Abstract
This work dealt with the study of growth parameters, pigments production, and bioenergetic aspects of the microalga Dunaliella tertiolecta in different culture media. For this purpose, cultures were carried out in Erlenmeyer flasks containing F/2 medium, Bold’s Basal medium, or an alternative medium made up of the same constituents of the Bold’s Basal medium dissolved in natural seawater instead of distilled water. D. tertiolecta reached the highest dry cell concentration (Xmax = 1223 mgDM·L−1), specific growth rate (µmax = 0.535 d−1), cell productivity (PX = 102 mgDM·L−1·d−1), and photosynthetic efficiency (PE = 14.54%) in the alternative medium, while the highest contents of carotenoids (52.0 mg·g−1) and chlorophyll (108.0 mg·g−1) in the biomass were obtained in Bold’s Basal medium. As for the bioenergetic parameters, the biomass yield on Gibbs energy dissipation was higher and comparable in both seawater-based media. However, the F/2 medium led to the highest values of moles of photons absorbed to produce 1 C-mol of biomass (nPh), total Gibbs energy absorbed by the photosynthesis (ΔGa) and released heat (Q), as well as the lowest cell concentration, thus proving to be the least suitable medium for D. tertiolecta growth. On the other hand, the highest values of molar development of O2 and consumption of H+ and H2O were obtained in the alternative medium, which also ensured the best kinetic parameters, thereby allowing for the best energy exploitation for cell growth. These results demonstrate that composition of culture medium for microalgae cultivation has different effects on pigments production, growth kinetics, and bioenergetics parameters, which should be taken into consideration for any use of biomass, including as raw material for biofuels production.
1. Introduction
Species belonging to the Dunaliella genus are unicellular and biflagellate green microalgae that grow under high salinity and irradiance conditions [1]. Large-scale Dunaliella sp. cultivation has been widely applied because its biomass is a source of valuable compounds such as polysaccharides, lipids, vitamins, proteins, and pigments, mainly carotenoids and chlorophyll, that can be used in several biotechnological applications [2,3,4,5]. Therefore, Dunaliella sp. have high potential to contribute for a sustainable industry through the generation of high added-value products [6,7].
Microalgae can be cultivated under different conditions, and several factors such as light intensity, photobioreactor type and configuration, mixing regime, temperature, and culture medium composition may influence cell growth and biomass composition [7]. Even though nutrient limitation reduces biomass concentration [8,9,10,11], it promotes the accumulation of carotenoids affecting the photosynthetic pathway [12]. Therefore, the composition of culture medium is one of the most important issues to be tackled in commercial production of microalgal biomass.
Microalgae biomass is considered a potential source of biofuels due to its high energy content and for being environmentally friendly compared to fossil fuels [13]. Nevertheless, new production methods have been proposed to improve its feasibility and cost effectiveness [14,15]. Among microalgae, Dunaliella sp. has stood out for being a halotolerant unicellular green alga, easily cultivable and resistant to contamination by other species. In addition, D. tertiolecta is highly adaptable to abiotic stress and able to accumulate high content of lipids as a potential feedstock to produce biofuels and pigments, especially carotenoids [16,17,18].
β-Carotene is the best-known compound of this class and has received a lot of attention due to antioxidant, anti-inflammatory, and anticarcinogenic activities [19], with a market value of $1.5 billion in 2017 and an estimated value for 2022 of $2.0 billion [20]. Chlorophyll is another important pigment found in Dunaliella sp. biomass, which is commercially used as a natural food coloring agent thanks to its green color and is consumed as nutraceutical due to beneficial properties such as antioxidant, anti-inflammatory, antimutagenic, and antibacterial activities [21,22].
Additionally, culture medium can influence other parameters such as photosynthetic efficiency (PE) and microalgal bioenergetics. PE is defined as the efficiency by which photons are absorbed, converted into chemical energy and stored as biomass [23], while bioenergetics refers to how energy is managed in living cells [24,25].
The aim of this work was to investigate D. tertiolecta growth, pigments production, and bioenergetic parameters in different culture media in order to select the best cultivation conditions to overproduce such added-value compounds. Moreover, within the framework of a biorefinery concept, the lipid fraction of the exhaust biomass will be exploited in the next effort as a raw material for energy production.
2. Materials and Methods
2.1. Microalgal Cultivations
Dunaliella tertiolecta (UTEX 999) was cultivated in three different media, namely the F/2 medium, the Bold’s Basal medium and an alternative saline medium. The F/2 medium contained mineral salts, trace elements, and vitamins dissolved in natural seawater previously filtered through membranes with 0.22 µm pore diameter [26]; the Bold’s Basal medium is a well-known broth made up of mineral salts dissolved in distilled water [27]; while the alternative medium was composed of the same mineral salts as the Bold’s Basal one but using seawater (Table 1) instead of distilled water. The compositions of these culture media are listed in Table 2.

Table 1.
Chemical composition of seawater.

Table 2.
