Role of Microbial Hydrolysis in Anaerobic Digestion
Abstract
1. Introduction
2. Biodegradable Waste for Anaerobic Digestion
3. Microbial Hydrolysis
3.1. Microbial Consortia and the Main Products in a Microbial Hydrolysis Stage
3.2. Microbial Hydrolysis in a Separate Stage
3.3. Comparison of Process Conditions for Hydrolysis
3.4. Combined Pretreatment
4. Measurement of Hydrolysis Efficiency
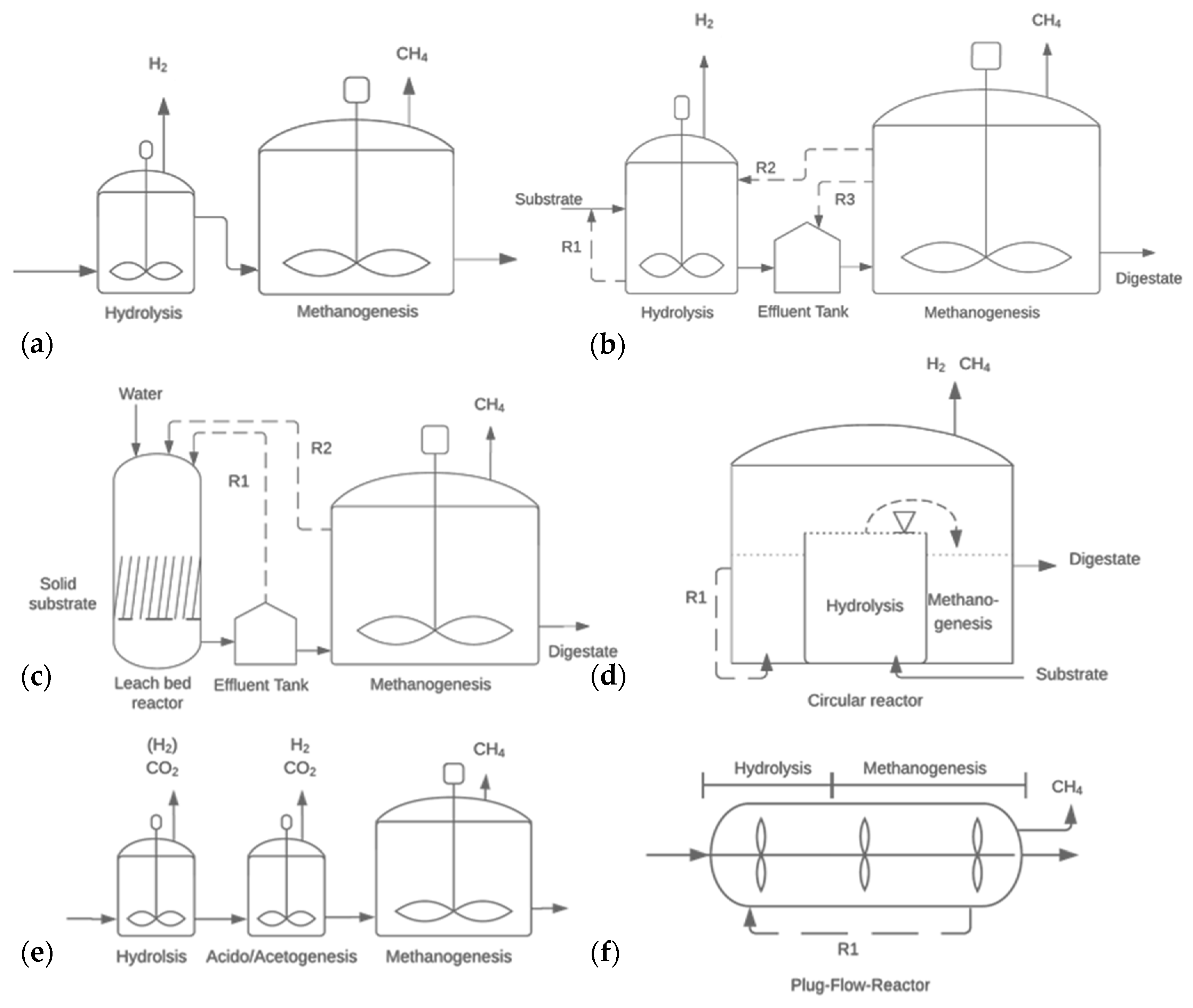
5. Impact of Reactor Design and Operational Parameters
5.1. Recirculation Systems
5.2. Gaseous Products of Hydrolytic Organisms
5.3. High-Solid Reactors
5.4. Three Stage Anaerobic Digestion
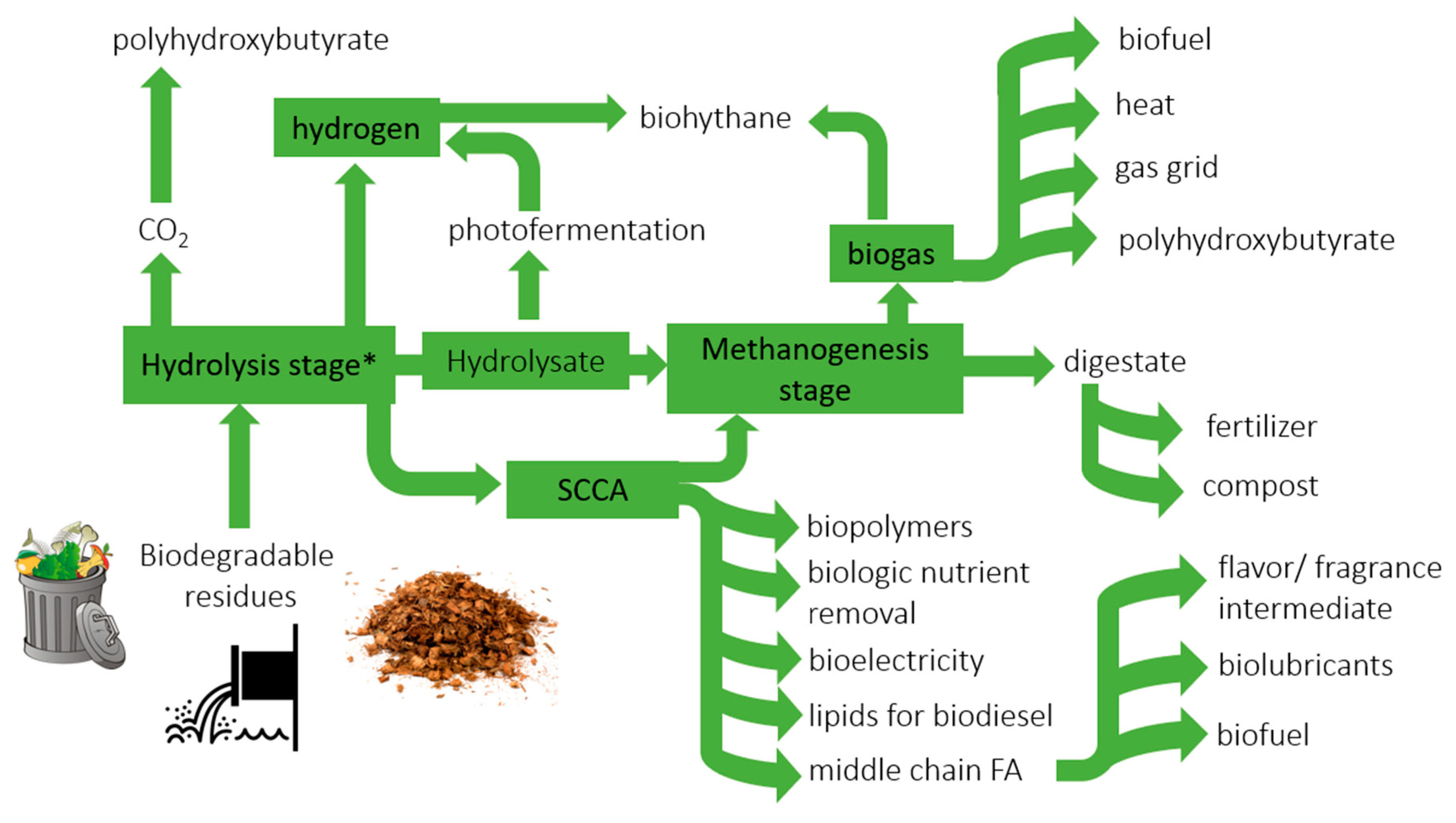
6. Perspective Applications in Biorefinery Systems
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| sCOD/tCOD | soluble/total chemical oxygen demand |
| CSTR | continuously stirred tank reactor |
| DOC/TOC | dissolved/total organic carbon |
| FW | food waste |
| HRT | hydraulic retention time |
| LBR | leach bed reactor |
| OFMSW | organic fraction of municipal solid waste |
| OLR | organic loading rate |
| TPAD | temperature-phased anaerobic digestion |
| SCCA | short-chain carboxylic acids |
| TVS | total volatile solids |
| VFA | volatile fatty acid |
| WAS | waste activated sludge |
References
- A European Green Deal. European Commission. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 25 August 2020).
- Carus, M.; Dammer, L. The “Circular Bioeconomy”—Concepts, Opportunities and Limitations; Nova-Institut: Hürth, Germany, 2018. [Google Scholar]
- Castellano-Hinojosa, A.; Armato, C.; Pozo, C.; González-Martínez, A.; González-López, J. New Concepts in Anaerobic Digestion Processes: Recent Advances and Biological Aspects. Appl. Microbiol. Biotechnol. 2018, 102, 5065–5076. [Google Scholar] [CrossRef] [PubMed]
- Van, D.P.; Fujiwara, T.; Leu Tho, B.; Song Toan, P.P.; Hoang Minh, G. A Review of Anaerobic Digestion Systems for Biodegradable Waste: Configurations, Operating Parameters, and Current Trends. Environ. Eng. Res. 2019, 25, 1–17. [Google Scholar] [CrossRef]
- Publications. European Biogas Association. Available online: https://www.europeanbiogas.eu/category/publications/ (accessed on 23 September 2020).
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of Feedstock Pretreatment Strategies for Improved Anaerobic Digestion: From Lab-Scale Research to Full-Scale Application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Brémond, U.; de Buyer, R.; Steyer, J.P.; Bernet, N.; Carrere, H. Biological Pretreatments of Biomass for Improving Biogas Production: An Overview from Lab Scale to Full-Scale. Renew. Sustain. Energy Rev. 2018, 90, 583–604. [Google Scholar] [CrossRef]
- Petracchini, F.; Liotta, F.; Paolini, V.; Perilli, M.; Cerioni, D.; Gallucci, F.; Carnevale, M.; Bencini, A. A Novel Pilot Scale Multistage Semidry Anaerobic Digestion Reactor to Treat Food Waste and Cow Manure. Int. J. Environ. Sci. Technol. 2018, 15, 1999–2008. [Google Scholar] [CrossRef]
- Micolucci, F.; Gottardo, M.; Pavan, P.; Cavinato, C.; Bolzonella, D. Pilot Scale Comparison of Single and Double-Stage Thermophilic Anaerobic Digestion of Food Waste. J. Clean. Prod. 2018, 171, 1376–1385. [Google Scholar] [CrossRef]
- Giuliano, A.; Zanetti, L.; Micolucci, F.; Cavinato, C. Thermophilic Two-Phase Anaerobic Digestion of Source-Sorted Organic Fraction of Municipal Solid Waste for Bio-Hythane Production: Effect of Recirculation Sludge on Process Stability and Microbiology over a Long-Term Pilot-Scale Experience. Water Sci. Technol. 2014, 69, 2200–2209. [Google Scholar] [CrossRef]
- Chatterjee, B.; Mazumder, D. Role of Stage-Separation in the Ubiquitous Development of Anaerobic Digestion of Organic Fraction of Municipal Solid Waste: A Critical Review. Renew. Sustain. Energy Rev. 2019, 104, 439–469. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.-C.; Zhang, J. Enhanced Biogas Production from Anaerobic Digestion of Solid Organic Wastes: Current Status and Prospects. Bioresour. Technol. Rep. 2019, 5, 280–296. [Google Scholar] [CrossRef]
