorithms and platforms customization, nowadays one of the key goals is to systematize the acquisition and processing of a huge amount of data sourced from crop images and auxiliary data [94,100], specifically in case of hyperspectral and LIDAR data.
Other trends and challenges have to do with the use of VI based on narrow-band hyperspectral imagery which is driving a redesign of the VI and will intense our ability to exploit the spectral information at leaf but particularly at canopy level. The potential in the use of bands in the range between 690–730 nm (red-edge) and 700–1100 nm (NIR, Near InfraRed) is being further explored but is specially the leverage of the range 1100–3000 nm (SWIR, Short Wave InfraRed) what deserves to be more widely studied [119]. Finally, another relevant and hardly developed challenge has to do with the combined use (and the holistic potential) of the different available resources and platforms which enable information on within-field spatial variation: from nanosatellites, to ground platforms going through UAS [118].
Author Contributions
Conceptualization, B.O. and E.D.M.; methodology, E.D.M., B.O., S.M. and A.K.; software, E.M. and S.M.; validation, E.D.M., B.O., S.M. and A.K.; investigation, E.D.M., B.O., S.M., A.K. and M.C.; resources, B.O.; data curation, E.D.M., B.O., S.M. and A.K.; writing—original draft preparation, E.D.M., B.O., S.M., A.K. and M.C.; writing—review and editing, E.D.M., B.O., S.M., A.K.; visualization, E.D.M., S.M. and A.K.; supervision, B.O.; project administration, B.O. and E.D.M.; funding acquisition, B.O. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this research was funded by the Spanish Plan for Research and Development (AGL2016-77628-R) and the European Regional Development Fund (FEDER).
Acknowledgments
Susana Martinez acknowledges the support from the State Research Agency of the Ministry of Economy, Industry and Competitiveness of the Government of Spain, which finances through the “Torres Quevedo” program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hörtensteiner, S.; Feller, U. Nitrogen metabolism and remobilization during senescence. J. Exp. Bot. 2002, 53, 927–937. [Google Scholar] [CrossRef]
- Aasen, H.; Honkavaara, E.; Lucieer, A.; Zarco-Tejada, J.P. Quantitative remote sensing at ultra-high resolution with UAV spectroscopy: A review of sensor technology, measurement procedures and data correction workflows. Remote Sens. 2018, 10, 1091. [Google Scholar] [CrossRef]
- Balazadeh, S. Stay-green not always stays green. Mol. Plant. 2014, 7, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Assotiates: Sunderland, MA, USA, 2015. [Google Scholar]
- Lim, P.O.; Nam, H.G. The molecular and genetic control of leaf senescence and longevity in Arabidopsis. Curr. Top. Dev. Biol. 2005, 67, 50–85. [Google Scholar]
- Schippers, J.H.; Schmidt, R.; Wagstaff, C.; Jing, H.-C. Living to die and dying to live: The survival strategy behind leaf senescence. Plant Physiol. 2015, 169, 914–930. [Google Scholar] [CrossRef]
- Schippers, J.H.M. Transcriptional networks in leaf senescence. Curr. Opin. Plant Biol. 2015, 27, 77–83. [Google Scholar] [CrossRef]
- Thomas, H. Senescence, ageing and death of the whole plant. New Phytol. 2013, 197, 696–711. [Google Scholar] [CrossRef]
- Jibran, R.; Hunter, D.A.; Dijkwel, P.P. Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol. Biol. 2013, 82, 547–561. [Google Scholar] [CrossRef]
