Molecular Dynamics Simulation of CO2 Diffusion in a Carbonated Water–Decane System
Abstract
1. Introduction
2. Simulation Method
2.1. Simulation Details
2.2. Calculation Methods
3. Results and Discussion
3.1. CO2 Diffusion Behavior after Phases Contact
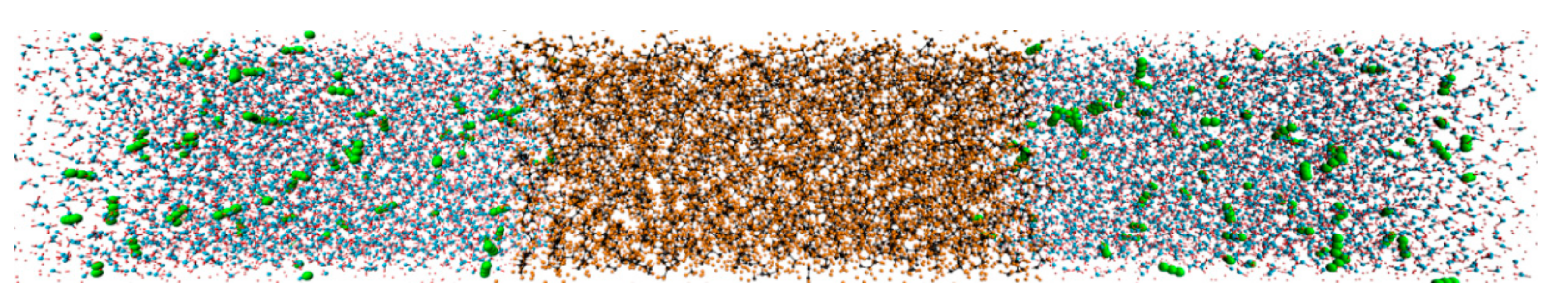
3.1.1. Diffusion Process and Distribution of CO2
3.1.2. Mechanism of CO2 Diffusion Process
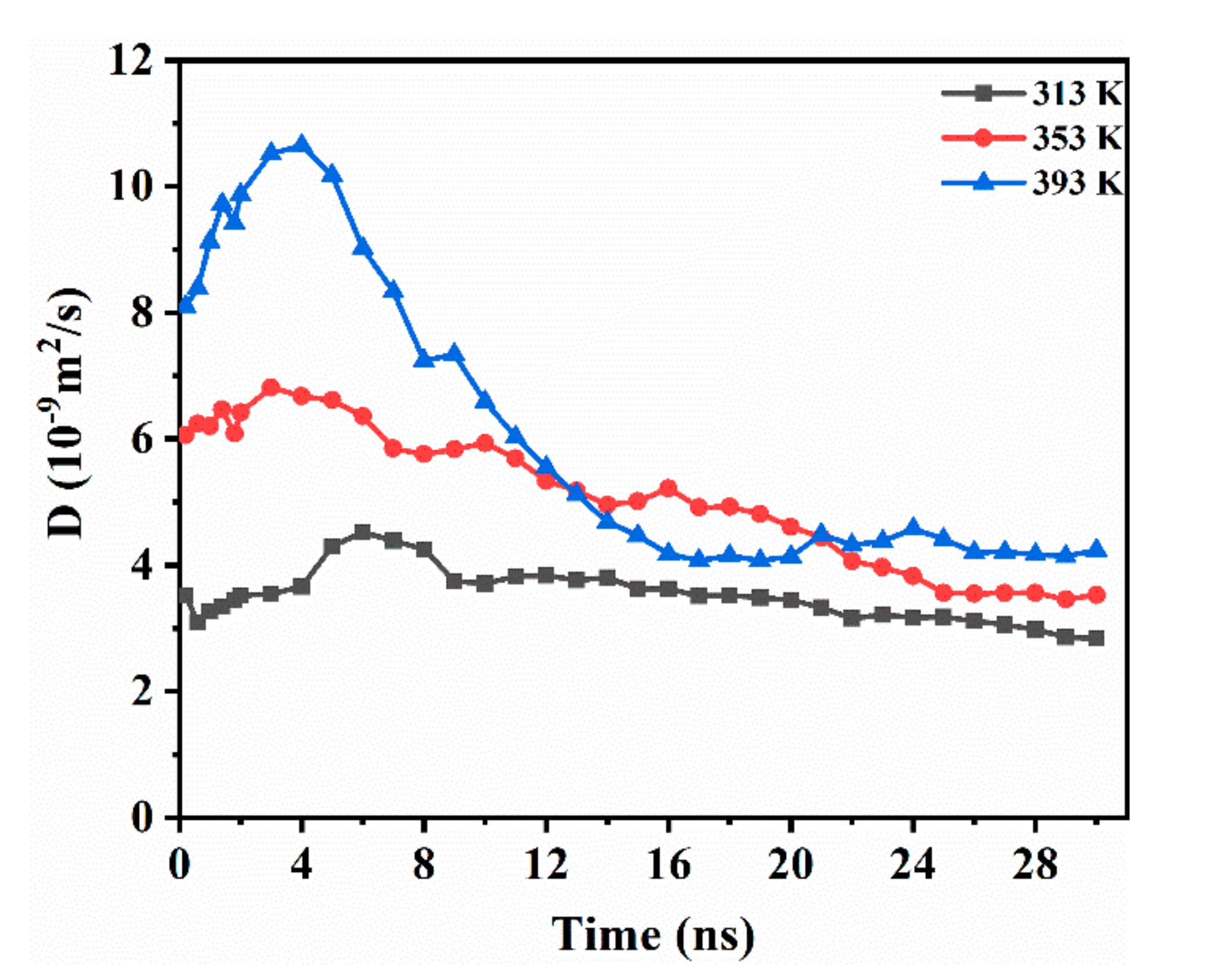
3.2. CO2 Diffusion Coefficient and Its Effect on Phase Diffusivity
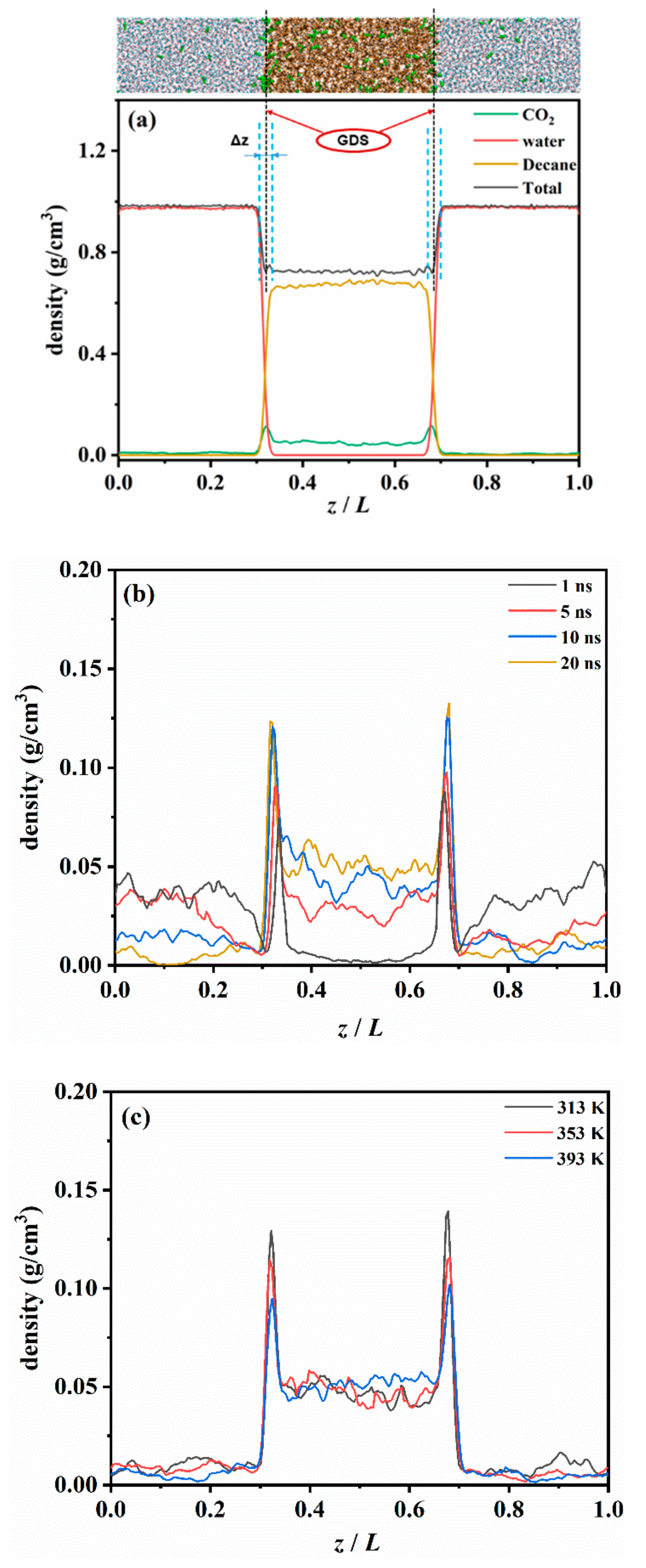
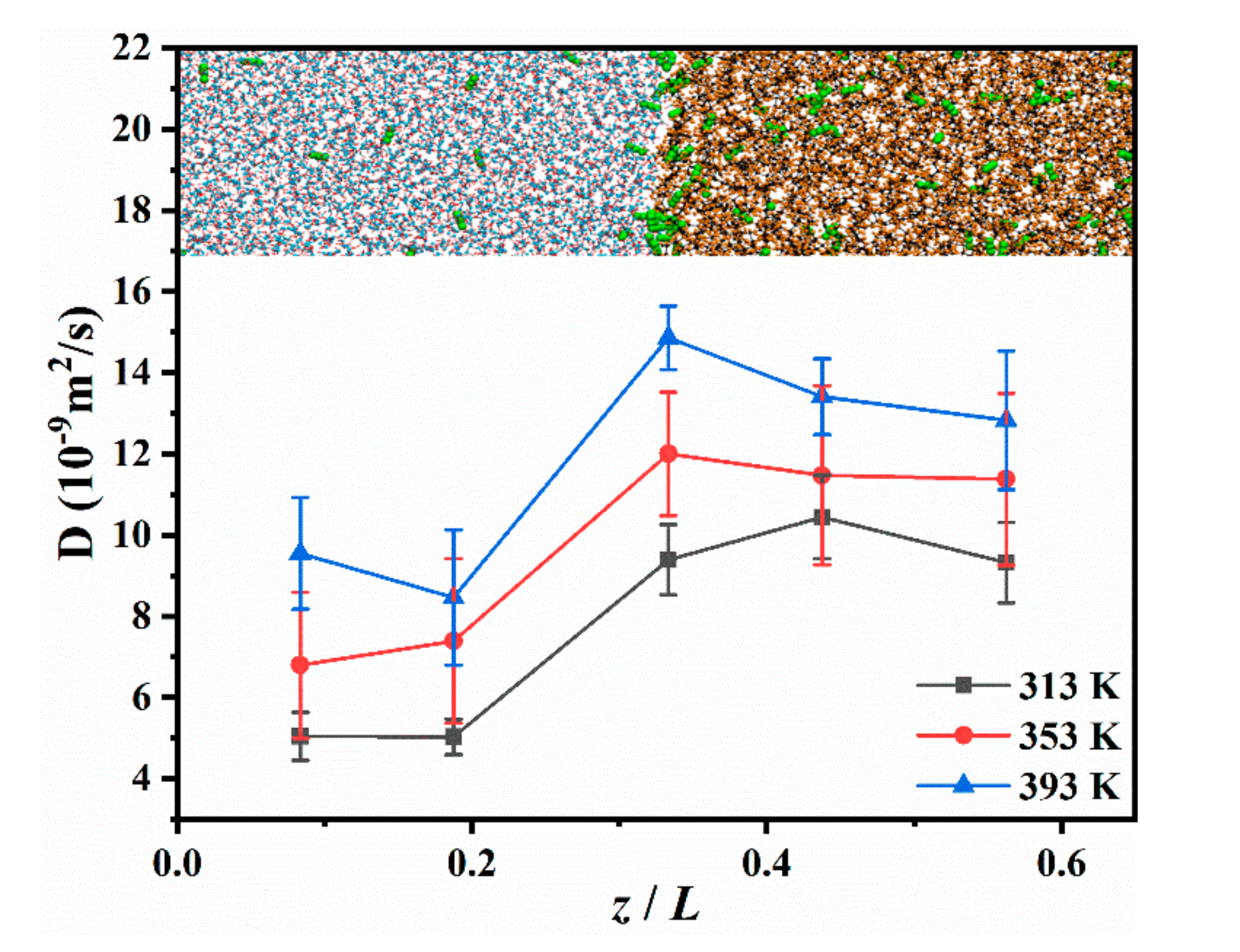
3.2.1. The DCO2 along the Vertical Interface Direction
3.2.2. The DCO2 in Different Regions of the CW–Decane System
3.2.3. Effect of CO2 on Diffusivity of Oleic Phase
3.3. CO2 Accumulation and Its Effect on the CW–Decane Interface
3.3.1. Interfacial Tension of the CW–Decane Interface
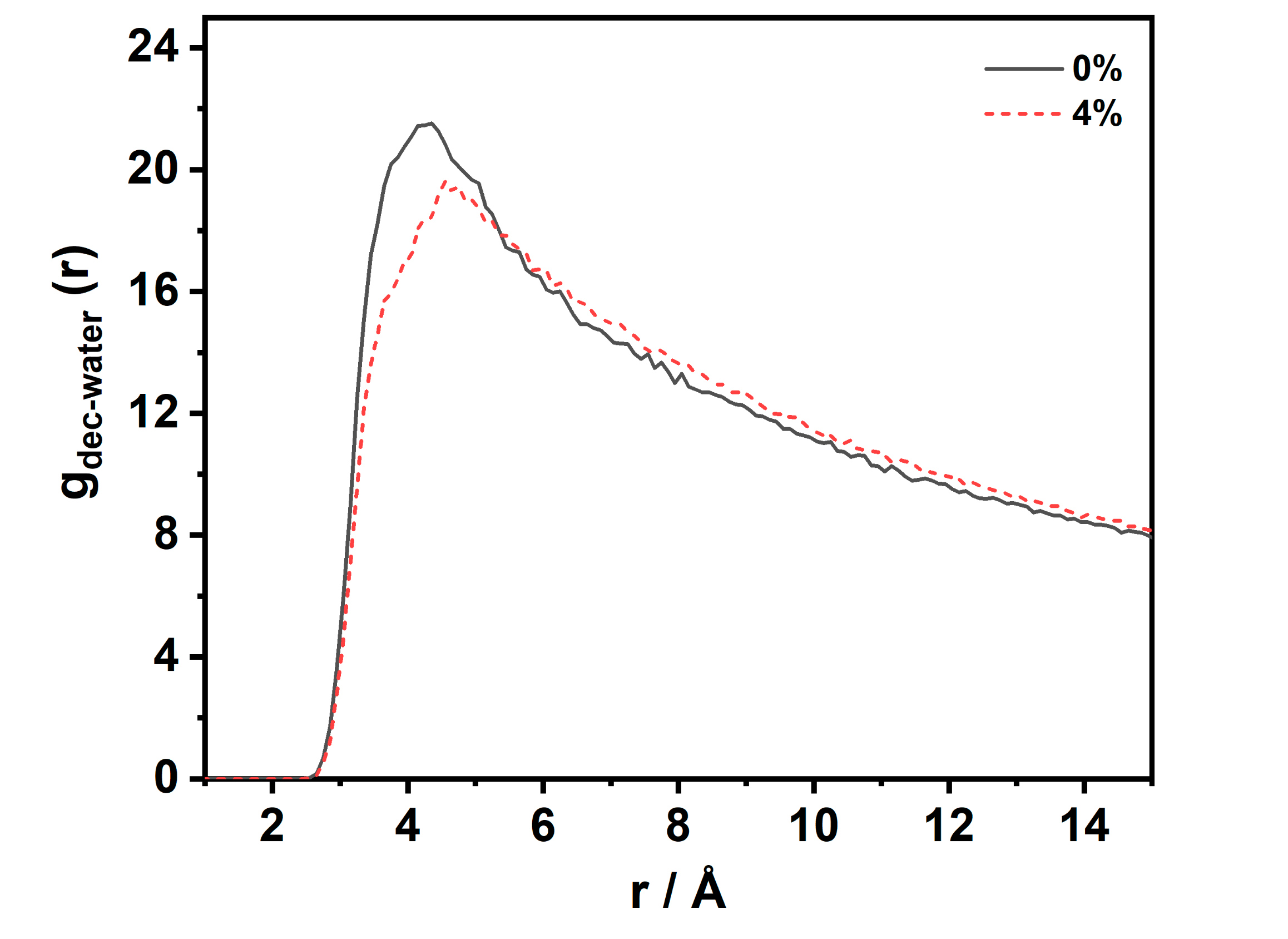
3.3.2. Structural Properties of CW–Decane Interface
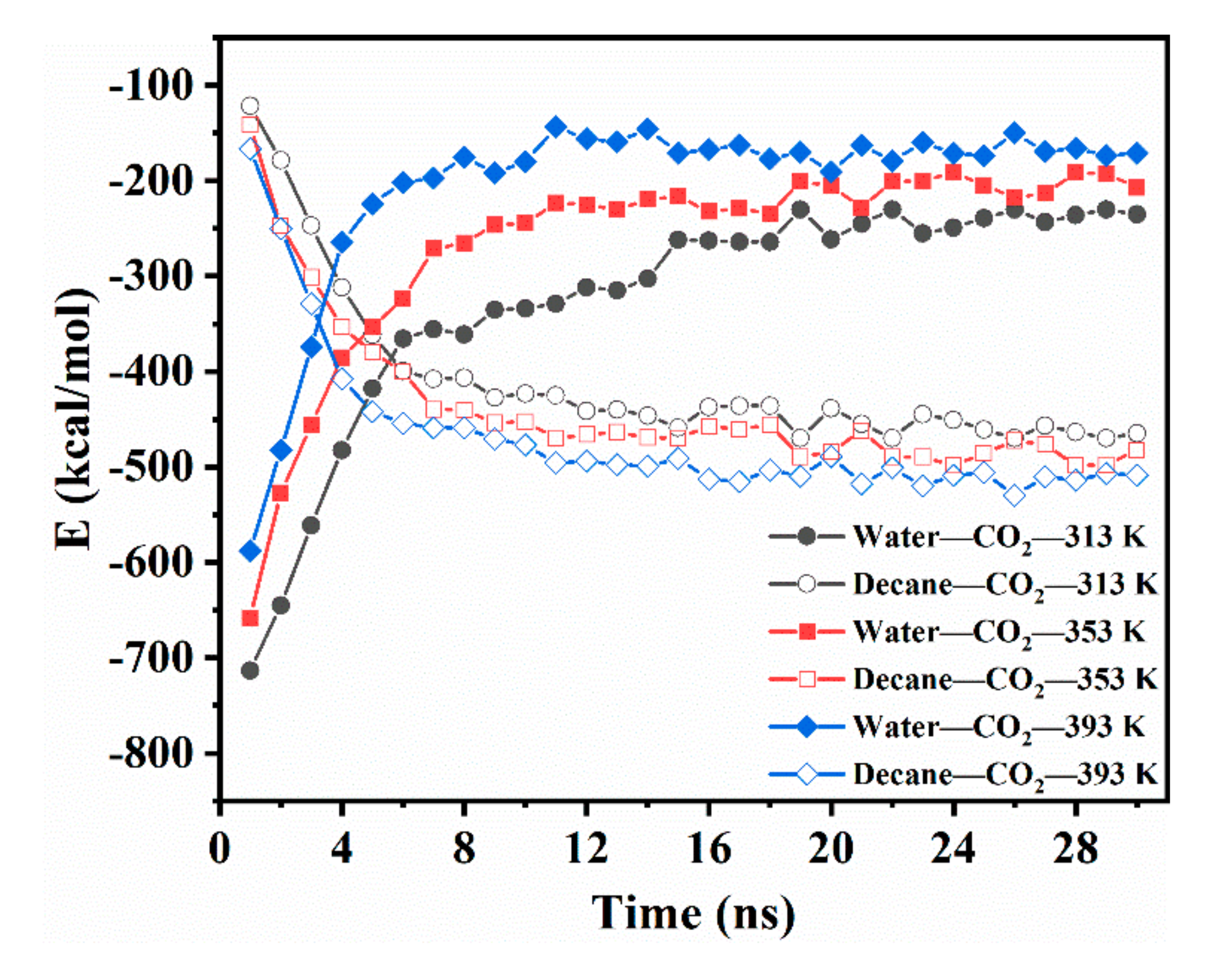
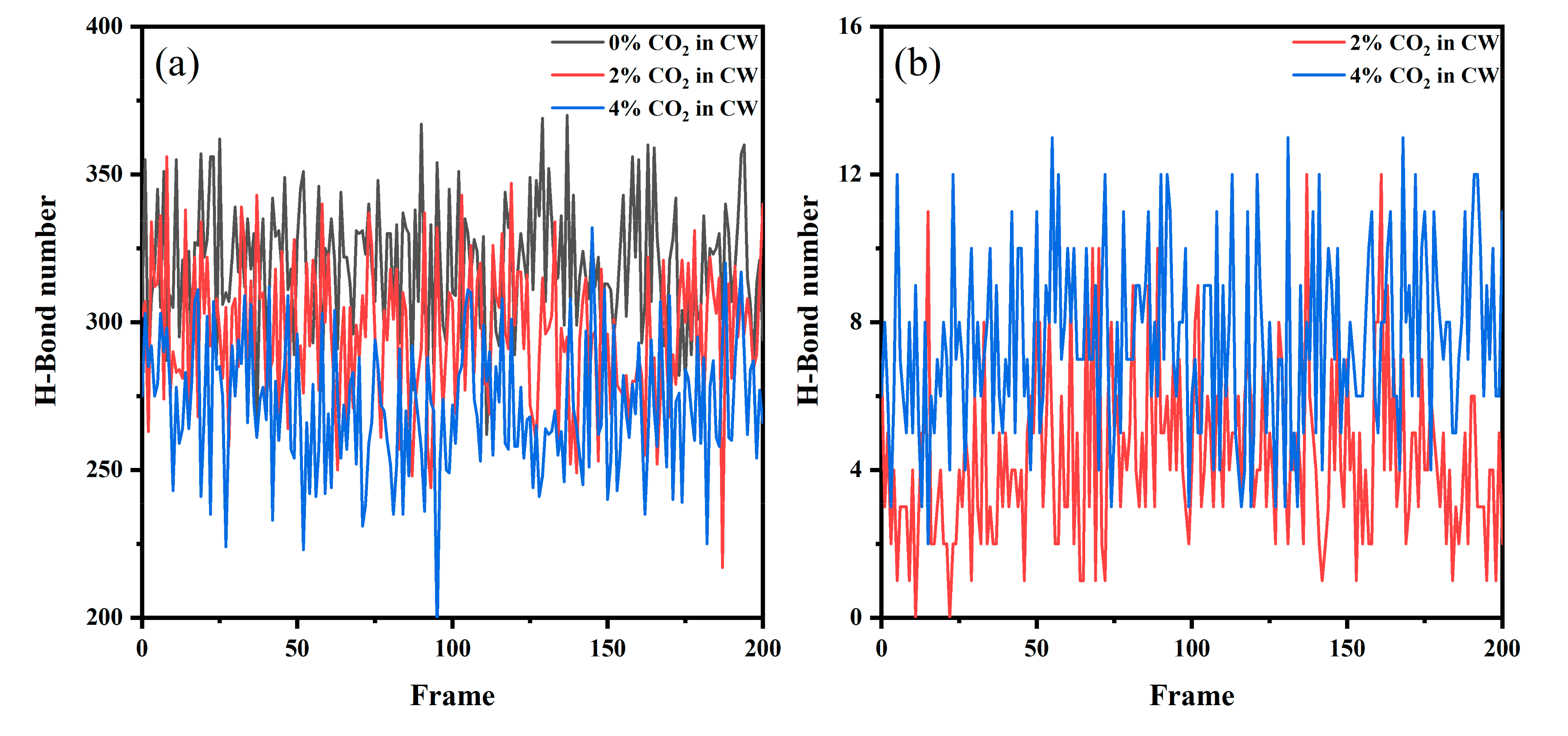
3.3.3. Intermolecular Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Riazi, M. Pore Scale Mechanisms of Carbonated Water Injection in Oil Reservoirs. Ph.D. Thesis, Heriot Watt University, Edinburgh, UK, 2011. [Google Scholar]
- Nevers, N.H. Carbonated waterflooding: Is it a lab success and a field failure. World Oil 1966, 163, 93–96. [Google Scholar]
