Application Study on a New Hybrid Canning Structure of After-Treatment System for Diesel Engine
Abstract
1. Introduction
2. Research Plan
2.1. Proposed New Mixer and Canning Design
2.2. Simulation Analysis
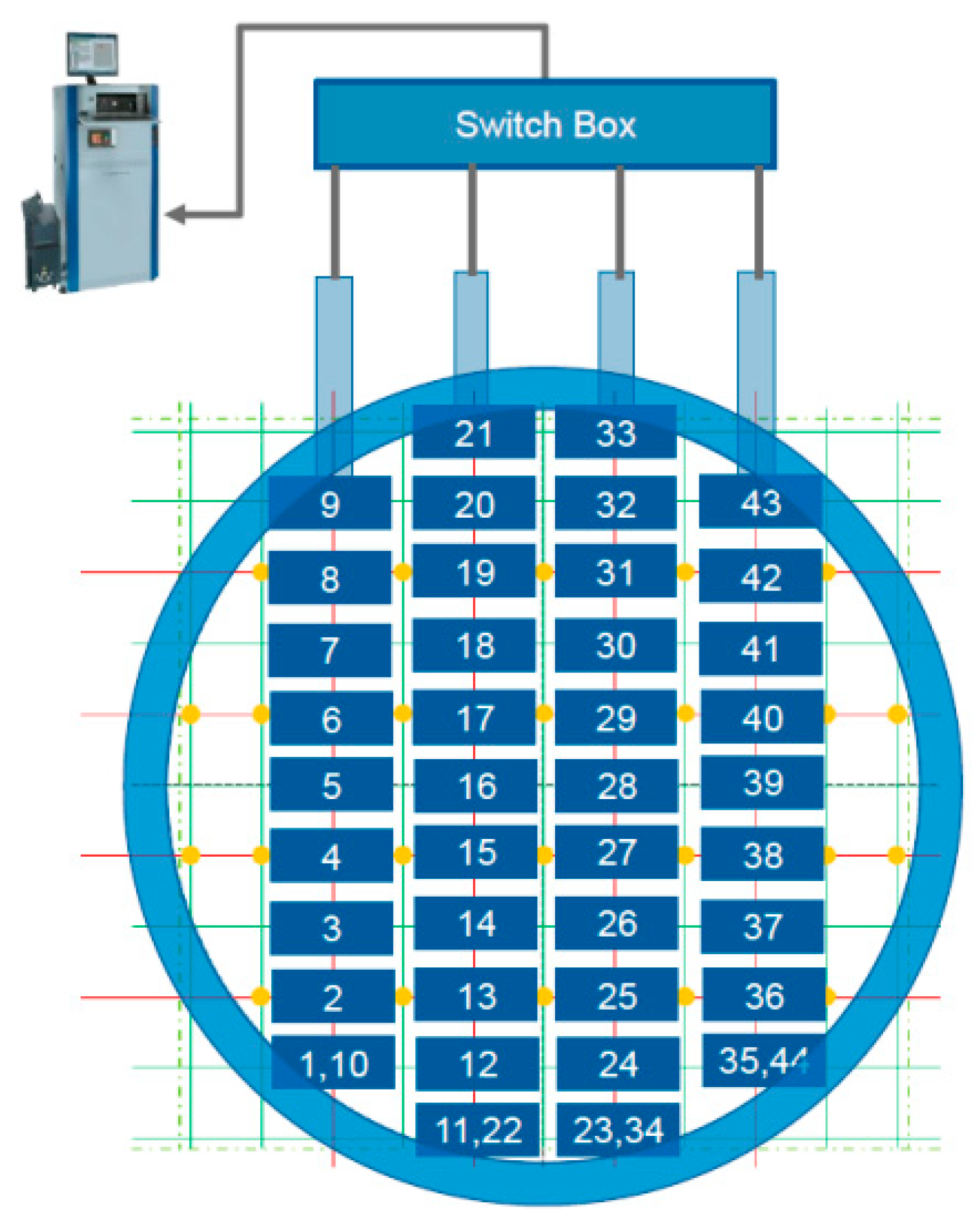
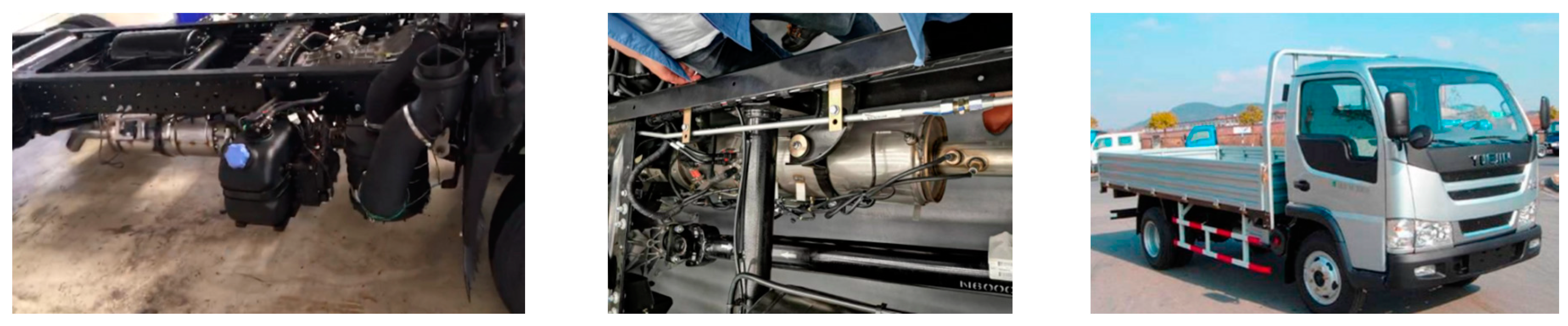
2.3. Experimental Validation
2.3.1. Engine Bench Test
2.3.2. Fleet Test
