Kinetics of Biotic and Abiotic CO Production during the Initial Phase of Biowaste Composting
Abstract
1. Introduction
- kco—CO consumption constant rate (s−1),
- m—the mass of CO (mg),
- t—time (s),
- kp—CO production rate (mg.s−1).
- m0, me—equilibrium values (mg).
2. Materials and Methods
2.1. Waste Characteristics
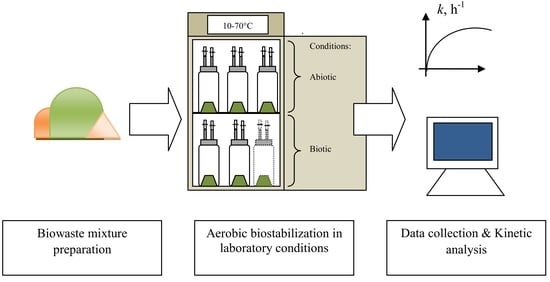
2.2. The Experiment Design and Procedure
2.3. Gas Production Monitoring
2.4. CO Production Kinetics Determination
- P—total production or consumption (CO, CO2, or O2), (mg·g−1d.m.),
- K—production or consumption rate (CO, CO2, or O2), (mg·g−1d.m.·h−1),
- T—time, (h).
- P—total production or consumption (CO, CO2, or O2), (mg·g−1d.m.),
- P0—maximum production or consumption (CO, CO2, or O2), (mg·g−1d.m.),
- k—production or consumption (CO, CO2, or O2) constant rate, (h−1),
- t—time, (h).
- r—average production or consumption rate (r) of CO, CO2, or O2, (mg·g−1d.m.·h−1).
2.5. Statistical Analysis
- êi—the estimated residuals for a particular candidate model,
- K—total number of estimated regression parameters.
3. Results
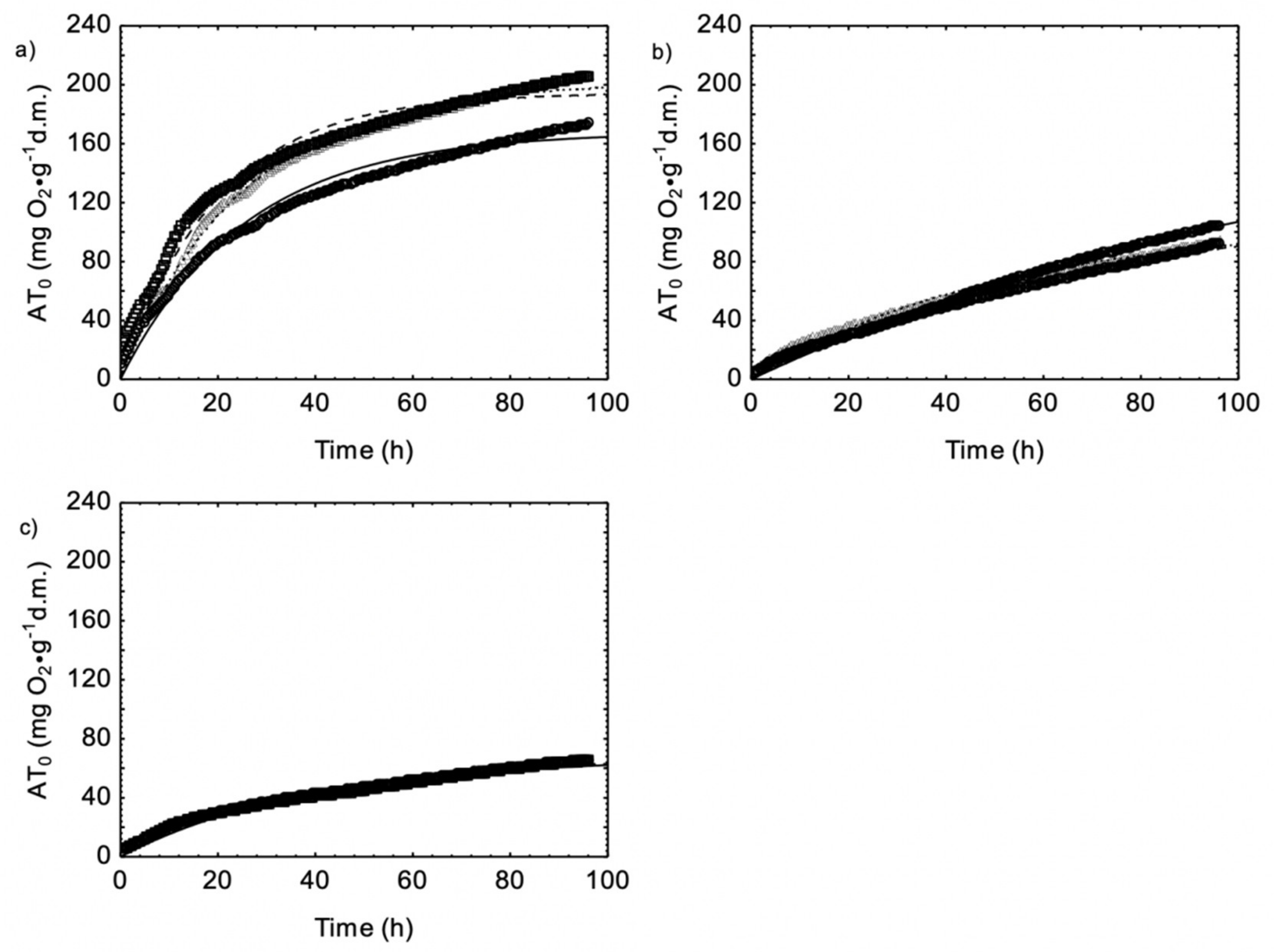
3.1. Material Properties before and after Composting
3.2. Dynamics of O2 Consumption and CO2 Production during Biowaste Aerobic Biostabilization
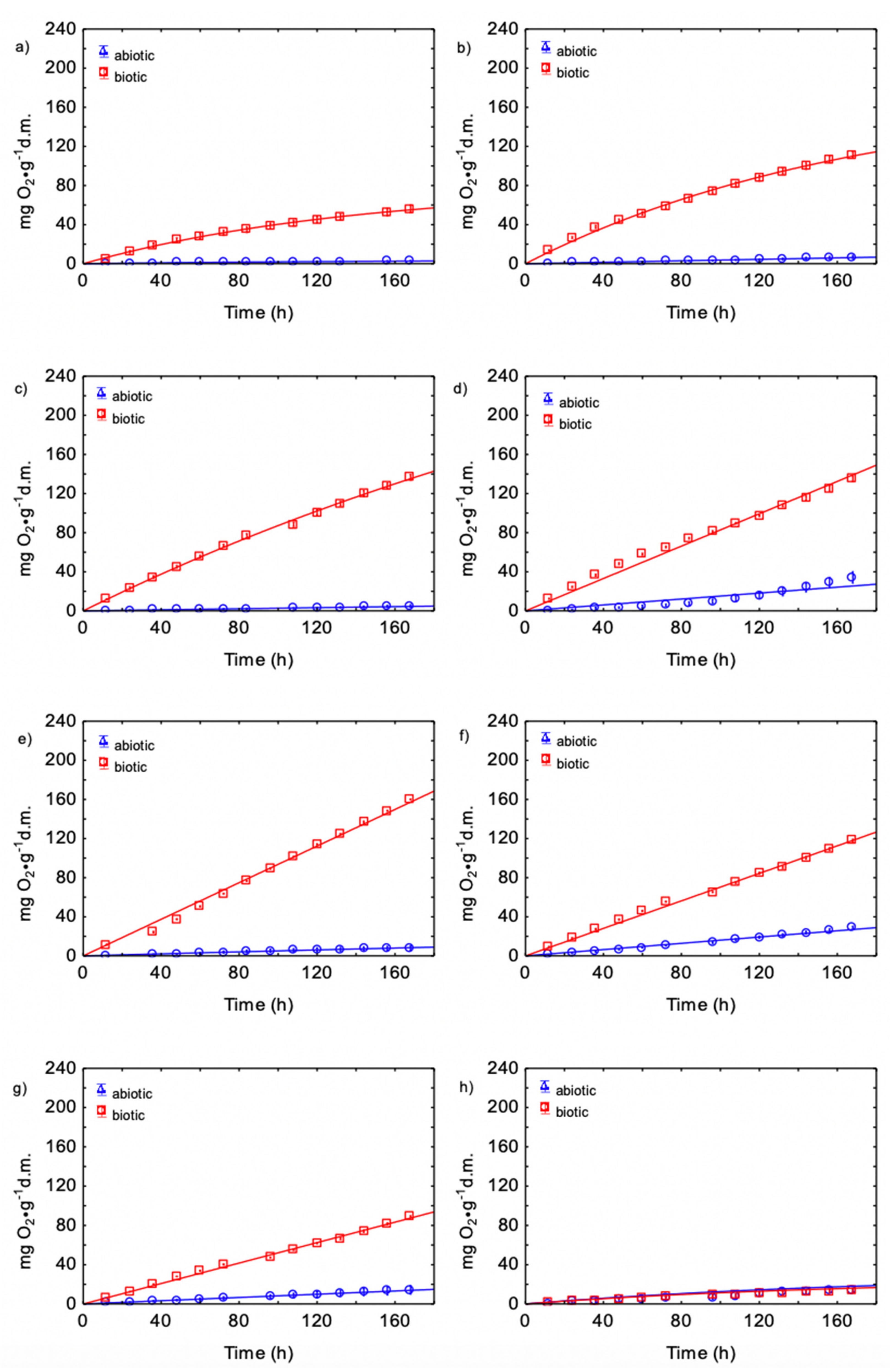
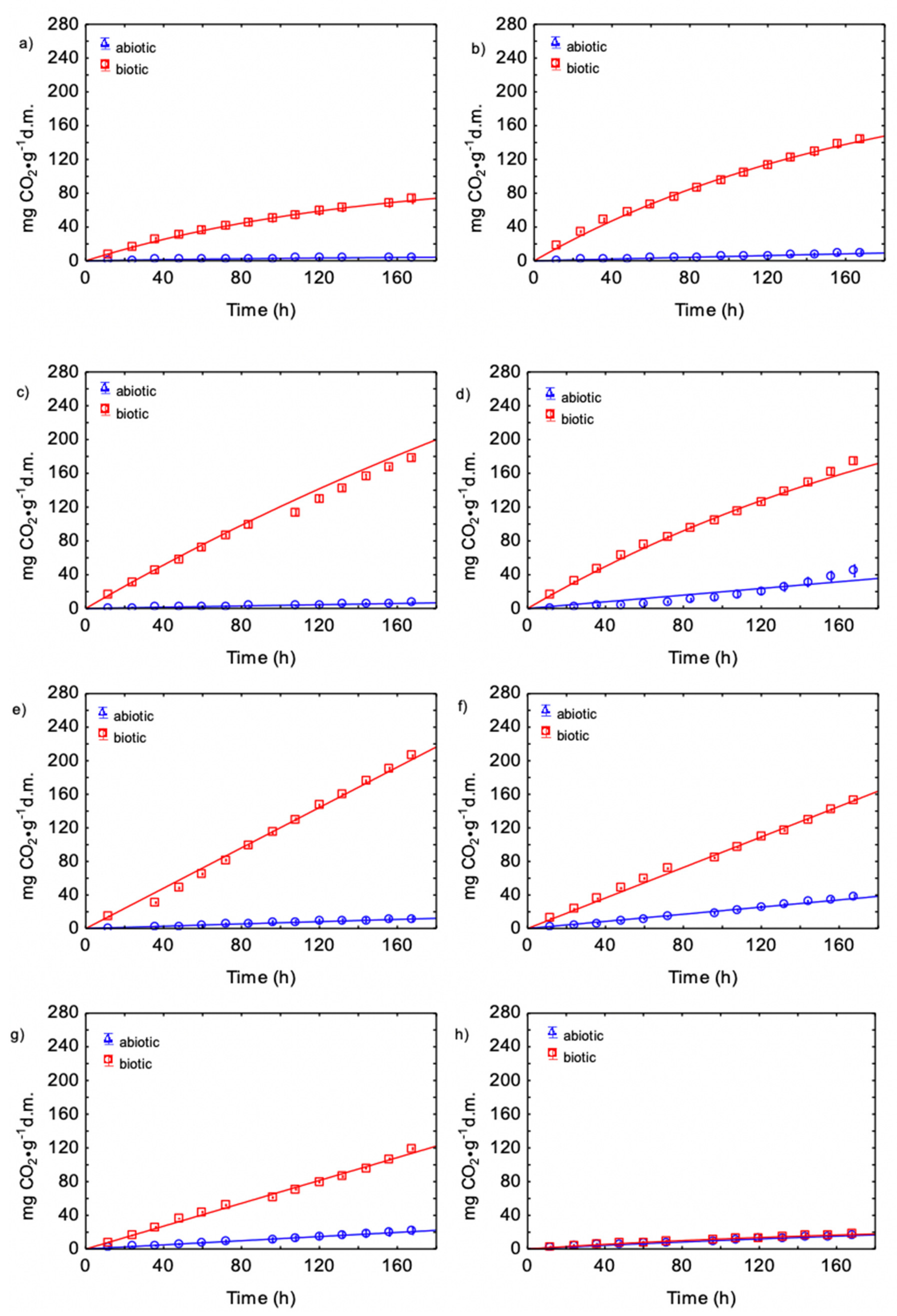
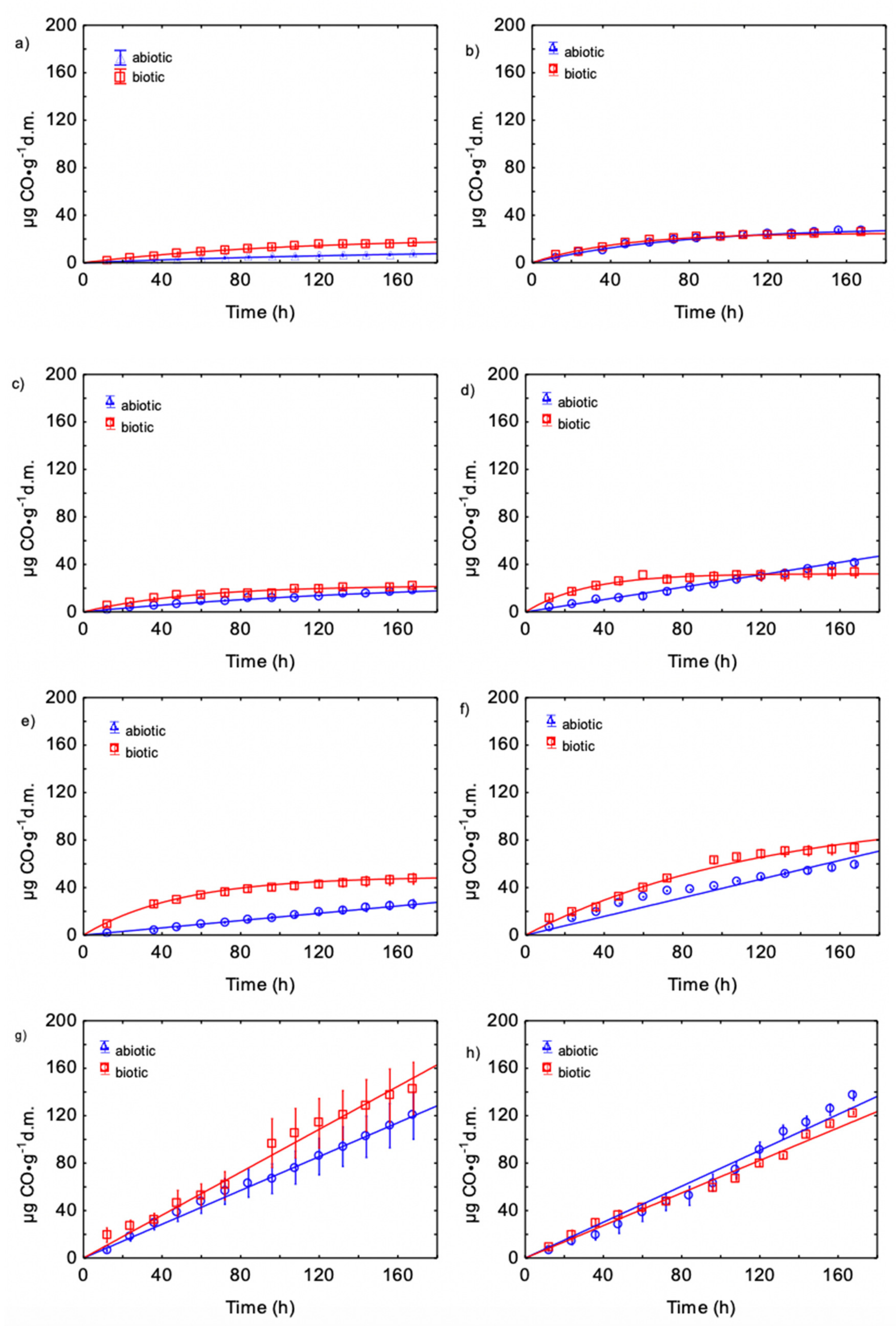
3.3. Kinetics of CO Production during Biowaste Aerobic Biostabilization
3.4. Kinetics of CO2 and CO Production and O2 Consumption during Biowaste Aerobic Biostabilization
4. Discussion
4.1. Biowaste Properties
4.2. O2 Consumption, CO2 and CO Production during Biowaste Aerobic Biostabilization
4.3. Kinetics of O2Consumption, CO2 and CO Production during Biowaste Aerobic Biostabilization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Production or Consumption | Temperature °C | Abiotic | Biotic |
|---|---|---|---|
| O2 | 10 | ||
| 20 | |||
| 30 | |||
| 37 | |||
| 40 | |||
| 50 | |||
| 60 | |||
| 70 | |||
| CO2 | 10 | ||
| 20 | |||
| 30 | |||
| 37 | |||
| 40 | |||
| 50 | |||
| 60 | |||
| 70 | |||
| CO | 10 | ||
| 20 | |||
| 30 | |||
| 37 | |||
| 40 | |||
| 50 | |||
| 60 | |||
| 70 |
References
- Techtmann, S.M.; Colman, A.S.; Robb, F.T. That which does not kill us only makes us stronger: The role of carbon monoxide in thermophilic microbial consortia. Environ. Microbiol. 2009, 11, 1027–1037. [Google Scholar] [CrossRef]
- Ahmad Farid, M.A.; Hassan, M.A.; Roslan, A.M.; Samsudin, M.H.; Mohamad, Z.J.J.; Othman, M.R.; Shirai, Y. Carbon monoxide reduction in the flue gas during biochar production from oil palm empty fruit bunch. J. Clean. Prod. 2020, 258, 120580. [Google Scholar] [CrossRef]