- Seyyedi, M.; Mahzari, P.; Sohrabi, M. A comparative study of oil compositional variations during CO2 and carbonated water injection scenarios for EOR. J. Pet. Sci. Eng. 2018, 164, 685–695. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Riazi, M.; Ayatollahi, S. Effect of CO2 and natural surfactant of crude oil on the dynamic interfacial tensions during carbonated water flooding: Experimental and modeling investigation. J. Pet. Sci. Eng. 2017, 159, 58–67. [Google Scholar] [CrossRef]
- Esene, C.; Rezaei, N.; Aborig, A.; Zendehboudi, S. Comprehensive review of carbonated water injection for enhanced oil recovery. Fuel 2019, 237, 1086–1107. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, C.; Chen, M.; Chi, Y.; Zhang, Y.; Zhao, J. Pore-scale visualization study on CO2 displacement of brine in micromodels with circular and square cross sections. Int. J. Greenh. Gas Control 2020, 95. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Rao, D.N. Experimental investigation of miscible and immiscible Water-Alternating-Gas (WAG) process performance. J. Pet. Sci. Eng. 2005, 48, 1–20. [Google Scholar] [CrossRef]
- Sohrabi, M.; Riazi, M.; Jamiolahmady, M.; Kechut, N.I.; Ireland, S.; Robertson, G. Carbonated Water Injection (CWI)—A productive way of using CO2 for oil recovery and CO2 storage. In Proceedings of the 10th International Conference on Greenhouse Gas Control Technologies, Amsterdam, The Netherlands, 19–23 September 2010; Gale, J., Hendriks, C., Turkenberg, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 2192–2199. [Google Scholar]
- Mosavat, N.; Torabi, F. Micro-optical analysis of carbonated water injection in irregular and heterogeneous pore geometry. Fuel 2016, 175, 191–201. [Google Scholar] [CrossRef]