- Jan, S.; Abbas, N.; Ashraf, M.; Ahmad, P. Roles of potential plant hormones and transcription factors in controlling leaf senescence and drought tolerance. Protoplasma 2019, 256, 313–329. [Google Scholar] [CrossRef]
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Bot. 2000, 51 (Suppl. 1), 329–337. [Google Scholar] [CrossRef]
- Peleg, Z.; Reguera, M.; Tumimbang, E.; Walia, H.; Blumwald, E. Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol. J. 2011, 9, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Reguera, M.; Peleg, Z.; Abdel-Tawab, Y.M.; Tumimbang, E.B.; Delatorre, C.A.; Blumwald, E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol. 2013, 163, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Guiboileau, A.; Sormani, R.; Meyer, C.; Masclaux-Daubresse, C. Senescence and death of plant organs: Nutrient recycling and developmental regulation. Comptes Rendus Biol. 2010, 333, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.-H.; Ma, X.; Luo, D.-X.; Gong, Z.-H.; Lu, M.-H. The plant heat stress transcription factors (HSFs): Structure, regulation and function in response to abiotic stresses. Front. Plant. Sci. 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Ougham, H. The stay-green trait. J. Exp. Bot. 2014, 65, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.R.; Aljadi, M.; Brewer, L. The importance of cosmetic Stay-Green in specialty crops. Plant Breed. Rev. 2018, 42, 219–256. [Google Scholar]
- Duvick, D.N. The contribution of breeding to yield advances in maize (Zea mays L.). Adv. Agron. 2005, 86, 83–145. [Google Scholar]
- Lee, E.; Tollenaar, M. Physiological basis of successful breeding strategies for maize grain yield. Crop Sci. 2007, 47 (Suppl. 3), S202–S215. [Google Scholar] [CrossRef]
- Robson, P.R.; Donnison, I.S.; Wang, K.; Frame, B.; Pegg, S.E.; Thomas, A.; Thomas, H. Leaf senescence is delayed in maize expressing the Agrobacterium IPT gene under the control of a novel maize senescence-enhanced promoter. Plant Biotechnol. J. 2004, 2, 101–112. [Google Scholar] [CrossRef]
- Joshi, S.; Choukimath, A.; Isenegger, D.; Panozzo, J.; Spangenberg, G.; Kant, S. Improved wheat growth and yield by delayed leaf senescence using developmentally regulated expression of a cytokinin biosynthesis gene. Front. Plant. Sci. 2019, 10, 1285. [Google Scholar] [CrossRef]
- Smidansky, E.D.; Meyer, F.D.; Blakeslee, B.; Weglarz, T.E.; Greene, T.W.; Giroux, M.J. Expression of a modified ADP-glucose pyrophosphorylase large subunit in wheat seeds stimulates photosynthesis and carbon metabolism. Planta 2007, 225, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.; Kim, S.-H.; Lee, B.-D.; An, G.; Sakuraba, Y.; Paek, N.-C. Rice transcription factor OsMYB102 delays leaf senescence by down-regulating abscisic acid accumulation and signaling. J. Exp. Bot. 2019, 70, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Lu, S.; Lv, B.; Zhang, B.; Shen, J.; He, J.; Luo, L.; Xi, D.; Chen, X.; Ming, F. A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiol. 2017, 174, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, M.; Ito, H.; Morita, R.; Iida, S.; Sato, Y.; Fujimoto, M.; Kawasaki, S.; Tanaka, R.; Hirochika, H.; Nishimura, M. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 2007, 19, 1362–1375. [Google Scholar] [CrossRef]
- Sato, Y.; Morita, R.; Katsuma, S.; Nishimura, M.; Tanaka, A.; Kusaba, M. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant J. 2009, 57, 120–131. [Google Scholar] [CrossRef]