Nutrient composition of different culture media. F/2 medium (A), Bold’s Basal medium (B), alternative medium (C).
The microalga was cultivated in 500 mL Erlenmeyer flasks containing 200 mL of the selected culture medium with initial cell concentration of 80 mg·L−1 at room temperature, under light intensity of 45 ± 5 μmol photon m−2· s−1 and continuous aeration at a flow rate of 2 L·min−1 for 13 days. Each culture was carried out in duplicate.
2.2. Analytical Methods
D. tertiolecta cell motility was examined using a light microscope (model Nikon Eclipse E200MV R, Nikon Instruments Inc, Shanghai, China) (magnification 400× g). Optical density (OD) was determined spectrophotometrically at a wavelength of 680 nm (OD680) by a UV/Visible spectrophotometer using a calibration curve correlating dry biomass concentration to OD680. Biomass concentration was measured daily along cultivations, and values were given as the average of triplicate determinations (n = 3) with standard deviation. The Equation (1) was derived from the standard curve:
Cell concentration (gDM·L−1) = 0.003 OD680 + 0.098
The contents of chlorophylls and carotenoids were quantified by the method described by Lichtenthaler [29]. Briefly, D. tertiolecta dry biomass was mixed with acetone and centrifuged at 500× g for 5 min to recover the pigment-containing supernatant, whose absorbance (Abs) was measured at 647, 663, and 470 nm. Chlorophyll and carotenoid contents were calculated using the following equations:
Chlorophyll a: Ca = [(12.25 Abs663 nm) − (2.79 Abs647 nm)]
Chlorophyll b: Cb = [(21.50 Abs647 nm) − (5.10 Abs663 nm)]
Carotenoids: [(1000 Abs470 nm) − (1.82 Ca) − (85.02 Cb)]/198
2.3. Growth Parameters
Cell productivity (PX) was calculated by dividing the variation in cell concentration (Xmax − Xi) by the time of cultivation (Tc), according to the equation:
where Xmax is the maximum cell concentration and Xi the initial cell concentration.
Maximum specific growth rate (μmax) was calculated as:
where Xj and Xj−1 are cell concentrations at the end and the beginning of each time interval (Δt = 1 day).
2.4. Photosynthetic Efficiency
Photosynthetic efficiency (PE) was determined by converting the photosynthetic photon flux density (PPFD) to photosynthetic active radiation (PAR). The input of PAR (IPAR) into the Erlenmeyer was obtained by multiplying PAR by the illuminated surface (m2). Therefore, PE was calculated by the equation:
where rG is the maximum daily growth rate (gDM·d−1) and HG = 21.01 kJ·gDM−1 dry biomass enthalpy [30].
2.5. Bioenergetic Parameters
The Gibbs energy dissipation for cell growth and maintenance (1/YGX) was estimated by the equation [31]:
in which μ is the specific growth rate, 1/. is the portion of 1/YGX referred only to growth in photoautotrophic cultivation using CO2 as a carbon source (986 kJ·C-molDM−1) and mG is the specific rate of Gibbs energy dissipation for cell maintenance (7.12 kJ C-molDM−1·h−1) [31].
Knowing 1/YGX, the average molar energy of photons at λ = 580 nm (ΔgPh = 206.2 kJ·mol−1) and the Gibbs energies of formation under biological standard conditions of the compounds involved in the growth (Δgf′i), it was possible to estimate the moles of photons to sustain the autotrophic growth of 1 C-mol of biomass (nPh) by the equation:
nPh = (ΔgfHCO3− + ΔgfNO3− + ΔgfH2O + ΔgfO2 + ΔgfH+ + ΔgfX + 1/YGX)/−ΔgPh
The average molar energy associated with the absorption of one mol of photons involved in the photosynthetic event was defined as [32]:
where h = 6.626·10−34 J·s is the Planck constant, c = 2.996·108 m·s−1 the light velocity, NA = 6.023 × 1023 mol−1 the Avogadro number.
The molar Gibbs energies of formation of compounds involved in the growth are, under biological standard conditions, Δgf′HCO3− = −587.2 kJ·mol−1, Δgf′NO3− = −111.4 kJ·mol−1, Δgf′H2O = −237.3 kJ·mol−1, Δgf′O2 = 0, Δgf′H+ = −39.87 kJ·mol−1, and Δgf′X = −67.0 kJ·mol−1 [31]. These values differ negligibly from those under experimental conditions [32], with exception of ΔgfH+, which was recalculated at the actual pH using the well-known equation of Gibbs:
where T and R are the absolute temperature and the ideal gas constant, respectively.
The molar rates of O2 production (qO2), H+ consumption (qH+) and H2O consumption or formation (qH2O) occurring during photosynthesis were calculated, according to Torre et al. [32], by multiplying cell concentration expressed in C-molDM L−1 by their respective stoichiometric coefficients expressed in mol C-molDM−1, using the experimental biomass elemental composition reported for D. tertiolecta by Kim et al. [33], and by dividing by Tc.
