Abstract
Biological treatments such as anaerobic digestion and composting are known to be the most widespread methods to deal with Organic Fraction of Municipal Solid Waste (OFMSW). The production of biogas, a mix of methane and carbon dioxide, is worth but alone cannot solve the problems of waste disposal and recovery; moreover, the digestate could be stabilized by aerobic stabilization, which is one of the most widespread methods. The anaerobic digestion + composting integration converts 10% to 14% of the OFMSW into biogas, about 35–40% into compost and 35–40% into leachate. The economic sustainability could be rather increased by integrating the whole system with lactic acid production, because of the high added value and by substituting the composting process with the hydrothermal carbonization process. The assessment of this integrated scenario in term of mass balance demonstrates that the recovery of useful products with a potentially high economic added value increases, at the same time reducing the waste streams outgoing the plant. The economic evaluation of the operating costs for the traditional and the alternative systems confirms that the integration is a valid alternative and the most interesting solution is the utilization of the leachate produced during the anaerobic digestion process instead of fresh water required for the hydrothermal carbonization process.
1. Introduction
Food waste (FW) represents an important fraction of biowaste posing increasing disposal costs and environmental challenges. Indeed, it represents a very severe environmental, social and economic problem both in developed and developing countries, accounting today for a production of over 2 Gton/year and expected to grow to more than 3.4 Gton/year in 2050 [1], when the world population will grow to around 9.7 billion inhabitants. In European Union (EU) countries, it has been estimated by De Laurentiis et al. [2] that around 50% of household waste is made up of vegetables and fruit, whereas in absolute terms another study [3] estimated about 100 kg per person per year of food squandered in the EU countries. The FW and losses account for around 58% at home and around 42% in the collective catering and restaurants [4]. Globally, each year around 88 million tons are estimated to be the food losses and waste in EU countries [5].
Based on these figures, the correct management of this peculiar waste is an extremely critical issue in the waste system—its landfilling increases the global warming potential by producing methane and carbon dioxide and it has been then prohibited in many developed countries [6].
Thermochemical energy recovery processes, as incineration, gasification or pyrolysis using FW require a preliminary drying step to remove the excess of moisture, which is usually larger than 70%, requiring large quantities of energy to accomplish this stage. Biochemical processes, on the opposite, do not require any drying stage but quite a limited yield of conversion is usually attained, with a corresponding low mass reduction of the starting substrate [6].
Anaerobic digestion (AD), a bio technique that breaks down organic matter in simpler chemicals components without oxygen and involves biowaste fermentation in controlled anaerobic bioreactors, for example, converts around 15% of FW mass into biogas while the remaining part needs to be treated to be mineralized and correctly disposed with economic costs [7]. This process, which produces biogas digestate and leachate, can be used to generate a source of income. Biogas can be used, for example, in a combined heat and power (CHP) unit to produce electricity and heat. The digestate can be further processed to produce compost [8].
Other biotechnological methods can be employed for production of value-added compounds [9], even in competition with traditional synthetic production of commodity chemicals. In particular, the FW can be used as substrate for fermentative production of a variety of chemicals including lactic acid (LA) [10]. Lactic acid has many applications in the food and beverage sectors as well as in the pharmaceutical, chemical and cosmetic industries and it can originate biodegradable polymer polylactic acid (PLA) through polymerization.
Many by-products, such as acetic, fumaric, formic and propionic acids and ethanol may be produced simultaneously in LA fermentation. These by-products can, anyway, significantly affect the yield of LA [11].
Fermentative production of LA from FW has already proved to be feasible [12]. Many of the studies reported by the scientific literature dealt with LA production on laboratory scale and attention has been paid to the optimization of fermentation conditions and systems, without considering a global technical as well as economic optimization.
Demichelis et al. [13] analyzed the sequential production of LA and biogas from FW. Lactic acid and Biogas were produced from FW via simultaneous saccharification and fermentation (SSF) and separate enzymatic hydrolysis and fermentation (SHF). To improve the overall process performance, the one-step production of LA (SSF) compared to the classical two-step process with (SHF) has several advantages in order to save time and costs. Artificial FW was used to mimic real FW. No external inoculum and nutrients were added and pH was not controlled. However, the mixture of enzymes added as cellulase, amylase and hemicellulose initiate the enzymatic hydrolysis [14]. In Reference [15] the authors studied thermal pre-treatment in co-fermentation of FW and waste activated sludge (WAS) to enhance LA production. First, sole FW was observed as the most suitable substrate employing thermal pre-treatment for the generation of LA. The investigation found that the enhancement of LA yield was in accordance with the acceleration of solubilization and hydrolysis. Furthermore, the physico-chemical characteristics of fermentative substrate and surface morphology of the fermentation mixture varied with the pre-treatment temperatures. Further investigations on microbial community structure were also accomplished. D’Amelia et al. [16] presented some preliminary results on a lab scale feasibility of LA extraction from tomato pomace residues using different bacteria mixtures. The time to attain high LA concentration values from the substrate was detected to be very short compared with that required to obtain biogas. Moreover, the variability in the chemical composition of the FW, the different bacterial communities, the required high temperature pre-treatments and pH variation represented the main critical points encountered in the many investigations previously carried out.
Production of biogas requires the selection of optimal conditions for bacteria growth, as it has been thoroughly discussed, for example, in Reference [17] and in Reference [18]. The optimization of the AD process must be carried out through the study of all the factors that can positively or adversely influence the overall process. In fact, changes in substrate composition, reaction environment, residence time and process conditions can change the equilibrium of the system and result in the buildup of intermediates that may inhibit or stop the overall process. The need of setting up the best operating conditions for specific substrates suggests studying a given substrate that should be as much as possible homogeneous, largely available along the year and with a suitable composition to obtain good yield of LA.
The Organic Fraction of Municipal Solid Waste (OFMSW) is often contaminated by other materials, mainly inorganics and plastics, with a fraction between 10% and 25%, depending on the waste collection system adopted for the separate collection [6]. One of the main severe limitation related to the organic waste utilization in AD process is the economic effort related to the pre-treatment operations required to obtain a digestate with low amounts of contaminants. These preliminary actions allow to use the digestate in composting (Co) and hydrothermal carbonization (HTC) processes for the production of high-quality compost and hydrochar (HC).