- Armstead, I.; Donnison, I.; Aubry, S.; Harper, J.; Hörtensteiner, S.; James, C.; Mani, J.; Moffet, M.; Ougham, H.; Roberts, L. From crop to model to crop: Identifying the genetic basis of the staygreen mutation in the Lolium/Festuca forage and amenity grasses. New Phytol. 2006, 172, 592–597. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Park, S.-Y.; Kim, Y.-S.; Wang, S.-H.; Yoo, S.-C.; Hörtensteiner, S.; Paek, N.-C. Arabidopsis STAY-GREEN2 is a negative regulator of chlorophyll degradation during leaf senescence. Mol. Plant. 2014, 7, 1288–1302. [Google Scholar] [CrossRef]
- Jiang, H.; Li, M.; Liang, N.; Yan, H.; Wei, Y.; Xu, X.; Liu, J.; Xu, Z.; Chen, F.; Wu, G. Molecular cloning and function analysis of the stay green gene in rice. Plant J. 2007, 52, 197–209. [Google Scholar] [CrossRef]
- Hu, Z.-L.; Deng, L.; Yan, B.; Pan, Y.; Luo, M.; Chen, X.-Q.; Hu, T.-Z.; Chen, G.-P. Silencing of the LeSGR1 gene in tomato inhibits chlorophyll degradation and exhibits a stay-green phenotype. Biol. Plant. 2011, 55, 27–34. [Google Scholar] [CrossRef]
- Zhang, J.; Fengler, K.A.; Van Hemert, J.L.; Gupta, R.; Mongar, N.; Sun, J.; Allen, W.B.; Wang, Y.; Weers, B.; Mo, H. Identification and characterization of a novel stay-green QTL that increases yield in maize. Plant Biotechnol. J. 2019, 17, 2272–2285. [Google Scholar] [CrossRef]
- Jagadish, K.S.; Kishor, K.; Polavarapu, B.; Bahuguna, R.N.; von Wirén, N.; Sreenivasulu, N. Staying alive or going to die during terminal senescence-an enigma surrounding yield stability. Front. Plant. Sci. 2015, 6, 1070. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Burch, D.; Badenhorst, P.; Palanisamy, R.; Mason, J.; Spangenberg, G. Regulated expression of a cytokinin biosynthesis gene IPT delays leaf senescence and improves yield under rainfed and irrigated conditions in canola (Brassica napus L.). PLoS ONE 2015, 10, e0116349. [Google Scholar] [CrossRef]
- Gentinetta, E.; Ceppl, D.; Lepori, C.; Perico, G.; Motto, M.; Salamini, F. A major gene for delayed senescence in maize. Pattern of photosynthates accumulation and inheritance. Plant Breed. 1986, 97, 193–203. [Google Scholar] [CrossRef]
- Beavis, W.; Smith, O.; Grant, D.; Fincher, R. Identification of quantitative trait loci using a small sample of topcrossed and F4 progeny from maize. Crop Sci. 1994, 34, 882–896. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Zhang, N.; Wang, X.; Zhang, Y.; Ding, Y.; Kuai, B.; Huang, X. Mapping and validation of the quantitative trait loci for leaf stay-green-associated parameters in maize. Plant Breed. 2017, 136, 188–196. [Google Scholar] [CrossRef]
- Guei, R.; Wassom, C. Genetic analysis of tassel size and leaf senescence and their relationships with yield in two tropical lowland maize populations. Afr. Crop Sci. J. 1996, 4, 275–281. [Google Scholar]
- Câmara, T.M.M.; Bento, D.A.V.; Alves, G.F.; Santos, M.F.; Moreira, J.U.V.; Souza Júnior, C.L.D. Genetic parameters of drought tolerance related traits in tropical maize. Bragantia 2007, 66, 595–603. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, A.; Zheng, C.; Wang, Y.; Cai, R.; Shen, X.; Xu, R.; Liu, P.; Kong, L.; Dong, S. QTL mapping of maize (Zea mays) stay-green traits and their relationship to yield. Plant Breed. 2009, 128, 54–62. [Google Scholar] [CrossRef]
- Wang, A.-Y.; Li, Y.; Zhang, C.-Q. QTL mapping for stay-green in maize (Zea mays). Can. J. Plant Sci. 2012, 92, 249–256. [Google Scholar] [CrossRef]
- Belícuas, P.R.; Aguiar, A.M.; Bento, D.A.V.; Camara, T.M.M.; de Souza Junior, C.L. Inheritance of the stay-green trait in tropical maize. Euphytica 2014, 198, 163–173. [Google Scholar] [CrossRef]
