Thermal and Economic Analysis of Multi-Effect Concentration System by Utilizing Waste Heat of Flue Gas for Magnesium Desulfurization Wastewater
Abstract
1. Introduction
2. Process Description and Methods
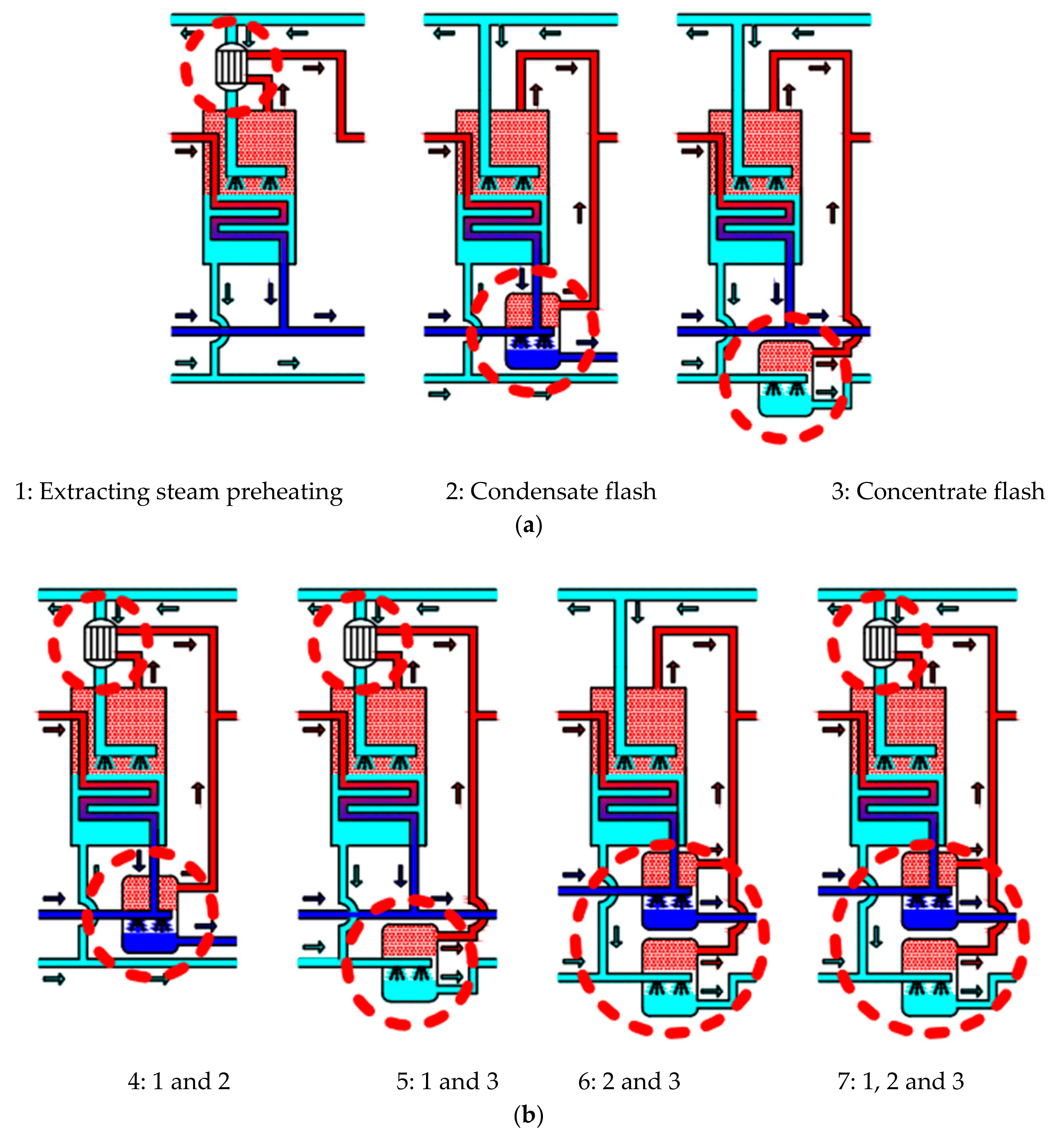
2.1. Process Description
2.2. Assumption and Validation
- (1)
- Steady-state operation.
- (2)
- The desulfurization wastewater entering the concentration system is regarded as a single component solution with only magnesium sulfate as the solute.
- (3)
- The water evaporated from the concentration system is treated as pure water.
- (4)
- No heat loss of any equipment of the concentration system.
- (5)
- No pressure drop loss of any equipment of the concentration system.
- (6)
- Ignore the influence of non-condensable gas.