A recent paper [19] dealt with techno-economic and profitability analysis of different FW biorefineries. The authors also included detailed flows of the investigated FW as feed for biorefineries in Europe. The results clearly showed that each FW type biorefinery presents different profitability results.
Notwithstanding the fact that conversion of FW into LA has great economic potential (around 1.5 $/kg), it has the drawback of many biochemical processes, in particular those that are highly specialized as in the case of LA production, that is the fate of residue remaining after the main and secondary processes (fermentation, purification, etc.). The yields are, in fact, quite small even though the obtained materials are economically valuable. This consideration drives toward the integration of the LA production with other processes dealing with the exploitation of residues (liquid and solid) in order to be considered sustainable.
These residues should undergo biochemical, mechanical and/or thermochemical treatments to increase the fraction of matter recovered for other uses. By assuming LA production as a step of a virtuous chain, which should be both economic and environmentally sustainable, the residue needs to be exploited by using other suitable methods; among the possible options to treat the biowaste the best are: (a) the biochemical fermentation under anaerobic conditions, that allows the conversion of a fraction of residual carbon into biogas/biomethane and (b) the thermochemical processes that allow the conversion of the residue into biochar (BC) or HC, depending on operating conditions of the process used and source composition (BC can be produced starting from high lignin biowaste).
The LA and HC production processes are not alternative nor in competition with each other and with AD. In fact, all these processes can be integrated by aiming at maximizing recovery of valuable chemicals and materials by applying a sequential process chain that, after the biowaste separate collection, can be summarized as: (1) LA extraction from FW; (2) AD with biogas production in series or parallel to LA production; (3) HTC of digestate (AD residue) to produce HC; (4) enhanced AD with HC addition to fresh biowaste; (5) HC utilization as soil conditioner.
Whatever the optimized order of application of LA production and AD, the further treatment of digestate is a sore point to face; in fact, digestate represents more than 65% mass of starting food/biowaste having 60–65% of water and around 17–20% of carbon. This “digestate” is generally mineralized under form of compost by using an aerobic treatment requiring 90 days with intense aeration and a cost (only electricity) of about 5 € per ton while the economic value does not overcome 3–5 € per ton of produced nitrogen (i.e., 1 €/t of digestate). The alternative to Co is the HTC process.
Hydrothermal carbonization is a water-based thermochemical process carried out at temperatures between 180 and 250 °C under autogenous pressure, promoting dehydration and decarboxylation reactions which reduce both the oxygen and hydrogen content of the feedstock. The HTC process is of great interest because allows to utilize wet biomass without pre-drying prior to use, thus reducing overall energy consumption.
The HTC process generates solid, liquid and small amounts of gas (mainly CO2). The characteristics and the yield of these products strongly depend on the process conditions (i.e., reaction temperature, reactor pressure, water/biomass ratio) and feedstock composition, which also affect their further utilization [20].
The solid product, known as HC, is a carbon-rich material of great interest because it can be used to produce high added-value products such as solid fuels, activated carbon, carbon-based catalysts and other useful carbonaceous materials. In Reference [21] the authors proved that HC production can be economically feasible on the industrial scale.
The aqueous fraction (or bio-oil) is rich in organic acids (acetic, formic, levulinic and glycolic acid) and hydroxy-methyl-furfural (HMF), and, as a consequence, shows high value of total organic carbon (TOC). The handling and disposal concerns related to this liquid by-product may outweigh the advantages of the HTC process from an economical and environmental point of view. A possible solution to this problem is the recirculation of process water which can increase the yield and the energy content of the HC [22].
In this paper the assessment of an integrated system composed by three processes combined in such a way to maximize the recovery of secondary materials lactic acid, biogas and hydrochar, is presented. The FW comes from public and private sources and it is collected and transported to the industrial scale plant performing the biological treatment. This plant operates a combined anaerobic (AD)—aerobic process of OFMSW producing biogas and compost as the main products. The plant is located near Naples, Southern Italy, owned by the CEA SpA company and processes around 35 kt of OFSMW per year [7].
The proposed integration, sketched in the Figure 1, includes the LA production starting from the biowaste and the substitution of HTC of digestate instead of Co.
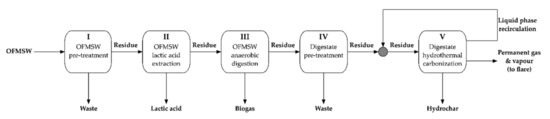
Figure 1.
Sketch of the proposed integrated system.
The technical and economic feasibility investigation is carried out, considering the additional running revenue/cost incurring in the integrated approach compared with the standard process. The results highlight that the integrated process is worth not only from the environmental but also from the economic side. The integrated process has been envisaged assuming that no more leachate should be treated and no more aerobic stabilization of digestate is required and that LA and HC are sold at market prices as high-added value products.
The approach employed in the paper combines data obtained from the management of the industrial plant with experimental results obtained by lab-scale apparatus. The experimental tests have been focused on LA production and the carbonization in sub-critical water environment.
The aim of the assessment is the evaluation of the effects of the industrial plant integration with the LA and HC production on the overall sustainability of the proposed combined plant, compared to the single biogas production plant.
2. Materials, Methods and Experimental Apparatus
The present study was carried out using FW collected between April and September 2019 and sampled directly as entering the AD plant.
2.1. Anaerobic Digestion Experimental Setup
The FW were collected the same day the lorries downloaded them at the plant location. They were placed in sterile plastic containers, transported to the laboratory and stored in the fridge at −4 °C [23] to slow down the bacteria metabolism and to preserve the chemical and physical properties of the sample. FW were used in the digestion experiments within few days and the entire storage period in the fridge was always less than a couple of weeks. Preliminary COD and BOD measurements were accomplished on the substrate before and after the storage to envisage if the storage period in the fridge did not significantly affect the substrate characteristics.
A 50:50 w/w mixture of the two different FW substrates was used in the experimental tests: FW from restaurants, containing food residues and reported in the next as FWR and the other from vegetables markets, reported as FWV.
Laboratory scale tests were carried out in reactors under thermophilic conditions (55 °C). Batch processing was adopted in this research. Each bottle has a total volume of 250 mL; the effective working volume was maintained at 150 mL and 100 mL were left to the gas as head volume.