3. Simulation Results and Discussion
3.1. Simulation on the Pathline (or Trajectory) of Flow Field
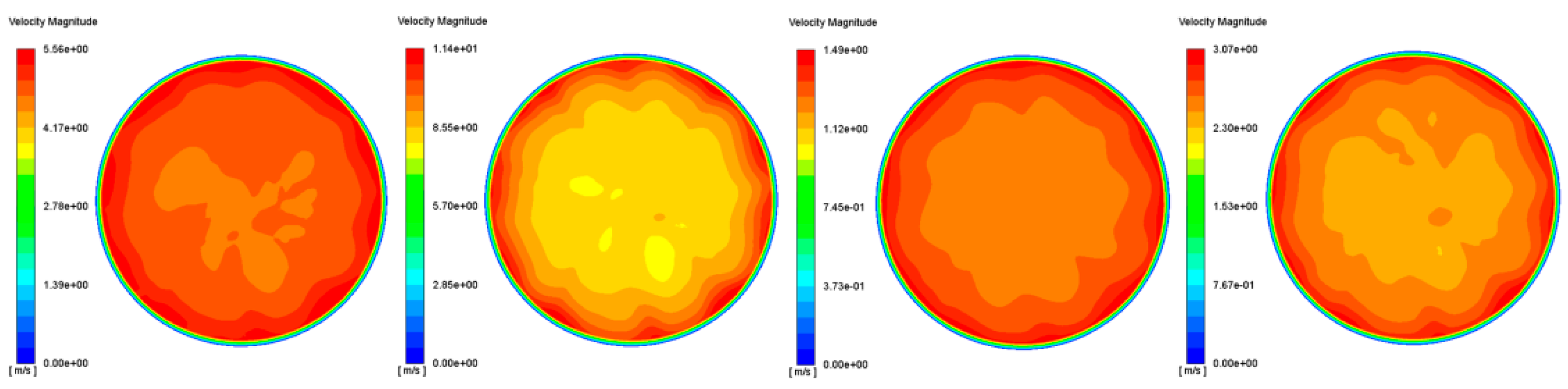
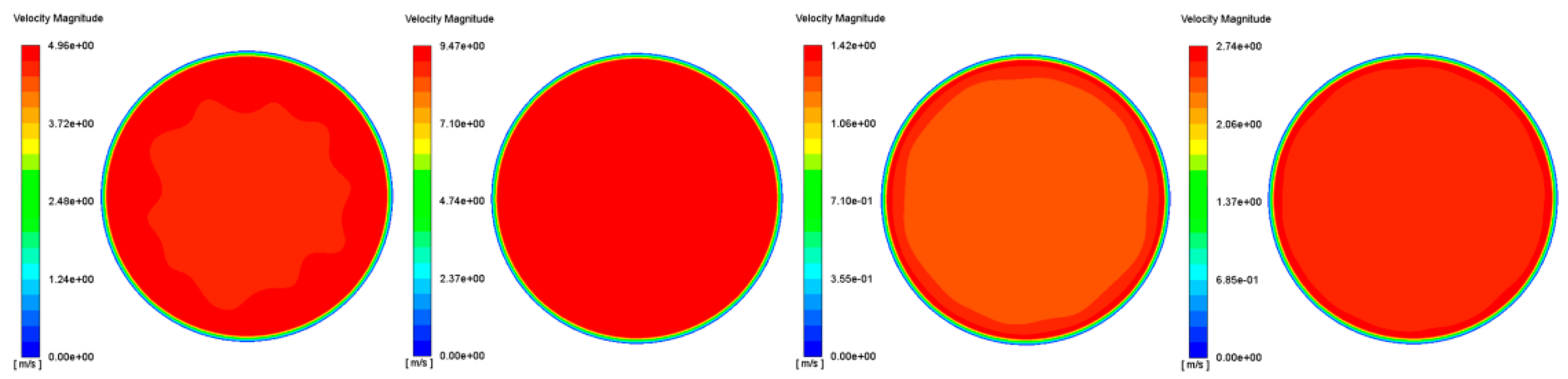
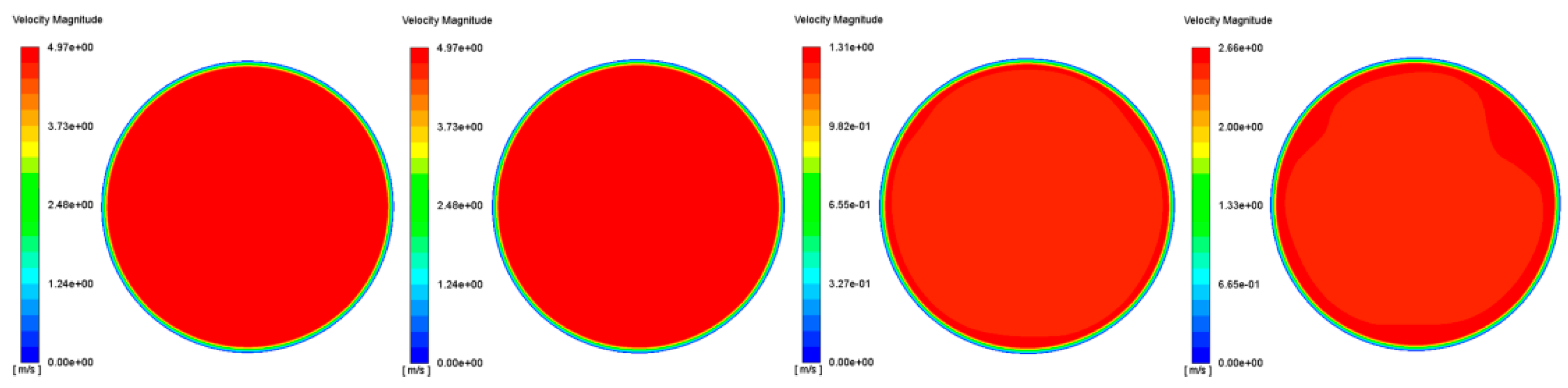
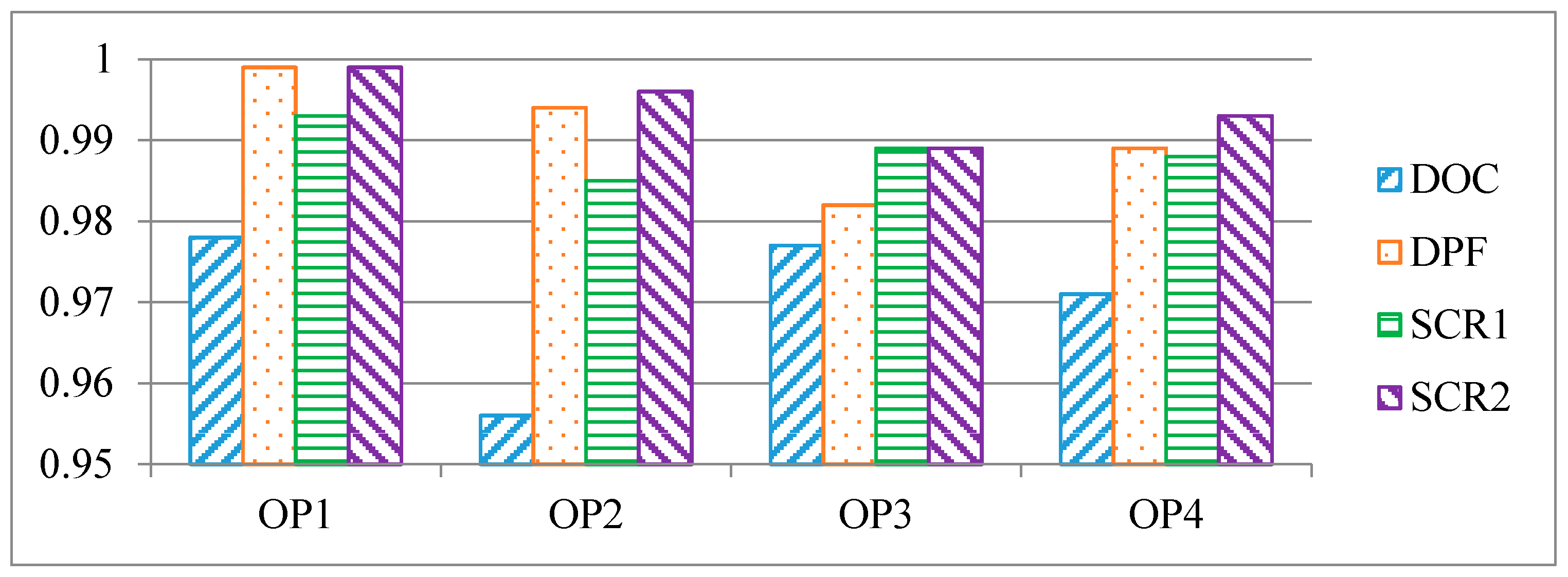
3.2. Uniformity Index
3.2.1. Velocity Uniformity Index
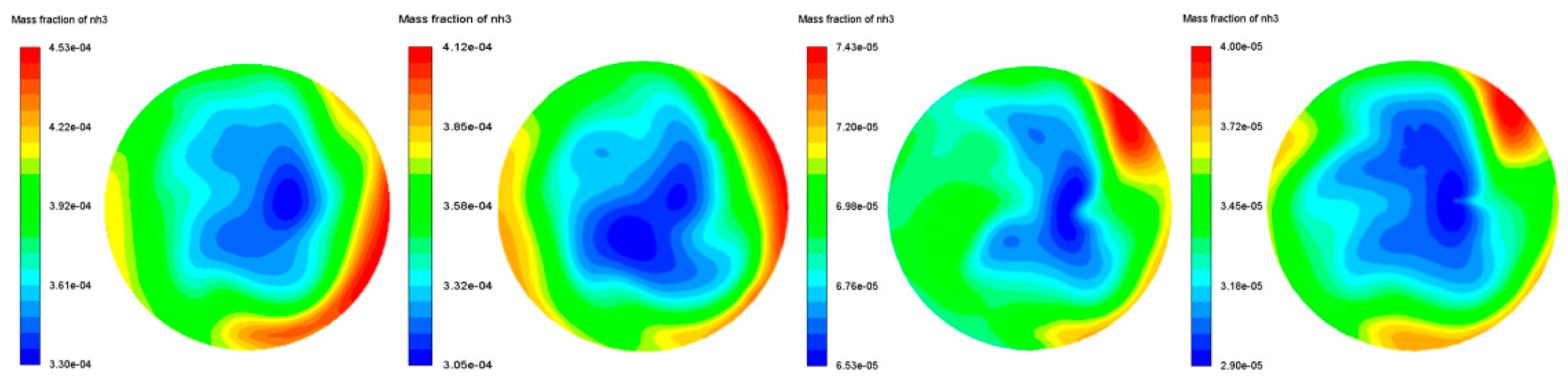
3.2.2. Ammonia Uniformity Index