- Almeida, G.D.; Nair, S.; Borém, A.; Cairns, J.; Trachsel, S.; Ribaut, J.-M.; Bänziger, M.; Prasanna, B.M.; Crossa, J.; Babu, R. Molecular mapping across three populations reveals a QTL hotspot region on chromosome 3 for secondary traits associated with drought tolerance in tropical maize. Mol. Breed. 2014, 34, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Kante, M.; Revilla, P.; De La Fuente, M.; Caicedo, M.; Ordás, B. Stay-green QTLs in temperate elite maize. Euphytica 2016, 207, 463–473. [Google Scholar] [CrossRef]
- Thomas, H. Sid: A Mendelian locus controlling thylakoid membrane disassembly in senescing leaves of Festuca pratensis. Theor. Appl. Genet. 1987, 73, 551–555. [Google Scholar] [CrossRef]
- Cha, K.-W.; Lee, Y.-J.; Koh, H.-J.; Lee, B.-M.; Nam, Y.-W.; Paek, N.-C. Isolation, characterization and mapping of the stay green mutant in rice. Theor. Appl. Genet. 2002, 104, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Guiamét, J.J.; Teeri, J.A.; Noodén, L.D. Effects of nuclear and cytoplasmic genes altering chlorophyll loss on gas exchange during monocarpic senescence in soybean. Plant Cell Physiol. 1990, 31, 1123–1130. [Google Scholar]
- Kumar, U.; Joshi, A.K.; Kumari, M.; Paliwal, R.; Kumar, S.; Röder, M.S. Identification of QTLs for stay green trait in wheat (Triticum aestivum L.) in the ‘Chirya 3’בSonalika’population. Euphytica 2010, 174, 437–445. [Google Scholar] [CrossRef]
- Tao, Y.; Henzell, R.; Jordan, D.; Butler, D.; Kelly, A.; McIntyre, C. Identification of genomic regions associated with stay green in sorghum by testing RILs in multiple environments. Theor. Appl. Genet. 2000, 100, 1225–1232. [Google Scholar] [CrossRef]
- Crasta, O.; Xu, W.; Rosenow, D.; Mullet, J.; Nguyen, H. Mapping of post-flowering drought resistance traits in grain sorghum: Association between QTLs influencing premature senescence and maturity. Mol. Gen. Genet. 1999, 262, 579–588. [Google Scholar] [CrossRef]
- Jiang, G.-H.; He, Y.-Q.; Xu, C.-G.; Li, X.-H.; Zhang, Q. The genetic basis of stay-green in rice analyzed in a population of doubled haploid lines derived from an indica by japonica cross. Theor. Appl. Genet. 2004, 108, 688–698. [Google Scholar] [CrossRef]
- Yoo, S.-C.; Cho, S.-H.; Zhang, H.; Paik, H.-C.; Lee, C.-H.; Li, J.; Yoo, J.-H.; Koh, H.-J.; Seo, H.S.; Paek, N.-C. Quantitative trait loci associated with functional stay-green SNU-SG1 in rice. Mol. Cells 2007, 24, 83–94. [Google Scholar]
- Yamamoto, T.; Suzuki, T.; Suzuki, K.; Adachi, S.; Sun, J.; Yano, M.; Ookawa, T.; Hirasawa, T. Characterization of a genomic region that maintains chlorophyll and nitrogen contents during ripening in a high-yielding stay-green rice cultivar. Field Crops Res. 2017, 206, 54–64. [Google Scholar] [CrossRef]
- Ramkumar, M.; Senthil Kumar, S.; Gaikwad, K.; Pandey, R.; Chinnusamy, V.; Singh, N.K.; Singh, A.K.; Mohapatra, T.; Sevanthi, A.M. A novel stay-green mutant of rice with delayed leaf senescence and better harvest index confers drought tolerance. Plants 2019, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Borrell, A.K.; Mullet, J.E.; George-Jaeggli, B.; van Oosterom, E.J.; Hammer, G.L.; Klein, P.E.; Jordan, D.R. Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth and water uptake. J. Exp. Bot. 2014, 65, 6251–6263. [Google Scholar] [CrossRef] [PubMed]