- Haarstad, K.; Bergersen, O.; Sorheim, R. Occurrence of carbon monoxide during organic waste degradation. J. Air Waste Manag. Assoc. 2006, 56, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Stegenta-Dąbrowska, S.; Drabczyński, G.; Sobieraj, K.; Koziel, J.A.; Białowiec, A. The biotic and abiotic carbon monoxide formation during aerobic co-digestion of dairy cattle manure with green waste and sawdust. Front. Bioeng. Biotechnol. 2019, 7, 283. [Google Scholar] [CrossRef] [PubMed]
- Hellebrand, H.J.; Kalk, W.D. Emission of carbon monoxide during composting of dung and green waste. Nutr. Cycl. Agroecosyst. 2001, 60, 79–82. [Google Scholar] [CrossRef]
- Stegenta, S.; Dębowski, M.; Bukowski, P.; Randerson, P.F.; Białowiec, A. The influence of perforation of foil reactors on greenhouse gas emission rates during aerobic biostabilization of the undersize fraction of municipal wastes. J. Environ. Manag. 2018, 207, 355. [Google Scholar] [CrossRef]
- Hellebrand, H.J. Emission of nitrous oxide and other trace gases during composting of grass and green waste. J. Agric. Eng. Res. 1998, 69, 365–375. [Google Scholar] [CrossRef]
- Boldrin, A.; Andersen, J.K.; Moller, J.; Christensen, T.H.; Favoino, E. Composting and compost utilization: Accounting of greenhouse gases and global warming contributions. Waste Manag. Res. 2009, 27, 800–812. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sanchez-Monedero, M.A.; Roig, A.; Sinicco, T.; Mondini, C.; Luz Cayuela, M.; Angel Sanchez-Monedero, M.; Roig, A.; Sinicco, T.; Mondini, C. Biochemical changes and GHG emissions during composting of lignocellulosic residues with different N-rich by-products. Chemosphere 2012, 88, 196–203. [Google Scholar] [CrossRef]
- Colon, J.; Cadena, E.; Pognani, M.; Barrena, R.; Sanchez, A.; Font, X.; Artola, A. Determination of the energy and environmental burdens associated with the biological treatment of source-separated Municipal Solid Wastes. Energy Environ. Sci. 2012, 5, 5731–5741. [Google Scholar] [CrossRef]
- Ermolaev, E.; Pell, M.; Smårs, S.; Sundberg, C.; Jönsson, H.; Smars, S.; Sundberg, C.; Jonsson, H. Greenhouse gas emission from covered windrow composting with controlled ventilation. Waste Manag. Res. 2012, 30, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Daou, A.; Mallat, C.; Chammas, G.; Cerantola, N.; Kayed, S.; Saliba, N.A. The Ecocanvas as a business model canvas for a circular economy. J. Clean. Prod. 2020, 258, 120938. [Google Scholar] [CrossRef]
- Sironi, S.; Botta, D. Biofilter efficiency in odor abatement at composting plants. Compost Sci. Util. 2001, 9, 149–155. [Google Scholar] [CrossRef]
- Stegenta, S.; Sobieraj, K.; Pilarski, G.; Koziel, J.; Białowiec, A. The spatial and temporal distribution of process gases within the biowaste compost. Data 2019, 4, 37. [Google Scholar] [CrossRef]
