Abstract
Vegetable oil is extracted from oil rich seeds, such as soybeans. Genetic engineering of green plants to accumulate oil in vegetative tissue is a future source of oil that promises increased land productivity and the use of marginal lands. However, the low concentration of lipids in current engineered plant biomass samples makes the oil extraction process challenging and expensive. In this study, liquid hot water (LHW) pretreatment was investigated to enhance oil recovery from the solids and increase enzymatic hydrolysis efficiency of such feedstocks. Corn germ meal was chosen as a model feedstock representing lipid-producing energy crops. Germ meal was pretreated at 160 and 180 °C for 10 and 15 min at 20% w/w solids loading. Enzymatic hydrolysis on the pretreated solid was performed. After pretreatment, the oil concentration increased by 2.2 to 4.2 fold. The most severe pretreatment condition of LHW, at 180 °C for 15 min, gave the maximum oil concentration (9.7%, w/w), the highest triacylglycerol (TAG) content of the extracted oil (71.6%), and the highest conversions of glucose and xylose (99.0% and 32.8%, respectively). This study demonstrates that the optimal pretreatment condition for corn germ meal is 180 °C LHW for 15 min. Pretreatment improves lipids recovery from oil bearing biomass with little or no effect on the lipid profile.
1. Introduction
Biodiesel is an energy dense biofuel that is commonly produced from vegetable oils. Compared to fossil diesel fuels, biodiesel supports comparable engine performance while emitting less particulates, hydrocarbons, and carbon monoxide [1,2]. It aids the security of energy and environmental protection. Its miscibility with fossil diesel allows the utilization of biodiesel blended diesel fuel, which demonstrates some benefits from both fuels [3]. However, the biodiesel industry still faces a few challenges: biodiesel has been reported to give a significantly higher NOx emission when combusted in the engine [1]; the low oxidation stability of biodiesel imposes difficulties on its storage and supply chain [3,4]. A major limitation of biodiesel production and commercialization is induced by the feedstocks. In the US, biodiesel is primarily produced from soybean oil. Although 1.86 billion gallons of biodiesel was produced in 2018 [5], the production level falls far short of US petroleum consumption, which was 8.69 billion gallons (567 thousand barrels per day) in 2018 [6]. Larger production of biodiesel is constrained by low production yield of soybean and other oilseed crops and limited arable lands.
A proposed solution to the feedstock limitation is to target fatty synthesis for lipid accumulation in vegetative tissue of plants with enhanced production yields and/or ability to grow on marginal farmlands. The oil could be used for biodiesel production and the residual cellulosic biomass converted to sugars for fermentation to other high value products and fuels. With advances in bioengineering, lipid-producing energy crops have been successfully cultivated through metabolic engineering, including sugarcane, tobacco, and potato plants [7,8,9,10,11]. These crops accumulate triacylglycerol (TAG) in their vegetative tissues such as leaves and stems [7]. The success of this genetic manipulation makes the lipid-producing energy crop a promising future feedstock that can be processed for the simultaneous production of bioethanol from cell wall saccharides and biodiesel from vegetable lipids.
These crops, however, are still in an early stage of development. The challenge of utilizing these feedstocks depends on efficient recoveries of oil and cell wall saccharides, which can be converted to biofuels or chemicals. The low concentration of lipids in biomass would make it difficult and expensive to extract oil. Lignocellulosic feedstocks consist of an entangled structure of cellulose, hemicellulose, and lignin polymers. Pretreatment of feedstock is a necessary step to solubilize hemicellulose and lignin and increase porosity and exposed surface area, and thus enhance enzymatic recovery of monosaccharides [12]. Most of the current pretreatments in commercial development require the use of acids and alkalis at high temperatures and pressures. These methods are likely not suitable for pretreatment of oil-producing crops as the use of chemicals would degrade the oil. For example, use of alkali can lead to oil saponification. The use of acids will require addition of base for neutralization and can also cause saponification. Hydrothermal pretreatment, also known as liquid hot water pretreatment, is a process where biomass is treated with liquid water at high temperatures (160 to 240 °C) [13] without an added catalyst. Auto-ionization of water generates hydronium ions that catalyze the solubilization of hemicelluloses. This method also generates fewer amounts of inhibitors compared to other methods [14].
The objective of this work is to investigate hydrothermal processing of biomass to improve oil concentrations and the saccharification of pretreated solids. The hypothesis is that the pretreatment of biomass would solubilize hemicellulose and partially remove lignin, producing oil-concentrated solids that should be more amenable to efficient and inexpensive oil extraction. Corn germ meal, solid residues after oil extraction from corn germ, was used as a model feedstock. Corn germ meal is the solid residue after oil extraction from corn germ in a wet milling facility [15]. It is commonly combined with corn fiber to produce corn gluten feed, which is used as a nutrient in ruminant animal diets [16]. There is typically 2.5% oil content on a dry basis (db) remaining in commercial corn germ meal [15], which corresponds to an oil content smaller than 3% (db) generally expected for lipid-producing energy crops [10,17,18]. Lipids exist as lipid droplets (LD) in both germ meal and vegetative tissue [19,20]. Thus, corn germ meal can be modeled as a lignocellulosic biomass with vegetative lipid, representing innovative energy crops accumulating lipids. Effects of hot-water pretreatment temperature and residence time on the fate of oil (both quantity and quality) and subsequent enzymatic digestion of the residual material to sugars from corn germ meal were investigated.