- Moreau, L.; Charcosset, A.; Gallais, A. Use of trial clustering to study QTL × environment effects for grain yield and related traits in maize. Theor. Appl. Genet. 2004, 110, 92–105. [Google Scholar] [CrossRef]
- Bogard, M.; Jourdan, M.; Allard, V.; Martre, P.; Perretant, M.R.; Ravel, C.; Heumez, E.; Orford, S.; Snape, J.; Griffiths, S. Anthesis date mainly explained correlations between post-anthesis leaf senescence, grain yield and grain protein concentration in a winter wheat population segregating for flowering time QTLs. J. Exp. Bot. 2011, 62, 3621–3636. [Google Scholar] [CrossRef]
- Kim, J.; Woo, H.R.; Nam, H.G. Towards systems understanding of leaf Senescence: An integrated multi-omics perspective on leaf senescence research. Mol. Plant. 2016, 9, 813–825. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Syaifullah, S.J.; Roitsch, T. Integration of multi-omics techniques and physiological phenotyping within a holistic phenomics approach to study senescence in model and crop plants. J. Exp. Bot. 2017, 69, 825–844. [Google Scholar] [CrossRef]
- Sade, N.; del Mar Rubio-Wilhelmi, M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2017, 69, 845–853. [Google Scholar] [CrossRef]
- Penfold, C.A.; Buchanan-Wollaston, V. Modelling transcriptional networks in leaf senescence. J. Exp. Bot. 2014, 65, 3859–3873. [Google Scholar] [CrossRef]
- Rodríguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering quantitative trait variation for crop improvement by genome editing. Cell 2017, 171, 470–480.e8. [Google Scholar] [CrossRef]
- Gregersen, P.L. Senescence and Nutrient Remobilization in Crop Plants. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops; Wiley Online Library: Hoboken, NJ, USA, 2011; pp. 83–102. [Google Scholar]
- Bernardo, R. Bandwagons I, too, have known. Theor. Appl. Genet. 2016, 129, 2323. [Google Scholar] [CrossRef] [PubMed]
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; de los Campos, G.; Burgueño, J.; González-Camacho, J.M.; Pérez-Elizalde, S.; Beyene, Y.; et al. Genomic selection in plant Breeding: Methods, models and perspectives. Trends Plant Sci. 2017, 22, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Rajcan, I.; Tollenaar, M. Source:sink ratio and leaf senescence in maize: II. Nitrogen metabolism during grain filling. Field Crops Res. 1999, 60, 255–265. [Google Scholar] [CrossRef]
- Gregersen, P.; Holm, P.; Krupinska, K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008, 10, 37–49. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Chen, K.; Kumudini, S.V.; Tollenaar, M.; Vyn, T.J. Plant biomass and nitrogen partitioning changes between silking and maturity in newer versus older maize hybrids. Field Crops Res. 2015, 183, 315–328. [Google Scholar] [CrossRef]
- Chen, K.; Camberato, J.J.; Tuinstra, M.R.; Kumudini, S.V.; Tollenaar, M.; Vyn, T.J. Genetic improvement in density and nitrogen stress tolerance traits over 38 years of commercial maize hybrid release. Field Crops Res. 2016, 196, 438–451. [Google Scholar] [CrossRef]
- Arriola, K.G.; Kim, S.C.; Huisden, C.M.; Adesogan, A.T. Stay-green ranking and maturity of corn hybrids: 1. Effects on dry matter yield, nutritional value, fermentation characteristics and aerobic stability of silage hybrids in Florida. J. Dairy Sci. 2012, 95, 964–974. [Google Scholar] [CrossRef]
- Mangold, A.; Lewandowski, I.; Hartung, J.; Kiesel, A. Miscanthus for biogas production: Influence of harvest date and ensiling on digestibility and methane hectare yield. GCB Bioenergy 2019, 11, 50–62. [Google Scholar] [CrossRef]