- Szanto, G.L.; Hamelers, H.M.; Rulkens, W.H.; Veeken, A.H.M. NH3, N2O and CH4 emissions during passively aerated composting of straw-rich pig manure. Bioresour. Technol. 2007, 98, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.; Cuhls, C. Greenhouse gas emissions from mechanical and biological waste treatment of municipal waste. Environ. Technol. 2003, 24, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Amlinger, F.; Peyr, S.; Cuhls, C. Green house gas emissions from composting and mechanical biological treatment. Waste Manag. Res. 2008, 26, 47–60. [Google Scholar] [CrossRef]
- Boldrin, A.; Andersen, J.K.; Christensen, T.H. Environmental assessment of garden waste management in the Municipality of Aarhus, Denmark. Waste Manag. 2011, 31, 1560–1569. [Google Scholar] [CrossRef]
- Hellebrand, H.; Schade, G.W.; Idler, C.; Kern, J. Carbon monoxide from composting due to thermal oxidation of biomass: An additional pathway for co in agricultural and forest ecosystems. In Proceedings of the Workshop on Agricultural Air Quality: State of the Science, Potomac, MA, USA, 4–8 June 2006; pp. 693–705. [Google Scholar]
- Conrad, R.; Seiler, W. Role of microorganisms in the consumption and production of atmospheric carbon monoxide by soil. Appl. Environ. Microbiol. 1980, 40, 437–445. [Google Scholar] [CrossRef]
- Zepp, R.G.; Miller, W.L.; Tarr, M.A.; Burke, R.A.; Stocks, B.J. Soil-atmosphere fluxes of carbon monoxide during early stages of postfire succession in upland Canadian boreal forests. J. Geophys. Res. Atmos. 1997, 102, 29301–29311. [Google Scholar] [CrossRef]
- Fraser, W.T.; Blei, E.; Fry, S.C.; Newman, M.F.; Reay, D.S.; Smith, K.A.; McLeod, A.R. Emission of methane, carbon monoxide, carbon dioxide and short-chain hydrocarbons from vegetation foliage under ultraviolet irradiation. Plant Cell Environ. 2015, 38, 980–989. [Google Scholar] [CrossRef] [PubMed]
- van Asperen, H.; Warneke, T.; Sabbatini, S.; Nicolini, G.; Papale, D.; Notholt, J. The role of photo- and thermal degradation for CO2 and CO fluxes in an arid ecosystem. Biogeosciences 2015, 12, 4161–4174. [Google Scholar] [CrossRef]
- Rich, J.J.; King, G.M. Carbon monoxide consumption and production by wetland peats. FEMS Microbiol. Ecol. 1999, 28, 215–224. [Google Scholar] [CrossRef]
- Goldstein, J.; Goldstein, N. Controlling odors at composting facilities. Biocycle 2005, 46, 22. [Google Scholar]
- Bünger, J.; Schappler-Scheele, B.; Hilgers, R.; Hallier, E. A 5-year follow-up study on respiratory disorders and lung function in workers exposed to organic dust from composting plants. Int. Arch. Occup. Environ. Health 2007, 80, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.E.W.; Nieden, A.; Seitz, H.; Harpel, S.; Stinner, D.; Stilianakis, N.; Eikmann, T. Bioaerosols in outdoor air—Statement of environmental medical assessment criteria on the basis of an epidemiological cross sectional study. Gefahrst. Reinhalt. Luft 2004, 64, 143–152. [Google Scholar]