2. Materials and Methods
2.1. Corn Germ Meal Samples
The corn germ meal was obtained from commercial wet milling. The fresh samples were dried under 45 °C for 24 h, and the moisture content was reduced to below 5%. The dried samples were stored at 4 °C.
2.2. Lab-Scale Hot Water Pretreatment (LHW)
Corn germ meal (4 g) was mixed with a pre-calculated amount of deionized water to achieve 20% w/w solids, and loaded in 50 mL stainless steel tube reactor (316 stainless steel tubing with 1.905 cm outer diameter × 0.165 cm wall × 10.478 cm length, SS-T12-S-065–20, Swagelok, Chicago Fluid system Technologies, Chicago, IL, USA) capped with a stainless steel cap (316 stainless steel, SS-1210-C, Swagelok, Chicago Fluid system Technologies, Chicago, IL, USA). The reactors were submerged in a fluidized sand bath (IFB-51 Industrial Fluidized Bath, Techne Inc., Burlington, NJ, USA) to reach a target temperature and were held for the required residence time. Reaction temperatures were reached within 5 min by rapid heating. The temperature inside the reactor was determined using a thermocouple (Penetration/Immersion Thermocouple Probe Mini Conn (−250 to 900 °C), Mc Master-Carr, Robbinsville, NJ) inserted into one tube reactor filled with an equal amount of water, and connected to a data logger (HH306/306A, Datalogger Thermometer, Omega, Stamford, CT, USA). After holding the tubes at a set temperature for the desired time, the reaction was quenched by submerging pipe reactors into cold water.
Based on the literature studies [21], four pretreatment conditions (160 and 180 °C temperature for 10 min and 15 min residence time) were investigated in this work. The severity of the individual pretreatment condition was measured using Equation (1) from [22,23,24]:
where = the severity parameter; t = residence time (min); T = the pretreatment temperature (°C). Log is used to represent the log severity factor.
After the pretreatment, the mixture was separated into solid and liquid portions through centrifugation at 10,000 rpm for 15 min. The solid portion was air dried at 45 °C for 24 h, and ground using a blender (KitchenAid, St. Joseph, MI, USA). The liquid portion was filtered to separate the remaining solids. The filtrates were analyzed by HPLC (Bio-Rad Aminex HPX-87H, Biorad, Hercules, CA, USA) for monosaccharides concentration. Mass balance was performed to determine the amounts of liquids and solids recovered after pretreatment.
2.3. Lipid Extraction and Analysis
Lipids extraction from the raw germ meal, pretreated germ meal, and solid residues after hydrolysis was determined using the protocol used by [18] for lipid-producing sugarcane. Sample (~1 g dry weight) was mixed in a 50 mL centrifuge tube with hexane (15 mL) and isopropanol (10 mL) and homogenized twice for 1 min respectively using a homogenizer (LabGen 700, Cole Parmer, Vernon Hills, IL, USA). A wrist-action shaker (HB-1000 Hybridizer, UVP LLC, Upland, CA, USA) was used to shake the mixture for 10 min at ambient temperature. After sodium sulfate solution (16 mL, 6.7%, w/v) was added, the mixture was shaken for another 10 min. The mixture was separated through centrifugation for 20 min at 200 rpm, and then the top liquid layer was transferred into a 50 mL centrifuge tube and evaporated with nitrogen. The mass of the tube before and after evaporation of the solvent was weighed on an analytical balance. The method assumes isopropanol and hexane solvent extracted 100% oil from the sample [18].
For the oil quality analysis, the dried oil samples were dissolved at 10 mg/mL using 90% of hexane and 10% of 2-propanol, then filtered through a 0.45 μm teflon filter before injecting 10 μL onto the HPLC for analysis of hydrocarbons (HC), steryl esters (SE), triacylglycerols (TAG), diacylglycerols (DAG), monoacylglycerols (MAG), free fatty acids (FFA), and steryl ferulates (SF) according to a modification of the method of [25]. The HPLC consisted of a Shimadzu (Columbia, MD, USA) LC-20AT low pressure gradient elution pump with a 20AS autosampler, column oven, and SPD-M20A photodiode array (PDA), and a Model—LTII evaporative light scattering detector (ELSD) (Shimadzu, Columbia, MD, USA) operating in series. The PDA cell was set to 40 °C and was operated in scan mode (200–500 nm) as well as at set wavelength of 325 nm. The ELSD was operated at 40 °C and used 99.99% pure nitrogen at 3.5 Bar as the nebulizer gas. Sample peaks were separated on a YMC Diol 3.0 mm × 150 mm (5 μm) column (YMC America, Allentown, PA, USA) held in an oven at 40 °C. The mobile phase consisted of two solvents; solvent A is hexane with 0.4% acetic acid, solvent B is 2-propanol, the flow rate was 1 mL/min. The solvent program was 10 min with 100% solvent A, linear ramp at 10 to 12 min to 10% solvent B. This was held until 17 min., then returned to 100% A until the program end at 27 min. Data were collected and peaks were integrated using EZ-Start chromatography v. 7.3.