- Hoskinson, R.L.; Karlen, D.L.; Birrell, S.J.; Radtke, C.W.; Wilhelm, W.W. Engineering, nutrient removal and feedstock conversion evaluations of four corn stover harvest scenarios. Biomass Bioenergy 2007, 31, 126–136. [Google Scholar] [CrossRef]
- Fagernäs, L.; Brammer, J.; Wilén, C.; Lauer, M.; Verhoeff, F. Drying of biomass for second generation synfuel production. Biomass Bioenergy 2010, 34, 1267–1277. [Google Scholar] [CrossRef]
- Hartmann, A.; Presterl, T.; Geiger, H.H. Determination of the optimal harvest date of silage maize with low and fast stover ripening. Landbauforsch. Völkenrode Sonderh. 2000, 217, 86–93. [Google Scholar]
- Schlagheck, A.; Entrup, N.L.; Freitag, M. Effect of the ripening character (“Stay Green”/“Dry Down”) on the in vitro digestibility of maize genotypes with regard to different parts of the maize plant. Landbauforschung Völkenrode, Sonderheft 2000, 217, 94–101. [Google Scholar]
- Serrano, C.; Monedero, E.; Portero, H.; Jiménez, E.; Ordás, B. Efficient biofuel production from traditional maize under low input. Agron. Sustain. Dev. 2014, 34, 561–567. [Google Scholar] [CrossRef]
- Hudiburg, T.W.; Davis, S.C.; Parton, W.; Delucia, E.H. Bioenergy crop greenhouse gas mitigation potential under a range of management practices. GCB Bioenergy 2015, 7, 366–374. [Google Scholar] [CrossRef]
- Yang, J.; Udvardi, M. Senescence and nitrogen use efficiency in perennial grasses for forage and biofuel production. J. Exp. Bot. 2017, 69, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.; Mos, M.; Clifton-Brown, J.; Donnison, I. Phenotypic variation in senescence in Miscanthus: Towards optimising biomass quality and quantity. Bioenergy Res. 2012, 5, 95–105. [Google Scholar] [CrossRef]
- Mangold, A.; Lewandowski, I.; Möhring, J.; Clifton-Brown, J.; Krzyżak, J.; Mos, M.; Pogrzeba, M.; Kiesel, A. Harvest date and leaf:stem ratio determine methane hectare yield of miscanthus biomass. GCB Bioenergy 2019, 11, 21–33. [Google Scholar] [CrossRef]
- Jensen, E.; Robson, P.; Farrar, K.; Thomas Jones, S.; Clifton-Brown, J.; Payne, R.; Donnison, I. Towards Miscanthus combustion quality improvement: The role of flowering and senescence. GCB Bioenergy 2017, 9, 891–908. [Google Scholar] [CrossRef]
- Salehi Jouzani, G.; Sharafi, R.; Soheilivand, S. Fueling the future; plant genetic engineering for sustainable biodiesel production. Biofuel Res. J. 2018, 5, 829–845. [Google Scholar] [CrossRef]
- Bos, H.L.; Meesters, K.P.; Conijn, S.G.; Corré, W.J.; Patel, M.K. Comparing biobased products from oil crops versus sugar crops with regard to non-renewable energy use, GHG emissions and land use. Ind. Crops Prod. 2016, 84, 366–374. [Google Scholar] [CrossRef]
- Kinney, A.; Clemente, T. Modifying soybean oil for enhanced performance in biodiesel blends. Fuel Process. Technol. 2005, 86, 1137–1147. [Google Scholar] [CrossRef]
- Woyann, L.G.; Meira, D.; Zdziarski, A.D.; Matei, G.; Milioli, A.S.; Rosa, A.C.; Madella, L.A.; Benin, G. Multiple-trait selection of soybean for biodiesel production in Brazil. Ind. Crops Prod. 2019, 140, 111721. [Google Scholar] [CrossRef]
- Nath, U.K.; Kim, H.-T.; Khatun, K.; Park, J.-I.; Kang, K.-K.; Nou, I.-S. Modification of fatty acid profiles of rapeseed (Brassica napus L.) oil for using as food, industrial feed-stock and biodiesel. Plant Breed. Biotech. 2016, 4, 123–134. [Google Scholar] [CrossRef]
- Kumudini, S.; Hume, D.; Chu, G. Genetic improvement in short-season soybeans. Crop Sci. 2002, 42, 141–145. [Google Scholar] [CrossRef]