- Grimberg, S.J.; Hilderbrandt, D.; Kinnunen, M.; Rogers, S. Anaerobic Digestion of Food Waste through the Operation of a Mesophilic Two-Phase Pilot Scale Digester—Assessment of Variable Loadings on System Performance. Bioresour. Technol. 2015, 178, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Theuerl, S.; Herrmann, C.; Heiermann, M.; Grundmann, P.; Landwehr, N.; Kreidenweis, U.; Prochnow, A. The Future Agricultural Biogas Plant in Germany: A Vision. Energies 2019, 12, 396. [Google Scholar] [CrossRef]
- Yuan, T.; Cheng, Y.; Wang, X.; Yu, Y.; Zhang, Z.; Lei, Z.; Shimizu, K.; Utsumi, M.; Adachi, Y.; Lee, D.J. A Novel Anaerobic Digestion System Coupling Biogas Recirculation with MgCl2 Addition for Multipurpose Sewage Sludge Treatment. J. Clean. Prod. 2019, 230, 499–507. [Google Scholar] [CrossRef]
- Wu, L.J.; Kobayashi, T.; Li, Y.Y.; Xu, K.Q. Comparison of Single-Stage and Temperature-Phased Two-Stage Anaerobic Digestion of Oily Food Waste. Energy Convers. Manag. 2015, 106, 1174–1182. [Google Scholar] [CrossRef]
- Stabnikova, O.; Ang, S.-S.; Liu, X.-Y.; Ivanov, V.; Tay, J.-H.; Wang, J.-Y. The Use of Hybrid Anaerobic Solid-Liquid (HASL) System for the Treatment of Lipid-Containing Food Waste. J. Chem. Technol. Biotechnol. 2005, 80, 455–461. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment Methods to Enhance Anaerobic Digestion of Organic Solid Waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic Digestion of Food Waste—Challenges and Opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Richard Hess, J.; Yousuf, A.; Vivekanand, V.; Paritosh, K.; Yadav, M.; Mathur, S.; Balan, V.; Liao, W.; Pareek, N. Organic Fraction of Municipal Solid Waste: Overview of Treatment Methodologies to Enhance Anaerobic Biodegradability. Front. Energy Res. 2018, 1, 75. [Google Scholar]
- Nasir, I.M.; Mohd Ghazi, T.I.; Omar, R. Anaerobic Digestion Technology in Livestock Manure Treatment for Biogas Production: A Review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Burgess, J.E.; Pletschke, B.I. Hydrolytic Enzymes in Sewage Sludge Treatment: A Mini-Review. Water SA 2008, 34, 343–349. [Google Scholar] [CrossRef]
- Himmel, M.E.; Xu, Q.; Luo, Y.; Ding, S.Y.; Lamed, R.; Bayer, E.A. Microbial Enzyme Systems for Biomass Conversion: Emerging Paradigms. Biofuels 2010, 1, 323–341. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Cheng, H.; Wang, W.; Dong, R. Two-Phase Anaerobic Digestion of Municipal Solid Wastes Enhanced by Hydrothermal Pretreatment: Viability, Performance and Microbial Community Evaluation. Appl. Energy 2017, 189, 613–622. [Google Scholar] [CrossRef]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological Strategies for Enhanced Hydrolysis of Lignocellulosic Biomass during Anaerobic Digestion: Current Status and Future Perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Chufo, A.; Jaffar, M.; Li, X. Improving Biomethane Yield by Controlling Fermentation Type of Acidogenic Phase in Two-Phase Anaerobic Co-Digestion of Food Waste and Rice Straw. Chem. Eng. J. 2015, 273, 254–260. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A Review of the Production and Applications of Waste-Derived Volatile Fatty Acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Sträuber, H.; Schröder, M.; Kleinsteuber, S. Metabolic and Microbial Community Dynamics during the Hydrolytic and Acidogenic Fermentation in a Leach-Bed Process. Energy. Sustain. Soc. 2012, 2, 13. [Google Scholar] [CrossRef]
- Gottardo, M.; Micolucci, F.; Bolzonella, D.; Uellendahl, H.; Pavan, P. Pilot Scale Fermentation Coupled with Anaerobic Digestion of Food Waste—Effect of Dynamic Digestate Recirculation. Renew. Energy 2017, 114, 455–463. [Google Scholar] [CrossRef]
- Lindner, J.; Zielonka, S.; Oechsner, H.; Lemmer, A. Is the Continuous Two-Stage Anaerobic Digestion Process Well Suited for All Substrates? Bioresour. Technol. 2016, 200, 470–476. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Aymerich, E.; de González-Mtnez Goñi, J.; Esteban-Gutiérrez, M. Selective VFA Production Potential from Organic Waste Streams: Assessing Temperature and PH Influence. Bioresour. Technol. 2017, 244, 1081–1088. [Google Scholar] [CrossRef]