2.3. Boundary Conditions and Simulation Settings for a 300 MW Power Plant
2.4. Thermal Performance Analysis
2.5. Economic Analysis
3. Results and Discussion
3.1. Benchmark System of 300 MW Power Plant
3.1.1. Thermal Performance Analysis of Benchmark System
3.1.2. Economic Analysis of Benchmark System
3.2. Optimization System of 300 MW Power Plant
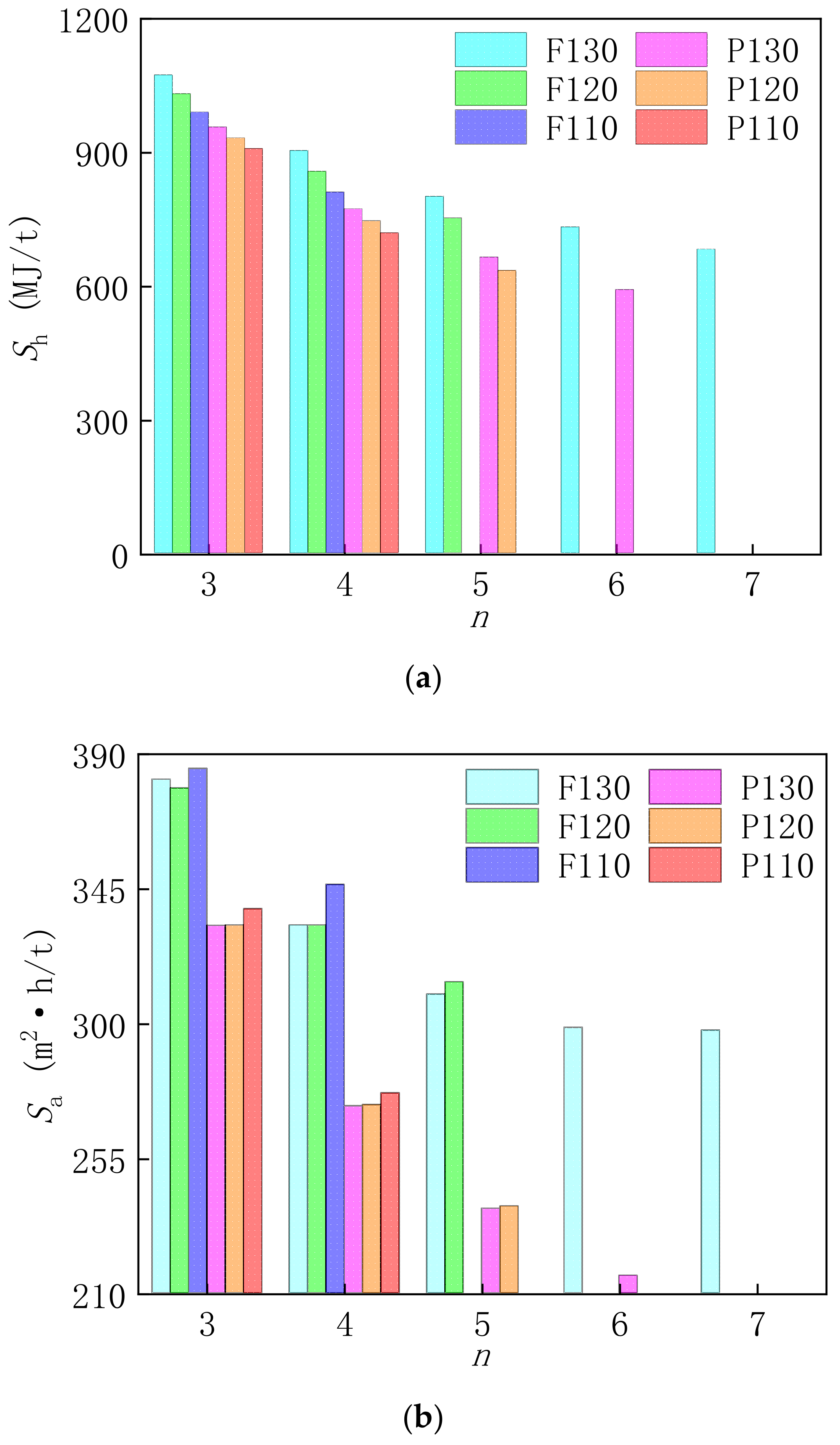
