Design and Fabrication of Solar Thermal Energy Storage System Using Potash Alum as a PCM
Abstract
1. Introduction
2. Design and Analysis
2.1. Design Requirements
2.2. Selection of Potash Alum as PCM and Heat Transfer Fluid
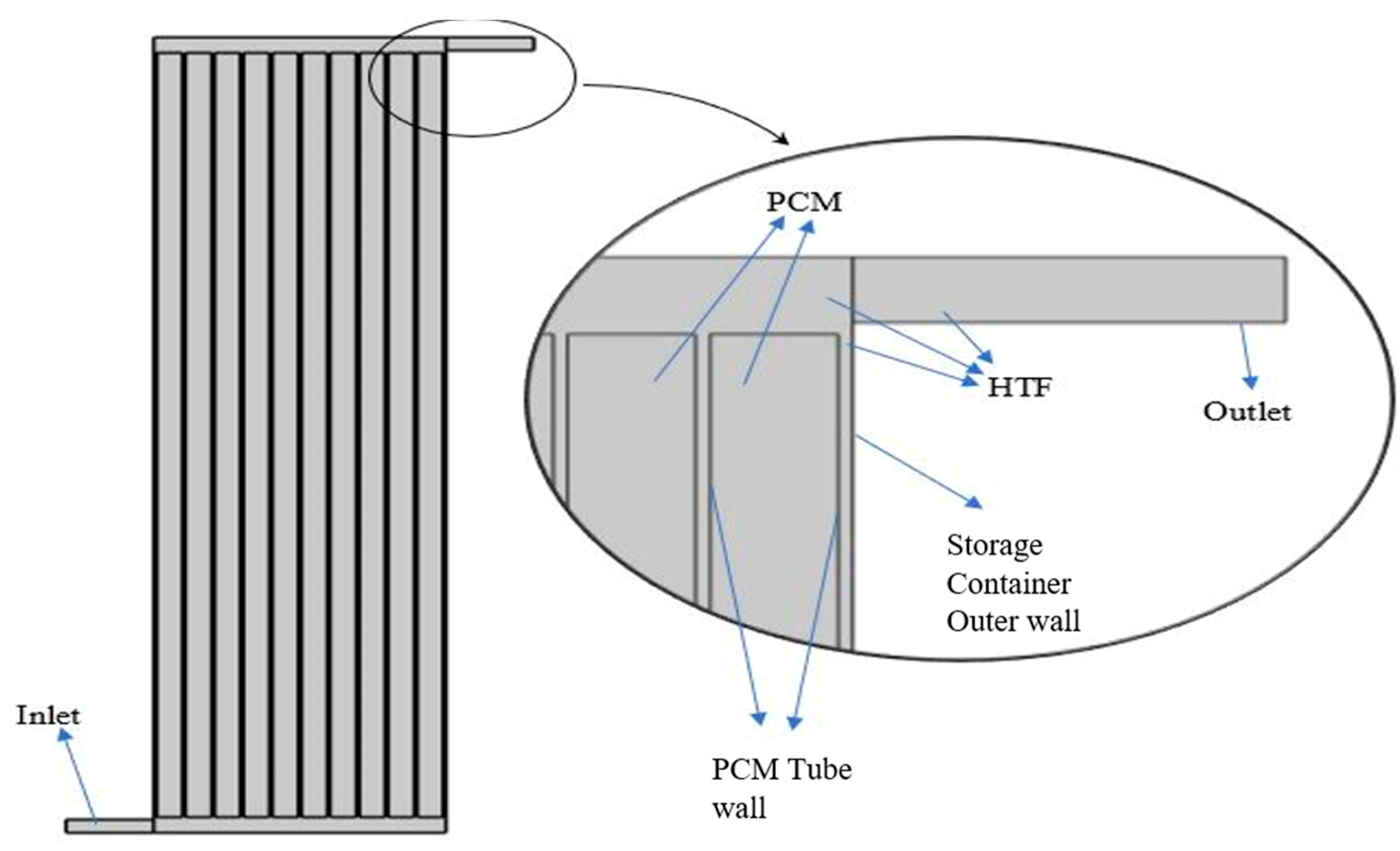
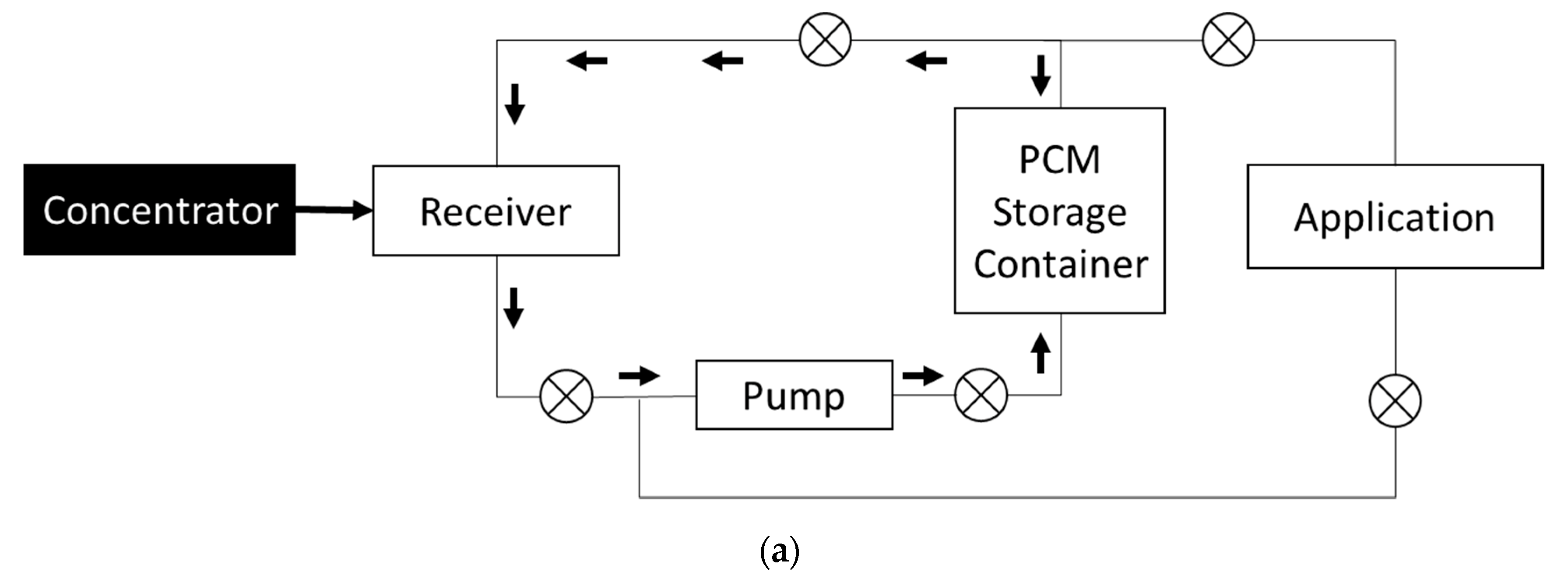
2.3. Modeling and Design of Storage Tank
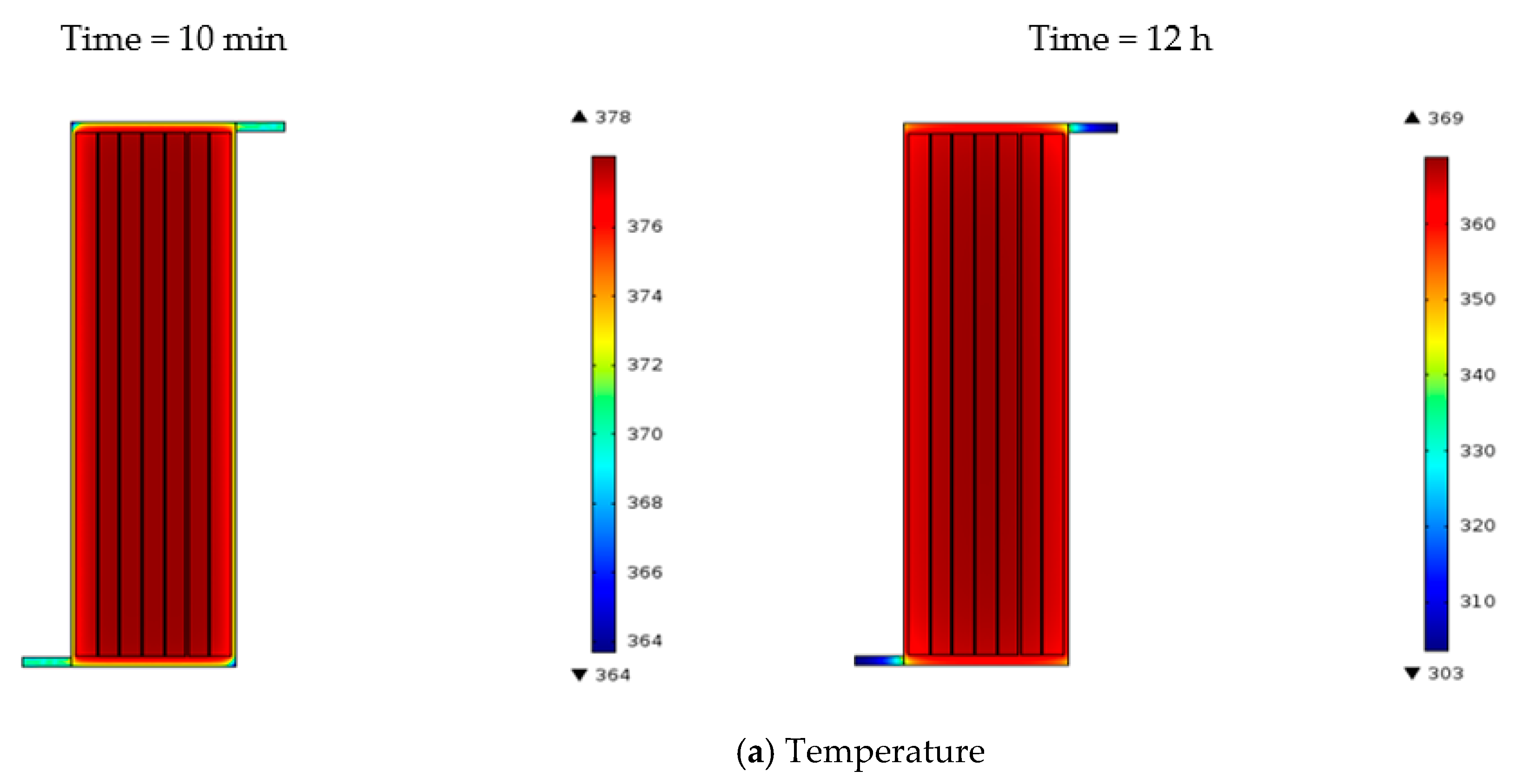
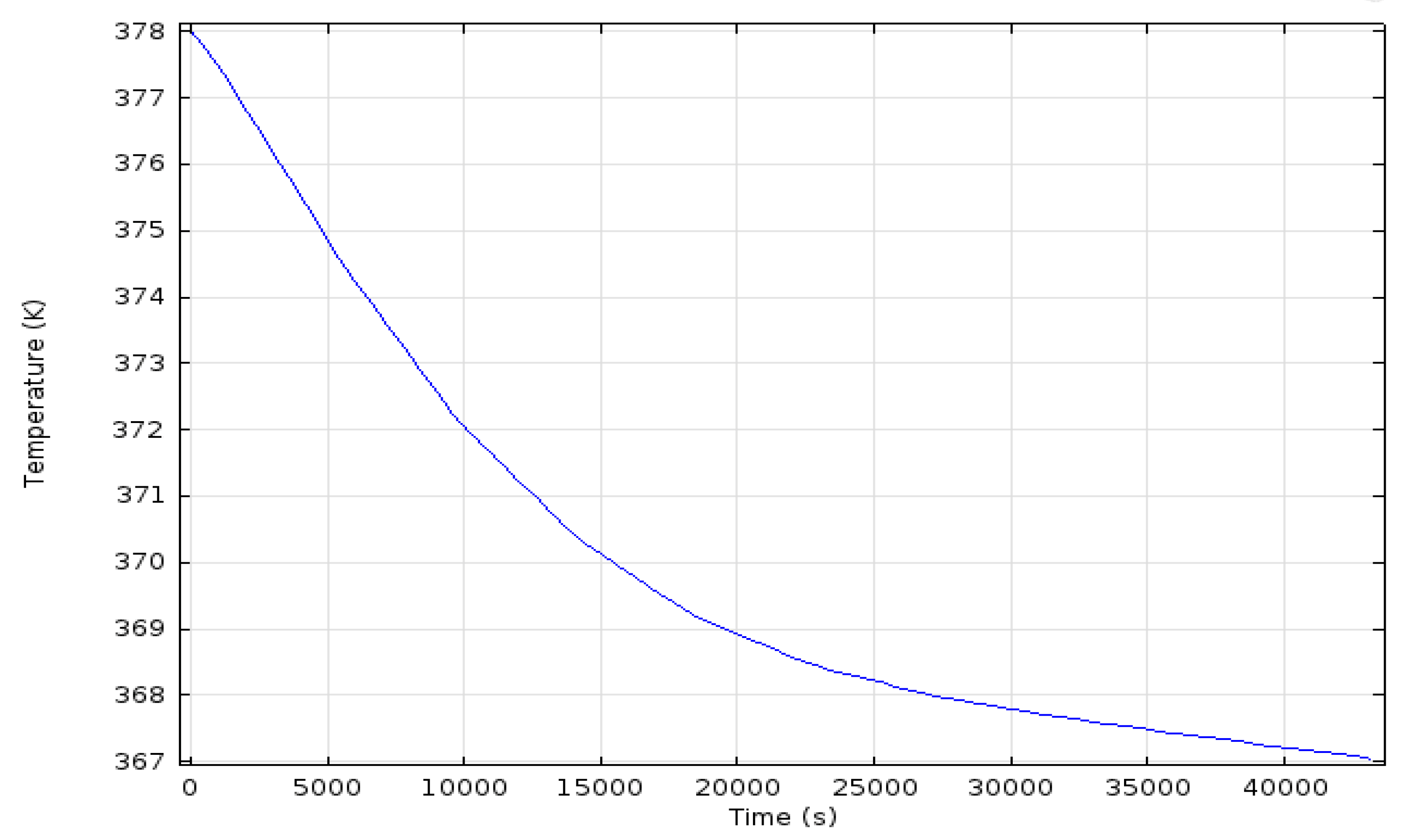
3. Simulation Results
Simulation for Charging Time
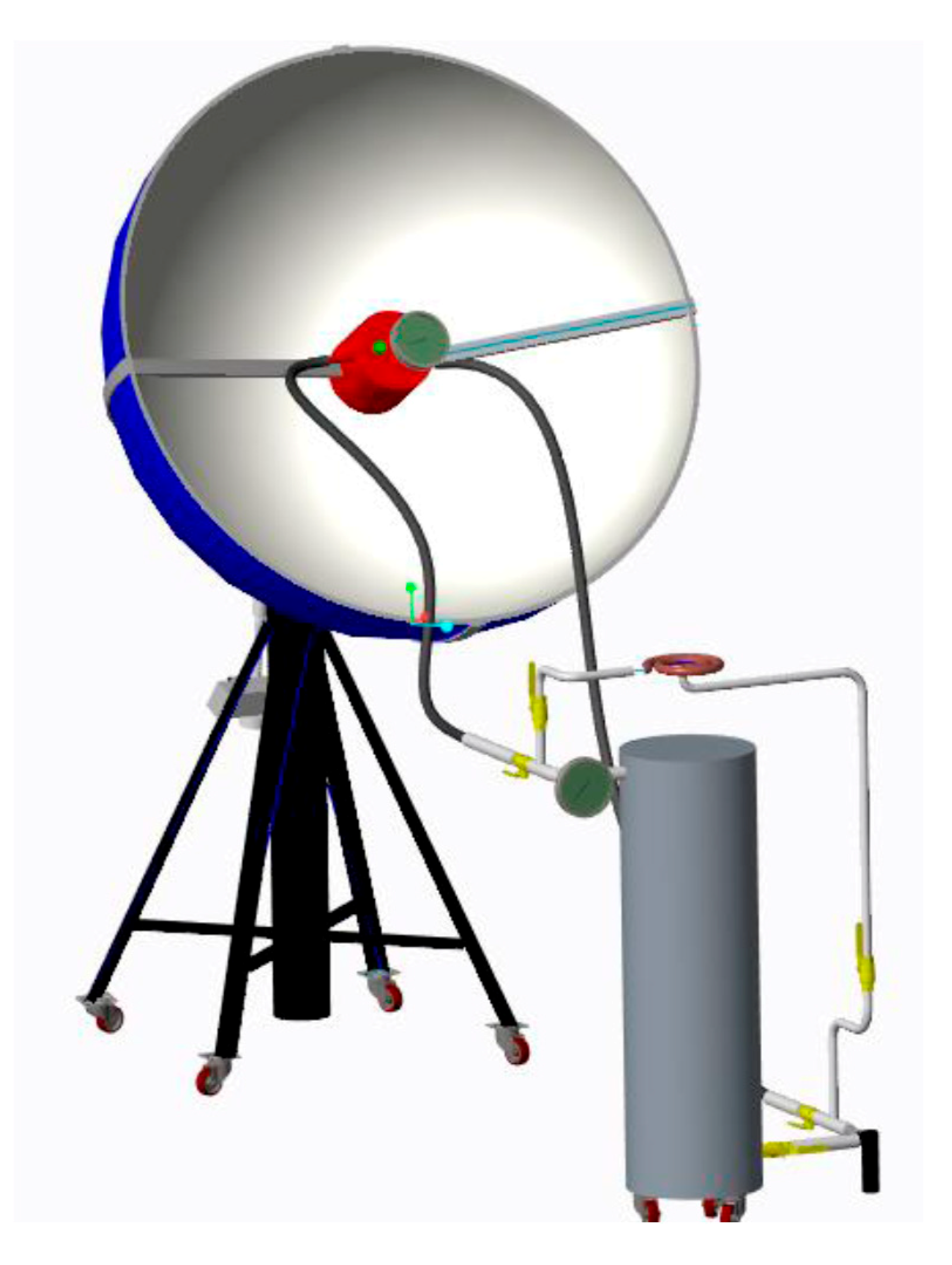
4. Fabrication
4.1. Prototype Modeling of Solar Concentrator
4.2. Design of Solar Concentrator
4.3. PCM Tubes
4.4. Storage Tank
4.5. Assembled Design
5. Experimental Results and Discussion
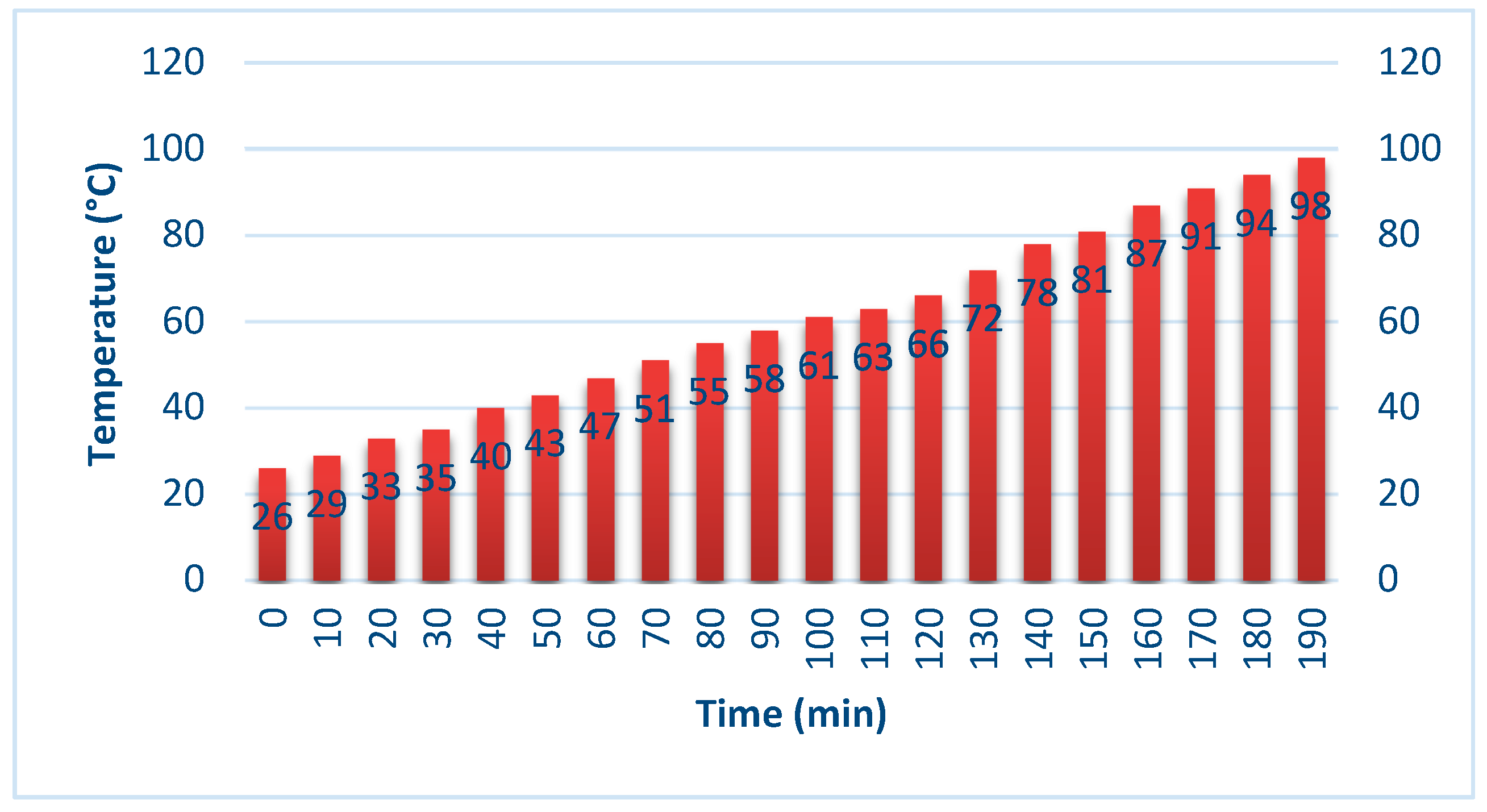
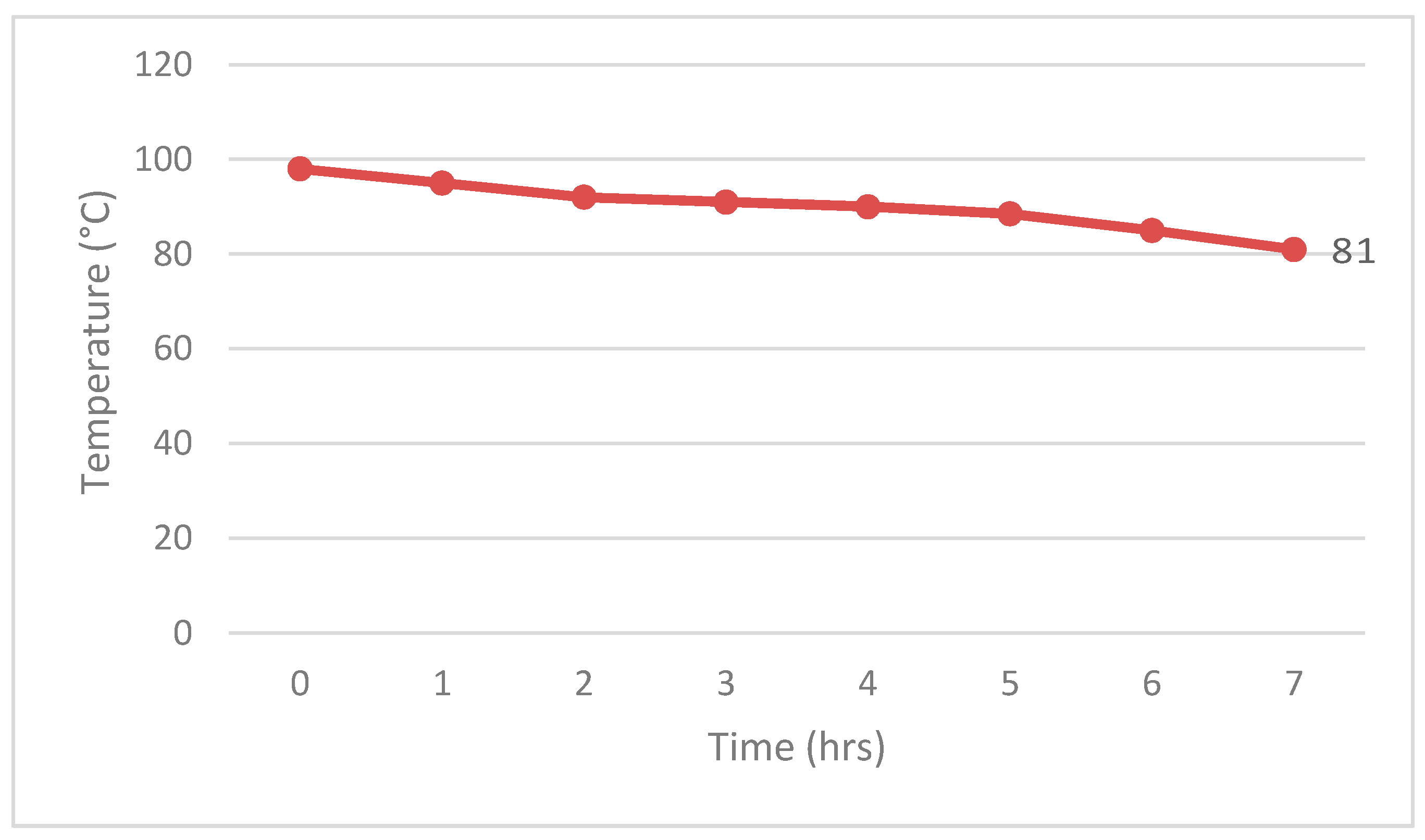
5.1. Experimental Results
5.2. Analysis and Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, M.-J.; Tao, W.-Q. Review of methodologies and polices for evaluation of energy efficiency in high energy-consuming industry. Appl. Energy 2017, 187, 203–215. [Google Scholar] [CrossRef]
- Ascione, F. Energy conservation and renewable technologies for buildings to face the impact of the climate change and minimize the use of cooling. Sol. Energy 2017, 154, 34–100. [Google Scholar] [CrossRef]