External standard curves were constructed by injecting triacontane, cholesteryl oleate, glyceryl tri-, di-, and mono-oleate, oleic acid, and cholesterol at concentrations ranging from 0.05 to 1 mg/mL, except for glyceryl tri-oleate, which ranged from 0.05 to 5 mg/mL. A standard mix at 0.25 mg/mL was injected after every four samples to ensure reliability of the detectors and consistency in retention times, along with solvent blank runs to ensure there was no carryover. The lower limit of quantitation based on the method parameters was determined to be 0.005 mg/mg for each lipid class.
2.4. Compositional Analysis of Raw and Pretreated Corn Germ Meal
Chemical composition of raw and pretreated corn germ meal was analyzed using the method from the Laboratory Analytical Procedure for biomass analysis from the National Renewable Energy Laboratory (NREL). Extractives were removed through deionized water and ethanol extraction following the Soxhlet method of NREL/TP-510-42619 [26]. Carbohydrates in samples were determined by two step acid hydrolysis with sulfuric acid according to NREL/TP-510-42618 [27]. Hydrolyzed samples were vacuum filtered through filtering crucibles. The filtrates were analyzed by HPLC (Bio-Rad Aminex HPX-87H, Biorad, Hercules, CA, USA) to determine carbohydrate content; acid soluble lignin (ASL) concentration was determined by a spectrophotometer (Evolution 60S UV-Visible Spectrophotometer, Thermo Scientific, Waltham, MA, USA). The solid residue was dried in a 105 °C oven and further turned into ash in a muffle furnace at 575 °C to determine acid insoluble lignin (AIL) and ash contents according to NREL/TP-510-42622 [28].
2.5. Enzymatic Hydrolysis of Raw and Pretreated Corn Germ Meal
Untreated germ meal and solids fraction obtained after pretreatment were hydrolyzed using commercial enzymes to determine the effect of pretreatment on the hydrolysis efficiency. The protocol for hydrolysis was adapted from [22]. Biomass was hydrolyzed at 10% (w/w) solid loading at total working volume of 25 mL in 50 mL centrifuge tubes. Citrate buffer (0.05M) was used to maintain the pH 5.0 during hydrolysis. A mixture of cellulase and hemicellulase, enzyme NS22,257 (Novozymes North America, Inc., Franklinton, NC, USA), whose enzyme activity is 231 FPU/mL, was added at 11.27 mg cellulase protein/g dry substrate. The hydrolysis was performed at 50 °C and 120 rpm for 72 h in an incubator. Hydrolysis for each pretreated biomass was conducted in duplicate, and enzyme blanks were used to eliminate background sugar concentration. Samples were withdrawn every 24 h, prepared and analyzed using HPLC for sugar concentrations. Sugar conversion was calculated as the ratio of actual sugar yields to theoretical yields from carbohydrate contents in the corresponding hydrolyzed samples.
2.6. HPLC Analysis for Compositional and Sugar Analysis
Monosaccharide concentration of filtrates from LHW, compositional analysis, and enzymatic hydrolysis were analyzed by HPLC (Bio-Rad Aminex HPX-87H, Biorad, Hercules, CA, USA). Filtrates were obtained via 0.2 μm PTFE filter.
2.7. Data Analysis
Analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) test were performed using (R software, 3.6.1, www.r-project.org) to compare means of lipid content, composition of samples, and sugar conversion. All experiments were conducted in duplicate. The level with statistically significant difference was chosen as 5% (p < 0.05).
3. Results and Discussions
3.1. Effect of LHW Pretreatment on Lipid Recovery and Solid Recovery Rate
Raw corn germ meal was found to contain 2.3% lipids. This lipid composition agrees with a previous study that reported 2 g of oil in 100 g of dry germ meal [15]. Partial solubilization of cellulose, hemicellulose, and lignin during LHW pretreatment was expected to enhance lipid recovery from pretreated solids. Temperature and residence time used for LHW are critical process factors for improving lipid content in the recovered pretreated solids (p < 0.05). Lipid content of pretreated solid (% w/w) from lipid extraction are graphed on Figure 1. As pretreatment severity was increased, lipid contents increased by 2.2 to 4.2 fold. LHW at 180 °C for 15 min gave the highest lipid content (9.7%). Detailed results of ANOVA and HSD test for lipid content are present in Table A1 in Appendix A.
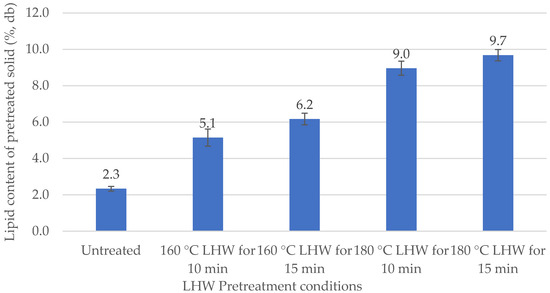
Figure 1.
