Comprehensive Experimental Study on the Thermophysical Characteristics of DI Water Based Co0.5Zn0.5Fe2O4 Nanofluid for Solar Thermal Harvesting
Abstract
1. Introduction
2. Nanoparticles and Experimental Methods
2.1. Co0.5Zn0.5Fe2O4 Nanofluid
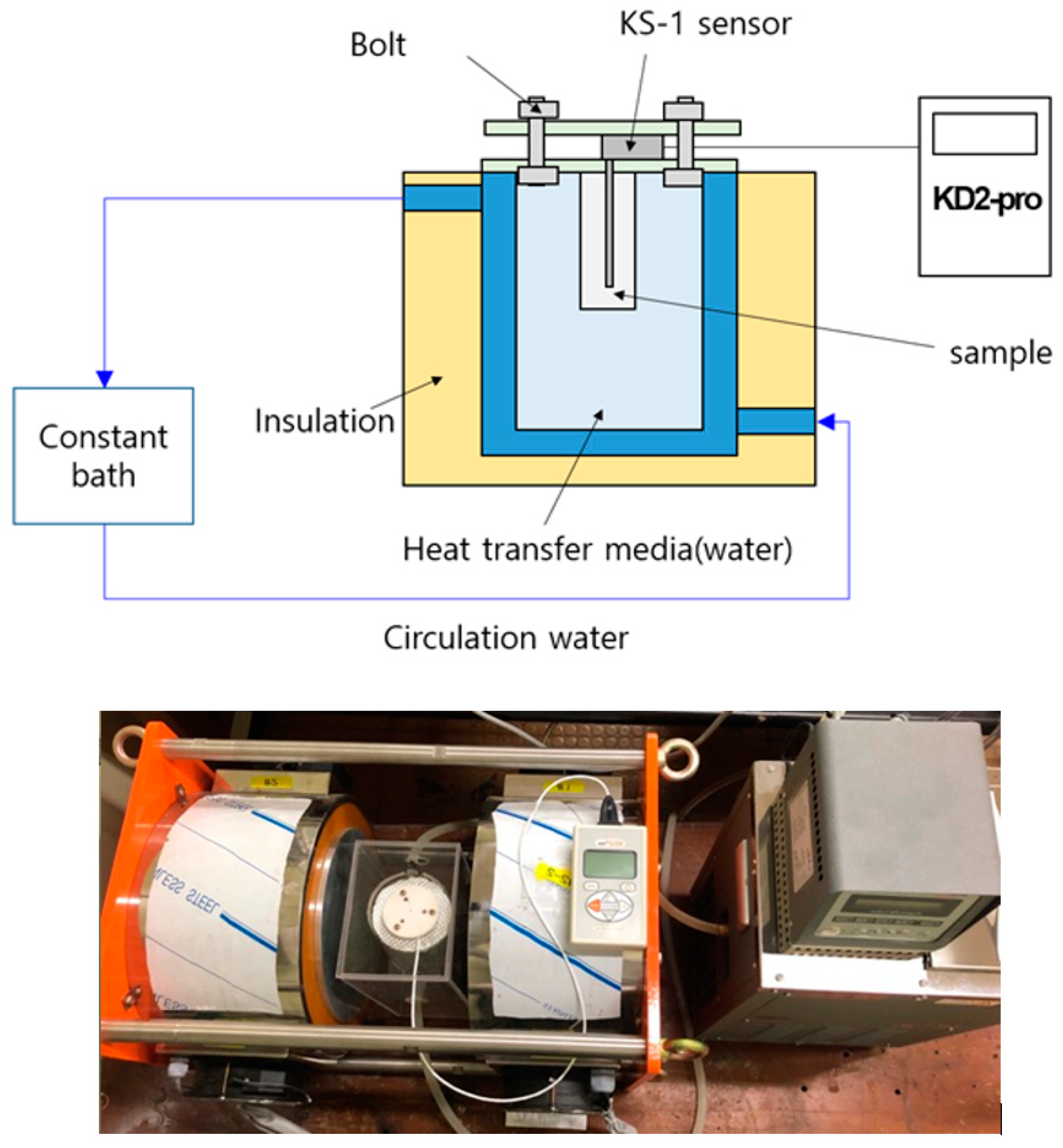
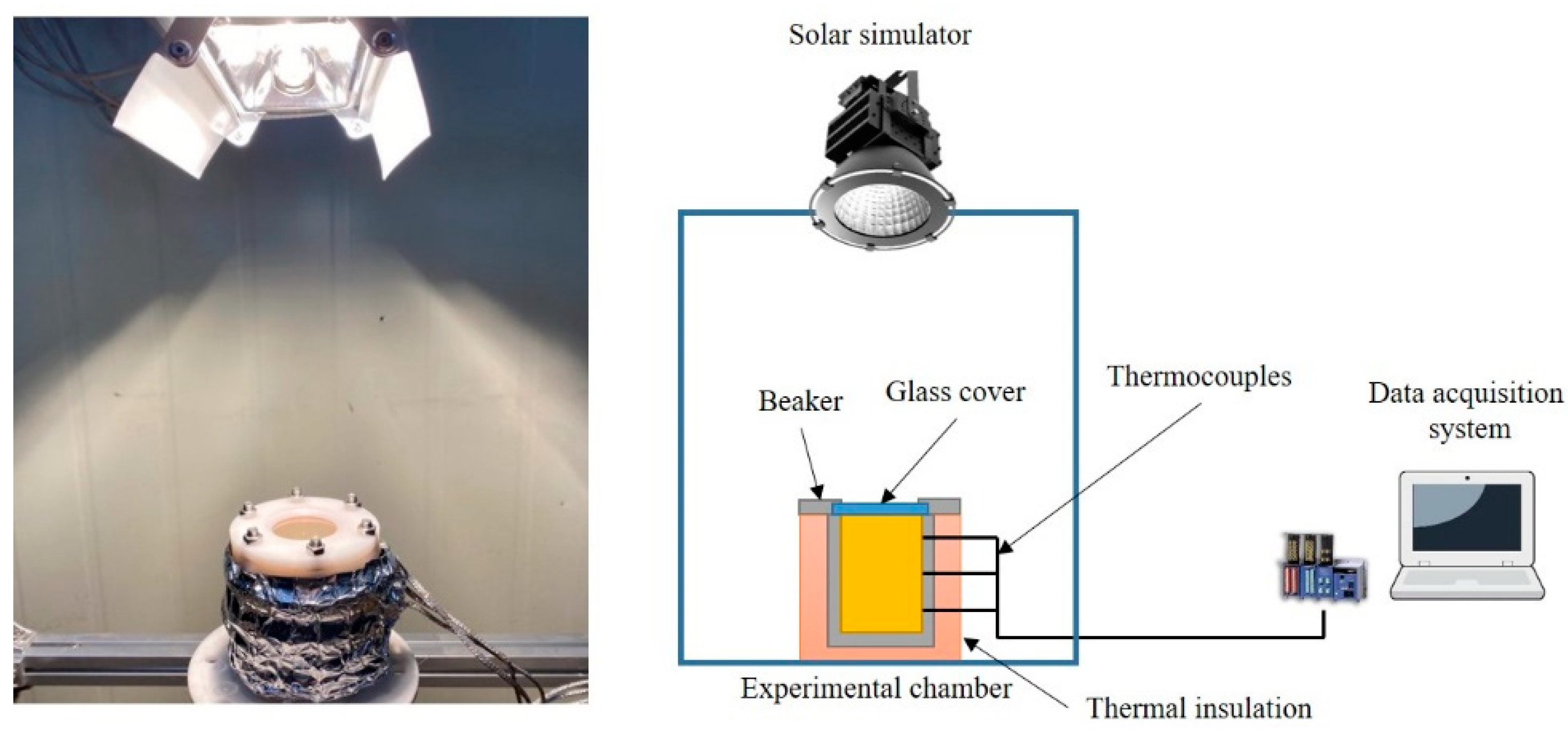
2.2. Experimental Setup
3. Results and Discussion