- Kittner, N.; Lill, F.; Kammen, D.M. Energy storage deployment and innovation for the clean energy transition. Nat. Energy 2017, 2, 17125. [Google Scholar] [CrossRef]
- Prasad, A.A.; Taylor, R.A.; Kay, M. Assessment of solar and wind resource synergy in Australia. Appl. Energy 2017, 190, 354–367. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Guo, S.; Zhou, H. Solar energy storage in the rechargeable batteries. Nano Today 2017, 16, 46–60. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Pelay, U.; Luo, L.; Fan, Y.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Esteves, L.; Magalhães, A.; Ferreira, V.M.; Pinho, C. Test of Two Phase Change Materials for Thermal Energy Storage: Determination of the Global Heat Transfer Coefficient. ChemEngineering 2018, 2, 10. [Google Scholar] [CrossRef]
- Zeinelabdein, R.; Omer, S.; Gan, G. Critical review of latent heat storage systems for free cooling in buildings. Renew. Sustain. Energy Rev. 2018, 82, 2843–2868. [Google Scholar] [CrossRef]
- Patankar, S. Numerical Heat Transfer and Fluid Flow; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- González-Roubaud, E.; Pérez-Osorio, D.; Prieto, C. Review of commercial thermal energy storage in concentrated solar power plants: Steam vs. molten salts. Renew. Sustain. Energy Rev. 2017, 80, 133–148. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, Z.; Zhu, H.; Wang, Y.L.; Peng, S. Melting heat transfer characteristics of a composite phase change material fabricated by paraffin and metal foam. Appl. Energy 2017, 185, 1971–1983. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sari, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Cao, Z.; Yang, D.W.; Sun, L.-X.; Zhang, L. Thermal conductivity enhancement of Ag nanowires on an organic phase change material. J. Therm. Anal. Calorim. 2009, 101, 385–389. [Google Scholar] [CrossRef]
- Shukla, A.; Buddhi, D.; Sawhney, R. Thermal cycling test of few selected inorganic and organic phase change materials. Renew. Energy 2008, 33, 2606–2614. [Google Scholar] [CrossRef]
- Singh, R.; Sadeghi, S.; Shabani, B. Thermal Conductivity Enhancement of Phase Change Materials for Low-Temperature Thermal Energy Storage Applications. Energies 2018, 12, 75. [Google Scholar] [CrossRef]
- Bushnell, D.L. Performance studies of a solar energy storing heat exchanger. Sol. Energy 1988, 41, 503–512. [Google Scholar] [CrossRef]
- Sharma, S.; Buddhi, D.; Sawhney, R.; Sharma, A. Design, development and performance evaluation of a latent heat storage unit for evening cooking in a solar cooker. Energy Convers. Manag. 2000, 41, 1497–1508. [Google Scholar] [CrossRef]