Lipid content of dry LHW-pretreated solid (%, w/w) from lipid extraction.
The absolute lipid yield (mg of lipid/g of raw biomass) was calculated as the product of dry solids recovery rate (%, w/w) and lipid content of the pretreated solids. Solids recovery (%, w/w) and lipid yield are presented in Table 1. Lipid content was inversely correlated to biomass recovery following LHW. As pretreatment severity increased residual solids decreased from 48.8 to 35.2%, while conversely lipid yield increased 1.1 to 1.5 fold. LHW at 180 °C for 15 min gave the highest lipid yield (34.0 mg of lipid/g of raw biomass). Therefore, 180 °C LHW for 15 min is the optimal processing condition to use for lipid extraction from germ meal.

Table 1.
Solid recovery (%, w/w), and lipid yield (mg of lipid/g of raw biomass) from mass balance and lipid extraction 1.
3.2. Effect of LHW Pretreatment on Lipid Components of Corn Germ Meal
The lipid compositions of the oil extracted from raw and pretreated corn germ meal solids are listed in Table 2. Major components in oil include triacylglycerols (TAG), diacylglycerols (DAG), monoacylglycerols (MAG), free fatty acids (FFA), steryl esters (SE), steryl ferulates (SF), and hydrocarbons (HC). TAG, MAG, and DAG are precursors for biodiesel production by transesterification.

Table 2.
Lipid components of the total extracted oil from untreated and dry LHW-pretreated solid 1.
Significant increase (26 to 60%) in the TAG, DAG, and MAG content was observed between untreated and pretreated corn germ samples. Additionally, a slight increase in these compounds was observed with increase in pretreatment severity. LHW at 180 °C for 15 min gave the highest TAG, DAG, and MAG concentrations (71.6%, 13.5%, and 2.4%, respectively). This increase primarily results from hydrolysis of some of the structural and storage carbohydrates into the liquor fraction due to the pretreatment. Khor et al., 2019, also reported that pretreatment at elevated temperatures (160, 170, and 180 °C) cause oxidation and polymerization of TAG, thus leading to TAGs with higher molecular weight. An increase in TAG, DAG, and MAG will be beneficial for biodiesel production.
FFA, including palmitic acid, stearic acid, oleic acid, and linoleic acid, were present in trace quantities, and thus specific fatty acids were not determined. FFAs form soaps and emulsify with alkali catalyst during transesterification, inhibiting biodiesel yield and the purification process [29,30,31]. A FFA content above 3% has been reported to hinder transesterification and needs to be neutralized via an acid pretreatment [29,31,32].
Small increase in minor constituents (steryl esters (SE), steryl ferulates (SF), and hydrocarbons (HC)) was also observed with pretreatment severity and was also primarily due to concentration effects. SE and SF were found at low concentrations, showing no remarkable differences under various pretreatment conditions. HC, identified from a peak with the same retention time as triacontane, was detected at low level in all the solids. Saponifiables and HC are undesired components since they would escalate precipitation in biodiesel [33]. LHW pretreatment had a negligible effect on saponifibales contents.
The sums of the major lipid components were below 100% for all the solids, and unknown peaks based upon different retention times from any of the analytical standards used for peak identifications were detected, indicating the existence of other unidentified lipids in the extracted oil. Overall, the lipid profile of pretreated corn germ meal samples improved with the pretreatment process. These results are indicative that LHW pretreatment of oil-bearing biomass improves the recovery of vegetative oil without detrimentally affecting the lipid profile.
3.3. Effect of LHW Preatment on Corn Germ Meal Composition
The chemical compositions of raw and pretreated corn germ meals are listed in Table 3. Major measured components include (ethanol/water) extractives, glucan, xylan, acid insoluble lignin (AIL), acid soluble lignin (ASL), and ash.

Table 3.
Compositions (% w/w) of raw and pretreated corn germ meal samples 1.
Raw corn germ meal sample consists on a mass basis of 13.6% extractives, 31.0% glucan, 22.3% xylan, 5.1% AIL, 8.4% ASL, and 0.03% ash. As the severity factor of pretreatment increased, extractives content increased, and samples pretreated at 180 °C LHW for 15 min contained 31.4% extractives. This trend was expected because more severe pretreatment conditions would hydrolyze and degrade greater amounts of carbohydrates and produce soluble sugars that end up as extractives [21,22]. Cellulose content of pretreated germ meal decreased from 28.9 to 20.9%; hemicellulose content decreased from 22.3% to 3.6%. AIL increased from 8.1 to 24.0%, while ASL decreased from 10.6 to 6.0%. Ash content slightly decreased at high pretreatment severity.