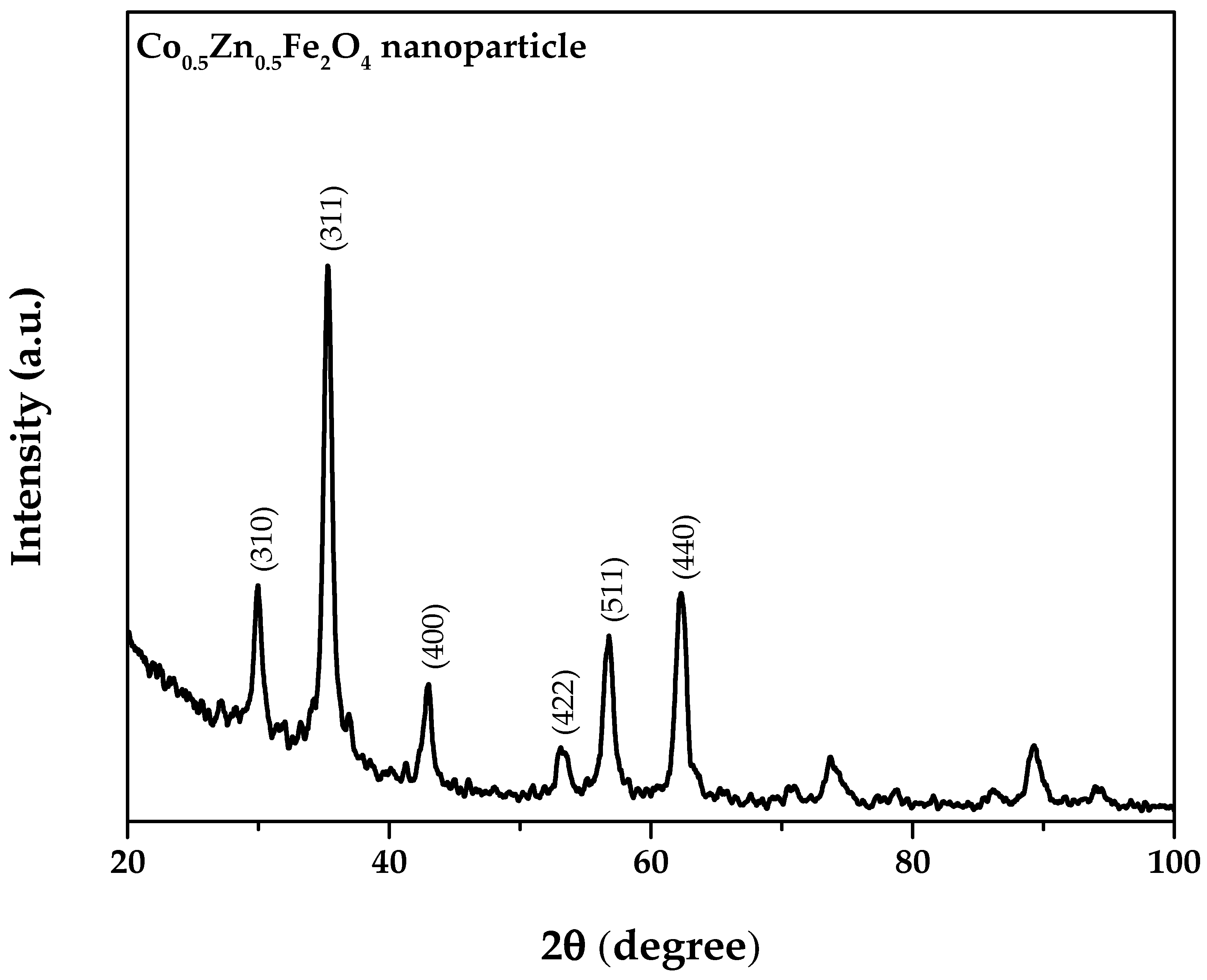
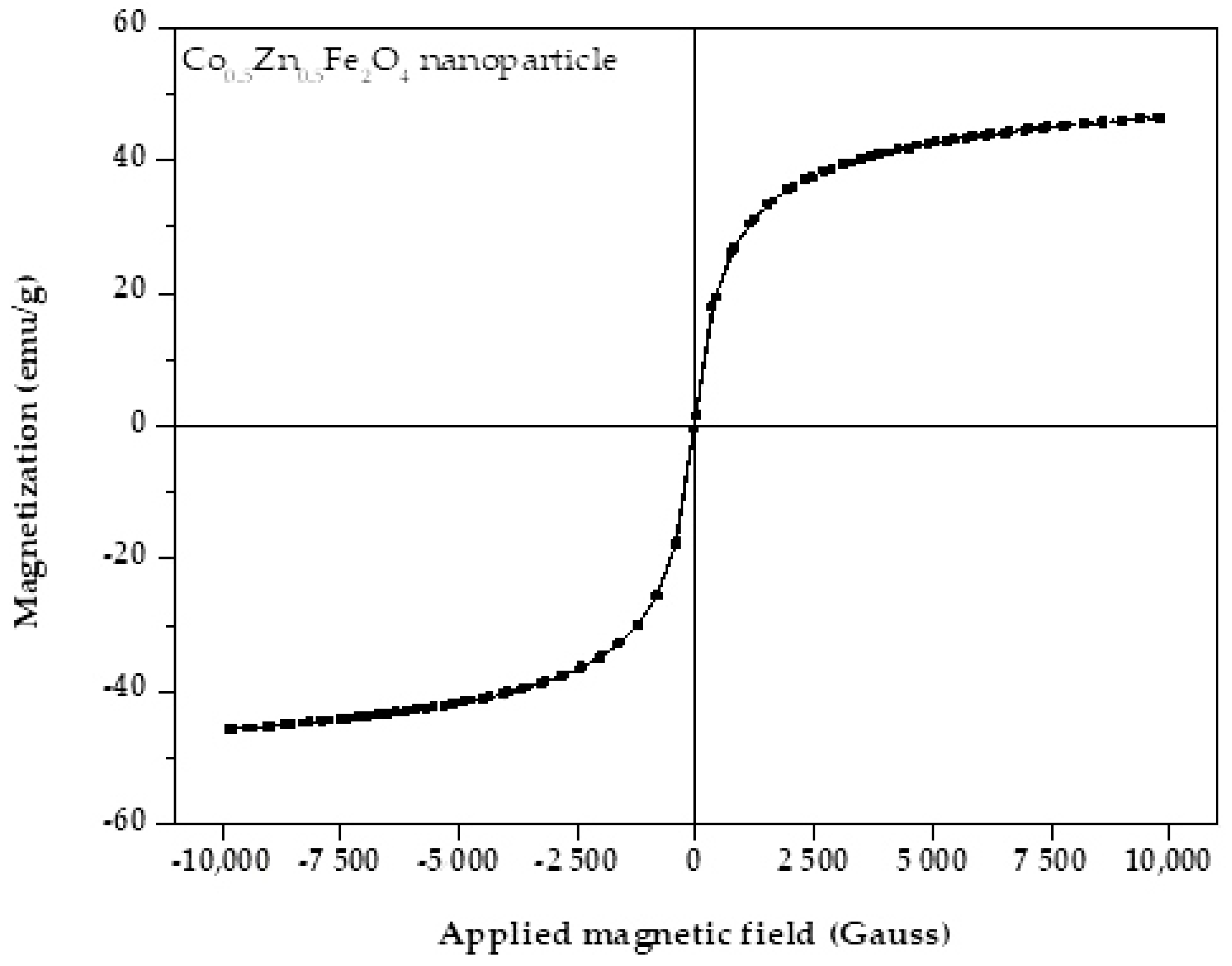
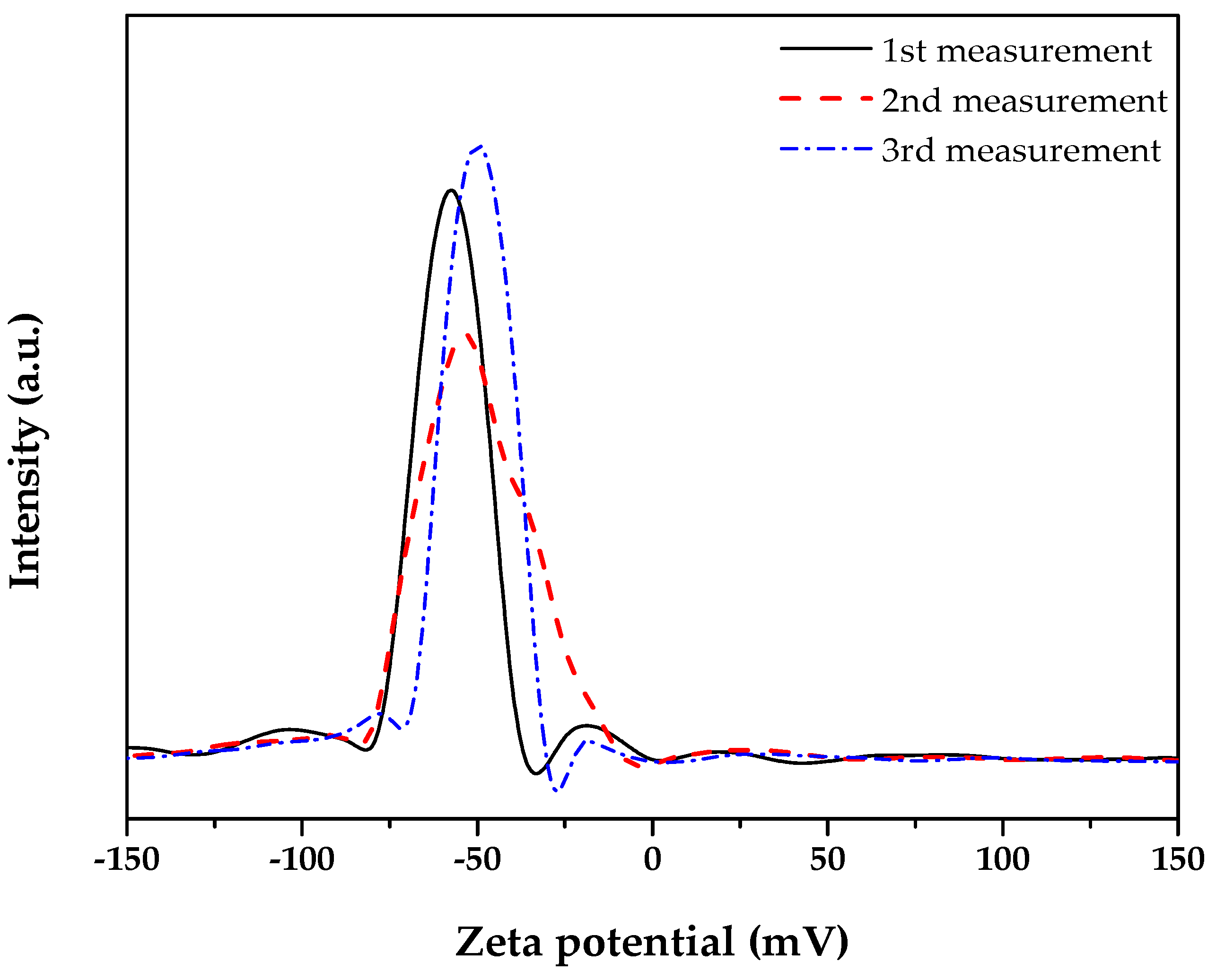
3.1. Characterization of Co0.5Zn0.5Fe2O4 Nanofluid
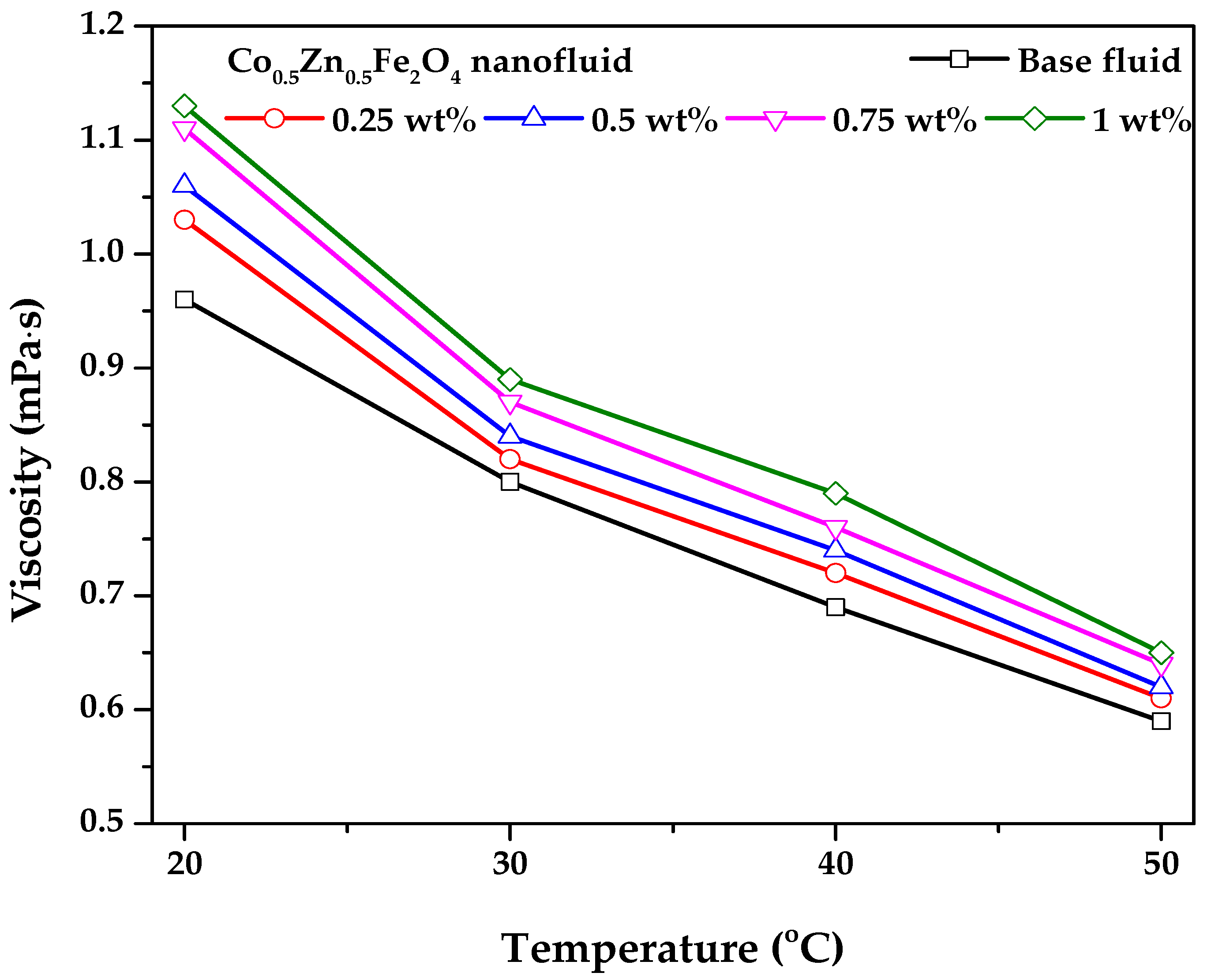
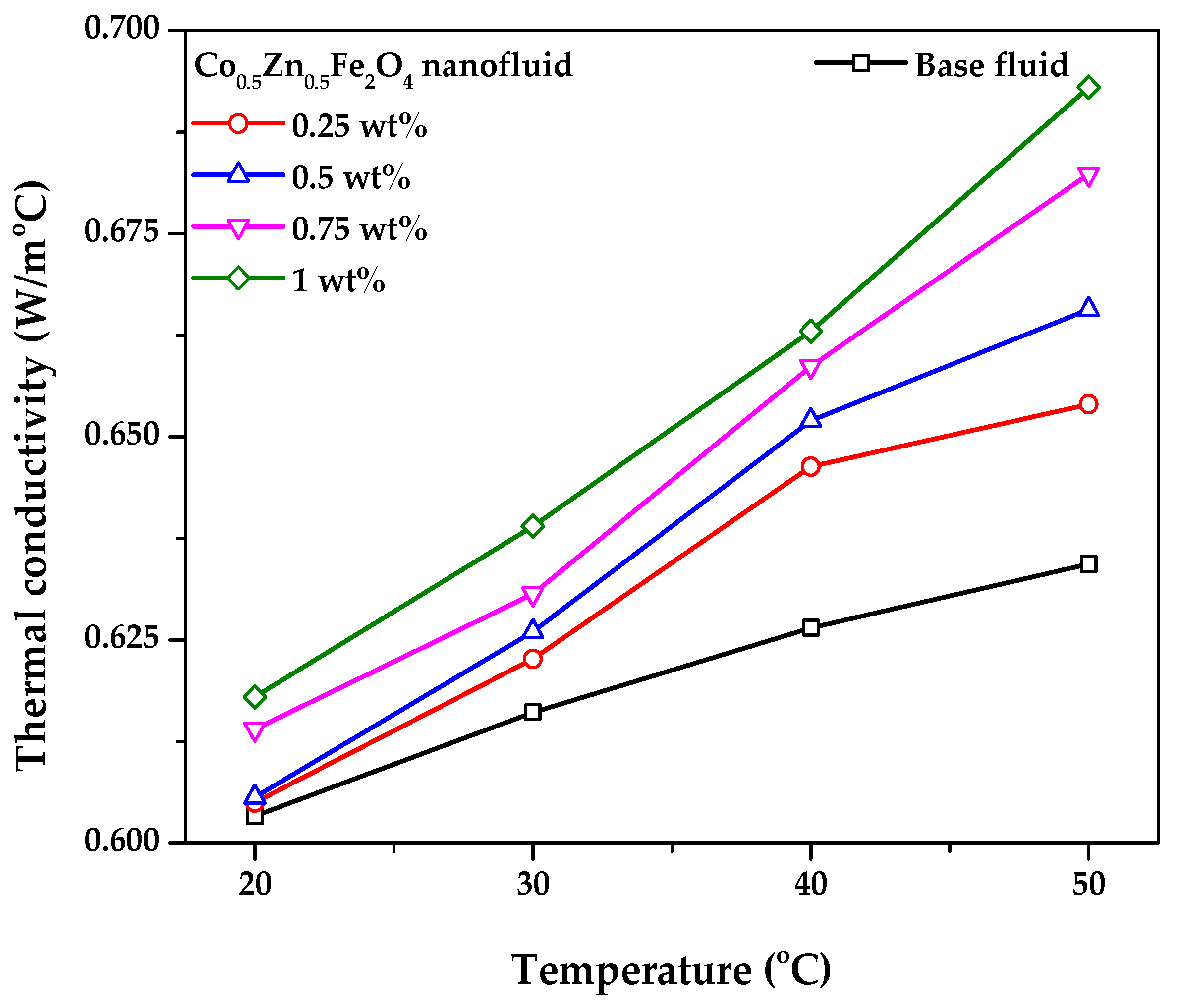
3.2. Viscosity and Thermal Conductivity of the Co0.5Zn0.5Fe2O4 Nanofluid
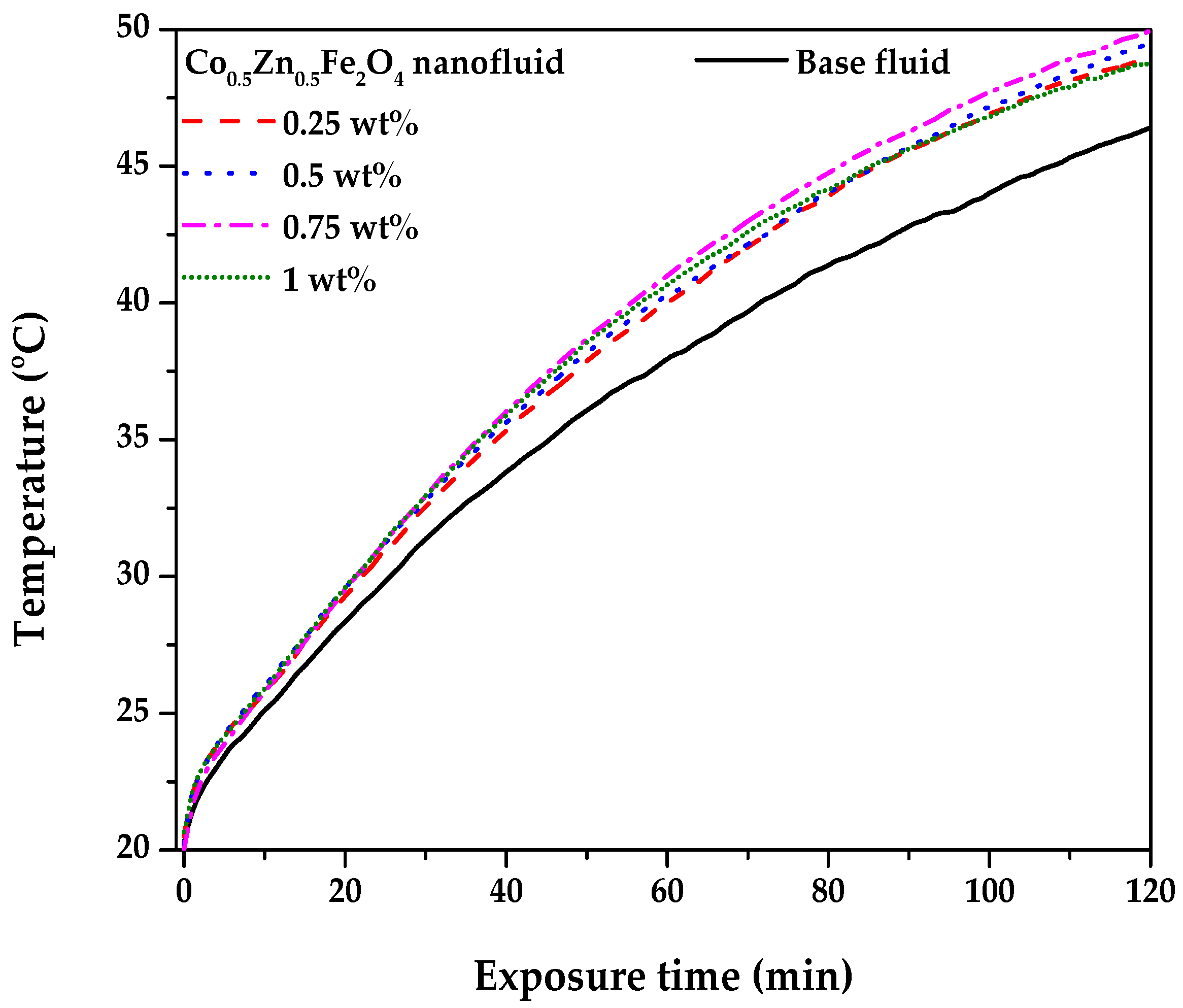