The total Gibbs energy absorbed by the photosynthesis (ΔGa), estimated by multiplying the moles of photons (nPh) by their average molar energy (ΔgPh), was considered to be equal to the sum of the energy fixed by the photosystems to increase its own enthalpic content (ΔH), that recovered as ATP (ΔGATP) and the released heat (Q), according to the equation:
ΔGa = nPh ΔgPh = −ΔGATP − ΔH − Q
It should be noticed that ΔGa, contrary to ΔGATP, ΔH and Q, conventionally assumes negative values being an energy entering the system.
2.6. Statistical Analysis
Cultures were done in duplicate, while pigments extraction from biomass was performed in triplicate. Results were expressed as means ± standard deviations (SD) and compared by one-way analysis of variance (ANOVA) with a confidence interval of 95%.
3. Results and Discussion
3.1. Cell Growth Profile
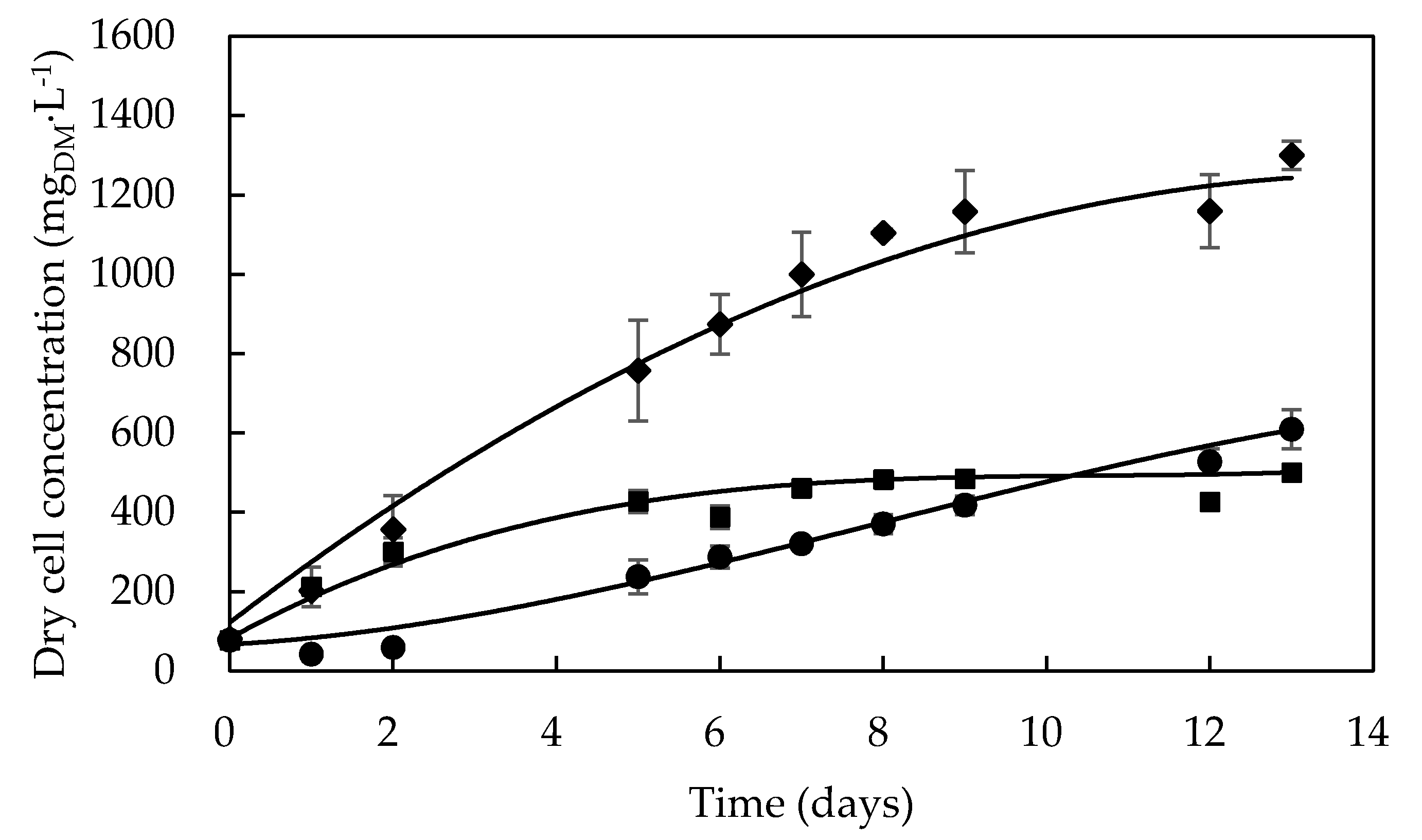
As shown in Figure 1, Dunaliella tertiolecta cultivation was simultaneously investigated in the three selected culture media, and its growth profile was followed for 13 days. One can see that, in F/2 medium, the exponential growth phase started after about 1 day of cultivation, and cell density achieved a value of 452 mgDM·L−1 on the fifth day, after which the microalga entered the stationary growth phase that continued until the end of cultivation. This may have been the result of phosphorus limitation, which has been reported to significantly affect the growth of Dunaliella sp. [34,35]. Kumar et al. [36] observed the same growth profile for the D. tertiolecta CCAP 19/27 strain in the same medium, where it reached a maximum cell concentration of around 700 mgDM·L−1 after 8 days of cultivation.