- De La Rubia, M.A.; Raposo, F.; Rincón, B.; Borja, R. Evaluation of the Hydrolytic-Acidogenic Step of a Two-Stage Mesophilic Anaerobic Digestion Process of Sunflower Oil Cake. Bioresour. Technol. 2009, 100, 4133–4138. [Google Scholar] [CrossRef]
- Qin, Y.; Higashimori, A.; Wu, L.J.; Hojo, T.; Kubota, K.; Li, Y.Y. Phase Separation and Microbial Distribution in the Hyperthermophilic-Mesophilic-Type Temperature-Phased Anaerobic Digestion (TPAD) of Waste Activated Sludge (WAS). Bioresour. Technol. 2017, 245, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, J.; Pérez, M.; Romero, L.I. Semicontinuous Temperature-Phased Anaerobic Digestion (TPAD) of Organic Fraction of Municipal Solid Waste (OFMSW). Comparison with Single-Stage Processes. Chem. Eng. J. 2016, 285, 409–416. [Google Scholar] [CrossRef]
- Zhang, B.; He, P.J. Performance Assessment of Two-Stage Anaerobic Digestion of Kitchen Wastes. Environ. Technol. 2014, 35, 1277–1285. [Google Scholar]
- Kinnunen, M.; Hilderbrandt, D.; Grimberg, S.; Rogers, S.; Mondal, S. Comparative Study of Methanogens in One- and Two-Stage Anaerobic Digester Treating Food Waste. Renew. Agric. Food Syst. 2015, 30, 515–523. [Google Scholar] [CrossRef]
- Hameed, S.A.; Riffat, R.; Li, B.; Naz, I.; Badshah, M.; Ahmed, S.; Ali, N. Microbial Population Dynamics in Temperature-phased Anaerobic Digestion of Municipal Wastewater Sludge. J. Chem. Technol. Biotechnol. 2019, 94, 1816–1831. [Google Scholar] [CrossRef]
- Albini, E.; Pecorini, I.; Ferrara, G. Improvement of Digestate Stability Using Dark Fermentation and Anaerobic Digestion Processes. Energies 2019, 12, 3552. [Google Scholar] [CrossRef]
- Bolzonella, D.; Battista, F.; Cavinato, C.; Gottardo, M.; Micolucci, F.; Lyberatos, G.; Pavan, P. Recent Developments in Biohythane Production from Household Food Wastes: A Review. Bioresour. Technol. 2018, 257, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food Waste Biorefinery: Sustainable Strategy for Circular Bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef] [PubMed]
- De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Energy Recovery from One- and Two-Stage Anaerobic Digestion of Food Waste. Waste Manag. 2017, 68, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, H.; Warith, M.; Hamoda, M.; Kennedy, K. Evaluation of Single vs. Staged Mesophilic Anaerobic Digestion of Kitchen Waste with and without Microwave Pretreatment. J. Environ. Manag. 2013, 125, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.; Hoffmann, E. Upgrading of a Co-Digestion Plant by Implementation of a Hydrolysis Stage. Waste Manag. Res. 2011, 29, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Lukitawesa; Wikandari, R.; Millati, R.; Taherzadeh, M.J.; Niklasson, C. Effect of Effluent Recirculation on Biogas Production Using Two-Stage Anaerobic Digestion of Citrus Waste. Molecules 2018, 23, 3380. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.; Krautkremer, B.; Hartmann, K.; Wachendorf, M. Review of Concepts for a Demand-Driven Biogas Supply for Flexible Power Generation. Renew. Sustain. Energy Rev. 2014, 29, 383–393. [Google Scholar] [CrossRef]
- Linke, B.; Rodríguez-Abalde, Á.; Jost, C.; Krieg, A. Performance of a Novel Two-Phase Continuously Fed Leach Bed Reactor for Demand-Based Biogas Production from Maize Silage. Bioresour. Technol. 2015, 177, 34–40. [Google Scholar] [CrossRef]
- Wall, D.M.; Allen, E.; O’Shea, R.; O’Kiely, P.; Murphy, J.D. Investigating Two-Phase Digestion of Grass Silage for Demand-Driven Biogas Applications: Effect of Particle Size and Rumen Fluid Addition. Renew. Energy 2016, 86, 1215–1223. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Cavinato, C.; Da Ros, C.; Pavan, P.; Bolzonella, D. Influence of Temperature and Hydraulic Retention on the Production of Volatile Fatty Acids during Anaerobic Fermentation of Cow Manure and Maize Silage. Bioresour. Technol. 2017, 223, 59–64. [Google Scholar] [CrossRef]