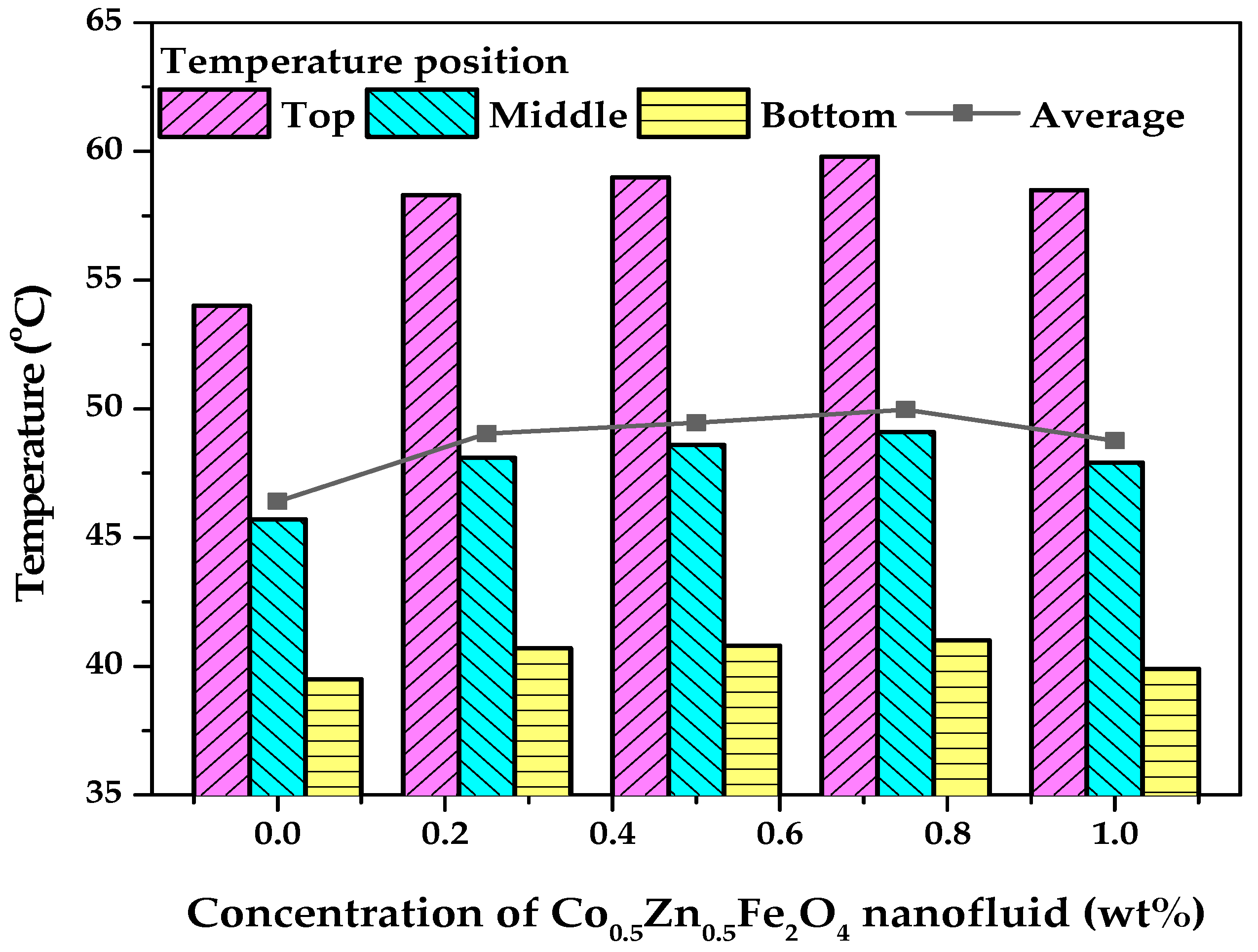
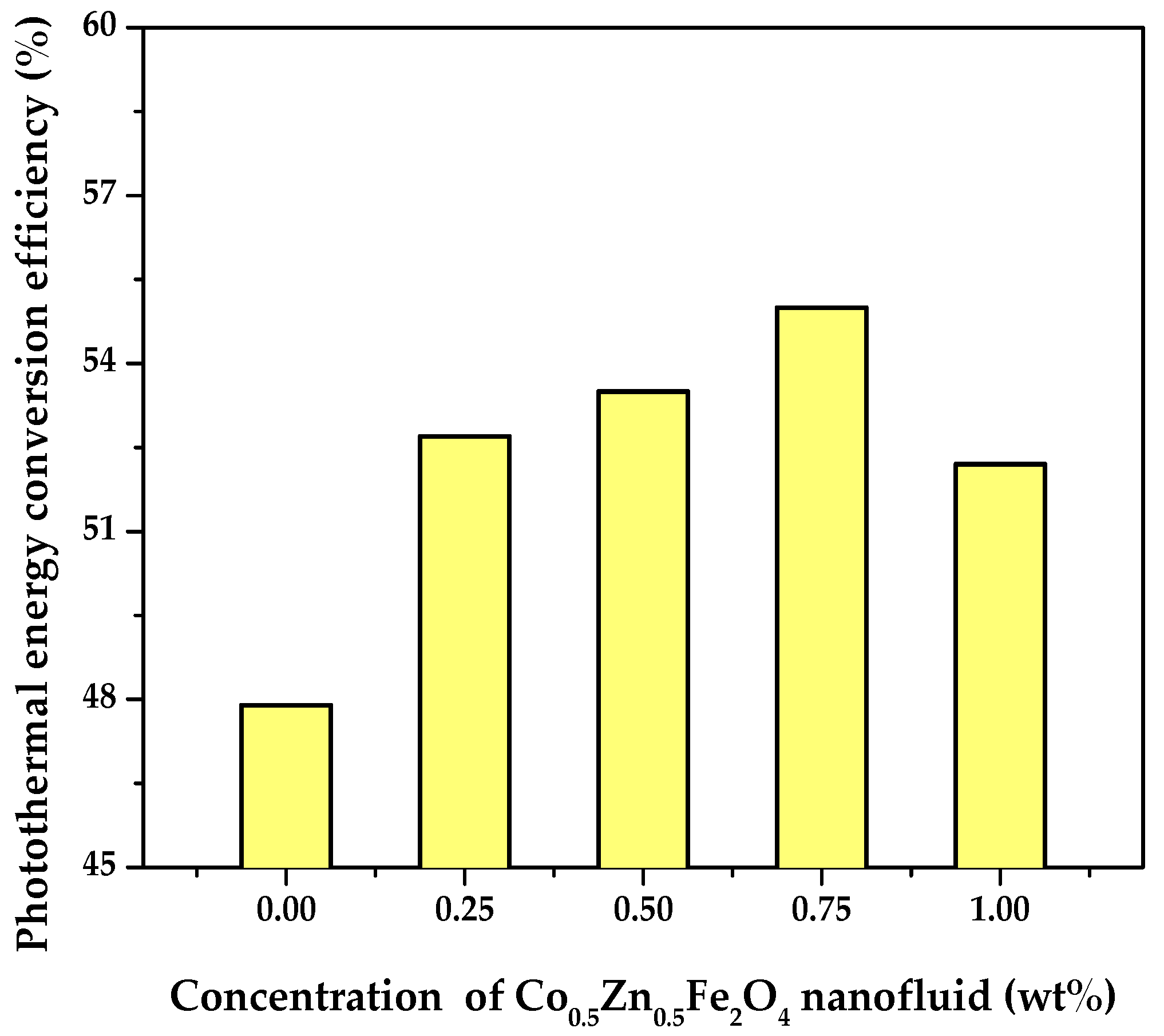
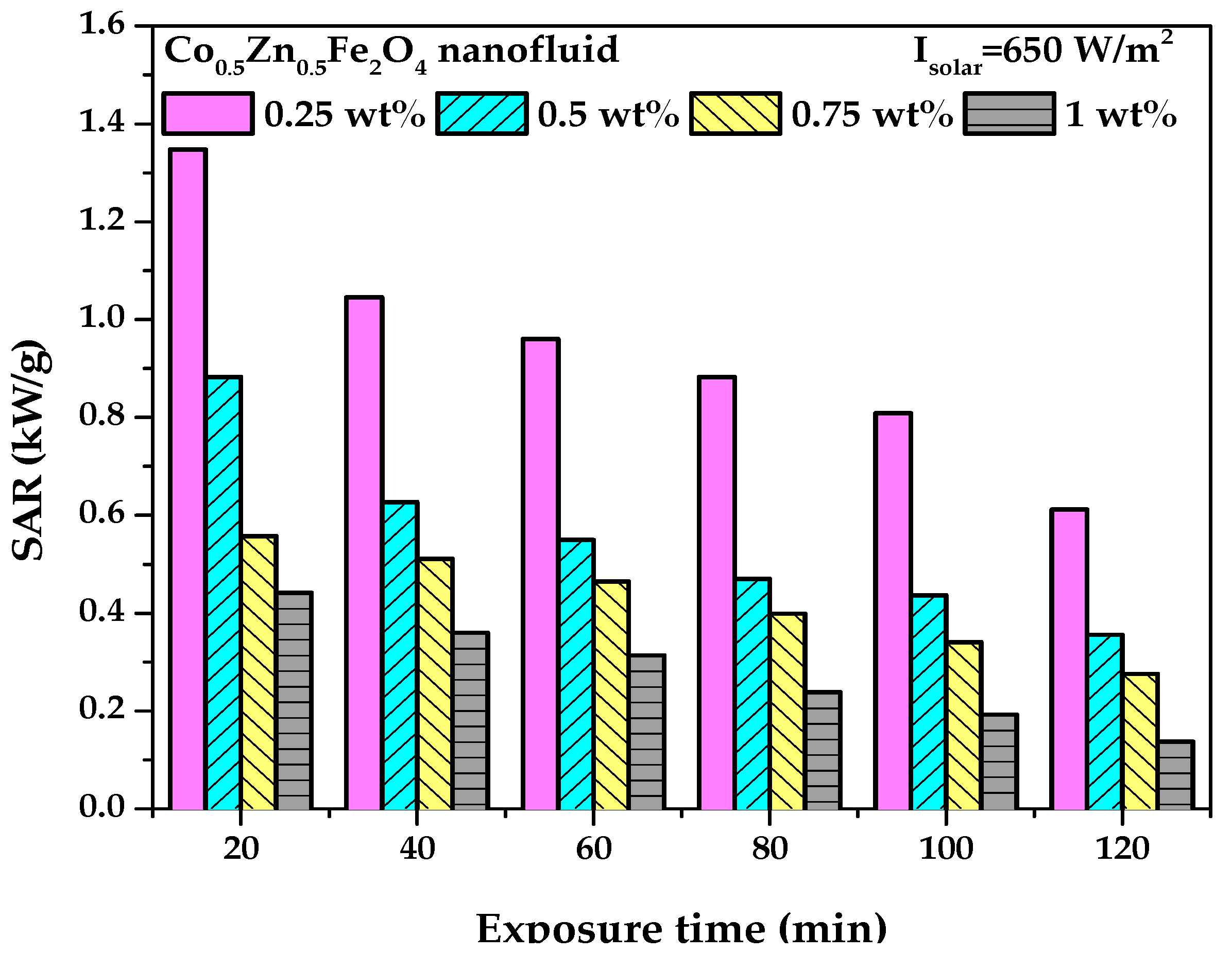
3.3. PTEC Characterization of the Co0.5Zn0.5Fe2O4 Nanofluid
4. Conclusions
- From the XRD results, the average and maximum diameter of the Co0.5Zn0.5Fe2O4 particles were 14.73 nm and 18.7 nm, respectively. The saturation