- Buddhi, D.; Sharma, S.; Sharma, A. Thermal performance evaluation of a latent heat storage unit for late evening cooking in a solar cooker having three reflectors. Energy Convers. Manag. 2003, 44, 809–817. [Google Scholar] [CrossRef]
- Schmerse, E.; Ikutegbe, C.A.; Auckaili, A.; Farid, M.M. Using PCM in Two Proposed Residential Buildings in Christchurch, New Zealand. Energies 2020, 13, 6025. [Google Scholar] [CrossRef]
- Nemś, A.; Puertas, A.M. Model for the Discharging of a Dual PCM Heat Storage Tank and its Experimental Validation. Energies 2020, 13, 5687. [Google Scholar] [CrossRef]
- Leang, E.; Tittelein, P.; Zalewski, L.; Lassue, S. Design Optimization of a Composite Solar Wall Integrating a PCM in a Individual House: Heating Demand and Thermal Comfort Considerations. Energies 2020, 13, 5640. [Google Scholar] [CrossRef]
- Pasupathi, M.K.; Alagar, K.; Mm, M.; Aritra, G. Characterization of Hybrid-nano/Paraffin Organic Phase Change Material for Thermal Energy Storage Applications in Solar Thermal Systems. Energies 2020, 13, 5079. [Google Scholar] [CrossRef]
- Wei, G.; Wang, G.; Xu, C.; Ju, X.; Xing, L.; Du, X.; Yang, Y. Selection principles and thermophysical properties of high temperature phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2018, 81, 1771–1786. [Google Scholar] [CrossRef]