3.2.1. Thermal Performance Analysis of Optimization System
3.2.2. Economic Analysis of Optimization System
3.2.3. Cost Reductions and Influence Factors of the Optimization System
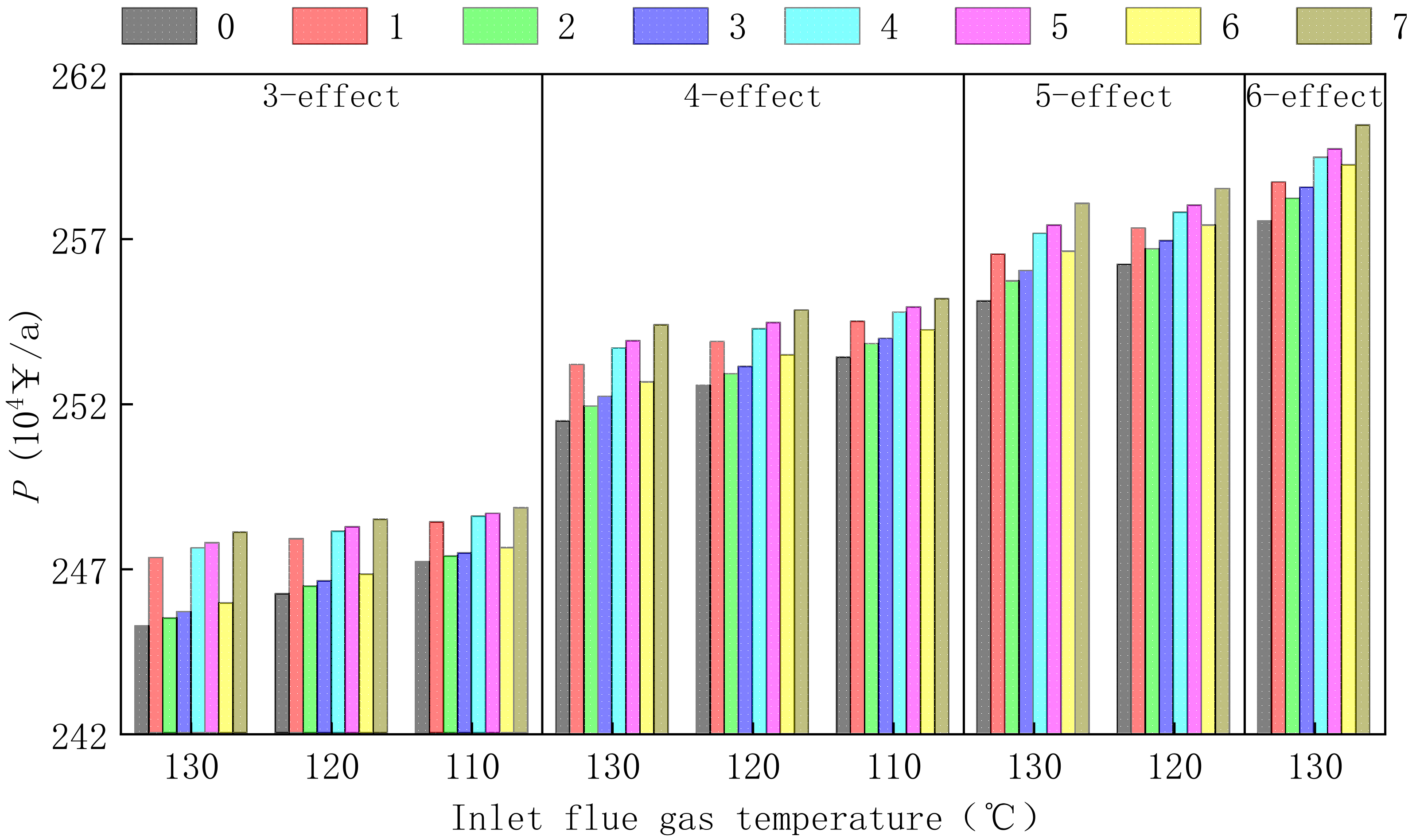
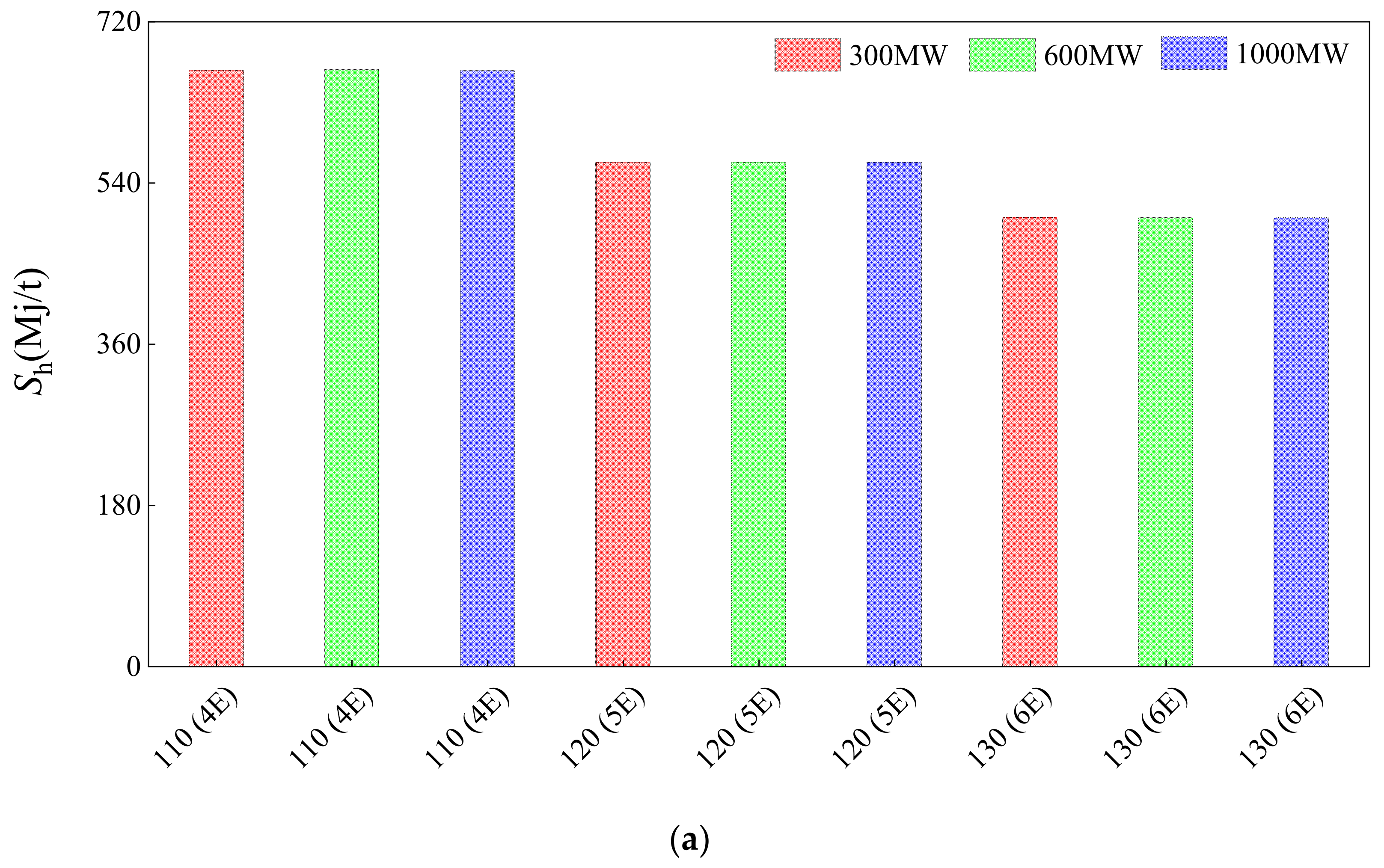
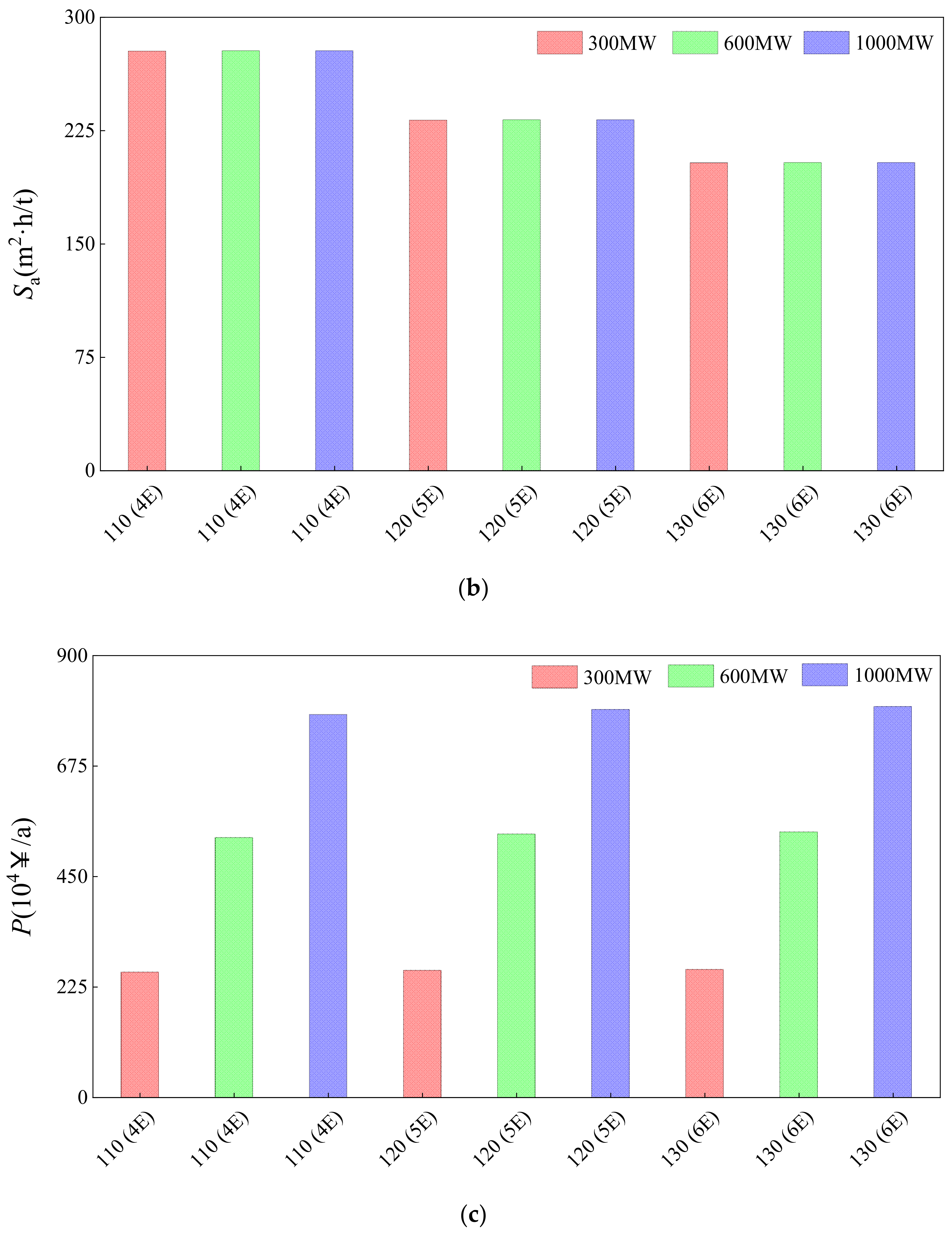
3.3. Comparison of Parallel-Feed Optimization System with 7-Process of 300, 600, and 1000 MW Power Plant
4. Conclusions
- (1)
- In a 300 MW power plant, the concentration benchmark process should adopt parallel feed. Compared with the forward-feed benchmark system, the parallel-feed benchmark system reduces about 15%, 20%, and 13% in specific flue gas heat, specific heat transfer area, and annual cost, respectively.