- Kumudini, S.; Hume, D.J.; Chu, G. Genetic improvement in short season soybeans. Crop Sci. 2001, 41, 391–398. [Google Scholar] [CrossRef]
- Koeslin-Findeklee, F.; Horst, W. Contribution of nitrogen uptake and retranslocation during reproductive growth to the nitrogen efficiency of winter oilseed-rape cultivars (Brassica napus L.) differing in leaf senescence. Agronomy 2016, 6, 1. [Google Scholar] [CrossRef]
- Wang, K.; Froehlich, J.E.; Zienkiewicz, A.; Hersh, H.L.; Benning, C. A plastid phosphatidylglycerol lipase contributes to the export of acyl groups from plastids for seed oil biosynthesis. Plant Cell 2017, 29, 1678–1696. [Google Scholar] [CrossRef]
- Troncoso-Ponce, M.A.; Cao, X.; Yang, Z.; Ohlrogge, J.B. Lipid turnover during senescence. Plant Sci. 2013, 205, 13–19. [Google Scholar] [CrossRef]
- Harbach, C.; Allen, T.; Bowen, C.; Davis, J.; Hill, C.; Leitman, M.; Leonard, B.; Mueller, D.; Padgett, G.; Phillips, X. Delayed senescence in soybean: Terminology, research update and survey results from growers. Plant Health Prog. 2016, 17, 76–83. [Google Scholar] [CrossRef]
- Singh, A.; Ganapathysubramanian, B.; Singh, A.K.; Sarkar, S. Machine Learning for High-Throughput Stress Phenotyping in Plants. Trends Plant Sci. 2016, 21, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y.; Yu, H.; Xu, B.; Yang, X.; Zhu, D.; Zhang, X.; et al. Unmanned Aerial Vehicle Remote Sensing for Field-Based Crop Phenotyping: Current Status and Perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Kefauver, S.C. Breeding to adapt agriculture to climate change: Affordable phenotyping solutions. Curr. Opin. Plant Biol. 2018, 45, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, P.; Zou, C.; Lu, Y.; Xie, C.; Zhang, X.; Prasanna, B.M.; Olsen, M.S. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating high-throughput phenotyping into genetic gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, F.; Kirchgessner, N.; Schneider, D.; Walter, A.; Hund, A. Remote, aerial phenotyping of maize traits with a mobile multi-sensor approach. Plant Methods 2015, 11, 9. [Google Scholar] [CrossRef]
- Fahlgren, N.; Gehan, M.A.; Baxter, I. Lights, camera, action: High-throughput plant phenotyping is ready for a close-up. Curr. Opin. Plant Biol. 2015, 24, 93–99. [Google Scholar] [CrossRef]
- Câmara, T. Mapeamento de QTLs de caracteres relacionados à tolerância ao estresse hídrico em milho tropical. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2006. [Google Scholar]
- Caicedo Villafuerte, M. Mejora genética de maíz para senescencia retrasada “Stay Green”. Ph.D. Thesis, Universidad de Santigo de Compostela, Santiago de Compostela, Spain, 2018. [Google Scholar]
- Gracia-Romero, A.; Kefauver, C.S.; Fernandez-Gallego, A.J.; Vergara-Díaz, O.; Nieto-Taladriz, T.M.; Araus, L.J. UAV and Ground Image-Based Phenotyping: A Proof of Concept with Durum Wheat. Remote Sens. 2019, 11, 1244. [Google Scholar] [CrossRef]
- Makanza, R.; Zaman-Allah, M.; Cairns, E.J.; Magorokosho, C.; Tarekegne, A.; Olsen, M.; Prasanna, M.B. High-Throughput Phenotyping of Canopy Cover and Senescence in Maize Field Trials Using Aerial Digital Canopy Imaging. Remote Sens. 2018, 10, 330. [Google Scholar] [CrossRef]
- Deery, D.M.; Rebetzke, G.J.; Jimenez-Berni, J.A.; James, R.A.; Condon, A.G.; Bovill, W.D.; Hutchinson, P.; Scarrow, J.; Davy, R.; Furbank, R.T. Methodology for High-Throughput Field Phenotyping of Canopy Temperature Using Airborne Thermography. Front. Plant. Sci. 2016, 7, 1808. [Google Scholar] [CrossRef] [PubMed]