- Qiu, S.; Solomon, L.; Fang, M. Study of Material Compatibility for a Thermal Energy Storage System with Phase Change Material. Energies 2018, 11, 572. [Google Scholar] [CrossRef]
- Palomba, V.; Brancato, V.; Palomba, G.; Borsacchi, S.; Forte, C.; Freni, A.; Frazzica, A. Latent Thermal Storage for Solar Cooling Applications: Materials Characterization and Numerical Optimization of Finned Storage Configurations. Heat Transf. Eng. 2018, 40, 1033–1048. [Google Scholar] [CrossRef]
- Prabhu, A.N.; Shetty, P.K.; Vali, I.; Kumar, H.; Udupa, S. Gamma and neutron irradiation effects on the structural and optical properties of potash alum crystals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2017, 413, 37–41. [Google Scholar] [CrossRef]
- Benoit, H.; Spreafico, L.; Gauthier, D.J.; Flamant, G. Review of heat transfer fluids in tube-receivers used in concentrating solar thermal systems: Properties and heat transfer coefficients. Renew. Sustain. Energy Rev. 2016, 55, 298–315. [Google Scholar] [CrossRef]
- Lorenzin, N.; Abánades, A. A review on the application of liquid metals as heat transfer fluid in Concentrated Solar Power technologies. Int. J. Hydrog. Energy 2016, 41, 6990–6995. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Hu, Y.; Jiang, B.; Huang, Y. Thermophysical and natural convection characteristics of ethylene glycol and water mixture based ZnO nanofluids. Int. J. Heat Mass Transf. 2015, 91, 385–389. [Google Scholar] [CrossRef]









| S. No | Description, Symbol (Units) | Values |
|---|---|---|
| 1 | Temperature requirement, T (°C) | 95–97 |
| 2 | Cooking time, t (minutes) | 55 |
| 3 | Power of electric Heater, Ph (W) | 1000 |
| 4 | Required heat, Q (kJ) | 3300 |
| 5 | Heat losses, Ql (kJ) | 100 |
| 6 | Total Heat, QT (kJ) | 3400 |
| 7 | Steel pipe inner diameter (inches) | 1 |
| 8 | Steel pipe length (inches) | 30 |
| Thermal Properties | Physical Properties | Kinetic Properties | Chemical Properties | Economics |
|---|---|---|---|---|
| Suitable phase-transition temperature | Favorable phase equilibrium | No super cooling | Long-term chemical stability | Abundant |
| High latent heat of transition | High density | Sufficient crystallization rate | Compatibility with materials of construction | Available |
| Good heat transfer | Small volume change | No toxicity | Cost effective | |
| Low vapor pressure | No fire hazard |
| S.No | Description, Symbol (Units) | Values |
|---|---|---|