Two experimental conditions were tested using the two mixed FW substrates, both accomplished in technical triplicate and results are presented using t-Student distribution confidence interval equal to 95% [24]. The first condition is FW mixed substrate with tap water as control test, with a concentration of 63.5 g substrate for 100 mL water. The second tested condition comprises the FW mixed substrate, the inoculum bacteria and tap water with a volume ratio 1:100 and gently mixed with a stirrer at low speed.
The inoculated exogenous bacteria were obtained from buffalo dung and previously grown and selected on a specific Clostridial nutrient medium (Sigma-Aldrich, St. Louis, MO, USA). Mediterranean water buffalo manure was collected in a commercial buffalo farm located in Villa Literno, Caserta, South West Italy. More detailed description of the bacteria present in the inoculum and the techniques employed to detect them can be found in Reference [17].
The initial pH for all the tested conditions was set equal to 6.0 using an NaOH 4% solution. All the samples were shredded by a kitchen mill and, immediately after, stored in the fridge at −4 °C.
Tests were performed with a substrate concentration of 103.6 gVS/l. After assembling the substrate in the fermenter bottles, the reactors were flushed with N2 for about 10 min to obtain anaerobiosis and finally placed in an incubator at controlled temperature of 55 ± 1 °C for the fermentation process. Continuous vigorous magnetic mixing was applied also with the aim at breaking up the forming foam or crust on the upper surface of the liquid bulk.
2.2. Determination of Sugars, Starch and Lactic Acid
Soluble sugars were quantified as described by Carillo et al. [25].
The determination of the LA is based on the transformation of LA to pyruvate using the enzyme lactate dehydrogenase (LDH). A spectrophometric method, using a Synergy HT spectrophotometer (Bio-Tek, Bad Friedrichshall, Germany), was employed to measure both the sugar and LA concentrations in the substrate by evaluating the increase of absorbance at 340 nm compared with the control sample. The calibration line for LA was obtained by using several known concentrations as the reference standard [26], resulting in a correlation coefficient R2 = 0.995.
2.3. BOD and COD Measurements
Respirometric methods, by VELP-BOD apparatus, provide direct measurements of the oxygen consumed by microorganisms. Carbon dioxide produced by the bacteria is chemically bound by the potassium hydroxide solution contained in the seal cup in the bottle. The result is a pressure drop in the system, directly proportional to the BOD value and is measured by the BOD sensor VELP. Measuring the COD is obtained by refluxing the sample in a boiling mixture of sulphuric acid and a known excess of potassium dichromate (K2 Cr2 O7). The remaining unreduced potassium dichromate is titrated with ferrous ammonium sulphate, allowing the determination of the consumed oxygen equivalents.
2.4. Ultimate Analysis and C/N Ratio
Typically, the optimum value for methane production is assumed to be between 20 and 30 [18,23,27]. C, N and H substrate content were determined using the analyzer LECO CHN-S 628 (USA). The measurement method is based on the complete and instantaneous oxidation using pure oxygen (dynamic flash combustion) of the samples [28] with their conversion into gaseous products. Their quantitative estimation is obtained either by non-dispersive IR or thermal conductivity cells. Oxygen is evaluated as difference.
2.5. Total and Volatile Solids
According to the standard procedure, five replicates are prepared, placed in ceramic crucibles and dried in oven at 105 ± 3 °C until constant solid mass is detected. The calculation of the total solids (TS), sometimes called dry matter (DM), is specified by the European Standard (UNI EN 14,346, 2007) as extensively reported in Guarino et al. [29].
Volatile solids (VS) are determined according to UNI EN 15,169 (2007) standard. After drying the substrate for the determination of TS content, the replicates are heated in a furnace up to 550 ± 25 °C for 60 min, till constant mass is attained.
2.6. Determination of Volatile Fatty Acids
The analyses were conducted on gas chromatography mass spectrometry (GC–MS Thermo Scientific TRACE 1300/ISQ-QD). A TG-WAXMS capillary column was used (30 m × 0.25 mm × 0.25 mm) for all the separation of standards and extracts. Helium was used as the carrier gas at a flow rate of 1.5 mL/min and temperature program used: 50–230 °C (10 °C/min), injector at 240 °C, detector at 230 °C. Volatile fatty acid (VFA) standards were purchased from Sigma Aldrich (St Louis, MO, USA). Calibration curves for each compound displayed good linearity with R2 values between 0.98 and 0.99.
2.7. Hydrothermal Carbonization Experimental Apparatus
The experimental work was carried out by using a bench-scale HTC apparatus composed of three main sections, a HTC reactor, a heat exchanger and a condenser, as shown in Figure 2. The HTC reactor is a stirred batch reactor made of AISI 316L with a reaction volume of 3 L. It is heated-up by two electric heating elements of 1.2 kW each. The temperature at the reactor bottom is guaranteed by a control loop that includes a thermocouple (T1) connected to the reactor bottom, a comparator that receives the temperature set-point and the voltage controller for tuning the current into the resistance. To minimize the reactor heat dissipation a 3 cm thick insulating layer of glass wool was used. The reactor top ends with a flange where different connectors are located: three are for the thermocouples (T1, T2 at bottom and T3 at top), one is dedicated to the shaft of the mechanical agitator, one for the reaction mixture input and one for the gas exit. The reactor can be operated at a maximum reaction temperature of 300 °C and a pressure of about 100 bar.
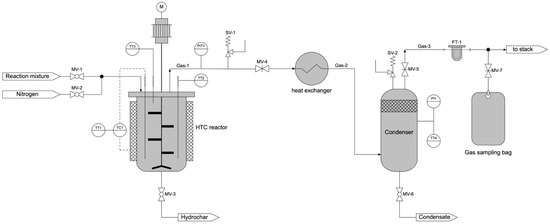
Figure 2.
Sketch of the hydrothermal carbonization (HTC) reactor.
2.8. Hydrothermal Carbonization Testing Procedure
The experimental procedure for producing HC can be divided into the following phases: reaction mixture preparation, biomass conversion and products recovery.
The digestate from the AD process usually contains coarse organic matter (fruits, vegetables, branches, etc.) and inorganic foreign materials (glass, stones, plastics, etc.). In order to obtain a high-quality feedstock, the digestate is pulped, for recovering the organic matter and sieved, to remove the inorganic foreign materials. Then, the pre-treated digestate is mixed with a defined amount of water in order to obtain a specific ratio water/dry pre-treated digestate, displayed as R. The R value is determined by applying the following Equation (1):
being wdig the weight of the digestate, weight of the added water and the moisture of the digestate.