Hot water pretreatment with high severity factor solubilizes large amounts of xylan and lignin and provides access to glucan [34]. As the severity factor increased, xylan concentration decreased by 62.9 to 83.9%. A similar trend has been observed in other studies with sugarcane bagasse [21] and biomass sorghum [22]. This is a desirable effect of LHW since hydrolysis of hemicellulose exposes cellulose to enzymatic saccharification. Total lignin content (AIL + ASL) increased from 18.7 to 30.0% with increased severity. Lignin was concentrated due to the greater solubilization of hemicellulose and cellulose during the pretreatment. A similar observation was reported after hydrothermal pretreatment of corn stover [23]. A large amount of solubilized xylan could also be further degraded to pseudo-lignin from reaction of released sugars and lignin components, which likely aids in explaining the trend of total lignin content [35]. As pretreatment conditions became more severe, cellulose concentration declined by 6.8 to 32.6%. Cellulose in corn germ meal should be more accessible and sensitive to pretreatment conditions because of the physical and chemical treatment during wet milling process and oil extraction process [36]. Although this increasing solubilization of glucan demonstrates a partial loss of fermentable sugars, it indicates LHW greatly improved accessibility of cellulose to enzymatic hydrolysis.
3.4. Effect of LHW Pretreatment on Sugar Conversion
Sugar yields from hydrolysis of raw and pretreated solid samples are graphed in Figure 2. Detailed results of ANOVA and HSD test for lipid content are present in Table A1 in Appendix A. With greater pretreatment severity, glucose and xylose yields increased by 1.1 to 1.5 and 2.9 to 4.4 fold, respectively. At 180 °C for 10 min, LHW gave glucose conversion (99.2%) and xylose conversion (34.5%); at 180 °C for 15 min, LHW gave glucose conversion (99.0%) and xylose conversion (32.8%). The difference between LHW at 180 °C for 10 min and 15 min was insignificant (p > 0.05), so both conditions led to maxima glucose and xylose conversions.
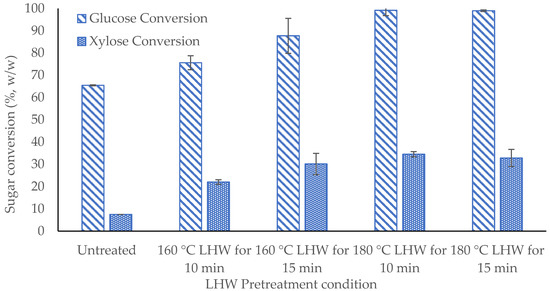
Figure 2.
Sugar conversion (%, w/w) of LHW-pretreated solid.
LHW pretreatment is intended to solubilize hemicellulose and lignin, thus increasing accessibility of cellulose to cellulases. Increased yields of monosaccharides indicate a pretreatment condition that leads to higher accessibility of cellulose. Therefore, LHW at 180 °C for both 10 and 15 min are the optimal pretreatment conditions to promote cellulose accessibility.
3.5. Structure of Corn Germ Meal and LD
Corn germ meal has a structural carbohydrate different from typical lignocellulosic biomass. Corn germ physiologically stores oil, nutrients, and minerals in a corn kernel required for growth of the seedling. It is mainly composed of embryonic axis and scutellum. Embryonic axis, where the new plant originates, contains the main root. Scutellum shields and transfers nutrients for embryonic axis [37]. The scutellum is mainly composed of four tissues, including epithelium, parenchyma, epidermis, and provascular tissue. The physical characteristic of these tissues enables scutellum to act as a storage organ where 90% of the lipids present in a kernel is stored [38]. Lipids are stored within LDs in germ. A structure model of LDs from corn was proposed by [20]: lipids are surrounded by a phospholipid monolayer and oleosin molecules (alkaline proteins) that interact and cover the phospholipid molecules. The LDs from corn have been reported to present an average diameter of 1.45 μm and to consist of 97.6% neutral lipids including TAG, DAG, and SE, 1.4% proteins and 0.9% phospholipids [19,39,40]. LDs need to be disrupted chemically or physically to release the lipids [41]. Theoretically, the major effect of LHW pretreatment on oil in germ meal is to break the oil bodies and release more lipids, which partially explains the enhanced lipid recovery after LHW.
Lipids accumulated in various vegetative tissues also exist as LDs that have a similar basic structure as LDs from corn and other seeds. This similarity implies LHW could have a comparable effect on oil-bearing energy crops. However, LDs from various vegetative tissues have been observed with some distinctions: they vary substantially in size [42]; lipid droplet-associated proteins (LDAP) instead of oleosins shape and stabilize the droplets [19,42]; the function of LDs in tissues are dynamic and poorly understood [19,43,44]; compositions of neutral lipids in LDs in various tissues are diverse [45,46]. The distinctions with respect to biomass structure and LD characteristics impose some uncertainties on how LHW pretreatment would affect the lipid recovery from lipid-enriched energy crops.