| 1 | Heat of fusion of potash alum (PCM), hm (kJ/kg) | 184 |
| 2 | Required mass of potash alum (PCM), mpcm (kg) | 18.4 |
| 3 | Specific heat of potash alum in the liquid phase (melted), Clp (kJ/kg.K) | 2.76 |
| 4 | Specific heat of potash alum in liquid phase, Csp (kJ/kg.K) | 1.38 |
| 5 | Melted fraction, am | 1 |
| 6 | Melting point of potash alum, Tm (°C) | 92 |
| 7 | Initial temperature, Ti (°C) | 10 |
| 8 | Final temperature, Tf (°C) | 140 |
| 9 | Total heat storage capacity, Qpcm (kJ) | 4591 |
| 10 | Density of potash alum in the liquid phase (melted), ρi (kg/m3) | 1300 |
| 11 | Volume of potash alum, V (m3) | 0.0079 |
| 12 | Outer diameter of cylinder, doo (m) | 0.201 |
| 13 | Inner diameter of cylinder, dii (m) | 0.2 |
| 14 | Inner diameter of tube, dit (m) | 0.0254 |
| 15 | Number of tubes, n | 89 |
| 16 | Length of PCM tube, Lpcmt (m) | 0.74 |
| 17 | Length of storage tank, Lst (m) | 0.77 |
| 18 | Inner radius of storage tank inlet, rii (m) | 0.00635 |
| 19 | Inner inlet area, Ai (m2) | 0.000125 |
| 20 | Volume flow rate, q` (m3/s) | 1.235 × 10−5 |
| 21 | Mass flow rate, m` (kg/s) | 0.013 |
| 22 | Velocity of HTF at the inlet, u (m/s) | 0.1 |
| 23 | Viscosity of HTF (at 20 °C), µhtf (Pa.S) | 2.1671 × 10−3 |
| 24 | Reynold’s number, Re | 605 |