The reactor is heated immediately after its filling with the pre-treated digestate. Once the reaction temperature is reached and kept for the desired reaction time, the process is stopped by switching off the electric power to the heater and by opening the valve of gas vent. The gas is sampled in a tedlar bag from the condenser by using a suction pump. Then, it is analyzed by means of a micro gas-chromatograph. The liquid remaining in the reactor is drained from a bottom nozzle. Both liquid drained from reactor and the condensed one are mixed and analyzed. The HC is recovered by removing the flange from the top of the reactor and then dried and analyzed.
2.9. Gaschromatography for HTC Gas Characterization
The composition of the gases produced during the HTC process are analyzed with the Agilent 490 Micro GC QUAD gas chromatograph equipped with two capillary columns: a MolSieve 5 A and a Poraplot Q. The former is used to separate H2, O2, N2, CH4 and CO; the injector and column temperature are respectively set at 90 °C and 110 °C with Ar as gas carrier. The latter is used for CO2 and light hydrocarbons measurements; the injector and column temperatures are set at 90 °C and 85 °C, respectively and He is the gas carrier.
3. Experimental Results
3.1. Characterization of the Input Substrates
As previously described, the investigated substrate is made up of FW from restaurants, FWR and the other from vegetables markets, FWV. The two substrates are mixed with a 1:1 weight ratio.
Characterization of the mixed substrate is accomplished by proximate and ultimate analyses reported in Table 1, Table 2 and Table 3. As it can be observed by Table 1, the moisture content is quite large but the value is in the classical range for FW. The ultimate analysis accounts for a C/N value of 27.8 which is in the optimal range, as reported in literature, for LA and biogas yields [27]. The ash content, which represents the inert component of the substrate, is around 15% on dry basis.

Table 1.
Proximate analysis of the food waste (FW) investigated substrate, as a mixture of FWR and FWV.

Table 2.
Ultimate analysis (daf) of the FW investigated substrate.

Table 3.
BOD- and COD of the two FW investigated substrates, FWR, FWV and FW mixture.
These data are utilized during the experimental results discussion in order to evaluate the performance of the processes; in particular, the LA production results in the loss of carbon in the solid phase while the HTC promote the loss of hydrogen, oxygen and nitrogen into the liquid and gas phases with a corresponding carbon accumulation in the solid residue, that is, the HC.
An important information for evaluating the LA production process is the initial sugar content of the biomass. Starch content is reported in Figure 3a for the two biomass substrates, FWV and FWR. They are compared to tomato pomace in terms of sugars content, used as a reference substrate, produced during the tomato industrial processing. It is evident that complex sugar as starch (reported in figure in terms of equivalent mg of glucose per g of substrate) are abundant in FWV, with a concentration of around 52 mg/g, followed by the FWR which display a similar concentration value. The reference value of tomato pomace is very low, less than 5 mg/g. The situation is completely reversed when considering the simple sugars (glucose, fructose, sucrose). The sum of the concentration of the three sugars is reported in Figure 3b. It evident that the investigated substrates, FWV and FWR, show very low or no simple sugars concentration, also compared with tomato pomace, which is instead rich in simple sugars. These data suggest that an efficient hydrolysis phase should proceed the effective LA fermentation.
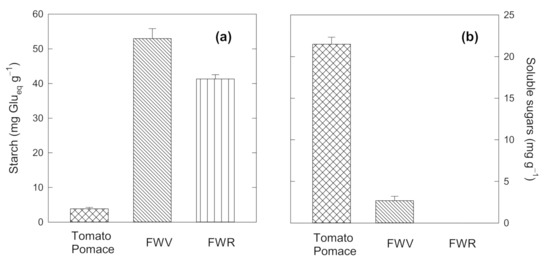
Figure 3.
Starch (a) and Soluble sugars (b) for the two FW substrates and Tomato Pomace as reference value.
3.2. Characterization of the Digestate
Downstream the AD process, once the 10–14% of matter is transformed into biogas, the residue has the ultimate and proximate analyses reported in Table 4. This solid residue is fed into the apparatus shown in Figure 2 and transformed into hydrochar, whose main characteristics are reported in a next paragraph. The ultimate analysis data show that the digestate has a very high ash content and a C/N ratio around 15. The humidity is still around 70% indicating quite a high water content still present after the AD process.

Table 4.
Ultimate analysis (dry basis) and moisture content of digestate.
3.3. Lactic Acid Production
No pretreatments of the substrate nor preliminary SHF process have been carried out in these experiments to mimic as much as possible the operating conditions in a OFMSW plant. The LA production assessment is accomplished by anaerobically fermenting the substrate using two different bacteria consortia, as reported in the previous section.
Figure 4 shows the cumulative and hourly LA production as a function of the time. It is evident that the case with exogenous inoculum shows lower LA production, compared with the blank case. Both the conditions show the maximum hourly production after around 20 h from the start of the fermentation process. The case without inoculum displays a maximum LA production of 6.2 g h −1 kg −1TS whereas the case with inoculum presents a value of around one third of the previous one. Once the maximum values are attained, the production strongly decreases and after around 80 h essentially no more LA is produced. In addition, bacteria from buffalo dung are not suitable for LA production, whereas they are very well fitted for biogas production [17]. The cumulative production shows a typical Gompertz function trend for both the cases. In addition, as for the hourly production, the cumulative values are larger when considering the case without inoculum and a yield of 12 g kg −1TS is reached whereas the case with inoculum shows a yield of 4.0 g kg−1TS. The vertical bars on the data point show the standard deviation for the three replicates.
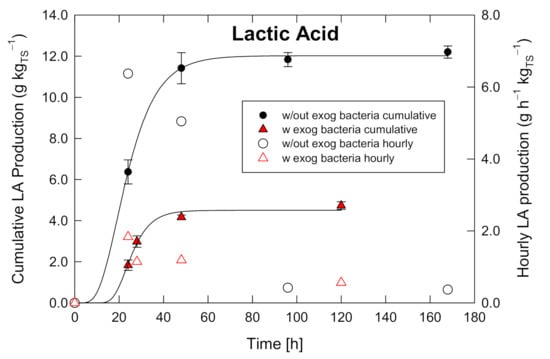
Figure 4.