- Müller, T.; Jörres, R.; Scharrer, E.; Hessel, H.; Nowak, D.; Radon, K. Acute blood neutrophilia induced by short-term compost dust exposure in previously unexposed healthy individuals. Int. Arch. Occup. Environ. Health 2006, 79, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Thissen, R.; Braun, S.; Dott, W.; Fischer, G. (M)VOC and composting facilities—Part 2: (M)VOC dispersal in the environment. Environ. Sci. Pollut. Res. Int. 2004, 11, 152. [Google Scholar] [CrossRef]
- Koskela, R.S.; Mutanen, P.; Sorsa, J.; Klockars, M. Factors predictive of ischemic heart disease mortality in foundry workers exposed to carbon monoxide. Am. J. Epidemiol. 2000, 152, 628–632. [Google Scholar] [CrossRef]
- Pikoń, K.; Rejman, R. Carbon cycle in municipal solid waste managements. Arch. Gospod. Odpad. 2009, 11, 27–46. [Google Scholar]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Yu, Q.; Li, H. Moderate separation of household kitchen waste towards global optimization of municipal solid waste management. J. Clean. Prod. 2020, 277, 123330. [Google Scholar] [CrossRef]
- Binner, E.; Bohm, K.; Lechner, P. Large scale study on measurement of respiration activity (AT(4)) by Sapromat and OxiTop. Waste Manag. 2012, 32, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Kilian, E.; Macedowska-Capiga, A. Parametr AT4 jako wskaźnik stopnia stabilizacji odpadów po mechaniczno-biologicznym przetworzeniu (AT4 parameter as an indicator of the degree of stabilization of waste after mechanical and biological treatment). Pr. Inst. Ceram. Mater. Bud. 2011, 4, 88–94. [Google Scholar]
- Stegenta, S.; Kałdun, B.; Białowiec, A. Model selection and estimation of kinetic parameters of oxygen consumption during biostabilization of under-size fraction of municipal solid waste. Rocz. Ochr. Sr. 2016, 18, 800–814. [Google Scholar]
- Burnham, K.P.; Anderson, D.R.; Burnham, K.P. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Jiang, T.; Schuchardt, F.; Li, G.; Guo, R.; Zhao, Y. Effect of C/N ratio, aeration rate and moisture content on ammonia and greenhouse gas emission during the composting. J. Environ. Sci. 2011, 23, 1754–1760. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Suzuki, K.; Kuroda, K.; Waki, M.; Yasuda, T. Effects of struvite formation and nitratation promotion on nitrogenous emissions such as NH3, N2O and NO during swine manure composting. Bioresour. Technol. 2011, 102, 1468–1474. [Google Scholar] [CrossRef]
- Albrecht, R.; Joffre, R.; Gros, R.; Le Petit, J.; Terrom, G.; Perissol, C. Efficiency of near-infrared reflectance spectroscopy to assess and predict the stage of transformation of organic matter in the composting process. Bioresour. Technol. 2008, 99, 448–455. [Google Scholar] [CrossRef]