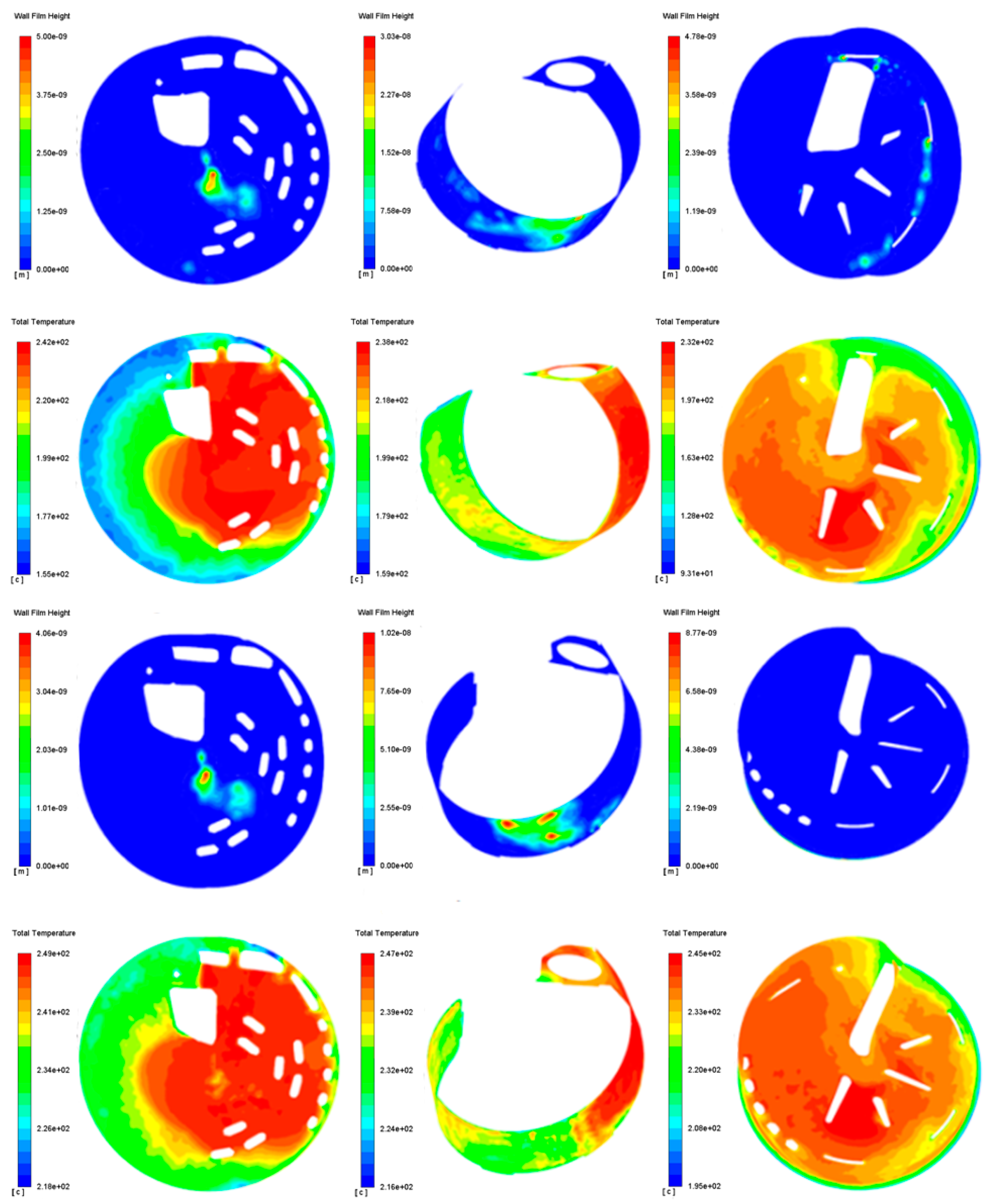
3.3. Crystallization Risk Analysis
4. Test Results and Discussion
4.1. Engine Bench Test
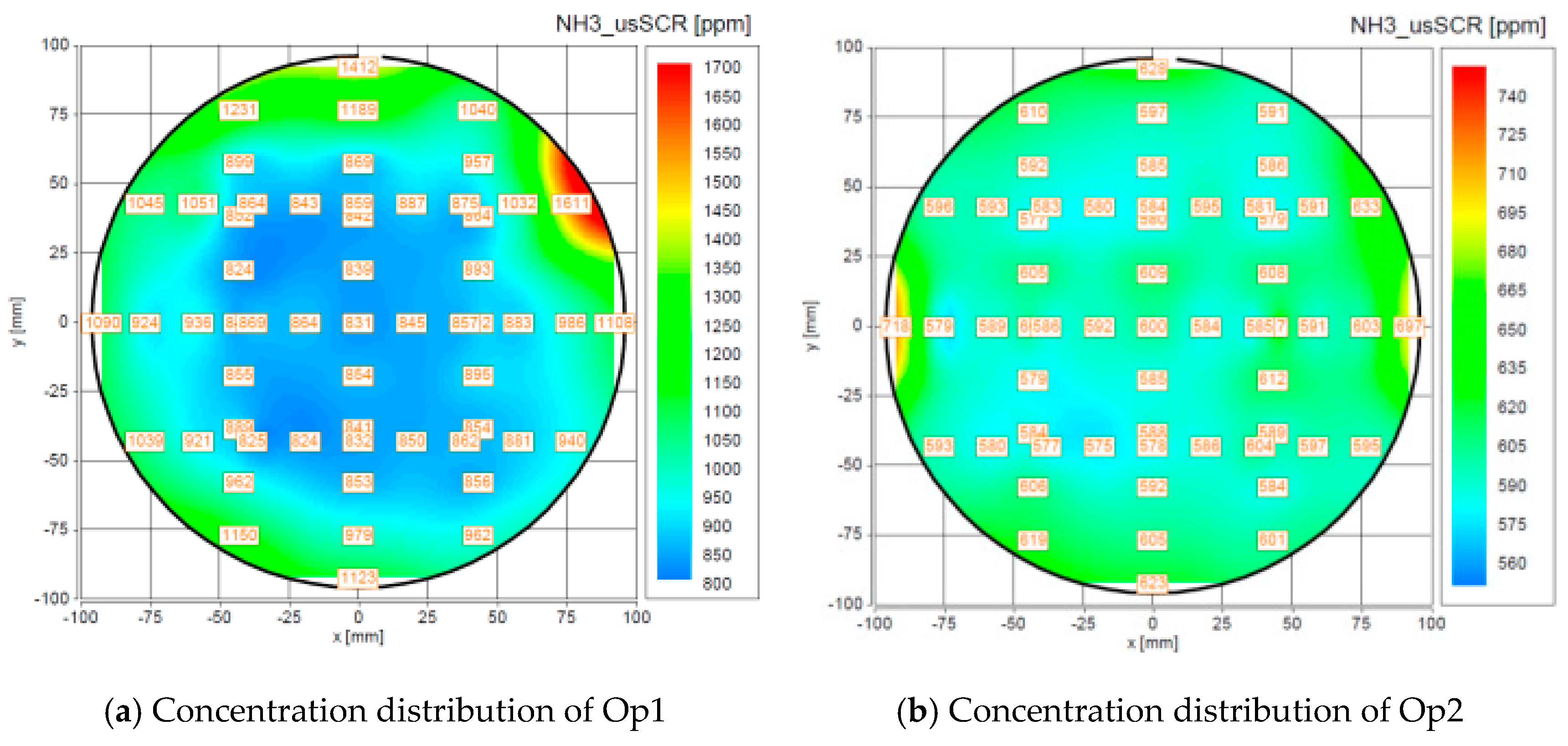
4.1.1. Ammonia Uniformity Test
4.1.2. Risk Assessment of Crystallization
4.1.3. Conversion Efficiency Test
4.2. Vehicle Road Test
5. Conclusions
- (1)
- The package structure provides sufficient flow uniformity on all catalytic modules (DOC, DPF, and SCR), and its speed uniformity is higher than 0.95.
- (2)
- The structure of the mixer has excellent ammonia concentration and gradient uniformity. The average ammonia uniformity value is 0.96, and the ammonia distribution gradient does not exceed 1.318, which is very important to improve the efficiency of the chemical reaction.