- (2)
- In a 300 MW power plant, 7-process should be adopted for the optimization of the concentration benchmark process. Compared with the parallel-feed benchmark system, the parallel-feed optimization system with 7-process reduces about 9%, 4%, and 9% in specific flue gas heat, specific heat transfer area, and annual cost, respectively.
- (3)
- Among the five factors influencing the annual profit of parallel-feed optimization system with 7-process of 300 MW power plant, the influence of magnesium sulfate concentrate price was the largest, annual utilization hours and steam price were the second, and ND steel price and 304 stainless steel price were the least.
- (4)
- The scheme of selling magnesium sulfate concentrate has good benefits in coal-fired power plants with different power generation capacities. The annual profit of a 300, 600, and 1000 MW power plant is about 2.58 million, 5.35 million, and 7.89 million CNY, respectively.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Ac | Heat transfer area of condenser, m2 |
| Ae | Total heat transfer area of all evaporators, m2 |
| Af | Heat transfer area of flue heat exchanger, m2 |
| Ai | Heat transfer area of the ith effect, m2 |
| Aj | Heat transfer area of the jth preheater, m2 |
| Ap | Total heat transfer area of all preheaters, m2 |
| C | Annual cost of concentration system, 104 ¥/a |
| Cc | Annual cost of cooling crystallization process, 104 ¥/a |
| Cd | Annual depreciation cost of concentration system, 104 ¥/a. |
| Ch | Annual heat source cost of concentration system, 104 ¥/a |
| Gc | Coal consumption of a 300 MW power generation unit, t/h |
| Gin | Inlet magnesium sulfate solution of concentration system, t/h |
| Gout, MgSO4 | Pure MgSO4 of concentration system outlet, t/h |
| Gs | Circulating steam generated by flue heat exchanger, t/h |
| Ic | Investment cost of condenser, 104 ¥ |
| Ie | Investment cost of evaporators, 104 ¥ |
| If | Investment cost of flue heat exchanger, 104 ¥ |
| Ip | Investment cost of preheats, 104 ¥ |
| i | The sequence number of evaporators |
| j | The sequence number of preheaters |
| k | The number of preheaters |
| MMgSO4 | Molar mass of magnesium sulfate, g/mol |
| MMgSO4·7H2O | Molar mass of magnesium sulfate heptahydrate, g/mol |
| MS | Molar mass of sulfur, g/mol |
| Mw | Evaporation water of concentration system, t/h |
| m | Flue heat exchanger weight, t |
| n | The effects number of evaporators |
| P | Annual profit of concentration system, 104 ¥/a |
| P3 | Unit price of 304 stainless steel, 104 ¥/t |
| PMgSO4·7H2O | Unit price of magnesium sulfate heptahydrate, 104 ¥/t |
| PN | Unit price of ND steel, 104¥/t |
| Ps | Steam price, 104¥/t |
| Qin | Input flue gas heat of concentration system, kW |
| S | Annual sale of concentration system, 104 ¥/a |
| Sa | Specific heat transfer area, m2·h/t |
| Sar | Sulfur content of coal on received basis, % |
| Sh | Specific flue gas heat, MJ/t |
| sMgSO4·7H2O | Cost factor magnesium sulfate heptahydrate, % |
| WMgSO4 | Mass fraction of magnesium sulfate in the solution, % |
| Y | Yield of magnesium sulfate heptahydrate, % |
| Zi | Investment increase coefficient of evaporator |
| α | Equipment investment cost coefficient of flue heat exchanger |
| βc | The condenser correction coefficient |
| βj | The jth preheater correction coefficient |
| γc | Annual depreciation rate of condenser, a−1 |
| γe | Annual depreciation rate of evaporators, a−1 |
| γf | Annual depreciation rate of flue heat exchanger, a−1 |
| γp | Annual depreciation rate of preheaters, a−1 |
| ηb | Combustion efficiency of boiler, % |
| ηd | Flue gas desulfurization efficiency, % |
| θ | Annual converted full load utilization hours of power plant, h/a |
References
- Cristóbal, J.; Guillén-Gosálbez, G.; Jiménez, L.; Irabien, A. Optimization of global and local pollution control in electricity production from coal burning. Appl. Energy 2012, 92, 369–378. [Google Scholar] [CrossRef]