3.6. Effect of Enhanced Lipid Recovery on Commercial Model
A few companies have developed and implemented technologies to produce cellulosic ethanol from corn fiber. Edeniq’s technology “Intellulose” was designed to produce ethanol from carbohydrates present in both corn fiber and corn starch in a dry grind process [47]. It increased ethanol yield from 2.8 to 3.0 gal bu−1. It also increased oil production, which added $5.1 M additional income for the plant. ICM’s “Gen 1.5” (Generation 1.5 Grain Fiber to Cellulosic Ethanol Technology) was designed to separate corn fiber from corn kernel and convert it to cellulosic ethanol in an additional cellulosic fermentation step in a dry grind process [48]. “Gen 1.5” also utilizes ICM’s patented technology Selective Milling Technology (SMT) to treat corn slurry, thus increasing accessibility to starch and oil during the process. This process increased ethanol yield by 7 to 10%. It also increased oil recovery rates [49]. In summary, current corn fiber conversion technologies that provide a higher oil production lead to higher profit from the plant. The LHW pretreatment described in this paper increased lipid concentration by 2.2 to 4.2 fold, thus delivering higher oil recovery rates. A commercial model utilizing the pretreatment process is expected to have higher total oil production and total profit. Detailed economic analysis could be performed in the future to investigate how much additional income could be gained from the lipid enhancement after pretreatment.
4. Conclusions
The optimal hydrothermal pretreatment condition for corn germ meal is at 180 °C with a holding time of 15 min, giving the maximum lipid yield of 34.0 mg of lipid/g of raw biomass, lipid concentration of 9.7%, 71.6% TAG purity of the extracted oil, glucose conversion of 99.0%, and xylose conversion of 32.8%.
For pretreated solids from corn germ meal, lipid content, and TAG purity of the extracted oil increased with the pretreatment severity. A similar trend of increased lipid recovery with more severe pretreatment is expected for vegetative lipid-producing engineered energy crops such as tobacco leaves, sugarcane, and sorghum. This research demonstrates an innovative approach to efficiently process these engineered energy crops by addressing the issue of low oil concentrations and simultaneously recovering sugars for biofuel production.
5. Patents
A provisional patent application has been filed from this work (U.S. Patent Application No.: 62/945,438).
Author Contributions
Y.J. conducted all the experiments, analyzed the data and prepared the manuscript; D.K.—helped formulate the study and edited the manuscript; J.K.W.-M.—conducted all the lipids analyses and edited the manuscript; B.D.—help formulate the study and edited manuscript; V.S.—is the PI of this project, help formulate the study and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the DOE Center for Advanced Bioenergy and Bioproducts Innovation (U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Number DE-SC0018420). Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the U.S. Department of Energy.
Acknowledgments
We would like to thank Novozymes North America for providing enzymes.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design, execution, interpretation, or writing of the study.
Appendix A
ANOVA and HSD test were performed to compare results from lipid content, compositional analysis and sugar conversion. Detailed test reults for compositional analysis are shown in Table 3 in Section 3.3 above. Detailed comparison results for lipid content and sugar conversion are present in Table A1 below.

Table A1.
Data analysis of lipid content (%, w/w), glucose conversion (%, w/w) and xylose conversion (%, w/w) of raw and pretreated corn germ meal samples 1.
Table A1.
Data analysis of lipid content (%, w/w), glucose conversion (%, w/w) and xylose conversion (%, w/w) of raw and pretreated corn germ meal samples 1.
| Experiment Data | Untreated | 160 °C LHW/10 min | 160 °C LHW/15 min | 180 °C LHW/10 min | 180 °C LHW/15 min |
|---|---|---|---|---|---|
| Lipid Content (%, w/w) | a | b | b | c | c |
| Glucose Conversion (%, w/w) | a | a | b | b | b |
| Xylose Conversion (%, w/w) | a | b | bc | c | c |
1 Results are represented as mean ± standard deviation. Data within one row without same letters were siginificantly different (p < 0.05).
References
- Graboski, M.S.; McCormick, R.L. Combustion of fat and vegetable oil derived fuels in diesel engines. Science 1998, 24, 125–164. [Google Scholar] [CrossRef]
- Haas, M.J.; McAloon, A.J.; Yee, W.C.; Foglia, T.A. A process model to estimate biodiesel production costs. Bioresour. Technol. 2006, 97, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Lapuerta, M.; Rodríguez-Fernández, J.; Ramos, Á.; Álvarez, B. Effect of the test temperature and anti-oxidant addition on the oxidation stability of commercial biodiesel fuels. Fuel 2012, 93, 391–396. [Google Scholar] [CrossRef]
- Tasić, I.; Mićić, R.; Tomić, M.; Aleksić, A.; Simikić, M. Storing, distribution and blending of biodiesel. Agric. Eng. Int. CIGR J. 2020, 22, 105–111. [Google Scholar]
- U.S. Energy Information Administration. Monthly Biodiesel Production Report; U.S. Energy Information Administration: Washington, DC, USA, 2019; p. 10.
- U.S. Energy Information Administration. September 2019 Monthly Energy Review; U.S. Energy Information Administration: Washington, DC, USA, 2019; Volume 24.