- Shu, G.; Dong, M.; Chen, S.; Hassanzadeh, H. Mass transfer of CO2 in a carbonated water-oil system at high pressures. Ind. Eng. Chem. Res. 2017, 56, 404–416. [Google Scholar] [CrossRef]
- Mosavat, N.; Torabi, F. Experimental evaluation of the performance of carbonated water injection (CWI) under various operating conditions in light oil systems. Fuel 2014, 123, 274–284. [Google Scholar] [CrossRef]
- Kechut, N.I.; Jamiolahmady, M.; Sohrabi, M. Numerical simulation of experimental Carbonated Water Injection (CWI) for improved oil recovery and CO2 storage. J. Pet. Sci. Eng. 2011, 77, 111–120. [Google Scholar] [CrossRef]
- Hickok, C.W.; Ramsay, H.J., Jr. Case histories of carbonated waterfloods in Dewey-Bartlesville field. Case histories of carbonated waterfloods in Dewey–Bartlesville field. In Proceedings of the SPE Secondary Recovery Symposium, Wichita Falls, TX, USA, 7–8 May 1962. [Google Scholar]
- Riazi, M.; Sohrabi, M.; Jamiolahmady, M.; Ireland, S.; Brown, C. Oil Recovery improvement using CO2-enriched water injection. In Proceedings of the EUROPEC/EAGE Conference and Exhibition, Amsterdam, The Netherlands, 8–11 June 2009. [Google Scholar]
- Mosavat, N.; Torabi, F. Application of CO2-saturated water flooding as a prospective safe CO2 storage strategy. In Proceedings of the 12th International Conference on Greenhouse Gas Control Technologies, Ghgt-12, Austin, TX, USA, 5–9 October 2014; Dixon, T., Herzog, H., Twinning, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 63, pp. 5408–5419. [Google Scholar]
- Seyyedi, M.; Mahzari, P.; Sohrabi, M. An integrated study of the dominant mechanism leading to improved oil recovery by carbonated water injection. J. Ind. Eng. Chem. 2017, 45, 22–32. [Google Scholar] [CrossRef]