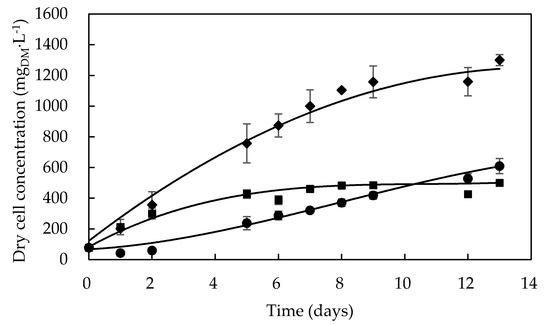
Figure 1.
Growth profiles of Dunaliella tertiolecta (UTEX 999) cultivated in different culture media: F/2 medium (■), Bold’s Basal medium (●), Alternative medium (◆).
When grown in Bold’s Basal medium, D. tertiolecta underwent a three-day lag phase likely due to the need to adapt itself to this synthetic medium, which is so different from its natural environment consisting of brackish or marine water [37]. Contrariwise, Dunaliella salina exhibited cell growth without any lag phase in a modified Bold’s Basal medium [38], possibly because it can adapt itself to more habitat types than D. tertiolecta, so that it is found in many different environments [37]. Nonetheless, after the lag phase, D. tertiolecta showed quicker growth in this medium rather than in the F/2 one, achieving higher cell concentration (607 mgDM·L−1) at the end of cultivation.
On the other hand, D. tertiolecta grew in the alternative medium without undergoing any lag phase as previously observed in the F/2 one, also prepared in seawater (Figure 1). The achievement of a much higher cell concentration (>1200 mgDM·L−1) as a result of an extension of the exponential growth phase suggests a synergistic effect of typical nutrients of seawater and some peculiar components of the Bold’s Basal medium such as H3BO3 and EDTA in significant levels.
To shed more light on these findings, mobility of D. tertiolecta cells was examined at the end of cultivations in the three different culture media as a rough, qualitative index of cell viability. Cells maintained in F/2 and alternative media showed normal motility, while that of cells cultivated in the Bold’s Basal one was significantly reduced. Considering the limited contents of S, O, Mg, Ca, and K in such a synthetic medium, this observation confirms the essential role of some seawater component as nutrient, whose shortage may have reduced the viability and consequently the motility of microalgal cells [39,40].
This influence of medium composition was confirmed by visual examination of the color of cell cultures. It can be seen in Figure 2 that only the culture grown in the alternative medium had an intense green color, while those grown either in the Bold’s Basal or the F/2 medium were yellowish in color, which confirms the above-supposed occurrence of some nutrient-limitation [41,42].

Figure 2.
Color of D. tertiolecta (UTEX 999) cultivations in different culture media: F/2 medium (A), Bold’s Basal medium (B), Alternative medium (C).
3.2. Kinetic Parameters
Kinetic parameters were greatly influenced by the culture medium composition, as the culture grown in the alternative medium showed almost twice the average values of maximum biomass concentration (Xmax = 1223 ± 91 mgDM·L−1), maximum specific growth rate (µmax = 0.535 d−1), and biomass productivity (PX = 102 mgDM·L−1·d−1) found in the Bold’s Basal medium, while intermediate results were observed in the F/2 one (Table 3). This result suggests that D. tertiolecta was able to quickly adapt itself to the composition of the first medium due to its high carbon (C), nitrogen (N) and phosphorus (P) levels. F/2 and alternative media contained bicarbonate as quickly-metabolizable C source provided by seawater (Table 1), whereas the only C source in the Bold’s Basal medium was the CO2 contained in air (about 400 ppm), which was probably the main reason for the poor performance of D. tertiolecta in it. This behavior appears to be typical of microalgae, in that Yeh et al. [43] observed a significant increase in biomass concentration (from 0.15 to 0.6 g·L−1) and specific growth rate (from 0.5 to 1.5 day−1) when 1.2 g·L−1 of NaHCO3 was added to a Chlorella vulgaris autotrophic culture.

Table 3.
Growth parameters and pigments production by Dunaliella tertiolecta (UTEX 999) cultivated in different culture media.