- Zale, J.; Jung, J.H.; Kim, J.Y.; Pathak, B.; Karan, R.; Liu, H.; Chen, X.; Wu, H.; Candreva, J.; Zhai, Z.; et al. Metabolic engineering of sugarcane to accumulate energy-dense triacylglycerols in vegetative biomass. Plant Biotechnol. J. 2016, 14, 661–669. [Google Scholar] [CrossRef]
- Vanhercke, T.; El Tahchy, A.; Liu, Q.; Zhou, X.R.; Shrestha, P.; Divi, U.K.; Ral, J.P.; Mansour, M.P.; Nichols, P.D.; James, C.N.; et al. Metabolic engineering of biomass for high energy density: Oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol. J. 2014, 12, 231–239. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Q.; Akbar, S.; Zhi, Y.; El Tahchy, A.; Mitchell, M.; Li, Z.; Shrestha, P.; Vanhercke, T.; Ral, J.P.; et al. Genetic enhancement of oil content in potato tuber (Solanum tuberosum L.) through an integrated metabolic engineering strategy. Plant Biotechnol. J. 2017, 15, 56–67. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yang, H.K.; Singh, S.P.; Sharp, P.J.; Liu, Q. Genetic Manipulation of Non-Classic Oilseed Plants for Enhancement of Their Potential as a Biofactory for Triacylglycerol Production. Engineering 2018, 4, 523–533. [Google Scholar] [CrossRef]
- Parajuli, S.; Kannan, B.; Karan, R.; Sanahuja, G.; Liu, H.; Garcia-Ruiz, E.; Kumar, D.; Singh, V.; Zhao, H.; Long, S.; et al. Towards oilcane: Engineering hyperaccumulation of triacylglycerol into sugarcane stems. GCB Bioenergy 2020, 12, 476–490. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels 2011, 4. [Google Scholar] [CrossRef]
- Mosier, N.S. Fundamentals of Aqueous Pretreatment of Biomass. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 129–143. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Rausch, K.D.; Belyea, R.L. The future of coproducts from corn processing. Appl. Biochem. Biotechnol. 2006, 128, 47–86. [Google Scholar] [CrossRef]
- Johnston, D.B.; McAloon, A.J.; Moreau, R.A.; Hicks, K.B.; Singh, V. Composition and economic comparison of germ fractions from modified corn processing technologies. J. Am. Oil Chem. Soc. 2005, 82, 603–608. [Google Scholar] [CrossRef]
- Hofvander, P.; Ischebeck, T.; Turesson, H.; Kushwaha, S.K.; Feussner, I.; Carlsson, A.S.; Andersson, M. Potato tuber expression of Arabidopsis WRINKLED1 increase triacylglycerol and membrane lipids while affecting central carbohydrate metabolism. Plant Biotechnol. J. 2016, 14, 1883–1898. [Google Scholar] [CrossRef]
- Huang, H.; Moreau, R.A.; Powell, M.J.; Wang, Z.; Kannan, B.; Altpeter, F.; Grennan, A.K.; Long, S.P.; Singh, V. Evaluation of the quantity and composition of sugars and lipid in the juice and bagasse of lipid producing sugarcane. Biocatal. Agric. Biotechnol. 2017, 10, 148–155. [Google Scholar] [CrossRef]
- McLachlan, D.H. Plant Lipid Droplets. In eLS; John Wiley & Sons Ltd., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 1–8. ISBN 978-0-470-01590-2. [Google Scholar]
- Huang, A.H.C. Structure of plant seed oil bodies. Curr. Opin. Struct. Biol. 1994, 4, 493–498. [Google Scholar] [CrossRef]
- Wang, Z.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Fermentation of undetoxified sugarcane bagasse hydrolyzates using a two stage hydrothermal and mechanical refining pretreatment. Bioresour. Technol. 2018. [Google Scholar] [CrossRef]
- Cheng, M.H.; Dien, B.S.; Lee, D.K.; Singh, V. Sugar production from bioenergy sorghum by using pilot scale continuous hydrothermal pretreatment combined with disk refining. Bioresour. Technol. 2019. [Google Scholar] [CrossRef]
- Kim, S.M.; Dien, B.S.; Tumbleson, M.E.; Rausch, K.D.; Singh, V. Improvement of sugar yields from corn stover using sequential hot water pretreatment and disk milling. Bioresour. Technol. 2016. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. A 1987, 321, 523–536. [Google Scholar]
- Moreau, R.A.; Powell, M.J.; Hicks, K.B. Extraction and Quantitative Analysis of Oil from Commercial Corn Fiber. J. Agric. Food Chem. 1996, 44, 2149–2154. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); Issue Date 7/17/2005, Technical Report NREL/TP-510-42619; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (NREL/TP-510-42618); National Renewable Energy Laboratory: Golden, CO, USA, 2012; p. 17.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Ribeiro, A.; Castro, F.; Carvalho, J. Influence of free fatty acid content in biodiesel production on non-edible oil. In Proceedings of the 1st International Conference on WASTES: Solutions, Treatmentsand Opportunities, Guimarães, Portugal, 12–14 September 2011. [Google Scholar]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Hood, E.E. Plant-based biofuels. F1000Research 2016, 5, 185. [Google Scholar] [CrossRef] [PubMed]