- Seyyedi, M.; Sohrabi, M. Pore-scale investigation of crude Oil/CO2 compositional effects on oil recovery by carbonated water injection. Ind. Eng. Chem. Res. 2017, 56, 1671–1681. [Google Scholar] [CrossRef]
- Hasanvand, M.Z.; Ahmadi, M.A.; Shadizadeh, S.R.; Behbahani, R.; Feyzi, F. Geological storage of carbon dioxide by injection of carbonated water in an Iranian oil reservoir: A case study. J. Pet. Sci. Eng. 2013, 111, 170–177. [Google Scholar] [CrossRef]
- Seyyedattar, M.; Zendehboudi, S.; Butt, S. Molecular dynamics simulations in reservoir analysis of offshore petroleum reserves: A systematic review of theory and applications. Earth Sci. Rev. 2019, 192, 194–213. [Google Scholar] [CrossRef]
- Chen, C.; Wan, J.; Li, W.; Song, Y. Water contact angles on quartz surfaces under supercritical CO2 sequestration conditions: Experimental and molecular dynamics simulation studies. Int. J. Greenh. Gas Control 2015, 42, 655–665. [Google Scholar] [CrossRef]
- Hu, H.; Li, X.; Fang, Z.; Wei, N.; Li, Q. Small-molecule gas sorption and diffusion in coal: Molecular simulation. Energy 2010, 35, 2939–2944. [Google Scholar] [CrossRef]
- Ma, Z.; Ranjith, P.G. Review of application of molecular dynamics simulations in geological sequestration of carbon dioxide. Fuel 2019, 255. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, Z.; Liu, K.; Burke, N. Molecular simulation of CO2 solubility and its effect on octane swelling. Energy Fuels 2013, 27, 2741–2747. [Google Scholar] [CrossRef]