magnetization of the Co0.5Zn0.5Fe2O4 nanoparticles was 46.57 emu/g, indicating that the Co0.5Zn0.5Fe2O4 nanofluid could be classified as a magnetic nanofluid. The thermal conductivity of Co0.5Zn0.5Fe2O4 nanofluid improved from 0.605 and 0.618 to 0.654 and 0.693 W/m·°C at concentrations of 0.25 wt% and 1 wt%, respectively, when the temperature increased from 20 to 50 °C. The maximum thermal conductivity of the Co0.5Zn0.5Fe2O4 nanofluid reached up to 0.693 W/m·°C, which was obtained at 1 wt% and 50 °C. Adding Co0.5Zn0.5Fe2O4 nanoparticles to DI water improved the optical absorption performance of the working fluid;
- The PTEC performance enhanced with the nanofluid concentration and exposure time. However, the highest temperature (50 °C) was observed in the 0.75 wt% Co0.5Zn0.5Fe2O4 nanofluid. After 120 min, the PTEC efficiency of the Co0.5Zn0.5Fe2O4 nanofluid was 52.7%, 53.5%, 55%, and 52.2% at concentrations of 0.25, 0.5, 0.75, and 1 wt%, respectively. The highest SAR of the Co0.5Zn0.5Fe2O4 nanofluid was 1.34 kW/g at a concentration of 0.25 wt% and an exposure time of 20 min.