- Biofuels: Prospects, Risks and Opportunities; The State of Food and Agriculture; FAO, Ed.; FAO: Rome, Italy, 2008; ISBN 978-92-5-105980-7. [Google Scholar]
- Wang, H.; Tang, H.; Salley, S.; Simon Ng, K.Y. Analysis of sterol glycosides in biodiesel and biodiesel precipitates. J. Am. Oil Chem. Soc. 2010, 87, 215–221. [Google Scholar] [CrossRef]
- Hashmi, M.; Sun, Q.; Tao, J.; Wells, T.; Shah, A.A.; Labbé, N.; Ragauskas, A.J. Comparison of autohydrolysis and ionic liquid 1-butyl-3-methylimidazolium acetate pretreatment to enhance enzymatic hydrolysis of sugarcane bagasse. Bioresour. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef]
- Dickey, L.C.; Kurantz, M.J.; Parris, N. Oil separation from wet-milled corn germ dispersions by aqueous oil extraction and aqueous enzymatic oil extraction. Ind. Crops Prod. 2008. [Google Scholar] [CrossRef]
- Tnani, H.; López Ribera, I.; Vicient Sánchez, C.M.; Ferrer Prats, A. The Structure and Function of Maize Scutellum during Early Stages of Germination. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2012. [Google Scholar]
- Swift, J.; O’Brien, T. Vascularization of the scutellum of wheat. Aust. J. Bot. 1970, 18, 45–53. [Google Scholar] [CrossRef]
- Tzen, J.; Cao, Y.; Laurent, P.; Ratnayake, C.; Huang, A. Lipids, Proteins, and Structure of Seed Oil Bodies from Diverse Species. Plant Physiol. 1993, 101, 267–276. [Google Scholar] [CrossRef]
- Pyc, M.; Cai, Y.; Greer, M.S.; Yurchenko, O.; Chapman, K.D.; Dyer, J.M.; Mullen, R.T. Turning over a New Leaf in Lipid Droplet Biology. Trends Plant. Sci. 2017, 22, 596–609. [Google Scholar] [CrossRef]
- Majoni, S.; Wang, T.; Johnson, L.A. Enzyme treatments to enhance oil recovery from condensed corn distillers solubles. J. Am. Oil Chem. Soc. 2011, 88, 523–532. [Google Scholar] [CrossRef]
- Lersten, N.R.; Czlapinski, A.R.; Curtis, J.D.; Freckmann, R.; Horner, H.T. Oil bodies in leaf mesophyll cells of angiosperms: Overview and a selected survey. Am. J. Bot. 2006, 93, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- van der Schoot, C.; Paul, L.K.; Paul, S.B.; Rinne, P.L.H. Plant lipid bodies and cell-cell signaling: A new role for an old organelle? Plant. Signal. Behav. 2011, 6, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.D.; Dyer, J.M.; Mullen, R.T. Biogenesis and functions of lipid droplets in plants: Thematic Review Series: Lipid Droplet Synthesis and Metabolism: From Yeast to Man. J. Lipid Res. 2012, 53, 215–226. [Google Scholar] [CrossRef]
- Van Wijk, K.J.; Kessler, F. Plastoglobuli: Plastid Microcompartments with Integrated Functions in Metabolism, Plastid Developmental Transitions, and Environmental Adaptation. Annu. Rev. Plant. Biol. 2017, 68, 253–289. [Google Scholar] [CrossRef]
- Lundquist, P.K.; Poliakov, A.; Giacomelli, L.; Friso, G.; Appel, M.; McQuinn, R.P.; Krasnoff, S.B.; Rowland, E.; Ponnala, L.; Sun, Q.; et al. Loss of Plastoglobule Kinases ABC1K1 and ABC1K3 Causes Conditional Degreening, Modified Prenyl-Lipids, and Recruitment of the Jasmonic Acid Pathway. Plant Cell 2013, 25, 1818–1839. [Google Scholar] [CrossRef]
- Woods, R.R.; Kacmar, J. Cellulose Co-feed For Dry Mill Corn Ethanol Operations. U.S. Patent 2014/0315259 A1, 23 October 2014. Available online: https://patentimages.storage.googleapis.com/b9/ca/ea/3b536adf160cb0/US20140315259A1.pdf (accessed on 14 July 2020).
- Javers, J.E.; Gerken, C.R.W.; Griend, S.V.; Spooner, J.; Licklider, J.P. Process for Producing Cellulosic Biofuel. CA Patent 2846489 A1, 29 May 2014. Available online: https://patentimages.storage.googleapis.com/64/f7/8f/95e6010ae0c7c5/CA2846489A1.pdf (accessed on 14 July 2020).
- ICM, Inc. Successfully Completes 1,000-Hour Run Proving Generation 1.5: Integrated Fiber to Cellulosic Ethanol Technology; ICM, Inc.: Colwich, KS, USA, 2012. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).