- Liu, Y.; Lafitte, T.; Panagiotopoulos, A.Z.; Debenedetti, P.G. Simulations of vapor–liquid phase equilibrium and interfacial tension in the CO2-H2O-NaCl system. Aiche J. 2013, 59, 3514–3522. [Google Scholar] [CrossRef]
- Makimura, D.; Kunieda, M.; Liang, Y.; Matsuoka, T.; Takahashi, S.; Okabe, H. Application of molecular simulations to CO2-enhanced oil recovery: Phase equilibria and interfacial phenomena. SPE J. 2013, 18, 319–330. [Google Scholar] [CrossRef]
- Bing, L.; Shi, J.; Wang, M.; Zhang, J.; Sun, B.; Yue, S.; Sun, X. Reduction in interfacial tension of water-oil interface by supercritical CO2 in enhanced oil recovery processes studied with molecular dynamics simulation. J. Supercrit. Fluids 2016, 111, 171–178. [Google Scholar]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Harris, J.G.; Yung, K.H. Carbon dioxide’s liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model. J. Phys. Chem. 1995, 99, 12021–12024. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; TiradoRives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Andersen, H.C. Rattle: A “velocity” version of the shake algorithm for molecular-dynamics calculations. J. Comput. Phys. 1983, 52, 24–34. [Google Scholar] [CrossRef]
- Hockney, R.W.; Eastwood, J.W. Computer Simulation Using Particles; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Shinoda, W.; Shiga, M.; Mikami, M. Rapid estimation of elastic constants by molecular dynamics simulation under constant stress. Phys. Rev. B 2004, 69, 8. [Google Scholar] [CrossRef]
- Chattoraj, D.K.; Birdi, K.S. Adsorption and the Gibbs Surface Excess; Experimental Methods and Procedures; Springer: New York, NY, USA, 1984. [Google Scholar]