| 25 | Required flow rate of pump, (m3/s) | 2.5 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, M.S.; Iftikhar, N.; Wadood, A.; Khan, M.O.; Asghar, M.U.; Khan, S.; Khurshaid, T.; Kim, K.-C.; Rehman, Z.; Rizvi, S.T.u.I. Design and Fabrication of Solar Thermal Energy Storage System Using Potash Alum as a PCM. Energies 2020, 13, 6169. https://doi.org/10.3390/en13236169
Malik MS, Iftikhar N, Wadood A, Khan MO, Asghar MU, Khan S, Khurshaid T, Kim K-C, Rehman Z, Rizvi STuI. Design and Fabrication of Solar Thermal Energy Storage System Using Potash Alum as a PCM. Energies. 2020; 13(23):6169. https://doi.org/10.3390/en13236169
Chicago/Turabian StyleMalik, Muhammad Suleman, Naveed Iftikhar, Abdul Wadood, Muhammad Omer Khan, Muhammad Usman Asghar, Shahbaz Khan, Tahir Khurshaid, Ki-Chai Kim, Zabdur Rehman, and S. Tauqeer ul Islam Rizvi. 2020. "Design and Fabrication of Solar Thermal Energy Storage System Using Potash Alum as a PCM" Energies 13, no. 23: 6169. https://doi.org/10.3390/en13236169
APA StyleMalik, M. S., Iftikhar, N., Wadood, A., Khan, M. O., Asghar, M. U., Khan, S., Khurshaid, T., Kim, K.-C., Rehman, Z., & Rizvi, S. T. u. I. (2020). Design and Fabrication of Solar Thermal Energy Storage System Using Potash Alum as a PCM. Energies, 13(23), 6169. https://doi.org/10.3390/en13236169