- (3)
- The mixer has excellent anti-crystallization performance, and the crystallization amount after regeneration is 0 g, which has been proved by bench tests and strict road verification.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Lou, D.; Tan, P.; Hu, Z. Experimental study on the durability of biodiesel-powered engine equipped with a diesel oxidation catalyst and a selective catalytic reduction system. Energy 2018, 159, 1024–1034. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, D.; Tan, P.; Hu, Z. Experimental study on the particulate matter and nitrogenous compounds from diesel engine retrofitted with DOC + CDPF + SCR. Atmos. Environ. 2018, 177, 45–53. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, C.; Wu, M.; Lu, K. Optimisation design of SCR mixer for improving deposit performance at low temperatures. Fuel 2019, 237, 465–474. [Google Scholar] [CrossRef]
- Choi, C.; Sung, Y.; Choi, G.M.; Kim, D.J. Numerical analysis of NOx reduction for compact design in marine urea-SCR system. Int. J. Nav. Archit. Ocean Eng. 2015, 7, 1020–1033. [Google Scholar] [CrossRef]
- Zhang, X.; Romzek, M. 3-D Numerical Study of Flow Mixing in Front of SCR for Different Injection Systems; No. 2007-01-1578; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- Zheng, G.; Palmer, G.; Salanta, G.; Kotrba, A. Mixer Development for Urea SCR Applications; No. 2009-01-2879; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2009. [Google Scholar]
- Seo, J.W.; Lee, K.I.; Oh, J.T.; Choi, Y.H.; Lee, J.H.; Park, J.I. The study on the effects of mixer configurations on fluid mixing characteristics in SCR systems. Trans. Korean Soc. Automot. Eng. 2008, 16, 192–199. [Google Scholar]
- Choi, C.; Sung, Y.; Choi, G.M.; Kim, D.J. Numerical analysis of urea decomposition with static mixers in marine SCR system. J. Clean Energy Technol. 2015, 3, 39–42. [Google Scholar] [CrossRef]
- Kusaka, J.; Sueoka, M.; Takada, K.; Ohga, Y.; Nagasaki, T.; Daisho, Y. A basic study on a urea-selective catalytic reduction system for a medium-duty diesel engine. Int. J. Engine Res. 2005, 6, 11–19. [Google Scholar] [CrossRef]
- Eichelbaum, M.; Farrauto, R.J.; Castaldi, M.J. The impact of urea on the performance of metal exchanged zeolites for the selective catalytic reduction of NOx: Part I. Pyrolysis and hydrolysis of urea over zeolite catalysts. Appl. Catal. B Environ. 2010, 97, 90–97. [Google Scholar] [CrossRef]
- Kuhnke, D. Spray/Wall Interaction Modelling by Dimensionless Data Analysis; Shaker: Düren, Germany, 2004. [Google Scholar]
- Wang, T.J.; Baek, S.W.; Lee, S.Y.; Kang, D.H.; Yeo, G.K. Experimental investigation on evaporation of urea-water-solution droplet for SCR applications. AIChE J. 2009, 55, 3267–3276. [Google Scholar] [CrossRef]
- Zheng, G.; Fila, A.; Kotrba, A.; Floyd, R.A. Investigation of Urea Deposits in Urea SCR Systems for Medium and Heavy Duty Trucks; No. 2010-01-1941; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2010. [Google Scholar]