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency. World Energy Outlook 2019; International Energy Agency: Paris, France, 2019. [Google Scholar]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particuology 2009, 7, 141–150. [Google Scholar] [CrossRef]
- Sun, B.; Guo, Y.; Yang, D.; Li, H. The effect of constant magnetic field on convective heat transfer of Fe3O4/water magnetic nanofluid in horizontal circular tubes. Appl. Therm. Eng. 2020, 171, 114920. [Google Scholar] [CrossRef]
- Esmaeili, E.; Ghazanfar, C.; Rounaghi, S.A. The influence of the alternating magnetic field on the convective heat transfer properties of Fe3O4-containing nanofluids through the Neel and Brownian mechanisms. Appl. Therm. Eng. 2017, 110, 1212–1219. [Google Scholar] [CrossRef]
- Ghasemian, M.; Najafian, Z.; Goharkhah, M.; Ashjaee, M. Heat transfer characteristics of Fe3O4 ferrofluid flowing in a mini channel under constant and alternating magnetic fields. J. Magn. Magn. Mater. 2015, 381, 158–167. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ganji, D. Nanofluid convective heat transfer using semi analytical and numerical approaches: A review. J. Taiwan Inst. Chem. Eng. 2016, 65, 43–77. [Google Scholar] [CrossRef]
- Soltani, F.; Toghraie, D.; Karimipour, A. Experimental measurements of thermal conductivity of engine oil-based hybrid and mono nanofluids with tungsten oxide (WO3) and MWCNTs inclusions. Powder Technol. 2020, 371, 37–44. [Google Scholar] [CrossRef]
- Sarbolookzadeh Harandi, S.; Karimipour, A.; Afrand, M.; Akbari, M.; D’Orazio, A. An experimental study on thermal conductivity of F-MWCNTs–Fe3O4/EG hybrid nanofluid: Effects of temperature and concentration. Int. Commun. Heat Mass Transf. 2016, 76, 171–177. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Rashidi, A.; Rashtchian, D.; Lotfi, R.; Amrollahi, A. Synthesis of spherical silica/multiwall carbon nanotubes hybrid nanostructures and investigation of thermal conductivity of related nanofluids. Thermochim. Acta 2012, 549, 87–94. [Google Scholar] [CrossRef]
- Bahrami, M.; Akbari, M.; Karimipour, A.; Afrand, M. An experimental study on rheological behavior of hybrid nanofluids made of iron and copper oxide in a binary mixture of water and ethylene glycol: Non-Newtonian behavior. Exp. Therm. Fluid Sci. 2016, 79, 231–237. [Google Scholar] [CrossRef]
- Afrand, M.; Toghraie, D.; Ruhani, B. Effects of temperature and nanoparticles concentration on rheological behavior of Fe3O4–Ag/EG hybrid nanofluid: An experimental study. Exp. Therm. Fluid Sci. 2016, 77, 38–44. [Google Scholar] [CrossRef]
- Afrand, M. Experimental study on thermal conductivity of ethylene glycol containing hybrid nano-additives and development of a new correlation. Appl. Therm. Eng. 2017, 110, 1111–1119. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the 1995 International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995; p. 66. [Google Scholar]
- Amjad, M.; Jin, H.; Du, X.; Wen, D. Experimental photothermal performance of nanofluids under concentrated solar flux. Solar Energy Mater. Solar Cells 2018, 182, 255–262. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Zhu, J.; Shuai, Y.; Jiang, B.; Huang, Y. An experimental investigation on sunlight absorption characteristics of silver nanofluids. Solar Energy 2015, 115, 85–94. [Google Scholar] [CrossRef]
- Rativa, D.; Gómez-Malagón, L.A. Solar radiation absorption of nanofluids containing metallic nanoellipsoids. Solar Energy 2015, 118, 419–425. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Zeng, S.; Zheng, Z. Experimental investigation on photothermal properties of nanofluids for direct absorption solar thermal energy systems. Energy Convers. Manag. 2013, 73, 150–157. [Google Scholar] [CrossRef]
- Qu, J.; Tian, M.; Han, X.; Zhang, R.; Wang, Q. Photo-thermal conversion characteristics of MWCNT-H2O nanofluids for direct solar thermal energy absorption applications. Appl. Therm. Eng. 2017, 124, 486–493. [Google Scholar] [CrossRef]
- He, Y.; Chen, M.; Wang, X.; Hu, Y. Plasmonic multi-thorny Gold nanostructures for enhanced solar thermal conversion. Solar Energy 2018, 171, 73–82. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Hu, Y.; Jin, G.; Jiang, B.; Huang, Y. Photothermal-conversion-enhanced photocatalytic activity of flower-like CuS superparticles under solar light irradiation. Solar Energy 2018, 170, 586–593. [Google Scholar] [CrossRef]
- Hazra, S.K.; Ghosh, S.; Nandi, T.K. Photo-thermal conversion characteristics of carbon black-ethylene glycol nanofluids for applications in direct absorption solar collectors. Appl. Therm. Eng. 2019, 163, 114402. [Google Scholar] [CrossRef]
- Tong, Y.; Boldoo, T.; Ham, J.; Cho, H. Improvement of PTEC performance of MWCNT/Fe3O4 hybrid nanofluid compared to Fe3O4 nanofluid. Energy 2020, 196, 117086. [Google Scholar] [CrossRef]
- Nkurikiyimfura, I.; Wang, Y.; Pan, Z. Heat transfer enhancement by magnetic nanofluids—A review. Renew. Sustain. Energy Rev. 2013, 21, 548–561. [Google Scholar] [CrossRef]
- Novopashin, S.A.; Serebryakova, M.A.; Khmel, S.Y. Methods of magnetic fluid synthesis (review). Thermophys. Aeromech. 2015, 22, 397–412. [Google Scholar] [CrossRef]
- Stephen Papell, S. Low Viscosity Magnetic Fluid Obtained by the Colloidal Suspension of Magnetic Particles. US Patent 3215572A, 2 November 1965. [Google Scholar]
- López, J.; González-Bahamón, L.F.; Prado, J.; Caicedo, J.C.; Zambrano, G.; Gómez, M.E.; Esteve, J.; Prieto, P. Study of magnetic and structural properties of ferrofluids based on cobalt–zinc ferrite nanoparticles. J. Magn. Magn. Mater. 2012, 324, 394–402. [Google Scholar] [CrossRef]
- Philip, J.; Damodaran, S.P.; Raj, B. Enhancement of thermal conductivity in magnetite based nanofluid due to chainlike structures. Appl. Phys. Lett. 2007, 91, 203108. [Google Scholar] [CrossRef]
- Abareshi, M.; Goharshadi, E.; Zebarjad, S.; Khandan Fadafan, H.; Youssefi, A. Fabrication, Characterization and Measurement of Thermal Conductivity of Fe3O4 Nanofluids. J. Magn. Magn. Mater. 2010, 322, 3895–3901. [Google Scholar] [CrossRef]
- Djurek, I.; Znidarsic, A.; Kosak, A.; Djurek, D. Thermal conductivity measurements of the CoFe2O4 and gamma-Fe2O3 based nanoparticle ferrofluids. Croat. Chem. Acta 2007, 80, 529–532. [Google Scholar]