- Dach, J. Kompostowanie trawy i liści (Grass and leaves composting). Przegląd Komunal. 2008, 10, 36–39. [Google Scholar]
- Kumar, M.; Ou, Y.; Lin, J. Co-composting of green waste and food waste at low C/N ratio. Waste Manag. 2010, 30, 602–609. [Google Scholar] [CrossRef]
- Adhikari, B.K.; Trémier, A.; Barrington, S.; Martinez, J. Biodegradability of municipal organic waste: A respirometric test. Waste Biomass. Valor. 2013, 4, 331–340. [Google Scholar] [CrossRef]
- Sun, W.; Huang, G.H.; Zeng, G.; Qin, X.; Sun, X. A stepwise-cluster microbial biomass inference model in food waste composting. Waste Manag. 2009, 29, 2956–2968. [Google Scholar] [CrossRef] [PubMed]
- Mason, I.G. Mathematical modelling of the composting process: A review. Waste Manag. 2006, 26, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, K.; Stegenta, S.; Białowiec, A. The use of respiration activity method for predicting easily biodegradable fractions in organic waste. Przem. Chem. 2017, 96. [Google Scholar] [CrossRef]
- Wetzel, S.; Volpe, S.; Damianopoulos, J.; Krigstin, S. Can biomass quality be preserved through tarping comminuted roadside biomass piles? Forests 2017, 8, 305. [Google Scholar] [CrossRef]
- de Guardia, A.; Petiot, C.; Rogeau, D.; Druilhe, C. Influence of aeration rate on nitrogen dynamics during composting. Waste Manag. 2008, 28, 575–587. [Google Scholar] [CrossRef]
- Mason, I.G.; Milke, M.W. Physical modelling of the composting environment: A review. Part 1: Reactor systems. Waste Manag. 2005, 25, 481–500. [Google Scholar] [CrossRef]
- Evangelou, A.; Calabrò, P.S.; Greco, R.; Sánchez, A.; Komilis, D. Biodegradation activity of eight organic substrates: A correlation study of different test methods. Waste Biomass. Valor. 2016, 7, 1067–1080. [Google Scholar] [CrossRef][Green Version]
- Barrena, R.; d’Imporzano, G.; Ponsa, S.; Gea, T.; Artola, A.; Vazquez, F.; Sanchez, A.; Adani, F. In search of a reliable technique for the determination of the biological stability of the organic matter in the mechanical-biological treated waste. J. Hazard. Mater. 2009, 162, 1065–1072. [Google Scholar] [CrossRef]
- Sadecka, Z.; Suchowska-Kisielewicz, M. The possibility of using organic substrates in the fermentation process. Rocz. Ochr. Sr. 2016, 18, 400–413. [Google Scholar]
- Manu, M.K.; Kumar, R.; Garg, A. drum composting of food waste: A kinetic study. Procedia Environ. Sci. 2016, 35, 456–463. [Google Scholar] [CrossRef]
- Fernandez, F.J.; Sanchez-Arias, V.; Villasenor, J.; Rodriguez, L. Evaluation of carbon degradation during co-composting of exhausted grape marc with different biowastes. Chemosphere 2008, 73, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, F.; Sano, Y.; Nakayama, A.; Nakasaki, K.; Fukazawa, T. Numerical modeling of a composting process with aeration. J. Porous Media 2009, 12, 927–938. [Google Scholar] [CrossRef]