- Matsumoto, M.; Kataoka, Y. Study on liquid vapor interface of water. I. Simulational results of thermodynamic properties and orientational structure. J. Chem. Phys. 1988, 88, 3233–3245. [Google Scholar] [CrossRef]
- Rowlinson, J.S.; Widom, B. Molecular Theory of Capillarity; Courier Corporation: Honolulu, HI, USA, 1982; 327p. [Google Scholar]
- Daniel, R.; Gaddy, V.L. Natural barriers formed in the region of hydrocarbon reservoirs. Bany. Kohasz Lapok Koolaj Floidgaz 1972, 5, 37–45. [Google Scholar]
- Balint, V. Methode de prevision du comportement d’un gisement d’huile balaye par du gaz carbonique. In Proceedings of the Communication 27, Colloque ARTEP, Rueil-Malmaison, France, 7–9 June 1971. [Google Scholar]
- Shu, G.; Dong, M.; Hassanzadeh, H.; Chen, S. Effects of operational parameters on diffusion coefficients of CO2 in a carbonated water-oil system. Ind. Eng. Chem. Res. 2017, 56, 12799–12810. [Google Scholar] [CrossRef]
- Fang, T.; Shi, J.; Sun, X.; Shen, Y.; Yan, Y.; Zhang, J.; Liu, B. Supercritical CO2 selective extraction inducing wettability alteration of oil reservoir. J. Supercrit. Fluids 2016, 113, 10–15. [Google Scholar] [CrossRef]
- Mohammed, S.; Gadikota, G. The influence of CO2 on the structure of confined asphaltenes in calcite nanopores. Fuel 2019, 236, 769–777. [Google Scholar] [CrossRef]
- Harris, K.R.; Woolf, L.A. Pressure and temperature dependence of the self diffusion coefficient of water and oxygen-18 water. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1980, 76, 377–385. [Google Scholar] [CrossRef]