- Cerrudo, D.; González Pérez, L.; Mendoza Lugo, A.J.; Trachsel, S. Stay-Green and Associated Vegetative Indices to Breed Maize Adapted to Heat and Combined Heat-Drought Stresses. Remote Sens. 2017, 9, 235. [Google Scholar] [CrossRef]
- Sagan, V.; Maimaitijiang, M.; Sidike, P.; Eblimit, K.; Peterson, K.T.; Hartling, S.; Esposito, F.; Khanal, K.; Newcomb, M.; Pauli, D. Uav-based high resolution thermal imaging for vegetation monitoring and plant phenotyping using ici 8640 p, flir vue pro r 640 and thermomap cameras. Remote Sens. 2019, 11, 330. [Google Scholar] [CrossRef]
- Condorelli, G.E.; Maccaferri, M.; Newcomb, M.; Andrade-Sanchez, P.; White, J.W.; French, A.N.; Sciara, G.; Ward, R.; Tuberosa, R. Comparative Aerial and Ground Based High Throughput Phenotyping for the Genetic Dissection of NDVI as a Proxy for Drought Adaptive Traits in Durum Wheat. Front. Plant. Sci. 2018, 9, 893. [Google Scholar] [CrossRef]
- Dodig, D.; Božinović, S.; Nikolić, A.; Zorić, M.; Vančetović, J.; Ignjatović-Micić, D.; Delić, N.; Weigelt-Fischer, K.; Junker, A.; Altmann, T. Image-Derived Traits Related to Mid-Season Growth Performance of Maize Under Nitrogen and Water Stress. Front. Plant. Sci. 2019, 10, 814. [Google Scholar] [CrossRef]
- Makanza, R.; Zaman-Allah, M.; Cairns, J.E.; Eyre, J.; Burgueño, J.; Pacheco, Á.; Diepenbrock, C.; Magorokosho, C.; Tarekegne, A.; Olsen, M.; et al. High-throughput method for ear phenotyping and kernel weight estimation in maize using ear digital imaging. Plant Methods 2018, 14, 49. [Google Scholar] [CrossRef]
- Miller, N.D.; Haase, N.J.; Lee, J.; Kaeppler, S.M.; de Leon, N.; Spalding, E.P. A robust, high-throughput method for computing maize ear, cob and kernel attributes automatically from images. Plant J. 2017, 89, 169–178. [Google Scholar] [CrossRef]
- Christopher, J.T.; Veyradier, M.; Borrell, A.K.; Harvey, G.; Fletcher, S.; Chenu, K. Phenotyping novel stay-green traits to capture genetic variation in senescence dynamics. Funct. Plant Biol. 2014, 41, 1035–1048. [Google Scholar] [CrossRef]
- Salas Fernandez, M.G.; Bao, Y.; Tang, L.; Schnable, P.S. A high-throughput, field-based phenotyping technology for tall biomass crops. Plant Physiol. 2017, 174, 2008. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Jimenez-Berni, J.A.; Bovill, W.D.; Deery, D.M.; James, R.A. High-throughput phenotyping technologies allow accurate selection of stay-green. J. Exp. Bot. 2016, 67, 4919–4924. [Google Scholar] [CrossRef]
- Berger, K.; Atzberger, C.; Danner, M.; D’Urso, G.; Mauser, W.; Vuolo, F.; Hank, T. Evaluation of the PROSAIL model capabilities for future hyperspectral model environments: A Review Study. Remote Sens. 2018, 10, 85. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT + SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Parent, B.; Leclere, M.; Lacube, S.; Semenov, M.A.; Welcker, C.; Martre, P.; Tardieu, F. Maize yields over Europe may increase in spite of climate change, with an appropriate use of the genetic variability of flowering time. Proc. Natl. Acad. Sci. USA 2018, 115, 10642–10647. [Google Scholar] [CrossRef] [PubMed]
- Maes, W.H.; Steppe, K. Perspectives for remote sensing with unmanned aerial vehicles in precision agriculture. Trends Plant Sci. 2019, 24, 152–164. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of spectral remote sensing for agronomic decisions. Agron. J. 2008, 100, S117–S131. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).