- Jiang, B.; Xie, Y.; Xia, D.; Liu, X. A potential source for PM2.5: Analysis of fine particle generation mechanism in Wet Flue Gas Desulfurization System by modeling drying and breakage of slurry droplet. Environ. Pollut. 2019, 246, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Lin, C.; Chen, W.; Hong, J.; Chang, J.; Dong, Y.; Zhang, Y. Environmental effect of current desulfurization technology on fly dust emission in China. Renew. Sust. Energ. Rev. 2017, 72, 1–9. [Google Scholar] [CrossRef]
- Ma, S.; Bie, X.; Sun, Y.; Chen, K.; Zhu, Z. Present research situation and prospect of magnesium desulfurization in coal-fired power plant. Chem. Ind. Eng. Prog. 2018, 37, 3609–3617. [Google Scholar]
- Ma, Y.; Nie, Q.; Xiao, R.; Hu, W.; Han, B.; Polaczyk, P.A.; Huang, B. Experimental investigation of utilizing waste flue gas desulfurized gypsum as backfill materials. Constr. Build. Mater. 2020, 245, 118393. [Google Scholar] [CrossRef]
- Koralegedara, N.H.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Recent advances in flue gas desulfurization gypsum processes and applications—A review. J. Environ. Manag. 2019, 251, 109572. [Google Scholar] [CrossRef]
- Ren, C.; Wang, W.; Mao, Y.; Yuan, X.; Song, Z.; Sun, J.; Zhao, X. Comparative life cycle assessment of sulfoaluminate clinker production derived from industrial solid wastes and conventional raw materials. J. Clean Prod. 2017, 167, 1314–1324. [Google Scholar] [CrossRef]
- Wu, S.; Wang, W.; Ren, C.; Yao, X.; Yao, Y.; Zhang, Q.; Li, Z. Calcination of calcium sulphoaluminate cement using flue gas desulfurization gypsum as whole calcium oxide source. Constr. Build. Mater. 2019, 228, 116676. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Liu, Y. Investigation of water-energy-emission nexus of air pollution control of the coal-fired power industry: A case study of Beijing-Tianjin-Hebei region, China. Energy Policy 2018, 115, 291–301. [Google Scholar] [CrossRef]
- Zhu, L.; Men, Y.; Kuang, Z.; Tian, B.; Yu, C.; Ye, F. Practice of ultra-low emission of 350 MW unit by magnesium desulphurization process. Electr. Power Technol. Environ. Prot. 2019, 35, 30–32. [Google Scholar]
- Feng, Y.; Liao, S.; Li, H.; Wu, H.; Wang, Y.; Wang, J.; Zhang, G. Research status of magnesium desulphurization and diversified utilization of desulfurization products. Inorg. Chem. Ind. 2019, 51, 1–6. [Google Scholar]
- Shi, L.; Pang, L.; Zhao, L.; Wu, X.; Li, D. The current treatment situation of magnesium sulfite from the magnesium FGD. Environ. Eng. 2014, 32, 533–536. [Google Scholar]
- Yan, L.; Lu, X.; Guo, Q.; Wang, Q.; Ji, X. Research on the thermal decomposition and kinetics of byproducts from MgO wet flue gas desulfurization. Adv. Powder Technol. 2014, 25, 1709–1714. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Xing, L.; Yang, L.; Qi, T.; Xu, P.; Zhang, S.; Wang, L. Cobalt-based metal-organic frameworks promoting magnesium sulfite oxidation with ultrahigh catalytic activity and stability. J. Colloid Interf. Sci. 2020, 559, 88–95. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; He, W.; Song, B.; Wang, Z. The phase diagram study of FGD by magnesium oxide scrubbing byproduct system. Environ. Eng. 2013, 31, 368–370. [Google Scholar]
- Ma, S.; Chen, J.; Liu, N.; Wan, Z.; Liang, J.; Yue, P.; Zhang, J.; Liang, W. Experimental study on the concentration of FGD wastewater using low-temperature flue gas. J. Chin. Soc. Power Eng. 2020, 40, 51–57. [Google Scholar]
- Shao, G.; Liu, Y.; Yong, J. Experimental study on near zero discharge of desulphurization wastewater treated with nanofiltration membrane. Membr. Sci. Technol. 2019, 39, 124–128. [Google Scholar]
- Jin, Y.; Gao, N.; Zhu, T. Techno-economic analysis on a new conceptual design of waste heat recovery for boiler exhaust flue gas of coal-fired power plants. Energy Convers. Manag. 2019, 200, 112097. [Google Scholar] [CrossRef]