- Prielmeier, F.X.; Lang, E.W.; Speedy, R.J.; Lüdemann, H.-D. The pressure dependence of self diffusion in supercooled light and heavy water. Ber. Bunsenges. Phys. Chem. 1988, 92, 1111–1117. [Google Scholar] [CrossRef]
- Bachl, F.; Lüdemann, H.-D. Pressure and temperature dependence of self-diffusion in liquid linear hydrocarbons. Ztschrift Nat. A 1986, 41, 963–970. [Google Scholar] [CrossRef]
- Mohammed, S.; Mansoori, G.A. Molecular insights on the interfacial and transport properties of supercritical CO2/brine/crude oil ternary system. J. Mol. Liq. 2018, 263, 268–273. [Google Scholar] [CrossRef]
- Wiegand, G.; Franck, E.U. Interfacial tension between water and non-polar fluids up to 473 K and 2800 bar. Ber. Bunsenges. Phys. Chem. 1994, 86, 809–817. [Google Scholar] [CrossRef]
- Georgiadis, A.; Maitland, G.; Trusler, J.P.M.; Bismarck, A. Interfacial tension measurements of the (H2O + n-Decane + CO2) ternary system at elevated pressures and temperatures. J. Chem. Eng. Data 2011, 56, 4900–4908. [Google Scholar] [CrossRef]
- Honarvar, B.; Azdarpour, A.; Karimi, M.; Rahimi, A.; Karaei, M.A.; Hamidi, H.; Ing, J.; Mohammadian, E. Experimental investigation of interfacial tension measurement and oil recovery by Carbonated Water Injection: A case study using core samples from an Iranian carbonate oil reservoir. Energy Fuels 2017, 31, 2740–2748. [Google Scholar] [CrossRef]
- Kunieda, M.; Nakaoka, K.; Liang, Y.F.; Miranda, C.R.; Ueda, A.; Takahashi, S.; Okabe, H.; Matsuoka, T. Self-accumulation of aromatics at the oil-water interface through weak hydrogen bonding. J. Am. Chem. Soc. 2010, 132, 18281–18286. [Google Scholar] [CrossRef] [PubMed]
| T (K) | |