- Yuan, Y.; Hu, X.; Chen, H.; Zhou, Y.; Zhou, Y.; Wang, D. Advances in Enhanced Volatile Fatty Acid Production from Anaerobic Fermentation of Waste Activated Sludge. Sci. Total Environ. 2019, 694, 133741. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Venkata Mohan, S. Acidogenic Fermentation of Food Waste for Volatile Fatty Acid Production with Co-Generation of Biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile Fatty Acid Production from Mesophilic Acidogenic Fermentation of Organic Fraction of Municipal Solid Waste and Food Waste under Acidic and Alkaline PH. Environ. Sci. Pollut. Res. 2019, 26, 35509–35522. [Google Scholar] [CrossRef] [PubMed]
- Aslanzadeh, S.; Rajendran, K.; Taherzadeh, M.J. A Comparative Study between Single- and Two-Stage Anaerobic Digestion Processes: Effects of Organic Loading Rate and Hydraulic Retention Time. Int. Biodeterior. Biodegrad. 2014, 95, 181–188. [Google Scholar] [CrossRef]
- Voelklein, M.A.; Jacob, A.; O’ Shea, R.; Murphy, J.D. Assessment of Increasing Loading Rate on Two-Stage Digestion of Food Waste. Bioresour. Technol. 2016, 202, 172–180. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Qiao, D.; Yue, L.; Li, Y.Y.; Zhou, J.; Cen, K. Investigating Hydrothermal Pretreatment of Food Waste for Two-Stage Fermentative Hydrogen and Methane Co-Production. Bioresour. Technol. 2017, 241, 491–499. [Google Scholar] [CrossRef]
- Rafieenia, R.; Girotto, F.; Peng, W.; Cossu, R.; Pivato, A.; Raga, R.; Lavagnolo, M.C. Effect of Aerobic Pre-Treatment on Hydrogen and Methane Production in a Two-Stage Anaerobic Digestion Process Using Food Waste with Different Compositions. Waste Manag. 2017, 59, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.F.; Liu, R.; Sun, W.X.; Zhu, R.; Zou, H.; Zheng, Y.; Wang, Z.Y. Enhancing Energy Recovery from Corn Straw via Two-Stage Anaerobic Digestion with Stepwise Microaerobic Hydrogen Fermentation and Methanogenesis. J. Clean. Prod. 2020, 247, 119651. [Google Scholar] [CrossRef]
- Akobi, C.; Yeo, H.; Hafez, H.; Nakhla, G. Single-Stage and Two-Stage Anaerobic Digestion of Extruded Lignocellulosic Biomass. Appl. Energy 2016, 184, 548–559. [Google Scholar] [CrossRef]
- Martin-Ryals, A.; Schideman, L.; Li, P.; Wilkinson, H.; Wagner, R. Improving Anaerobic Digestion of a Cellulosic Waste via Routine Bioaugmentation with Cellulolytic Microorganisms. Bioresour. Technol. 2015, 189, 62–70. [Google Scholar] [CrossRef]
- Poszytek, K.; Ciężkowska, M.; Skłodowska, A.; Drewniak, Ł. Microbial Consortium with High Cellulolytic Activity (MCHCA) for Enhanced Biogas Production. Front. Microbiol. 2016, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Nkemka, V.N.; Gilroyed, B.; Yanke, J.; Gruninger, R.; Vedres, D.; McAllister, T.; Hao, X. Bioaugmentation with an Anaerobic Fungus in a Two-Stage Process for Biohydrogen and Biogas Production Using Corn Silage and Cattail. Bioresour. Technol. 2015, 185, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kvesitadze, G.; Sadunishvili, T.; Dudauri, T.; Zakariashvili, N.; Partskhaladze, G.; Ugrekhelidze, V.; Tsiklauri, G.; Metreveli, B.; Jobava, M. Two-Stage Anaerobic Process for Bio-Hydrogen and Bio-Methane Combined Production from Biodegradable Solid Wastes. Energy 2012, 37, 94–102. [Google Scholar] [CrossRef]
- Miryahyaei, S.; Olinga, K.; Ayub, M.S.; Jayaratna, S.S.; Othman, M.; Eshtiaghi, N. Rheological Measurements as Indicators for Hydrolysis Rate, Organic Matter Removal, and Dewaterability of Digestate in Anaerobic Digesters. J. Environ. Chem. Eng. 2020, 8, 103970. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Selvam, A.; Wong, J.W.C. Hydrolysis-Acidogenesis of Food Waste in Solid-Liquid-Separating Continuous Stirred Tank Reactor (SLS-CSTR) for Volatile Organic Acid Production. Bioresour. Technol. 2016, 200, 366–373. [Google Scholar] [CrossRef]
- Panico, A.; d’Antonio, G.; Esposito, G.; Frunzo, L.; Iodice, P.; Pirozzi, F. The Effect of Substrate-Bulk Interaction on Hydrolysis Modeling in Anaerobic Digestion Process. Sustainability 2014, 6, 8348–8363. [Google Scholar] [CrossRef]