The lower N content of the F/2 medium (0.8823 mM instead of 2.9442 mM) (Table 2) led to lower Xmax value (452 ± 28 mgDM·L−1) than in Bold’s Basal (567 ± 33 mgDM·L−1) or alternative (1223 ± 91 mgDM·L−1) medium. Similarly, Chen et al. [44] observed that, in a medium containing 23 mM NaNO3, D. tertiolecta grew up to an OD680 of 2.8, whereas, when this salt was 10-fold more diluted, its growth was strongly affected, thereby confirming how this microalga is sensitive to N availability. Moreover, the P content was higher in F/2 and alternative media compared to the Bold’s Basal one (Table 2); it has been reported that depletion of this element inhibited D. salina growth, stopped cell duplication, and reduced the photosynthetic rate [45]. Resuming, C, N, and P are the fundamental elements for microalgae growth, and their higher availability in the alternative medium probably contributed to improve the kinetic parameters of D. tertiolecta growth.
Tammam et al. [46] reported that both D. salina and D. tertiolecta grew better at high (2.5 to 4.0 M NaCl) rather than at low (0.05 to 1.0 M NaCl) salt concentrations. Similarly, in the present work D. tertiolecta grew better in the alternative medium containing higher salt concentrations. In addition, as suggested by Katz and Pick [47], the Dunaliella genus has a singular capacity to remove Na+ ions in hypersaline environments through a redox-driven sodium pump, and this transfer process results in enhanced photosynthetic CO2 uptake [48]. Some Dunaliella species, such as D. salina, are able to survive in media containing NaCl in concentration ranging from about 0.05 to 5.5 M [49].
Likewise, Jiang et al. [50] observed that N-depletion significantly reduced D. tertiolecta growth, because under nitrogen limitation the microalga slowed its cell division, redirecting the flow of carbon from the formation of proteins and chlorophyll to those of carbohydrates and lipids. Chlorophyll is in fact an easily accessible N-rich compound, which is used as an intracellular nitrogen pool to hold up cell growth and biomass production as the N source in the medium becomes the limiting factor [51]. Therefore, we can infer that N limitation in the F/2 medium was responsible for marked decreases in both the chlorophyll content of D. tertiolecta biomass and its final Xmax value.
On the other hand, in Bold’s Basal medium, cells showed lower biomass volumetric productivity (PX = 47 mgDM·L−1·d−1) and maximum specific growth rate (µmax = 0.269 d−1) than in the other two media, which suggests that the overall salt concentration and, then, the medium osmolarity may have also played a key role in D. tertiolecta growth. For instance, under limitation by sulfur (S), which is one of the most abundant elements of seawater, the microalga Chlamydomonas reinhardtii showed inhibition of both cell division and photosynthesis [52,53,54]. A similar sulfur limitation may have occurred with D. tertiolecta in Bold’s Basal medium prepared using distilled water. Considering all these growth parameters together, it can be said that the alternative medium was the most suitable for D. tertiolecta growth, thanks to the simultaneous presence of seawater and Bold’s Basal medium components.
Table 3 also lists the values of the maximum photosynthetic efficiency (PEmax), which was significantly influenced by the medium composition. The highest value of this parameter was observed in the alternative medium (14.54%), whereas in the F/2 and Bold’s Basal ones it was only 9.14 and 5.13%, respectively. These results may have also been due to different availability of nutrients in the selected media. Particularly, the absence of seawater affected cell division and photosynthesis in the Bold’s Bold medium, while the N-limitation responsible for the reduced chlorophyll content of biomass was the likely reason for the poor performance of the F/2 one. According to Srinivasan et al. [55], who investigated the combined effect of sodium bicarbonate and macronutrient starvation stress on D. salina V-101 physiological and biochemical responses, observed that the photosynthesis efficiency decreased in all N-, P-, or S-deficient cultures compared to the control containing all nutrients. A similar reduction of PE accompanied by lipid content increase was observed by Gao et al. [56] in an unspecified strain of D. salina under conditions of complete nutrient deprivation. This means that nitrogen depletion in general affects the synthesis of proteins, including those involved in the reaction centers, and results in a decrease of chlorophyll content reducing the functioning of photosystem II (PSII) [55]. PE values (7.25%) close to those observed in the F/2 (9.14%) and Bold’s Basal (5.13%) media were reported for Chlorella sorokiniana cultivation in a conical helical tubular photobioreactor [57].
3.3. Pigments Production
Contents of chlorophyll and total carotenoids were determined for D. tertiolecta in the three selected culture media (Table 3). Likely due to the early supposed N-limitation in the F/2 medium, the contents of carotenoids (18.2 ± 2.1 mg·g−1) and chlorophyll (65.7 ± 4.8 mg·g−1) in biomass were significantly lower than those detected in cells cultured in the alternative medium (33.4 ± 5.7 and 108.0 ± 11.3 mg·g−1, respectively) and especially in the Bold’s Basal one (52.0 ± 7.2 and 162.6 ± 15.7 mg·g−1, respectively). As described by Li et al. [51], when the N source runs out in the medium chlorophyll is used as an internal N source to sustain cell division, until its content drops to a critical value below which growth ceases. Likewise, Lai et al. [58] observed that, under N-limited conditions, the chlorophyll content of Dunaliella viridis progressively decreased causing cell growth to stop. Furthermore, it was described that, although the photosynthesis can continue even under N-limited conditions, biomass has smaller contents of N-rich components and accessory pigments such as carotenoids but higher contents of energy-rich components such as lipids and sugars [59].