- Stevanovic, V.D.; Petrovic, M.M.; Wala, T.; Milivojevic, S.; Ilic, M.; Muszynski, S. Efficiency and power upgrade at the aged lignite-fired power plant by flue gas waste heat utilization: High pressure versus low pressure economizer installation. Energy 2019, 187, 115980. [Google Scholar] [CrossRef]
- Xu, X.; Wu, L.; Ju, G. Structural optimization and experimental study of plate low pressure economizer with intermediate passage. Appl. Therm. Eng. 2019, 151, 328–334. [Google Scholar] [CrossRef]
- Xue, Y.; Du, X.; Ge, Z.; Yang, L. Study on multi-effect distillation of seawater with low-grade heat utilization of thermal power generating unit. Appl. Therm. Eng. 2018, 141, 589–599. [Google Scholar] [CrossRef]
- Liponi, A.; Tempesti, C.; Baccioli, A.; Ferrari, L. Small-Scale Desalination Plant Driven by Solar Energy for Isolated Communities. Energies 2020, 13, 3864. [Google Scholar] [CrossRef]
- Papapetrou, M.; Kosmadakis, G.; Giacalone, F.; Ortega-Delgado, B.; Cipollina, A.; Tamburini, A.; Micale, G. Evaluation of the Economic and Environmental Performance of Low-Temperature Heat to Power Conversion using a Reverse Electrodialysis—Multi-Effect Distillation System. Energies 2019, 12, 3206. [Google Scholar] [CrossRef]
- Deymi-Dashtebayaz, M.; Tayyeban, E. Multi objective optimization of using the surplus low pressure steam from natural gas refinery in the thermal desalination process. J. Clean. Prod. 2019, 238, 117945. [Google Scholar] [CrossRef]
- Ma, S.; Chai, J.; Chen, G.; Yu, W.; Zhu, S. Research on desulfurization wastewater evaporation: Present and future perspectives. Renew. Sustain. Energy Rev. 2016, 58, 1143–1151. [Google Scholar]
- Hao, J. Study on Simulation and Optimization Design of 60,000 t/a NaOH Multi Effect Evaporation System. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2014. [Google Scholar]
- Mu, G. Design and Control of High Salt Water Low-Temperature Multi-Effect Distillation. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2016. [Google Scholar]
- Ng, K.C.; Thu, K.; Oh, S.J.; Ang, L.; Shahzad, M.W.; Ismail, A.B. Recent developments in thermally-driven seawater desalination: Energy efficiency improvement by hybridization of the MED and AD cycles. Desalination 2015, 356, 255–270. [Google Scholar] [CrossRef]
- Castro, M.; Esparcia, E.A., Jr.; Ocon, J.D. A Comparative Techno-Economic Analysis of Different Desalination Technologies in Off-Grid Islands†. Energies 2020, 13, 2261. [Google Scholar] [CrossRef]
- Zhao, D.; Xue, J.; Li, S.; Sun, H.; Zhang, Q. Theoretical analyses of thermal and economical aspects of multi-effect distillation desalination dealing with high-salinity wastewater. Desalination 2011, 273, 292–298. [Google Scholar] [CrossRef]
- Ullah, I.; Rasul, M.G. Recent Developments in Solar Thermal Desalination Technologies: A Review. Energies 2019, 12, 119. [Google Scholar] [CrossRef]
- Druetta, P.; Aguirre, P.; Mussati, S. Minimizing the total cost of multi effect evaporation systems for seawater desalination. Desalination 2014, 344, 431–445. [Google Scholar] [CrossRef]
- Yılmaz, I.H.; Söylemez, M.S. Design and computer simulation on multi-effect evaporation seawater desalination system using hybrid renewable energy sources in Turkey. Desalination 2012, 291, 23–40. [Google Scholar] [CrossRef]
- Zhou, S.; Gong, L.; Liu, X.; Shen, S. Mathematical modeling and performance analysis for multi-effect evaporation/multi-effect evaporation with thermal vapor compression desalination system. Appl. Therm. Eng. 2019, 159, 113759. [Google Scholar] [CrossRef]
- Darwish, M.A.; Alsairafi, A. Technical comparison between TVC/MEB and MSF. Desalination 2004, 170, 223–239. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Wang, X.; Cong, Y. Magnesium FGD technology in engineering and operation of a number of questions. Guangdong Chem. Ind. 2009, 36, 121–123. [Google Scholar]