- Oh, J.; Lee, K. Spray characteristics of a urea solution injector and optimal mixer location to improve droplet uniformity and NOx conversion efficiency for selective catalytic reduction. Fuel 2014, 119, 90–97. [Google Scholar] [CrossRef]
- Schmid, J.; Zarikos, I.; Terzis, A.; Roth, N.; Weigand, B. Crystallization of urea from an evaporative aqueous solution sessile droplet at sub-boiling temperatures and surfaces with different wettability. Exp. Therm. Fluid Sci. 2018, 91, 80–88. [Google Scholar] [CrossRef]
- Birkhold, F.; Meingast, U.; Wassermann, P.; Deutschmann, O. Modeling and simulation of the injection of urea-water-solution for automotive SCR DeNOx-systems. Appl. Catal. B Environ. 2007, 70, 119–127. [Google Scholar] [CrossRef]
- Birkhold, F.; Meingast, U.; Wassermann, P.; Deutschmann, O. Analysis of the Injection of Urea-Water-Solution for Automotive SCR DeNOx-Systems: Modeling of Two-Phase Flow and Spray/Wall-Interaction; No. 2006-01-0643; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2006. [Google Scholar]
- Liao, Y.; Eggenschwiler, P.D.; Furrer, R.; Wang, M.; Boulouchos, K. Heat transfer characteristics of urea-water spray impingement on hot surfaces. Int. J. Heat Mass Transf. 2018, 117, 447–457. [Google Scholar] [CrossRef]
- Wanker, R.; Raupenstrauch, H.; Staudinger, G. A fully distributed model for the simulation of a catalytic combustor. Chem. Eng. Sci. 2000, 55, 4709–4718. [Google Scholar] [CrossRef]
- Park, T.; Sung, Y.; Kim, T.; Lee, I.; Choi, G.; Kim, D. Effect of static mixer geometry on flow mixing and pressure drop in marine SCR applications. Int. J. Nav. Archit. Ocean Eng. 2014, 6, 27–38. [Google Scholar] [CrossRef]
- Tan, L.; Feng, P.; Yang, S.; Guo, Y.; Liu, S.; Li, Z. CFD studies on effects of SCR mixers on the performance of urea conversion and mixing of the reducing agent. Chem. Eng. Process. Process Intensif. 2018, 123, 82–88. [Google Scholar] [CrossRef]
- Capetillo, A.; Ibarra, F. Multiphase injector modelling for automotive SCR systems: A full factorial design of experiment and optimization. Comput. Math. Appl. 2017, 74, 188–200. [Google Scholar] [CrossRef]
- Ström, H.; Lundström, A.; Andersson, B. Choice of urea-spray models in CFD simulations of urea-SCR systems. Chem. Eng. J. 2009, 150, 69–82. [Google Scholar] [CrossRef]
- Lockyer, T.O.; Reid, B.A.; Hargrave, G.K.; Gaynor, P.; Wilson, J. Optical Investigation on the Ability of a Cordierite Substrate Mixing Device to Combat Deposits in SCR Dosing Systems; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2015. [Google Scholar]
- Smith, H.; Lauer, T.; Schimik, V.; Gabel, K. Evaluation and prediction of deposit severity in SCR systems. SAE Int. J. Engines 2016, 9, 1735–1750. [Google Scholar] [CrossRef]
- Shilimkan, R.V.; Stepanek, J.B. Effect of tube size on liquid side mass transfer in co-current gas-liquid upward flow. Chem. Eng. Sci. 1977, 32, 1397–1400. [Google Scholar] [CrossRef]
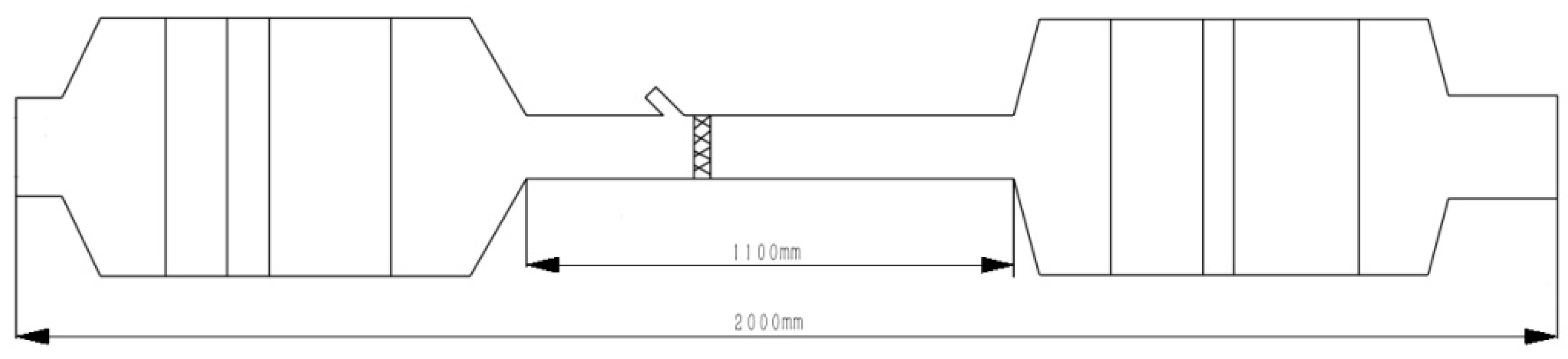