Hourly and cumulative LA production for the two samples: without and with inoculated exogenous bacteria.
VFA are shown in Figure 5 for the two cases: (a) without inoculum and (b) with inoculum. The case (a) without inoculum shows a constant descent of total VFA concentration, with acetic acid being the predominant one in the first 48 h. After from 72 h butyric acid is the predominant one with a concentration nearly double the acetic acid one. After 24 h the total VFA concentration is about 0.40 g/L and after 96 h they decrease to 0.147 g/L.
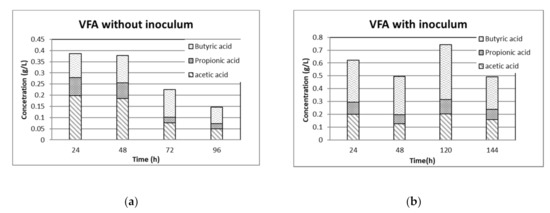
Figure 5.
VFA concentrations during fermentation with main acids: (a) without inoculum; (b) with inoculum.
Case (b) displays higher total VFAs concentration values with a maximum at 120 h of about 0.75 g/L. In this case butyric acid shows a larger concentration than that of acetic acid. Clostridium Spp that are present in case (b), they prefer butyric fermentation by transforming LA into butyric acid, H2 and CO2 [30]. This explains why in this case large butyric acid concentrations are observed. VFA increase matched decrease in LA indicating that LA can be transformed into VFA, also in accordance with the previous studies [31]. However, in both cases the behavior of bacteria can be related to the uncontrolled pH value. The low pH values and presence of VFA in its non-dissociated form, may contribute to inhibit microbial growth by passing through the cell membrane of microorganisms [32].
As far as the concentration of LA is concerned, the fermentation without inoculum shows a maximum value of 1.41 g/L at the end of the AD process, whereas the case with inoculum has a maximum LA concentration of 0.531 g/L after 120 h. In agreement with [33], the exogenous bacteria follow the heterolactic pathway producing low concentrations of LA and also VFA.
3.4. Hydrochar Production
The residue from the AD has been pre-treated in order to prepare a homogeneous and clean slurry. The foreign matter present in the input biowaste is removed in order to obtain a high-quality hydrochar. The removal of this fraction is obtained just after the pulping process since the mechanical filtration becomes effective when the organic matter is in slurry phase. The slurry phase is then fed to the batch reactor and transformed into solid hydrochar, liquid phase and gas.
Tests have been carried out with two different reaction temperatures (180 and 250 °C), in the next displayed as Trxn, while R has been kept at about 10. The reaction temperature is hold for about 6 h. The ultimate analysis of the produced HC on dry basis is reported in Table 5.

Table 5.
Ultimate analysis (dry basis) of hydrochar (HC) for the two investigated temperatures.
The composition of the HC obtained for the two reaction temperatures shows differences in ashes and carbon content. The ultimate analysis at 180 °C shows a 39% of ashes and 37.5% of Carbon content, whereas at 250 °C ashes are around 50% and Carbon at 33%. This circumstance modifies the Higher Heating Value (HHV) of the hydrochar, which has been calculated using the relation given by Fredl et al. in Reference [34]. The obtained results are shown in Table 6.

Table 6.
Higher Heating Value [MJ/kg] obtained by using the ultimate composition by Reference [34].
It can be observed a reduction of the HHV of about 4.1% when a temperature of 250 °C is used as process temperature. This is due to the lower reaction conversion degree obtained at the lower temperature and at the same reaction time; the ratio H/C is in fact higher for the test at the lower temperature. These results can be explained considering the key role played by temperature in the HTC process. Temperature provides thermal energy to break up the biomass chemical bonds. As a consequence, higher temperature favors the migration of atoms from the solid biomass to the liquid and gaseous phases generated during the process. This behavior necessarily leads to a decrease in the Carbon, Hydrogen and Oxygen HC contents. On the other hand, the increase of ash fraction, due to its refractoriness to the reaction conditions, is obtained. Therefore, the chemical energy of the obtained HC reduces.
The HC yield is defined and calculated by using the following equation:
YHC = wHC (dry)/wdigestate (dry) (g/g).
The meaning of YHC needs to be clarified: this value decrease in mass with time because of the extraction of elements such as hydrogen, oxygen, nitrogen and carbon. It reaches a plateau value corresponding to the maximum weight reduction that is dependent on the process conditions. This means that a higher yield does not represent necessarily a positive outcome; this value needs to be coupled with the characterization of the HC quality so that at least the values H/C and O/C need to be associated to it for a complete evaluation. The temperature of the process affects the energy content as well as the HC yield. The yields obtained during the experimental campaign are reported in Table 7.

Table 7.
Hydrochar yield for the two investigated temperatures.
The results show that the increase of reaction temperature causes a decrease of the HC yield from 0.82 g/g to 0.72 g/g.
Other than the final results obtained by the experiments in term of yields and quality of hydrochar, the progression of the HTC process is also interesting. The time evolutions of temperature and pressure inside the reactor are reported in Figure 6a,b for two different reaction temperature and R values about 10, for a shorter time interval up to around 150 min. The effective pressure developing in the headspace of the reactor is also compared with the water vapor pressure in order to account for its possible vaporization. The reaction temperatures are attained after around 40 and 70 min from the beginning of the process, for 180 and 250 °C, respectively. It is observed that the pressure of the gases in the headspace, evolved during the heating process, increases and remains always greater than the vapor pressure of water, also shown in the plots for comparison purposes. Thus, the endogenous moisture and the water added to the digestate remain for the largest amount liquid during the heating process.
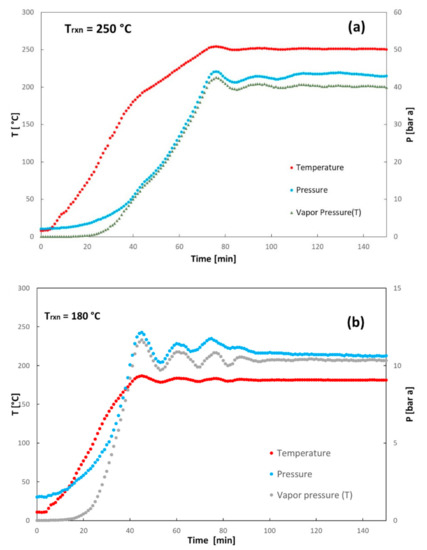
Figure 6.