On the other hand, literature reports suggest that the highest contents of both types of pigments in biomass cultured in the Bold’s Basal medium may be ascribed to its low S content. In fact, as already mentioned, the alternative medium is constituted by seawater that has high S content [40], which reduces the production of pigments. For example, when cultured under S deprivation, C. reinhardtii showed an increase in the fluorescence yield of chlorophyll [60], and D. salina a 20% higher carotenoid content compared to non-limited culture [61]. Similarly to our results in Bold’s Basal medium, Volgusheva et al. [54] observed for C. reinhardtii an increase in chlorophyll content accompanied by a decay in growth kinetic parameters.
Finally, the carotenoid and chlorophyll contents in biomass cultured in alternative medium were around 35% lower than in Bold’s Basal medium; this was probably due to the higher S concentration in the former medium, consistently with the previously-discussed results of Antal et al. [60].
As for the influence of medium salinity on the production of pigments, although Dunaliella is usually considered a highly salt-tolerant genus, some studies have shown that halophilicity can vary widely from one species to another and even from one strain to another [62]. For example, maximal carotenoid production by D. tertiolecta occurred at lower NaCl concentration (0.7 M) than by D. salina (2.0 M) [63]. β-Carotene accumulation triggered by reactive oxygen species has also been found to defend Dunaliella cells from the adverse effects of high salinity [64]. In another study, carotenoid production by D. salina increased continuously with increasing salinity [65]. Based on these results, we can infer that, due to the moderate halophilicity of D. tertiolecta, the NaCl concentration in the seawater used to prepare the alternative medium was excessive for the purpose of carotenoids’ production, thus leading to a value of their content significantly lower than that obtained in Bold’s Basal medium.
3.4. Bioenergetic Parameters
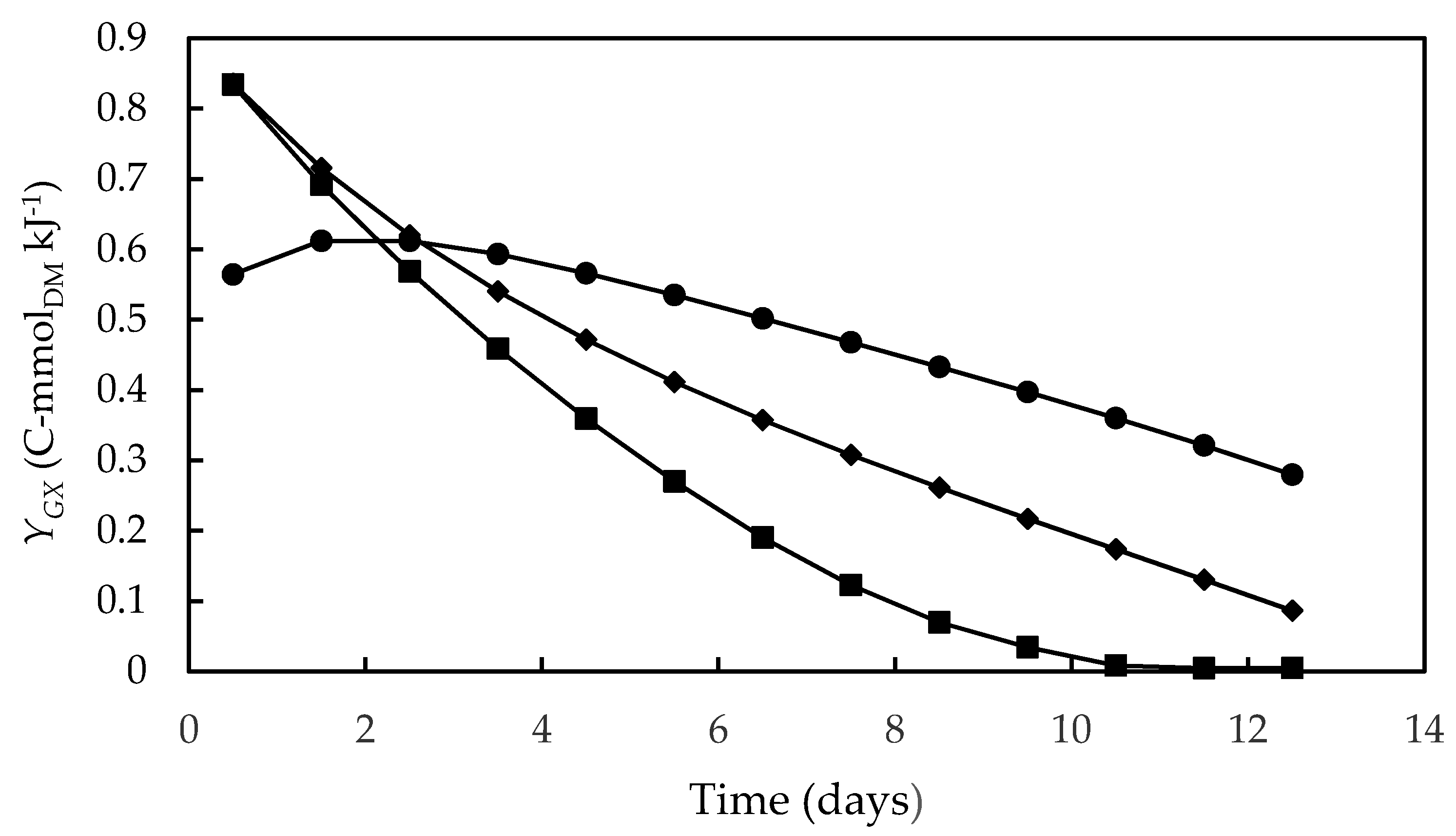
Figure 3 shows the time course of biomass yield on Gibbs energy dissipation (YGX), which corresponds to the reciprocal of the previously defined Gibbs energy dissipation for cell growth and maintenance. As a rule, this bioenergetic parameter is the highest at the beginning of cultivation, when cell concentration is low and there is a high metabolic rate favoring cell growth and maintenance. During cultivation, there was a progressive decrease in YGX, accompanied by a reduction in the specific growth rate, probably due to the onset of growth-limiting conditions resulting from the exhaustion of some nutrients or from shading. The same profile was observed by Silva et al. [66], Sassano et al. [67] and Torre et al. [32] in Arthrospira (Spirulina) platensis cultivations carried out in different photobioreactor configurations, using different light intensities and N-sources in the culture medium.