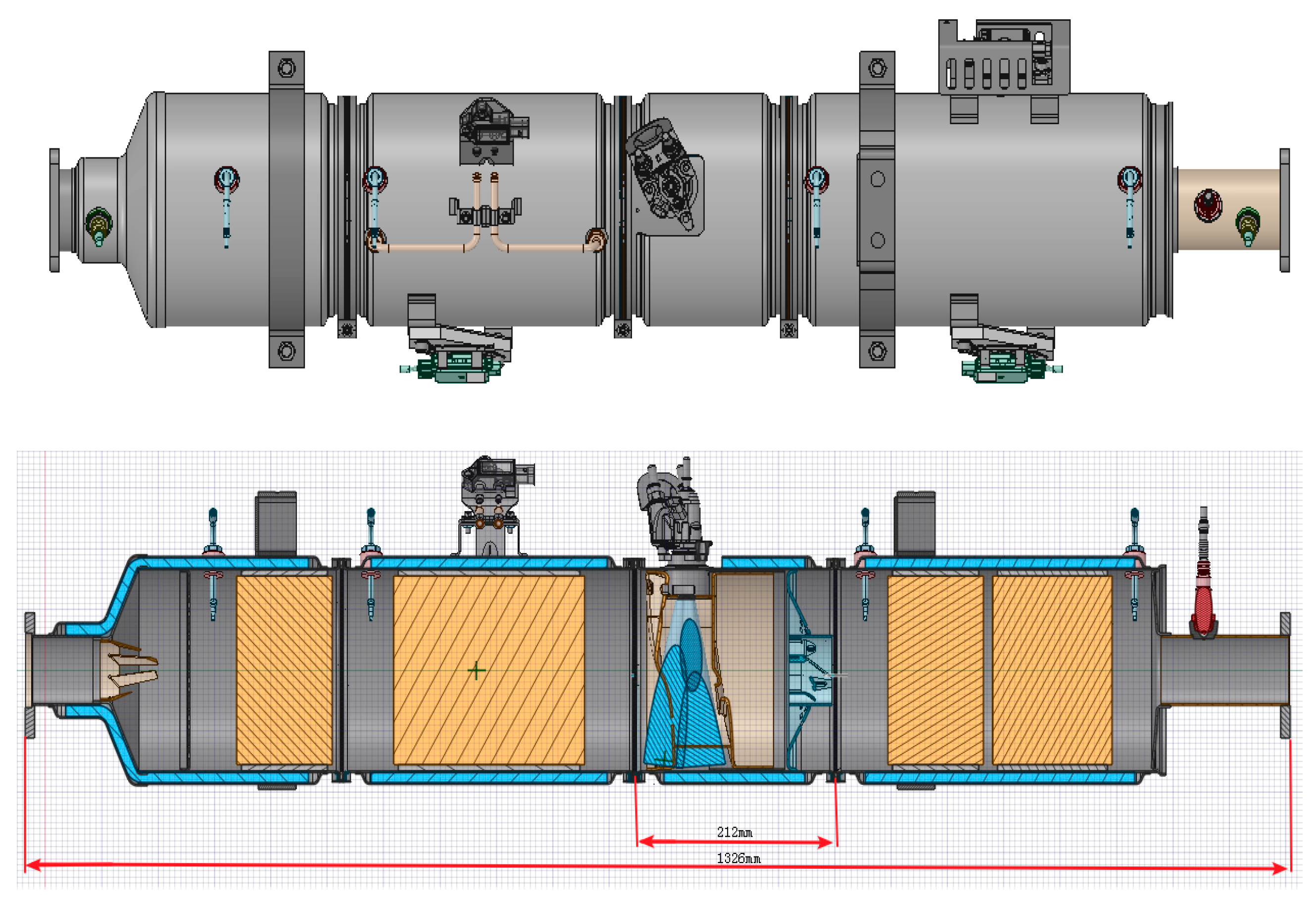
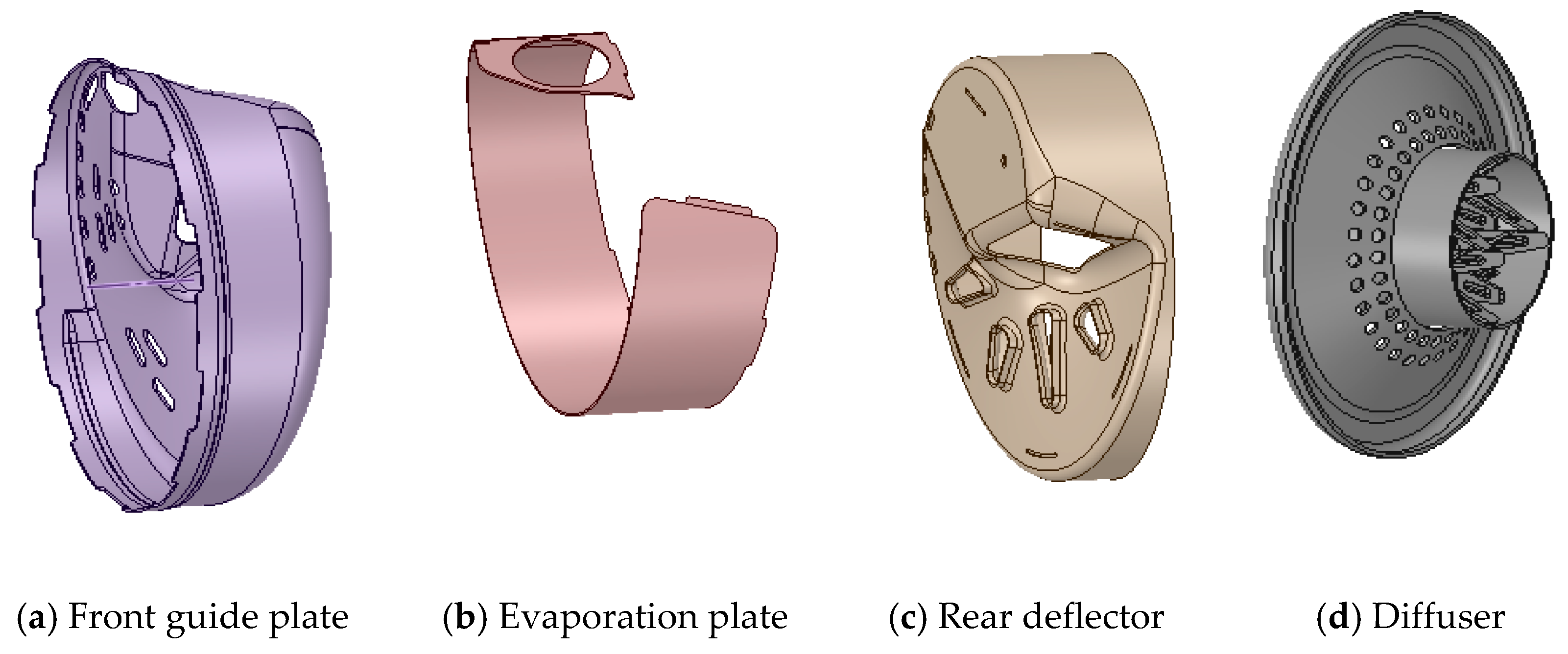

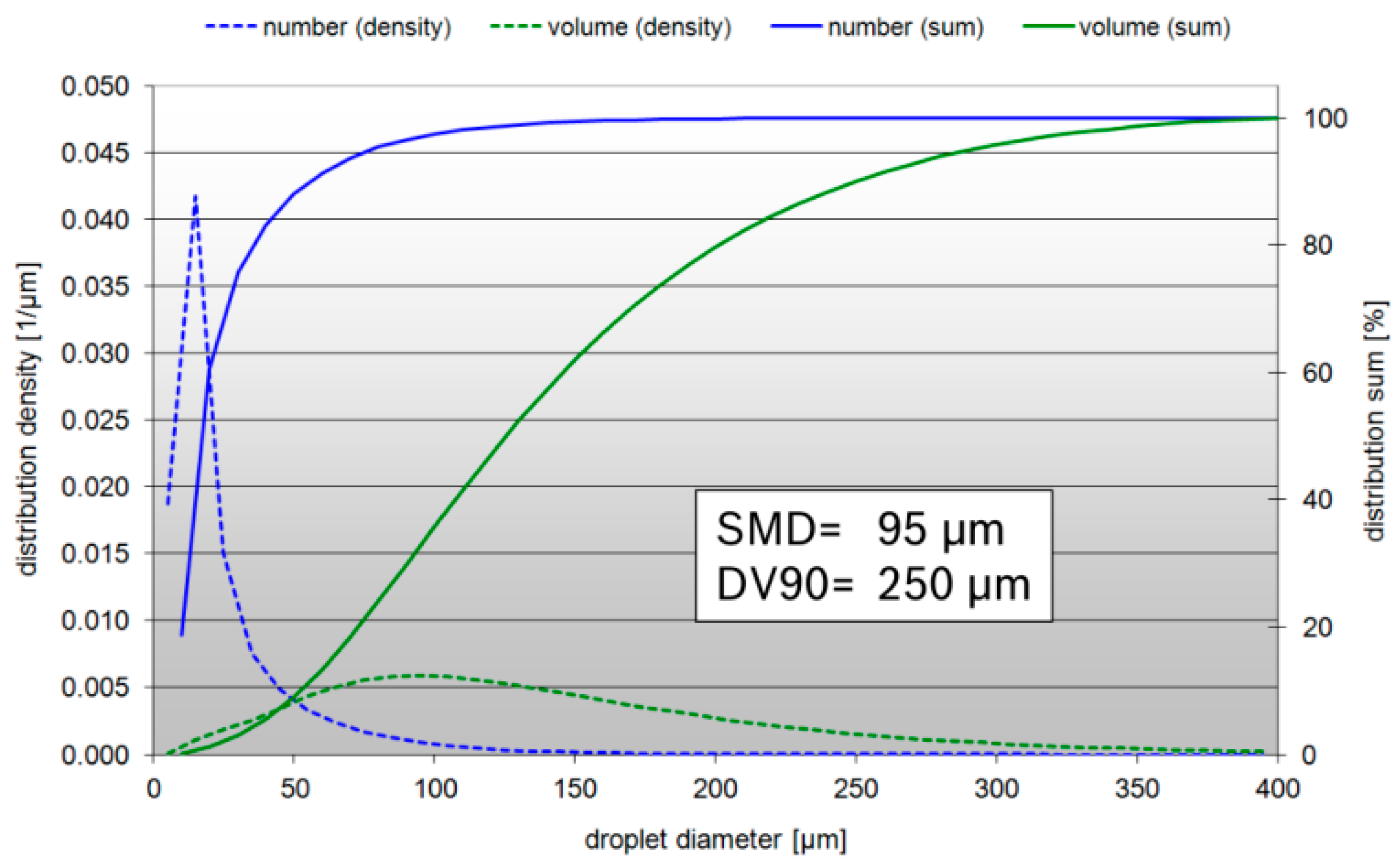










| Operating Point | Mass Flow (kg/h) | Inlet Temperature (°C) | NOX (ppm) |
|---|---|---|---|
| Op1 | 231 | 503 | 481 |
| Op2 | 505 | 550 | 414 |
| Op3 | 95 | 250 | 219 |
| Op4 | 190 | 250 | 87 |
| Number | 3 |
| Hole diameter (μm) | 120 |
| Initial droplet velocity (m/s) | 24 |
| Cone angle (°) | 8 |
| Spray angle (°) | 6 |
| Droplet diameter (sauter mean diameter, SMD) (μm) | 95 |
| Item | Value |
|---|---|
| Emission legislation | China VI |
| Engine type | 2.8L-DI Diesel |
| Cylinder number | 4 |
| Displacement/(L) | 2.776 |
| Rated Power/(kW @ r/min) | 110 @ 3000 |
| Exhaust Gas Recirculation, EGR | Cooled EGR |
| Conditions | Normalized Stoichiometric Ratio (NSR) Parameter: α | Stabilization Time/s | NOX Conversion Efficiency (α = 1) | NOX Maximum Conversion Efficiency (Ammonia Slip <5 ppm) |
|---|---|---|---|---|
| OP1 | 0, 0.5, 0.8, 0.9, 1.0, 1.1, 1.2, 1.3, 1.4 | 300 | >95% | >98% |
| OP2 | 300 | >95% | >98% | |
| OP3 | 2400 | >90% | >90% | |
| OP4 | 900 | >95% | >98% |
| Region | Temperate Zone | Desert Area | Tropical Area | Subarctic Region | |
|---|---|---|---|---|---|
| Natural climate | Air temperature Range (°C) | +40 −15 | +60 −10 | +50 +15 | +30 −25 |
| Temperature Variation range (°C) | 20 | 40 | 15 | 20 | |
| Relative humidity Range (%) | 90 20 | 15 5 | 100 20 | 80 20 | |
| Maximum solar Radiation Temperature (°C) | 800 | 1000 | 900 | 600 | |
| Air salt Concentration Requirements | Near the ocean (100–5000 m): 0.3~30 mg·m−2/day Ship area (within 100m from the shore): 0.3~300 mg·m−2/day | ||||
| Air pollutant | Sulfur dioxide | Industrial and urban areas: Monthly average: 0.014~0.052 ppm Daily average maximum:0.03~0.95 ppm | |||
| Nitrogen oxides | Industrial and urban areas: Monthly average: 0.06~0.47 ppm Daily average maximum: 0.10~0.81 ppm | ||||
| Carbon monoxide | Industrial and urban areas: Monthly average: 4~16 ppm Daily average maximum: 5.8~27.4 ppm | ||||
| P1 | P2 | P3 | P4 | |
|---|---|---|---|---|
| Subgrade | Flat | Uneven | ||
| Asphalt or concrete | Sand and/or stone | Asphalt or concrete | Asphalt or concrete | |
| Highest altitude (m) | 2000 | |||
| Operating Point/Surroundings | Temperate Zone, 30% (1,050,000 km) | Desert Area, 20% (70,000 km) | Tropical Area, 30% (1,050,000 km) | Subarctic Region, 20% (70,000 km) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Load | No Load, 30% | Half Load, 30% | Full Load, 40% | No Load, 30% | Half Load, 30% | Full Load, 40% | No Load, 30% | Half Load, 30% | Full Load, 40% | No Load, 30% | Half Load, 30% | Full Load, 40% |
| city | 6,3000 | 63,000 | 84,000 | 7350 | 7350 | 9800 | 110,250 | 110,250 | 147,000 | 7350 | 7350 | 9800 |
| suburb | 25,200 | 25,200 | 33,600 | 4200 | 4200 | 5600 | 94,500 | 94,500 | 126,000 | 4200 | 4200 | 5600 |
| highway | 94,500 | 94,500 | 126,000 | 6300 | 6300 | 8400 | 47,250 | 47,250 | 63,000 | 8400 | 8400 | 11,200 |