- Ermolaev, E.; Sundberg, C.; Pell, M.; Smårs, S.; Jönsson, H. Effects of moisture on emissions of methane, nitrous oxide and carbon dioxide from food and garden waste composting. J. Clean Prod. 2019, 240, 118165. [Google Scholar] [CrossRef]
- Beck-Friis, B.; Smårs, S.; Jönsson, H.; Eklind, Y.; Kirchmann, H. Composting of source-separated household organics at different oxygen levels: Gaining an understanding of the emission dynamics. Compost Sci. Util. 2003, 11, 41–50. [Google Scholar] [CrossRef]
- Nasini, L.; De Luca, G.; Ricci, A.; Ortolani, F.; Caselli, A.; Massaccesi, L.; Regni, L.; Gigliotti, G.; Proietti, P. Gas emissions during olive mill waste composting under static pile conditions. Int. Biodeterior. Biodegrad. 2016, 107, 70–76. [Google Scholar] [CrossRef]
- Hao, J.; Wang, H. Volatile fatty acids productions by mesophilic and thermophilic sludge fermentation: Biological responses to fermentation temperature. Bioresour. Technol. 2015, 175, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.G.; Moller, H.B. Emission of greenhouse gases during composting of deep litter from pig production—effect of straw content. J. Agric. Sci. 2000, 134, 327–335. [Google Scholar] [CrossRef]
- Kulcu, R.; Yaldiz, O. Effects of air flow directions on composting process temperature profile. Waste Manag. 2008, 28, 1766–1772. [Google Scholar] [CrossRef]
- Sun, X.; Ma, S.; Han, L.; Li, R.; Schlick, U.; Chen, P.; Huang, G. The effect of a semi-permeable membrane-covered composting system on greenhouse gas and ammonia emissions in the Tibetan Plateau. J. Clean Prod. 2018, 204, 778–787. [Google Scholar] [CrossRef]
- Puyuelo, B.; Gea, T.; Sánchez, A. A new control strategy for the composting process based on the oxygen uptake rate. Chem. Eng. J. 2010, 165, 161–169. [Google Scholar] [CrossRef]
- Stegenta, S.; Sobieraj, K.; Pilarski, G.; Koziel, J.A.; Białowiec, A. Analysis of the spatial and temporal distribution of process gases within municipal biowaste compost. Sustainability 2019, 11, 2340. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Sarsaiya, S.; Awasthi, M.K.; Liu, T.; Zhao, J.; Kumar, S.; Zhang, Z. Changes in global trends in food waste composting: Research challenges and opportunities. Bioresour. Technol. 2020, 299, 122555. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Wu, C.; Wang, K. New insights into the interactions between carbon dioxide and ammonia emissions during sewage sludge composting. Bioresour. Technol. 2013, 136, 385–393. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Muktadirul Bari Chowdhury, A.K.M.; Akratos, C.S.; Tekerlekopoulou, A.G.; Pavlou, S.; Vayenas, D.V. Mathematical modeling of olive mill waste composting process. Waste Manag. 2015, 43, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Tatano, F.; Pagliaro, G.; Di Giovanni, P.; Floriani, E.; Mangani, F. Biowaste home composting: Experimental process monitoring and quality control. Waste Manag. 2015, 38, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Petric, I.; Helić, A.; Avdić, E.A. Evolution of process parameters and determination of kinetics for co-composting of organic fraction of municipal solid waste with poultry manure. Bioresour. Technol. 2012, 117, 107–116. [Google Scholar] [CrossRef]
- Ge, J.; Huang, G.; Huang, J.; Zeng, J.; Han, L. Mechanism and kinetics of organic matter degradation based on particle structure variation during pig manure aerobic composting. J. Hazard Mater. 2015, 292, 19–26. [Google Scholar] [CrossRef]
- Siles-Castellano, A.B.; López, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López-González, J.A.; Estrella-González, M.J.; Moreno, J. Industrial composting of low carbon/nitrogen ratio mixtures of agri-food waste and impact on compost quality. Bioresour. Technol. 2020, 316, 123946. [Google Scholar] [CrossRef]
- Paillat, J.-M.; Robin, P.; Hassouna, M.; Leterme, P. Predicting ammonia and carbon dioxide emissions from carbon and nitrogen biodegradability during animal waste composting. Atmos. Environ. 2005, 39, 6833–6842. [Google Scholar] [CrossRef]
- Wu, C.; Li, W.; Wang, K.; Li, Y. Usage of pumice as bulking agent in sewage sludge composting. Bioresour. Technol. 2015, 190, 516–521. [Google Scholar] [CrossRef]
- Tosun, I.; Gonullu, M.T.; Arslankaya, E.; Gunay, A. Co-composting kinetics of rose processing waste with OFMSW. Bioresour. Technol. 2008, 99, 6143–6149. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wang, T.; Li, T.; Lü, F.; He, P. Comparison of sludge digestion under aerobic and anaerobic conditions with a focus on the degradation of proteins at mesophilic temperature. Bioresour. Technol. 2013, 140, 131–137. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Aziz, H.A. Evaluation of thermochemical pretreatment and continuous thermophilic condition in rice straw composting process enhancement. Bioresour. Technol. 2013, 133, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Lo, P.K.; Xu, J.; Li, M.; Jiang, Z.; Li, G.; Zhu, Q.; Li, X.; Leong, S.Y.; Li, Q. Molecular mechanisms underlying lignocellulose degradation and antibiotic resistance genes removal revealed via metagenomics analysis duringdifferent agricultural wastes composting. Bioresour. Technol. 2020, 314, 123731. [Google Scholar] [CrossRef] [PubMed]