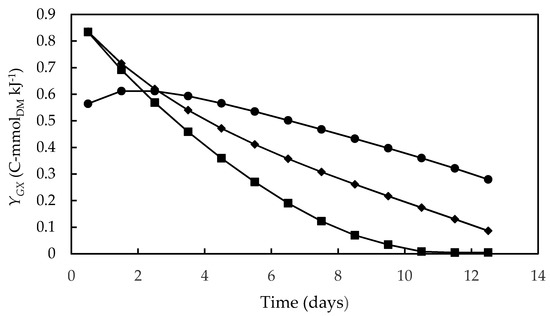
Figure 3.
Biomass yield on Gibbs energy dissipation during cultivations of D. tertiolecta carried out in different culture media. F/2 medium (■), Bold’s Basal medium (●), Alternative medium (◆).
The highest value of YGX (0.83 C-mmolDM·kJ−1), observed in both seawater-based F/2 and alternative media, is close to the maximum YGX values reported by Silva et al. [66] (0.7–0.9 C-mmolDM·kJ−1) and by Sassano et al. [67] (0.8–1.0 C-mmolDM·kJ−1) in bioenergetic studies on A. platensis. The significantly lower YGX value obtained in the Bold’s Basal medium (0.56 C-mmolDM·kJ−1) can be explained by the need for cells well adapted to the high salinity conditions of seawater to adapt to the new conditions, which increased the amount of Gibbs energy needed to produce a given amount of biomass. In addition, as previously mentioned in the section addressed to kinetic parameters, the high NaCl concentration in seawater-based media may have enhanced the photosynthetic CO2 assimilation favoring biomass production [48]. However, it can be seen that, after the third day of cultivation, YGX decreased in the F/2 medium more quickly than in the alternative one, probably due to growth limitation resulting from depletion of some nutrient or microelement instead present in the formulation of the latter.
As is known, it is possible to describe cell metabolism through a set of reaction equations using stoichiometric coefficients for the formation of products from substrates [68]. In particular, knowing the compositions of substrates, products, and biomass, we can write the following overall material balance for the formation of 1 C-mol of D. tertiolecta biomass:
a HCO3− + b NO3− + c H2O + d O2 + e H+ + CH1.944O0.873N0.249 = 0
To this purpose, we used the elemental composition of biomass reported by Kim et al. [33]. Since Bezerra et al. [69] observed for A. platensis a negligible influence of a different elemental biomass composition (CHNOS) on the results of such a bioenergetic model, it was considered that it was not necessary to determine it at the end of each experiment, being sufficient, for the purposes of this study, the biomass composition data available in the literature.
The stoichiometric coefficients estimated for the growth of D. tertiolecta through material balances of carbon, nitrogen, oxygen, charge, and reduction degree of biomass (γX) are listed in Table 4 together with the Gibbs energies of formation under biological standard conditions of the compounds involved in growth.

Table 4.
Gibbs energies of formation under biological standard conditions of the compounds involved in the growth of Dunaliella tertiolecta biomass, and stoichiometric coefficients estimated through material balances of carbon, nitrogen, oxygen, charge, and reduction degree of biomass (γX).