- Farno, E.; Baudez, J.C.; Parthasarathy, R.; Eshtiaghi, N. Impact of Temperature and Duration of Thermal Treatment on Different Concentrations of Anaerobic Digested Sludge: Kinetic Similarity of Organic Matter Solubilisation and Sludge Rheology. Chem. Eng. J. 2015, 273, 534–542. [Google Scholar] [CrossRef]
- Farno, E.; Baudez, J.C.; Parthasarathy, R.; Eshtiaghi, N. Impact of Thermal Treatment on the Rheological Properties and Composition of Waste Activated Sludge: COD Solubilisation as a Footprint of Rheological Changes. Chem. Eng. J. 2016, 295, 39–48. [Google Scholar] [CrossRef]
- Kapsokalyvas, D.; Wilbers, A.; Boogers, I.A.L.A.; Appeldoorn, M.M.; Kabel, M.A.; Loos, J.; Van Zandvoort, M.A.M.J. Biomass Pretreatment and Enzymatic Hydrolysis Dynamics Analysis Based on Particle Size Imaging. Microsc. Microanal. 2018, 24, 517–525. [Google Scholar] [CrossRef]
- Liu, Y.; Wachemo, A.C.; Yuan, H.R.; Li, X.J. Anaerobic Digestion Performance and Microbial Community Structure of Corn Stover in Three-Stage Continuously Stirred Tank Reactors. Bioresour. Technol. 2019, 287, 121339. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Rajendran, K.; Jeihanipour, A.; Taherzadeh, M.J. The Effect of Effluent Recirculation in a Semi-Continuous Two-Stage Anaerobic Digestion System. Energies 2013, 6, 2966–2981. [Google Scholar] [CrossRef]
- Micolucci, F.; Uellendhal, H. Two-Stage Dry Anaerobic Digestion Process Control of Biowaste for Hydrolysis and Biogas Optimization. Chem. Eng. Technol. 2018, 41, 717–726. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Ji, M. Effects of Two-Stage Operation on Stability and Efficiency in Co-Digestion of Food Waste and Waste Activated Sludge. Energies 2019, 12, 2748. [Google Scholar] [CrossRef]
- Michele, P.; Giuliana, D.; Carlo, M.; Sergio, S.; Fabrizio, A. Optimization of Solid State Anaerobic Digestion of the OFMSW by Digestate Recirculation: A New Approach. Waste Manag. 2015, 35, 111–118. [Google Scholar] [CrossRef]
- Bertin, L.; Grilli, S.; Spagni, A.; Fava, F. Innovative Two-Stage Anaerobic Process for Effective Codigestion of Cheese Whey and Cattle Manure. Bioresour. Technol. 2013, 128, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.H.; Selvam, A.; Wong, J.W.C. Innovative Method for Increased Methane Recovery from Two-Phase Anaerobic Digestion of Food Waste through Reutilization of Acidogenic off-Gas in Methanogenic Reactor. Bioresour. Technol. 2016, 217, 3–9. [Google Scholar] [CrossRef]
- Masse, L.; Massé, D.I.; Kennedy, K.J.; Chou, S.P. Neutral Fat Hydrolysis and Long-Chain Fatty Acid Oxidation during Anaerobic Digestion of Slaughterhouse Wastewater. Biotechnol. Bioeng. 2002, 79, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Filiatrault, M.; Guiot, S.R. Acidogenic Digestion of Food Waste in a Thermophilic Leach Bed Reactor: Effect of PH and Leachate Recirculation Rate on Hydrolysis and Volatile Fatty Acid Production. Bioresour. Technol. 2017, 245, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, D.; Luo, Z.; Zeng, W.; Huang, H. The Role of Reflux Time in a Leach Bed Reactor Coupled with a Methanogenic Reactor for Anaerobic Digestion of Pig Manure: Reactor Performance and Microbial Community. J. Clean. Prod. 2020, 242, 118367. [Google Scholar] [CrossRef]
- Xie, S.; Lawlor, P.G.; Frost, J.P.; Wu, G.; Zhan, X. Hydrolysis and Acidification of Grass Silage in Leaching Bed Reactors. Bioresour. Technol. 2012, 114, 406–413. [Google Scholar] [CrossRef]
- Xu, S.Y.; Karthikeyan, O.P.; Selvam, A.; Wong, J.W.C. Microbial Community Distribution and Extracellular Enzyme Activities in Leach Bed Reactor Treating Food Waste: Effect of Different Leachate Recirculation Practices. Bioresour. Technol. 2014, 168, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Browne, J.D.; Allen, E.; Murphy, J.D. Improving Hydrolysis of Food Waste in a Leach Bed Reactor. Waste Manag. 2013, 33, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.H.; Selvam, A.; Wong, J.W.C. Application of Rumen Microbes to Enhance Food Waste Hydrolysis in Acidogenic Leach-Bed Reactors. Bioresour. Technol. 2014, 168, 64–71. [Google Scholar] [CrossRef]