| Non-road | 31,500 | 31,500 | 42,000 | 3150 | 3150 | 4200 | 63,000 | 63,000 | 84,000 | 1050 | 1050 | 1400 |
| Operating Point | Op1 | Op2 | Op3 | Op4 |
|---|---|---|---|---|
| UI | 0.978 | 0.956 | 0.977 | 0.971 |
| Operating Point | Op1 | Op2 | Op3 | Op4 |
|---|---|---|---|---|
| UI | 0.999 | 0.994 | 0.982 | 0.989 |
| Operating Point | Op1 | Op2 | Op3 | Op4 |
|---|---|---|---|---|
| UI | 0.993 | 0.985 | 0.989 | 0.988 |
| Operating Point | Op1 | Op2 | Op3 | Op4 |
|---|---|---|---|---|
| UI | 0.999 | 0.996 | 0.989 | 0.993 |
| Operating Point | Op1 | Op2 | Op3 | Op4 |
|---|---|---|---|---|
| UI | 0.957 | 0.959 | 0.989 | 0.967 |
| Ratio | 1.318 | 1.297 | 1.101 | 1.259 |
| Operating Point | Op1 | Op2 | Op3 | Op4 |
|---|---|---|---|---|
| UI | 0.969 | 0.969 | 0.991 | 0.969 |
| Ratio | 1.234 | 1.230 | 1.091 | 1.250 |
| Risk | Wall-film Thickness ≥1 × 10−6 m | 1 × 10−7 m ≤ Wall-Film Thickness <1 × 10−6 m | Wall-Film Thickness <1 × 10−7 m |
|---|---|---|---|
| Temperature ≤ 160 °C | High | Medium | Low |
| 160 °C < Temperature < 250 °C | Medium | Medium | Low |
| Temperature ≥ 250 °C | Medium | Low | Low |
| Operating Point | Op1 | Op2 | Op3 | Op4 |
|---|---|---|---|---|
| Wall film, m | 1.22 × 10−8 | 2.82 × 10−7 | 3.03 × 10−8 | 1.02 × 10−8 |
| Temperature, °C | 403 | 384 | 155 | 195 |
| risk | low | low | low | low |
| Operating Point | Method | Exhaust Flow | Exhaust Gas Temperature | Ammonia Uniformity |
|---|---|---|---|---|
| Op1 | CFD | 231 | 503 | 0.963 |
| Test 1 | 0.944 | |||
| Test 2 | 0.948 | |||
| Op2 | CFD | 505 | 550 | 0.964 |
| Test 1 | 0.987 | |||
| Test 2 | 0.985 | |||
| Op3 | CFD | 95 | 250 | 0.990 |
| Test 1 | 0.990 | |||
| Test 2 | 0.993 | |||
| Op4 | CFD | 190 | 250 | 0.968 |
| Test 1 | 0.985 | |||
| Test 2 | 0.978 |
| Test Environment | Temperate Zone (1,050,000 km) | Desert Area (70,000 km) | Tropical Area (1,050,000 km) | Subarctic Region (70,000 km) |
|---|---|---|---|---|
| Crystallization description |  |  |  |  |
| Whether the crystal is present after regeneration | NO | NO | NO | NO |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Lou, D.; Zhang, Y.; Lu, K.; Liu, S. Application Study on a New Hybrid Canning Structure of After-Treatment System for Diesel Engine. Energies 2020, 13, 734. https://doi.org/10.3390/en13030734
Zhao C, Lou D, Zhang Y, Lu K, Liu S. Application Study on a New Hybrid Canning Structure of After-Treatment System for Diesel Engine. Energies. 2020; 13(3):734. https://doi.org/10.3390/en13030734
Chicago/Turabian StyleZhao, Chuang, Diming Lou, Yunhua Zhang, Kai Lu, and Shusen Liu. 2020. "Application Study on a New Hybrid Canning Structure of After-Treatment System for Diesel Engine" Energies 13, no. 3: 734. https://doi.org/10.3390/en13030734
APA StyleZhao, C., Lou, D., Zhang, Y., Lu, K., & Liu, S. (2020). Application Study on a New Hybrid Canning Structure of After-Treatment System for Diesel Engine. Energies, 13(3), 734. https://doi.org/10.3390/en13030734