- Aksenov, V.L.; Avdeev, M.V.; Balasoiu, M.; Bica, D.; Rosta, L.; Török, G.; Vekas, L. Aggregation in non-ionic water-based ferrofluids by small-angle neutron scattering. J. Magn. Magn. Mater. 2003, 258–259, 452–455. [Google Scholar] [CrossRef]
- Karimi, A.; Afghahi, S.S.S.; Shariatmadar, H.; Ashjaee, M. Experimental investigation on thermal conductivity of MFe2O4 (M=Fe and Co) magnetic nanofluids under influence of magnetic field. Thermochim. Acta 2014, 598, 59–67. [Google Scholar] [CrossRef]
- Sundar, L.; Singh, M.; Ramana, E.; Singh, B.; Grácio, J.; Sousa, A. Enhanced Thermal Conductivity and Viscosity of Nanodiamond-Nickel Nanocomposite Nanofluids. Sci. Rep. 2014, 4, 4039. [Google Scholar] [CrossRef]
- Altan, C.; Elkatmış, A.; Aslan, N.; Bucak, S. Enhancement of thermal conductivity upon application of magnetic field to Fe3O4 nanofluids. J. Appl. Phys. 2011, 110, 093917. [Google Scholar] [CrossRef]
- Azizian, R.; Doroodchi, E.; Moghtaderi, B. Influence of Controlled Aggregation on Thermal Conductivity of Nanofluids. J. Heat Transf. 2015, 138. [Google Scholar] [CrossRef]
- Parekh, K.; Lee, H. Experimental Investigation of Thermal Conductivity of magnetic nanofluids. AIP Conf. Proc. 2012, 1447, 385–386. [Google Scholar]
- Arana, M.; Bercoff, P.G.; Jacobo, S.E. Thermomagnetic characterization of organic-based ferrofluids prepared with Ni ferrite nanoparticles. Mater. Sci. Eng. B 2017, 215, 1–8. [Google Scholar] [CrossRef]
- Nabeel Rashin, M.; Hemalatha, J. Magnetic and ultrasonic studies on stable cobalt ferrite magnetic nanofluid. Ultrasonics 2014, 54, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Nabeel Rashin, M.; Hemalatha, J. Magnetic and ultrasonic investigations on magnetite nanofluids. Ultrasonics 2012, 52, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Chopkar, M.; Sudarshan, S.; Das, P.K.; Manna, I. Effect of Particle Size on Thermal Conductivity of Nanofluid. Metall. Mater. Trans. A 2008, 39, 1535–1542. [Google Scholar] [CrossRef]
- Li, Q.; Xuan, Y.; Wang, J. Experimental investigations on transport properties of magnetic fluids. Exp. Therm. Fluid Sci. 2005, 30, 109–116. [Google Scholar] [CrossRef]
- Hatami, N.; Kazemnejad Banari, A.; Malekzadeh, A.; Pouranfard, A.R. The effect of magnetic field on nanofluids heat transfer through a uniformly heated horizontal tube. Phys. Lett. A 2017, 381, 510–515. [Google Scholar] [CrossRef]
- Zablotsky, D.; Mezulis, A.; Blums, E. Surface cooling based on the thermomagnetic convection: Numerical simulation and experiment. Int. J. Heat Mass Transf. 2009, 52, 5302–5308. [Google Scholar] [CrossRef]
- Dorofeev, G.A.; Streletskii, A.N.; Povstugar, I.V.; Protasov, A.V.; Elsukov, E.P. Determination of nanoparticle sizes by X-ray diffraction. Colloid J. 2012, 74, 675–685. [Google Scholar] [CrossRef]
- Jin, H.; Lin, G.; Bai, L.; Amjad, M.; Bandarra Filho, E.P.; Wen, D. Photothermal conversion efficiency of nanofluids: An experimental and numerical study. Solar Energy 2016, 139, 278–289. [Google Scholar] [CrossRef]
- Bandarra Filho, E.P.; Mendoza, O.S.H.; Beicker, C.L.L.; Menezes, A.; Wen, D. Experimental investigation of a silver nanoparticle-based direct absorption solar thermal system. Energy Conv. Manag. 2014, 84, 261–267. [Google Scholar] [CrossRef]
- Bragg, W.H. The Structure of Magnetite and the Spinels. Nature 1915, 95, 561. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Sendhilnathan, S.; Arulmurugan, R. Structural and magnetic properties of Co1−xZnxFe2O4 nanoparticles by co-precipitation method. J. Magn. Magn. Mater. 2007, 313, 293–299. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. 3—Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 43–58. [Google Scholar]
- Malekzadeh, A.; Pouranfard, A.; Hatami, N.; Banari, A.; Rahimi, M.R. Experimental Investigations on the Viscosity of Magnetic Nanofluids under the Influence of Temperature, Volume Fractions of Nanoparticles and External Magnetic Field. J. Appl. Fluid Mech. 2016, 9, 693–697. [Google Scholar] [CrossRef]




| Reference | Particles | Diameter (nm) | Base Fluid | Concentration |
|---|---|---|---|---|
| [27] | Fe3O4 | 6.7 | Kerosene | 0–7.8 vol% |
| [28] | α-Fe2O3 | 5 | Glycerol | 0.155–0.75 vol% |
| [29] | γ-Fe2O3 | 8–15 | H2O, n-decane | 65–300 g/L |
| [30] | Fe3O4, CoFe2O4 | le | H2O | 2–6.5 vol% |
| [31] | Fe3O4 | 10 | H2O | 0–4.8 vol% |
| [32] | ND, Ni | 30 | H2O/EG | 0–3.03 wt% |
| [33] | Fe3O4 | 10 | H2O, kerosene | 1–7 wt% |
| [34] | Fe3O4, Fe | 60, 35–45 | H2O | 0.86 vol% |
| [35] | Fe3O4, Mn-Zn | 6.7 | Kerosene | 0–10 vol% |
| [36] | Ni Fe2O3 | 50 | Kerosene | 5, 10 vol% |
| [37] | CoFe2O4 | 14 | H2O | 0.2–1 vol% |
| [38] | Fe3O4 | 11 | H2O | 0.2–1 vol% |
| Item | Thermal Conductivity Meter | Viscometer | Spectrometer | K-Type Thermocouple | Irradiance Meter |
|---|---|---|---|---|---|
| Measurement range | 0.02–2 W/m·°C | 0.3–10,000 mPa·s | 200–1300 nm | −200–1300 °C | 0–1500 W/m2 |
| Accuracy | ±5% | ±1% | 0.04–20 nm | ±2.2 °C | ±5% |
| Zeta Potential (mV) | Stability |
|---|---|
| 0–15 | No stability |
| 15–30 | Moderate stability |
| 30–45 | High dispersion stability, low tendency to settle to the bottom |
| 45–60 | Great stability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boldoo, T.; Ham, J.; Cho, H. Comprehensive Experimental Study on the Thermophysical Characteristics of DI Water Based Co0.5Zn0.5Fe2O4 Nanofluid for Solar Thermal Harvesting. Energies 2020, 13, 6218. https://doi.org/10.3390/en13236218
Boldoo T, Ham J, Cho H. Comprehensive Experimental Study on the Thermophysical Characteristics of DI Water Based Co0.5Zn0.5Fe2O4 Nanofluid for Solar Thermal Harvesting. Energies. 2020; 13(23):6218. https://doi.org/10.3390/en13236218
Chicago/Turabian StyleBoldoo, Tsogtbilegt, Jeonggyun Ham, and Honghyun Cho. 2020. "Comprehensive Experimental Study on the Thermophysical Characteristics of DI Water Based Co0.5Zn0.5Fe2O4 Nanofluid for Solar Thermal Harvesting" Energies 13, no. 23: 6218. https://doi.org/10.3390/en13236218
APA StyleBoldoo, T., Ham, J., & Cho, H. (2020). Comprehensive Experimental Study on the Thermophysical Characteristics of DI Water Based Co0.5Zn0.5Fe2O4 Nanofluid for Solar Thermal Harvesting. Energies, 13(23), 6218. https://doi.org/10.3390/en13236218