- Liu, T.; Ghosh, S. Phase Separation during Anaerobic Fermentation of Solid Substrates in an Innovative Plug-Flow Reactor. In Water Science and Technology; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1997; Volume 36, pp. 303–310. [Google Scholar]
- Liu, T. Anaerobic Digestion of Solid Substrates in an Innovative Two-Phase Plug-Flow Reactor (TPPFR) and a Conventional Single-Phase Continuously Stirred-Tank Reactor. In Water Science and Technology; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1998; Volume 38, pp. 453–461. [Google Scholar]
- Namsree, P.; Suvajittanont, W.; Puttanlek, C.; Uttapap, D.; Rungsardthong, V. Anaerobic Digestion of Pineapple Pulp and Peel in a Plug-Flow Reactor. J. Environ. Manag. 2012, 110, 40–47. [Google Scholar] [CrossRef]
- Escalante-Hernández, H.; Castro-Molano, L.D.P.; Besson, V.; Jaimes-Estévez, J. Feasibility of the Anaerobic Digestion of Cheese Whey in a Plug Flow Reactor (PFR) under Local Conditions. Ing. Investig. y Tecnol. 2017, 18, 264–277. [Google Scholar]
- Park, C.; Lee, C.; Kim, S.; Chen, Y.; Chase, H.A. Upgrading of Anaerobic Digestion by Incorporating Two Different Hydrolysis Processes. J. Biosci. Bioeng. 2005, 100, 164–167. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.C.; Lee, J.; Wang, C.H.; Dai, Y.; Wah Tong, Y. Three-Stage Anaerobic Co-Digestion of Food Waste and Horse Manure. Sci. Rep. 2017, 7, 1269. [Google Scholar] [CrossRef]
- Zuo, Z.; Wu, S.; Zhang, W.; Dong, R. Effects of Organic Loading Rate and Effluent Recirculation on the Performance of Two-Stage Anaerobic Digestion of Vegetable Waste. Bioresour. Technol. 2013, 146, 556–561. [Google Scholar] [CrossRef]
- Wu, L.J.; Qin, Y.; Hojo, T.; Li, Y.Y. Upgrading of Anaerobic Digestion of Waste Activated Sludge by Temperature-Phased Process with Recycle. Energy 2015, 87, 381–389. [Google Scholar] [CrossRef]
- Gómez, D.; Ramos-Suárez, J.L.; Fernández, B.; Muñoz, E.; Tey, L.; Romero-Güiza, M.; Hansen, F. Development of a Modified Plug-Flow Anaerobic Digester for Biogas Production from Animal Manures. Energies 2019, 12, 2628. [Google Scholar] [CrossRef]
- Liu, W.Y.; Liao, B. Anaerobic Co-Digestion of Vegetable and Fruit Market Waste in LBR + CSTR Two-Stage Process for Waste Reduction and Biogas Production. Appl. Biochem. Biotechnol. 2019, 188, 185–193. [Google Scholar] [CrossRef]
- Battista, F.; Frison, N.; Pavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; et al. Food Wastes and Sewage Sludge as Feedstock for an Urban Biorefinery Producing Biofuels and Added-value Bioproducts. J. Chem. Technol. Biotechnol. 2019, 95, 328–338. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile Fatty Acids Production from Food Wastes for Biorefinery Platforms: A Review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Colombo, B.; Villegas Calvo, M.; Pepè Sciarria, T.; Scaglia, B.; Savio Kizito, S.; D’Imporzano, G.; Adani, F. Biohydrogen and Polyhydroxyalkanoates (PHA) as Products of a Two-Steps Bioprocess from Deproteinized Dairy Wastes. Waste Manag. 2019, 95, 22–31. [Google Scholar] [CrossRef]
- Brigham, C.J.; Riedel, S.L. The Potential of Polyhydroxyalkanoate Production from Food Wastes. Appl. Food Biotechnol. 2019, 6, 7–18. [Google Scholar]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-Based Volatile Fatty Acid Production and Recovery from Waste Streams: Current Status and Future Challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Han, W.; He, P.; Shao, L.; Lü, F. Road to Full Bioconversion of Biowaste to Biochemicals Centering on Chain Elongation: A Mini Review. J. Environ. Sci. 2019, 86, 50–64. [Google Scholar] [CrossRef]
- De Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Medium Chain Carboxylic Acids from Complex Organic Feedstocks by Mixed Culture Fermentation. Molecules 2019, 24, 398. [Google Scholar] [CrossRef]
- Cavinato, C.; Giuliano, A.; Bolzonella, D.; Pavan, P.; Cecchi, F. Bio-Hythane Production from Food Waste by Dark Fermentation Coupled with Anaerobic Digestion Process: A Long-Term Pilot Scale Experience. Int. J. Hydrogen Energy 2012, 37, 11549–11555. [Google Scholar] [CrossRef]