Temperature and pressure time evolution during the heating and HTC production phases: (a) reaction temperature 250 °C; (b) reaction temperature 180 °C.
Once the desired reaction temperature, Trxn, is reached the process continues for almost 5–6 h to assure that the chemical reactions of breaking of chemical bonds and release of carboxyl groups and aromatization advance to the highest possible degree to obtain a high quality hydrochar.
Once the HC is produced, after the reaction temperature holding time, the gases are vented in a controlled way to the atmosphere. The sudden pressure drop allows part of the liquid phase to evaporate as steam.
From an industrial perspective, the control of steam evaporation at controlled rate, is technically feasible and economically interesting. In fact, one of the main costs for these plants to deal with is the running costs related to the disposal of leachate, which accounts for around 60 €/t in Italy but similar values are found in several European countries. The evaporation of a HTC liquid phase in the atmosphere allows a significant reduction its disposal costs.
The results from GC analyses showed that the largest component present in the gas mixture produced during the HTC process is CO2, which accounts for more than 90%. The remaining part is represented by CO, H2, CH4 and traces of light hydrocarbons (CnHm, sum of C2–C4 hydrocarbons such as ethane, ethylene, propane, propylene and butane).
The detailed composition of gases released during the formation of the HC in the reactor is given in Table 8.

Table 8.
Gas composition during HC production.
The large amounts of CO2 in the gas composition could be attributed to a large extension of decarboxylation reaction. At the higher temperature (250 °C), CO is produced in larger amount probably due to the occurrence of decarbonylation reaction of organic compounds containing carbonyl functional groups [35]. The composition of gas is in line with the other studies present in the literature and confirm the efficacy of the HTC process.
Detailed aspects of the large-scale process application are dealt with in the next section.
4. Preliminary Economic Assessment of Large-Scale Integrated System
The block diagram of the integrated process proposed in this paper is reported in Figure 1, with the main stages displayed.
The results obtained from the experimental tests carried out for LA extraction and HC productions together with the data related to the biogas production [7], obtained from the plant data, are used to evaluate the overall mass balance. The daily mass balance for a steady-state regime is represented by the block diagram displayed in Figure 7, together with the streams of matter, whose quantities are reported in t/d, related each stage of the process. The streams of matter are subdivided into Main Streams, High-Value Streams and Waste Streams. The names are representative of the economic values for each stream. For the main process stages, the moisture of the solid phase is also reported, since it represents an essential information.
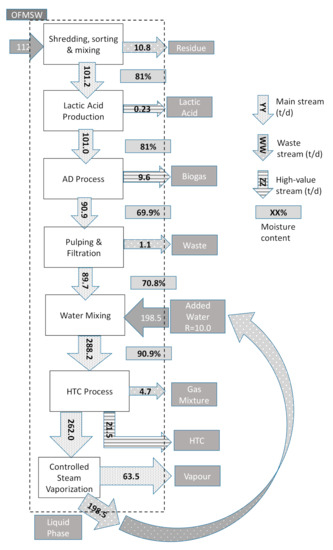
Figure 7.
Integrated process stages and daily mass balance for R = 10.0 and Trxn = 250 °C.
It can be observed that the integration of the process allows to recover different streams of matter when compared with a typical OFMSW biogas plant, avoiding to produce more waste to cope with. In addition, streams of water or leachate that usually represent an expensive cost to be dealt with by the plant owners, can represent a resource, since they are largely employed and recirculated during the HTC production stage. Figure 7 highlights that in any case the actual waste streams are represented by the extraneous matter included in the entering stream of the OFMSW. These streams are mainly represented by plastic and other inert materials always present in the entering municipal waste stream, which usually cannot be avoided. As an example, the case displayed in the figure occurs for an R value of 10.0 and a reaction temperature equal to 250 °C. In this figure, Lactic Acid, Biogas and HC main streams, accounting for an economic value, are of particular relevance. The entering stream of 112 t/d by using data from experimental investigations, the daily production of LA is estimated 0.23 t/d, whereas Biogas assessment is around 9.6 t/d and HC 21.5 t/d. These data are employed for the assessment of the economic performance of the integrated process.
The economic evaluation is assessed considering the additional running costs of the plant and taking into account exclusively the incremental economic cash flows, either positive or negative, not present in the typical municipal waste plant. For this reason, the revenue from biogas is not considered, since it is the specific product of a biogas plant and it does not change considering the integrated process. The economic savings obtained from the avoided treatment of leachate as well as the elimination of the aerobic stabilization of digestate, requiring large quantities of electrical energy, are taken into account.
Each stream of matter or energy is evaluated considering its economic value, being either a revenue or cost. Lactic acid on the market is worth around 1.5 €/kg [13]. Hydrochar is the other additional product that can be conveniently placed on the market at around 0.30 €/kg as soil conditioner or as solid fuel. As far as the water is concerned, the evaporation allows cost saving for its treatment. Thus, the avoided cost of treating the liquid phase is considered a revenue. Experimentally, it was determined that part of the liquid phase can evaporate in the flash process; in addition, reusing large part of it for the HTC process to obtain the right value of R, all the water would not undergo any expensive treatment, which is worth around 60 €/t [7]. The cost is an average value that has been obtained from the European market, in which it ranges between 10 and 80 €/t. Another process avoided is the stabilization of digestate after the biogas production. This process involves electrical energy for the aerobic stabilization by means of warm air, which is pushed over the digestate by large fans. The electrical energy consumption depends on the type of stabilization process but it can be estimated that around 0.022 kWh is required per kg of digestate to be stabilized [7].
The heating process to obtain HC is a cost to be accounted for, since it is required to bring the matter from 37 °C to carbonization temperature, 180 or 250 °C and then maintaining it at the reaction temperature. Considering that around 288 t/d of digestate with a moisture of around 90% need to be warmed up to the carbonization temperature, the cost related to this process is assessed considering heating of water which is around 75 MWh, in the least favorable case, that is, Trxn = 250 °C.