|---|---|
| 313 | 4.22 |
| 353 | 5.98 |
| 393 | 7.25 |
| T | ωCO2 (%) | Dwater (10−9 m2/s) | Ddecane (10−9 m2/s) | γ (mN/m) | Δz (Å) |
|---|---|---|---|---|---|
| 313 | 4 | 3.367 ± 0.019 | 0.954 ± 0.018 | 53.83 ± 1.68 | 5.72 ± 0.10 |
| 353 | 0 | 5.314 ± 0.026 | 1.329 ± 0.027 | 52.51 ± 1.53 | 4.85 ± 0.07 |
| - | 2 | 5.372 ± 0.016 | 1.386 ± 0.030 | 50.29 ± 1.61 | 5.42 ± 0.05 |
| - | 4 | 5.384 ± 0.027 | 1.497 ± 0.035 | 49.16 ± 1.91 | 6.00 ± 0.14 |
| 393 | 4 | 7.970 ± 0.051 | 2.142 ± 0.046 | 45.62 ± 2.11 | 6.41 ± 0.13 |
| ωCO2 (%) | Water–Water | Water–CO2 | Decane–Decane | Decane–CO2 | Water–Decane |
|---|---|---|---|---|---|
| 0.0 | −3349.2 | 0 | −167.2 | 0 | −67.1 |
| 2.0 | −3257.4 | −38.6 | −177.0 | −29.7 | −58.1 |
| 4.0 | −3266.9 | −71.2 | −176.1 | −47.4 | −50.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Zhao, C.; Xu, Y.; Zhang, Y. Molecular Dynamics Simulation of CO2 Diffusion in a Carbonated Water–Decane System. Energies 2020, 13, 6031. https://doi.org/10.3390/en13226031
Yuan L, Zhao C, Xu Y, Zhang Y. Molecular Dynamics Simulation of CO2 Diffusion in a Carbonated Water–Decane System. Energies. 2020; 13(22):6031. https://doi.org/10.3390/en13226031
Chicago/Turabian StyleYuan, Lei, Changzhong Zhao, Yongsheng Xu, and Yi Zhang. 2020. "Molecular Dynamics Simulation of CO2 Diffusion in a Carbonated Water–Decane System" Energies 13, no. 22: 6031. https://doi.org/10.3390/en13226031
APA StyleYuan, L., Zhao, C., Xu, Y., & Zhang, Y. (2020). Molecular Dynamics Simulation of CO2 Diffusion in a Carbonated Water–Decane System. Energies, 13(22), 6031. https://doi.org/10.3390/en13226031