- Niu, H. Study on the Process of Preparation Magnesium Sulfate by Medium Transformation and Low Temperature Freezing Crystation. Master’s Thesis, Graduate School of Chinese Academy of Sciences (Qinghai Salt Lake Research Institute), Xining, China, 2016. [Google Scholar]
- Xiao, C. Comparative analysis of operation cost of limestone gypsum and magnesia desulfurization processes. Energy Conserv. 2019, 38, 161–163. [Google Scholar]
- Zhu, T. Research on Technologies of Desulphurization and DeNOx by Magenesium FGD and Resourcing of Byproduct for Sintering Flue Gas. Ph.D. Thesis, Tsinghua University, Beijing, China, 2017. [Google Scholar]
- Lukowicz, H.; Kochaniewicz, A. Analysis of the use of waste heat obtained from coal-fired units in Organic Rankine Cycles and for brown coal drying. Energy 2012, 45, 203–212. [Google Scholar] [CrossRef]
- Wang, C.; He, B.; Yan, L.; Pei, X.; Chen, S. Thermodynamic analysis of a low-pressure economizer based waste heat recovery system for a coal-fired power plant. Energy 2014, 65, 80–90. [Google Scholar] [CrossRef]
- Ding, W. Thermo Economic Analysis of Steam System & Energy Medium Cost Assessment for Iron and Steel Plant. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2015. [Google Scholar]
- Yang, K. Simulation, Analysis and Upgrading of Sugar Multi-effects Evaporation. Master’s Thesis, South China University of Technology, Guangzhou, China, 2015. [Google Scholar]
- Wei, W.; Sun, F.; Shi, Y.; Ma, L. Theoretical prediction of acid dew point and safe operating temperature of heat exchangers for coal-fired power plants. Appl. Therm. Eng. 2017, 123, 782–790. [Google Scholar] [CrossRef]





| Parameter | ALBA Plant Operation Data | Ebsilon Model Simulation Data | Relative Error |
|---|---|---|---|
| Number of effects | 4 | 4 | – – |
| Motive steam pressure, MPa | 2.1 | 2.1 | – – |
| Motive steam temperature, °C | 224 | 224 | – – |
| Motive steam flow, t/h | 59.98 | 59.98 | – – |
| Top brine temperature, °C | 63 | 63 | – – |
| Minimum brine temperature, °C | 48 | 48 | – – |
| Feed flow, t/h | 1400.4 | 1400.4 | – – |
| Evaporation water, t/h | 455.04 | 454.93 | <1% |
| Specific heat consumption, MJ/t | 372.43 | 372.52 | <1% |
| Specific heat transfer area, m2·h/t | 61.39 | 61.54 | <1% |
| Gin/(t/h) | Gc/(t/h) | ηb/% | Sar/% | ηd/% | MS/(g/mol) | MMgSO4/(g/mol) | WMgSO4/% |
|---|---|---|---|---|---|---|---|
| 8.70 | 118.39 | 99 | 0.49 | 97 | 32 | 120 | 24 |
| System Configuration | Effects Number | Investment Cost (104 ¥) | Annual Heat Source Cost (104 ¥) | Annual Depreciation Cost (104 ¥) |
|---|---|---|---|---|
| Forward-feed benchmark system | 3 | 111.49 | 24.02 | 11.05 |
| 4 | 92.40 | 19.95 | 9.24 | |
| 5 | 82.57 | 17.51 | 8.26 | |
| Parallel-feed benchmark system | 3 | 98.61 | 21.71 | 9.86 |
| 4 | 78.97 | 17.36 | 7.9 | |
| 5 | 67.94 | 14.78 | 6.79 | |
| Parallel-feed optimization system with 7-process | 3 | 91.72 | 20.15 | 9.17 |
| 4 | 72.42 | 15.73 | 7.24 | |
| 5 | 61.71 | 13.11 | 6.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.; Shi, Y. Thermal and Economic Analysis of Multi-Effect Concentration System by Utilizing Waste Heat of Flue Gas for Magnesium Desulfurization Wastewater. Energies 2020, 13, 5384. https://doi.org/10.3390/en13205384
Yan M, Shi Y. Thermal and Economic Analysis of Multi-Effect Concentration System by Utilizing Waste Heat of Flue Gas for Magnesium Desulfurization Wastewater. Energies. 2020; 13(20):5384. https://doi.org/10.3390/en13205384
Chicago/Turabian StyleYan, Mingwei, and Yuetao Shi. 2020. "Thermal and Economic Analysis of Multi-Effect Concentration System by Utilizing Waste Heat of Flue Gas for Magnesium Desulfurization Wastewater" Energies 13, no. 20: 5384. https://doi.org/10.3390/en13205384
APA StyleYan, M., & Shi, Y. (2020). Thermal and Economic Analysis of Multi-Effect Concentration System by Utilizing Waste Heat of Flue Gas for Magnesium Desulfurization Wastewater. Energies, 13(20), 5384. https://doi.org/10.3390/en13205384