Then, simplistic assumptions have been made to evaluate the energy required to hold the maximum temperature. Assuming two cylindrical reactors for the HTC process which can hold the daily volume of digestate to process and using insulated walls with a 15 cm fiberglass thickness, the energy required to maintain the mass inside the reactor at 250 °C for 6 h is about 95 kWh/d, needed to counterbalance the heat dissipation from the lateral, upper and bottom surfaces of the reactors. Then, assuming a natural gas boiler with an energy efficiency of 90%, an amount of 9120 Smc/d of gas is required to accomplish the HTC process operation.
The LA extraction process has been assumed to occur by electrodialysis with the use of selectively ionic membranes. The operational cost is mainly ascribed to electrical energy used by electrodialyzer device and it has been estimated that the specific electrical energy consumption is equal to about 0.35 kWh kg−1 [36].
Table 9 shows in detail each stream in the economic assessment together with the corresponding yearly revenue or cost.

Table 9.
Economic flux evaluation with reference to Figure 7.
The mass balance of the integrated system shows that the yield of the recovered matter is 27.9% taking into account all the high-value streams.
The incremental yearly revenue considering the integrated process accounts for around 5.8 M€/year the largest part of which, about 70% comes from the saving related to not treating the leachate. Electrical energy for stabilization of digestate saving is worth about 1.0% and 29% from selling LA (1.8%) and HC (28.7%) to the market. The incremental operating costs are represented only by natural gas for HTC process, about 1.4 M€/year.
It is clear that a thorough economic assessment would require the increment of Capex, which mainly accounts for the HTC process reactors, which should sustain up to 50 bars and the equipment for extracting LA in the first stage of the process, which can account for large additional capital cost. The increments of Capex and of Opex revenue for the integrated process would, in any case, result in a positive economic indexes such as Discounted Pay-Back Period, Net Present Value and Internal Rate of Return, unveiling the economic potential of such solutions.
Thus, the integrated process is undoubtedly convenient not only economically but also in terms of reduced environmental impact, with a cleaner management of MSW and thus strongly suggested in order to reduce the pollution load to the environment.
5. Conclusions
Mixed data from experimental activities and actual plant survey, allowed to evaluate the feasibility of the proposed integrated process plant or biorefinery, using OFMSW and producing biogas, LA and HC as the outcome of the integrated process.
Not only a technical evaluation but also an economic feasibility study has been carried out, considering only the additional running (operating) revenue/cost compared with the standard process and showing that the integrated process is worth not only from the environmental but also from the economic side. Incremental capital cost is present since reactors where HC is produced need to be installed together with the LA extraction system. In addition, the estimated increase in yearly revenue, which is worth about 5.8 M€, is large. This figure has been estimated considering that no more leachate should be treated and no more aerobic stabilization of digestate is required and that LA and HC are sold at market prices as high-added value products.
From the experimental investigation the OFMSW substrate shows high quality endogenous bacteria population able to produce lactic acid. Although quite low quantities are produced, anyway the high economic value is worth extracting it. In addition, this process does not affect meaningfully the biogas production.
Hydrothermal carbonization of the digestate has been demonstrated to be an efficient process with a yield of 72% or 82% depending on the reaction temperature. The water required to perform the process is obtained from the same HTC production process, recirculating the liquid phase expelled from the digestate during its carbonization. The main findings are:
- The integrated system allows the largest part of carbon to be moved into useful secondary materials;
- The liquid phase production can be recirculated for the HTC process in an economic and sustainable way;
- The economic values of the products are increased since the LA has a large added value and the HC has a wider range of applications compared to compost;
- The substitution of Co with HTC reduces the local environmental impact because of zero odor emissions and the strongly reduced land use.
Thus, integrating different processes in an OFMSW plant has been demonstrated technically feasible and very promising both from economic and environmental perspectives.
Author Contributions
Conceptualization, M.L.M., L.Z. and B.M.; methodology, M.L.M., B.M., L.Z.; investigation, L.I.D., M.C., L.Z.; data curation, B.M., M.L.M., L.Z., M.C., L.I.D.; writing—original draft preparation, M.L.M., B.M., L.Z.; writing—review and editing, B.M., M.L.M., L.Z., M.C.; funding acquisition, M.L.M., B.M.; visualization L.Z., B.M.; validation L.Z. and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge Università degli studi della Campania “L. Vanvitelli” for funding the research project CHIMERA with V:ALERE 2019 grant and the CEA SpA for funding and supporting the project.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| P | Pressure (bar) |
| R | Water to digestate ratio (kg/kg) |
| T | Temperature (K) |
| w | Weight (kg) |
| X | Moisture content (%) |
| Y | Yield (kg/kg) |
| Subscripts | |
| H2O | Water |
| dig | Digestate |
| rxn | Reaction (Hydrothermal Carbonization) |
| Abbreviations | |
| AD | Anaerobic Digestion |
| BC | Biochar |
| BOD | Biochemical Oxygen Demand |
| COD | Chemical Oxygen Demand |
| Co | Composting |
| FW | Food Waste |
| FWR | Food Waste from restaurants |
| FWV | Food Waste from Vegetable market |
| HC | Hydrochar |
| HHV | Higher Heating Value (MJ/kg) |
| HTC | Hydrothermal Carbonization |
| LA | Lactic Acid |
| OFMSW | Organic Fraction of Municipal Solid Waste |
| SHF | Separate enzymatic hydrolysis and fermentation |
| SSF | Simultaneous saccharification and fermentation |
| TS | Total Solids (%) |
| VFA | Volatile Fatty Acids |
| VS | Volatile Solids (%) |
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; The World Bank: Washington, DC, USA, 2018; p. 292. [Google Scholar] [CrossRef]
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying household waste of fresh fruit and vegetables in the EU. Waste Manag. 2018, 77, 238–251. [Google Scholar] [CrossRef]
- Monier, V.; Escalon, V.; O’Connor, C. Preparatory Study on Food Waste Across EU 27; European Commission (DG ENV): Brussels, Belgium, 2010. [Google Scholar]
- Food Losses and Waste—Inventory and Management at Each Stage in the Food Chain; ADEME-INCOME Consulting, AK2C; Ademe: Strasbourg, France, 2016.