- Wang, L.; Shen, F.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Li, X. Anaerobic Co-Digestion of Kitchen Waste and Fruit/Vegetable Waste: Lab-Scale and Pilot-Scale Studies. Waste Manag. 2014, 34, 2627–2633. [Google Scholar] [CrossRef]

| Substrate | Compositional Characteristics | Challenges in Hydrolysis | Strategies to Improve Hydrolysis | |
|---|---|---|---|---|
| Liquid residues | Sludge/WAS |
|
|
|
| Animal manure |
|
|
| |
| Solid residues | FW |
|
|
|
| OFMSW/municipal solid waste |
|
|
| |
| Animal by-products |
|
|
| |
| Lignocellulosic biomass |
|
|
| |
| Substrate | T (°C) | pH | OLR (gVS L−1d−1) | HRT (d) | H2 Production (L kgVS−1) | Hydrolysis Yield * (%) | Acidification Yield (%) | SCCA Composition (%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAc | HBu | HPr | Other | |||||||||
| FW | M | 6.9 ± 0.1 (i) | - | 1.25 | 56.5 | 42.4 | 48.9 | 42 | 26 | 30 | - | [44] |
| 10.0 (i) ~ 6.0 (p) | 15 kg COD m3d | 2 | 30% v/v | - | 25.29 | >50 | - | - | - | [55] | ||
| 5.5 ± 0.3 (c) | 9 | 4 | 11.8 ± 2.1 | 39.9 ± 1.8 | 36.2 ± 4.6 | 41 | 18.3 | - | 25.6 HCa | [58] | ||
| 5.5 ± 0.5 (c) | 15 | 8.2 ± 0.9 | 45.6 ± 2.4 | 37.6 ± 0.1 | 34.2 | 47 | - | 7.5 HCa | ||||
| ~8.8 (c) | 17.5 | 3.5 | - | - | 21.39 | 80 | - | - | - | [56] | ||
| OFMSW | 9.8–10.0 (c) | - | - | 18.15 | 40 | 16 | 19.6 | - | ||||
| 5.5–6.0 | 13–18 | 5.5–7.5 | 29% of 20–28 Ld−1 | 17–22 | - | 45 | 31 | 5 | 10 HVa | [27] | ||
| FW, Rice straw ** | 6.0 | 4 | 8–10 | - | - | 36 | 56 | 42.6 | - | - | [29] | |
| Cow manure, maize silage | 5.5–5.6 | 17.9 | 4 | - | 20.4 | 18.3 | 44 | 29 | - | - | [53] | |
| Sunflower oil cake | 5.1–5.4 (c) | 8 | 8–10 | - | 30 | 60–65 | 25–27 | - | 35 | 18 HVa | [35] | |
| 6 | - | 22.5 | 81.6–83.8 | 25–30 | 20 | 25–27 | - | |||||
| OFMSW | 55 | 10 (i) | 10,000 mg O2 L−1 | 10 | - | 39 | 83 | ~73 | ~7 | ~7 | - | [34] |
| Sewage sludge | 40 | 49 | ~50 | ~12 | ~15 | ~15 isoHVa | ||||||
| Meat/bone meal | 54 | 53 | ~47 | ~8 | ~8 | ~12 isoHVa | ||||||
| WAS | 70 | 5.8 (i) | - | 6 | - | 32 | 18.3 | 50.3 | - | 19.8 | 15.7 isoHVa | [36] |
| Pretreatment Conditions | Substrate | Main Conditions Hydrolysis Stage | Process Performance | Reference |
|---|---|---|---|---|
| 24 h aerobic aeration with 5 Lh−1 | FW: C-rich P-rich L-rich | Batch, 35 °C, initial pH 6.0, OLR 5 gVS L−1 |
| [60] |
| Microaeration 5–10 mLO2 gVS−1, daily | Corn straw | Batch, 37 °C, initial pH 7.5, 5 d |
| [61] |
| NaOH pretreatment of rice straw | Rice straw + FW | Semi-continuous, 35 °C, pH 6.0, OLR 4 gVS L−1d−1 |
| [29] |
| HTP, 140 °C, 20 min | FW | Batch, 37 °C, initial pH 6.0, OLR 20 gVS L−1, HRT 2 d/24 d |
| [59] |
| HTP, 170–175 °C, 60 min | OFMSW | Semi-continuous, 37 °C, pH 5.5–6.0, various OLR |
| [27] |
| Freeze explosion, milling | Corn stalk | Batch, 55 °C, pH 8.0–9.0, coculture of 2 Clostridia strains |
| [66] |
| OFMSW |
| |||
| Twin screw extrusion | Poplar wood | Batch, 37 °C, initial pH 5.5 |
| [62] |
| Bioaugmentation (Piromyces rhizinflata) | Corn silage, cattail | Batch, LBR, external recirculation |
| [65] |
| Bioaugmentation, 16 strains (Bacillus, Providencia, Ochrobactrum) | Maize silage | Batch, 30 °C, initial pH 7.2, HRT 3 d |
| [64] |
| Bioaugmentation, daily (Clostrida) | Sweet corn processing residues | Continuous, 37–40 °C pH 7.2–5.2, HRT 2 d |
| [63] |
| Reactor Config. | Recirc. Ratio | Substrate | T (°C) | pH | OLR (kgVS m−3d−1) | HRT (d) | Hydro-Lysis Yield (%) | Acidifi-Cation Yield (%) | SCCA Composition (%) | H2 Yield (m3kg−1 TVS) | Removal Efficiency (%) | Ref. | ||
| HAc | Hbu | HPr | ||||||||||||
| CSTR | Dynamic to NH3 conc. | FW | T | 5.3 ± 0.2 | 16–18 | 3.3 | - | 13.2 gCOD L−1 | 14.9 | 30.3 | 7.9 | ~0.06–0.07 ** | - | [11] |
| 0.5 | FW + WAS | 5.0–5.5 | 29.3 /39.6 | 0.8 /1.1 | - | 20 /14 | ~37–40 | ~37–40 | 0.064–0.076 | VS 38–51.7 | [76] | |||
| 9.1 | 3.3 | - | 46 | 5-51 | 38–89 | 0.031 | ||||||||
| 0.5–0.7 | FW | 5.3 | 17 | 3.3 | - | 26.7 * | 0.068 | - | [32] | |||||
| Dynamic to pH 5.75 | Sugar beet | 5.5–5.8 | 5.75 | 2.7 | - | - | 61.2 | 34.3 | 12.4 EtOH | - | - | [33] | ||
| Maize silage | 7.3 | 60.9 | 27.2 | |||||||||||
| Hay, Straw | 28.6 | 76.5 | 11.4 | |||||||||||