- Tremier, A.; De Guardia, A.; Massiani, C.; Martel, J.L. Influence of the airflow rate on heat and mass transfers during sewage sludge and bulking agent composting. Environ. Technol. 2005, 26, 1137–1149. [Google Scholar] [CrossRef]
- Tang, J.C.; Shibata, A.; Zhou, Q.; Katayama, A. Effect of temperature on reaction rate and microbial community in composting of cattle manure with rice straw. J. Biosci. Bioeng. 2007, 104, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Clark, O.G.; Leonard, J.J. Influence of free air space on microbial kinetics in passively aerated compost. Bioresour. Technol. 2009, 100, 782–790. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Liu, H.; Xie, S.; Abbas, F. Impact of different nitrogen source on the compost quality and greenhouse gas emissions during composting of garden waste. Process Saf. Environ. Prot. 2019, 124, 326–335. [Google Scholar] [CrossRef]
- Burtraw, D.; Toman, M. The Benefits of Reduced Air Pollutants in the U.S. from Greenhouse Gas Mitigation Policies; Discussion Paper 98-01-REV; University of Minnesota: St. Paul, MN, USA, 1997; p. 10496. [Google Scholar] [CrossRef]


| Material | Repetition | AT4 | k | r | R2 | |||
|---|---|---|---|---|---|---|---|---|
| (mg O2·g d.m.−1) | Mean ± SD | (h−1) | Mean ± SD | (mg O2·g d.m.−1·h−1) | Mean ± SD | - | ||
| Grass | 1 | 167.92 | 188.11 ± 17.9 | 0.0392 | 0.0432 ± 0.006 | 6.5751 | 8.1500 ± 1.61 | 0.9642 |
| 2 | 194.59 | 0.0503 | 9.7958 | 0.9675 | ||||
| 3 | 201.83 | 0.0400 | 8.0792 | 0.9898 | ||||
| Manure | 1 | 190.74 | 140.63 ± 44.3 | 0.0082 | 0.0136 ± 0.006 | 1.5707 | 1.7558 ± 0.28 | 0.9959 |
| 2 | 124.44 | 0.0130 | 1.6192 | 0.9897 | ||||
| 3 | 106.70 | 0.0195 | 2.0775 | 0.9787 | ||||
| Mixture | 1 | 67.86 | 67.50 ± 0.5 | 0.0267 | 0.0263 ± 0.001 | 1.8138 | 1.7762 ± 0.05 | 0.9763 |
| 2 | 67.15 | 0.0259 | 1.7385 | 0.9648 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stegenta-Dąbrowska, S.; Sobieraj, K.; Koziel, J.A.; Bieniek, J.; Białowiec, A. Kinetics of Biotic and Abiotic CO Production during the Initial Phase of Biowaste Composting. Energies 2020, 13, 5451. https://doi.org/10.3390/en13205451
Stegenta-Dąbrowska S, Sobieraj K, Koziel JA, Bieniek J, Białowiec A. Kinetics of Biotic and Abiotic CO Production during the Initial Phase of Biowaste Composting. Energies. 2020; 13(20):5451. https://doi.org/10.3390/en13205451
Chicago/Turabian StyleStegenta-Dąbrowska, Sylwia, Karolina Sobieraj, Jacek A. Koziel, Jerzy Bieniek, and Andrzej Białowiec. 2020. "Kinetics of Biotic and Abiotic CO Production during the Initial Phase of Biowaste Composting" Energies 13, no. 20: 5451. https://doi.org/10.3390/en13205451
APA StyleStegenta-Dąbrowska, S., Sobieraj, K., Koziel, J. A., Bieniek, J., & Białowiec, A. (2020). Kinetics of Biotic and Abiotic CO Production during the Initial Phase of Biowaste Composting. Energies, 13(20), 5451. https://doi.org/10.3390/en13205451