- Caldeira, C.; Corrado, S.; Sala, S. Food Waste Accounting—Methodologies, Challenges and Opportunities; JRC: Luxembourg, 2017. [Google Scholar]
- Mastellone, M.L. Waste Manage and Clean Energy Production from Municipal Solid Waste; Nova Science Publ.: New York, NY, USA, 2015; p. 172. [Google Scholar]
- Comunale, L. CEA Plant Data. Personal communication, 2019. [Google Scholar]
- Monnet, F. An Introduction to Anaerobic Digestion of Organic Wastes; Remade: Scotland, UK, 2003; pp. 5–42. [Google Scholar]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T.; Vandenberghe, L.P.; Mohan, R. Biotechnological potential of agro-industrial residues. II: Cassava bagasse. Bioresour. Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Kim, Y.H.; Moon, S.-H. Lactic acid recovery from fermentation broth using one-stage electrodialysis. J. Chem. Tech Biotech. 2001, 76, 169–178. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Sonomoto, K. Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J. Biotechnol. 2016, 236, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Pleissner, D.; Caruso, A.; Mariano, S.; Fiore, S.; Gutiérrez, I.M.N.; Schneider, R.; Venus, J. Direct production of lactic acid based on simultaneous saccharification and fermentation of mixed restaurant food waste. J. Clean. Prod. 2017, 143, 615–623. [Google Scholar] [CrossRef]
- Caruso, A.; Pleissner, D.; Fiore, S.; Mariano, S.; Gutiérrez, I.M.N.; Schneider, R.; Venus, J. Investigation of food waste valorization through sequential lactic acid fermentative production and anaerobic digestion of fermentation residues. Bioresour. Technol. 2017, 241, 508–516. [Google Scholar] [CrossRef]
- Yousuf, A.; Bastidas-Oyanedel, J.R.; Schmidt, J.E. Effect of total solid content and pretreatment on the production of lactic acid from mixed culture dark fermentation of food waste. Waste Manag. 2018, 77, 516–521. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Li, X.; Ye, T.; Gan, Y.; Zhang, A.; Chen, H.; Xue, G.; Liu, Y. Production of lactic acid from thermal pretreated food waste through the fermentation of waste activated sludge: Effects of substrate and thermal pretreatment temperature. Bioresour. Technol. 2018, 247, 890–896. [Google Scholar] [CrossRef]
- Amelia, L.D.; Aversana, E.D.; Faiella, D.; Cacace, D.; Woodrow, P.; Carillo, P.; Morrone, B. Lactic acid production from tomato pomace fermentable sugars using innovative biological treatments. Chem. Engineer Trans. 2018, 65, 595–600. [Google Scholar]
- Carillo, P.; Carotenuto, C.; Di Cristofaro, F.; Kafantaris, I.; Lubritto, C.; Minale, M.; Morrone, B.; Papa, S.; Woodrow, P. DGGE analysis of buffalo manure eubacteria for hydrogen production: Effect of pH, temperature and pretreatments. Mol. Biol. Rep. 2012, 39, 10193–10200. [Google Scholar] [CrossRef]
- Carotenuto, C.; Guarino, G.; Minale, M.; Morrone, B. Biogas Production from Anaerobic Digestion of Manure at Different Operative Conditions. Int. J. Heat Technol. 2016, 34, 623–629. [Google Scholar] [CrossRef]
- Cristóbal, J.; Caldeira, C.; Corrado, S.; Sala, S. Techno-economic and profitability analysis of food waste biorefineries at European level. Bioresour. Technol. 2018, 259, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dong, X.; Wu, T.; Zhu, C. Influence of reaction conditions and feedstock on hydrochar properties. Energy Convers. Manag. 2016, 123, 95–103. [Google Scholar] [CrossRef]
- Lucian, M.; Fiori, L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process Water from the Hydrothermal Carbonization of Biomass: A Waste or a Valuable Product? Waste Biomass-Valorization 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Zeshan; Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar] [CrossRef]
- Fowler, J.; Cohen, L. Statistics for Ornithologists, 2nd ed.; British Trust for Ornithology: Norfolk, UK, 1990. [Google Scholar]
- Carillo, P.; Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Dell’Aversana, E.; D’Amelia, L.; Kyriacou, M.C.; Caruso, G.; De Pascale, S.; Rouphael, Y. Sensory and functional quality characterization of protected designation of origin ‘Piennolo del Vesuvio’ cherry tomato landraces from Campania-Italy. Food Chem. 2019, 292, 166–175. [Google Scholar] [CrossRef]
- Borshchevskaya, L.N.; Gordeeva, T.L.; Kalinina, A.N.; Sineokii, S.P. Spectrophotometric determination of lactic acid. J. Anal. Chem. 2016, 71, 755–758. [Google Scholar] [CrossRef]
- Carotenuto, C.; Guarino, G.; D’Amelia, L.I.; Morrone, B.; Minale, M. The peculiar role of C/N and initial pH in anaerobic digestion of lactating and non-lactating water buffalo manure. Waste Manag. 2020, 103, 12–21. [Google Scholar] [CrossRef]
- Friis, J.; Holm, C.; Halling-Sørensen, B. Evaluation of elemental composition of algal biomass as toxical endpoint. Chemosphere 1998, 37, 2665–2676. [Google Scholar] [CrossRef]
- Guarino, G.; Carotenuto, C.; Di Cristofaro, F.; Papa, S.; Morrone, B.; Minale, M. Does the C/N ratio really affect the biomethane yield? A three years investigation of buffalo manure digestion. Chem. Engineer Trans. 2016, 49, 463–468. [Google Scholar]
- Liu, S.; Bischoff, K.M.; Leathers, T.D.; Qureshi, N.; Rich, J.O.; Hughes, S.R. Butyric acid from anaerobic fermentation of lignocellulosic biomass hydrolysates by Clostridium tyrobutyricum strain RPT-4213. Bioresour. Technol. 2013, 143, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, X.C.; Hu, Y.; Zhang, Y.; Li, Y. Effect of pH on lactic acid production from acidogenic fermentation of food waste with different types of inocula. Bioresour. Technol. 2017, 224, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Wright, A. (Eds.) Lactic Acid Bacteria, Microbiological and Functional Aspects, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 656. [Google Scholar]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Basso, D.; Weiss-Hortala, E.; Patuzzi, F.; Castello, D.; Baratieri, M.; Fiori, L. Hydrothermal carbonization of off-specification compost: A byproduct of the organic municipal solid waste treatment. Bioresour. Technol. 2015, 182, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Komesu, A.; Maciel, M.R.W.; Filho, R.M. Separation and Purification Technologies for Lactic Acid—A Brief Review. Bioresources 2017, 12, 6885–6901. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).