| 0.5 | FW | 5.36 | 14.2 | 3 | 16.2 | 40.3 | - | 0.04 | COD 35.3 | [17] | ||||
| none | 3.61 | 12 | 6 | 88 ** | 90 ** | 0.00 | 0.00 | |||||||
| External (5%) | Citrus waste | 5.0–6.0 | 5 | 15 | - | 47 * of sCOD | 84 | 1 | 8.5 | - | VS 32–34 | [47] | ||
| 0.6 | VW | M | 5.5 | 2.6 | - | - | - | 58 ** | 30 ** | - | - | [93] | ||
| 0.5 | FW + cow manure | 5.5–7.5 | 16.8 | 10 | 12 * of sCOD | - | 30 ** | 30 ** | 25 ** | COD 38 | [9] | |||
| 0.5 | WAS | T | 7.65 ± 0.05 | 9.07 | 3 | 37.4 | 23.2 | - | - | - | - | VS 23.1 COD 26.3 | [94] | |
| SLS-CSTR | - | FW | M | ~8.0 | 2 | 15 | 60–80 | 61–89 | 32 | 60 | - | - | - | [68] |
| 6.0 | 85–93 | 95 | - | - | ||||||||||
| CSTR–shared gas phase | - | Cheese whey, cattle manure | 5.1 ± 0.3 | 1.7 kgCOD m−3d−1 | 5 | - | - | - | - | sCOD 30 | [78] | |||
| Plug-flow reactor | 0.2 | Dry animal feed | M | 4.4–7.0 | 15.2 *** | 5.7 *** | - | Max 9.6 gL−1 | - | none | - | [87] | ||
| 0.4 | Pineapple pulp | - | 0.97 kgCOD m−3d−1 | 10 | - | - | - | - | 80.9 COD | [89] | ||||
| 0.4 | Pig manure | 8.0 ± 0.1 | 1.61 ± 0.33 | 27.06 ± 4.37 | - | - | 94 ± 9.7 | - | - | - | VS 46.47 ± 20.03 | [95] | ||
| Reactor Config. | Reflux | Substrate | T (°C) | pH | Liquid/Solid Ratio | SRT (d) | Hydro-Lysis Yield (%) | Acidifi-Cation Yield (%) | SCCA Composition (%) | H2 Yield (m3kg−1 TVS) | Removal Efficiency (%) | Ref. | ||
| Hac | Hbu | HPr | ||||||||||||
| LBR | 1.7 | FW | T | 7.2 | 0.65 | 14 | 53 | 57 | 27 | 54 | 8.4 | - | - | [81] |
| 2.6 | 56.5 | 75 | 25 | 55.5 | 10 | |||||||||
| 7.2 L/d | FW | M | 5.1 ± 1.4 | 1 | 16 | - | 51 | - | - | VS 69.4 | [84] | |||
| 2.2 | OFMSW | 5.2 ± 0.2 | 13.4 | 24 | 71 | 83 * | - | - | - | [75] | ||||
| external | VFW | 6.5–5.0 | - | 5.8 | - | - | 31.5 | 0 | 51 | - | VS 70.9 | [96] | ||
| 2, 1:1 leachate dilution | Grass silage | 6.5–6.2 | 5-10 | 24 | 52–58 | 57–60 | 38–41 | 25–28 | 27–30.6 | - | VS 62-66 | [83] | ||
| 2 | Pig manure | 7.0–7.7 | 18 | 42 | - | - | - | VS 97.53 | [82] | |||||
| LBR, off-gas recirc. | 0.5 | FW | 5.0–6.0 | 1 | 21 | 60 | - | 18 | 43 | 6 | - | - | [86] | |
| Process Parameters | Unit | [32] | [11] | [104] | [9] | [40] | [14] | [10] | [105] |
|---|---|---|---|---|---|---|---|---|---|
| Substrate | FW | FW | FW | FW, cow manure | municipal sludge | FW | FW | KW, FVW | |
| TS | % | 5.3/2.3 | 8/ | 8/ | 15/<10 | 6.5/ | 7.32/2.84 | /1.5 | |
| OLR | kgTVS m−3d−1 | 17/3.5 | 16–18/4–5 | 16–18/4–6 | 16.8/0.86–1.28 | - | 0.78 ± 0.42 | 3.5 | 3 |
| HRT | d | 3.3/12.6 | 3.3/12.6 | 3.3/12.6 | 10/9 | 2.5/10 | 20-30 | 3.3/16.7 | 10/20 |
| T | °C | 55 | 55 | 55 | -/37 | 45/35 | 37.4 ± 3.6 | 55 | 35 |
| pH * | 5.3/8.15 | 5.3/8.2 | 5.7/8.4 | 7.2/8.03 | 7.4/7.3 | 5.2/8.4 | 4.6/8.0 | /7.0 | |
| Recirc. | 0.5–0.7 ratio | external | external | internal | internal gas | none | none | none | |
| VFA yield | gL−1 | 13.9 ± 0.5/ 0.63 | 13.2 ± 5/ 0.53 ± 0.4 | 11.7 ± 3.1 /1.1 ± 1.1 | /0.137 | 3.42 ± 0.6/ 1.85 ± 0.2 | 38.9 ± 4.8/ 6.3 ± 3.6 *** | 9.99 ± 3.9/ 0.55 | - |
| SBP | m3 kgTVS−1 | 0.17/0.75 | 0.65 ± 0.1 | 0.8 | /0.68–0.92 | - | - | /0.88 | - |
| SMP | m3 kgTVS−1 | - | - | /0.72 | /0.54–0.82 | 0.289/0.89 | 0.446 | /0.55 | 0.61 |
| SHP | m3 kgTVS−1 | - | - | 0.067/ | - | - | - | - | - |
| CH4 content | % | 7/67 | 53 | /67 ± 3.7 | 85 | 64/63.2 | 59 | 62 ± 2 | 65.7 |
| H2 content | % | 40/ | 8 | 38.5 ± 9.7/ | - | - | - | - | - |
| COD removal | % | 98 ** | - | - | 73 | 65 | 91 | - | VS 89.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzel, T.; Neubauer, P.; Junne, S. Role of Microbial Hydrolysis in Anaerobic Digestion. Energies 2020, 13, 5555. https://doi.org/10.3390/en13215555
Menzel T, Neubauer P, Junne S. Role of Microbial Hydrolysis in Anaerobic Digestion. Energies. 2020; 13(21):5555. https://doi.org/10.3390/en13215555
Chicago/Turabian StyleMenzel, Theresa, Peter Neubauer, and Stefan Junne. 2020. "Role of Microbial Hydrolysis in Anaerobic Digestion" Energies 13, no. 21: 5555. https://doi.org/10.3390/en13215555
APA StyleMenzel, T., Neubauer, P., & Junne, S. (2020). Role of Microbial Hydrolysis in Anaerobic Digestion. Energies, 13(21), 5555. https://doi.org/10.3390/en13215555