The reduction degree of D. tertiolecta biomass (γX = 6.06) was slightly higher than that reported for A. platensis (4.89–5.80) [69], because of a higher H content of its biomass compared to that of this cyanobacterium (CH1.59O0.50N0.10), i.e., of a higher number of equivalents available electrons per C-mol of biomass.
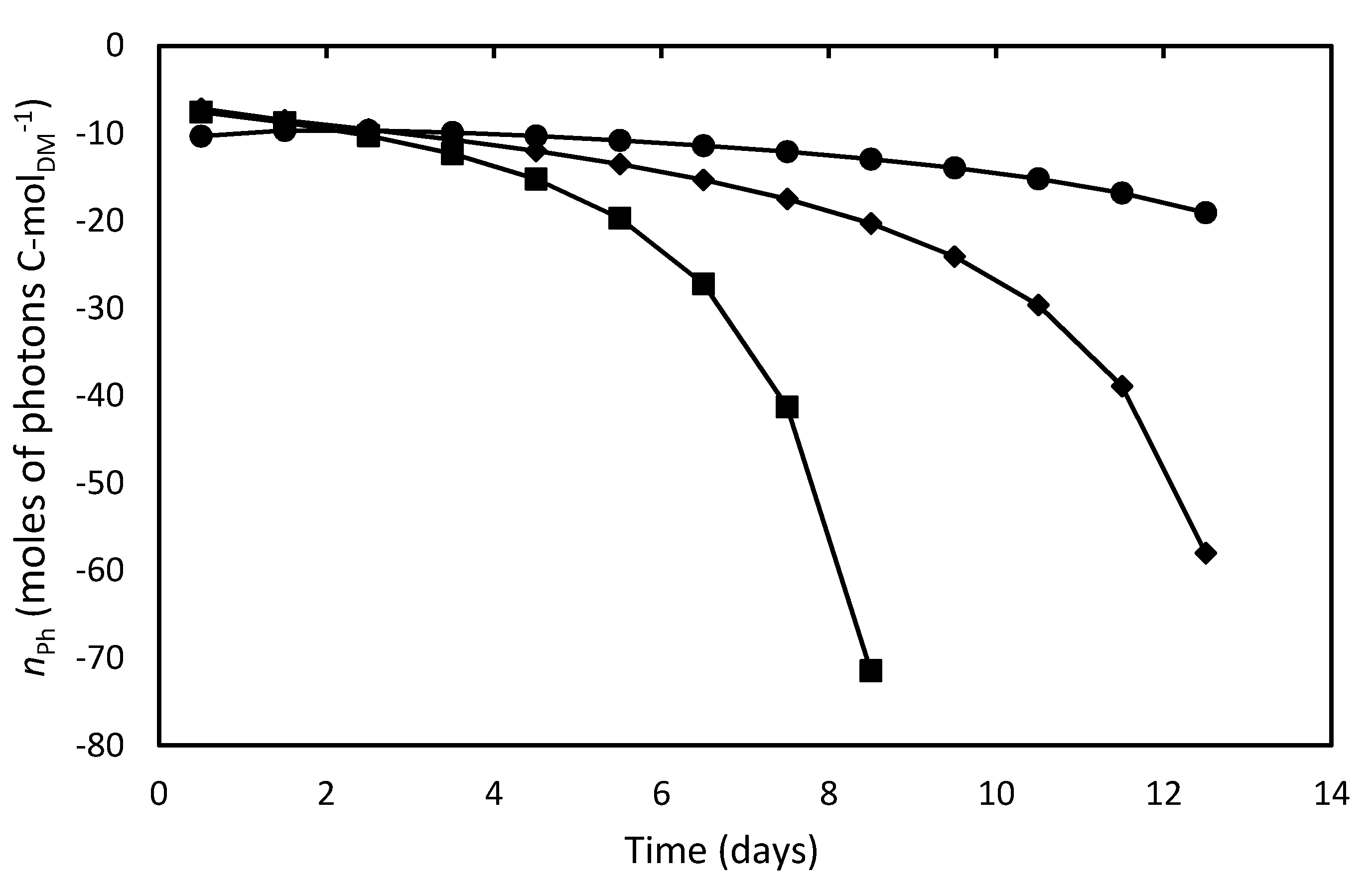
Estimating the above stoichiometric coefficients by Equation (13), YGX by Equation (8) and using the standard Gibbs energies of formation of reactants, products and biomass listed in Table 4 [31], it was possible to calculate, by Equation (9), the moles of photons to sustain the autotrophic growth of 1 C-mol biomass (nPh), whose time course is illustrated in Figure 4. It can be observed that this bioenergetic parameter increased (in absolute value) throughout the cultivations, with the negative values indicating that photons were taken up by the system. In particular, at the beginning of cultures, when cell concentration was low, the nPh absolute values were very close to the theoretical one reported by Richmond [70] to support phototrophic growth under ideal conditions (8 moles of photons per C-mol of biomass). According to the widely accepted two-step model of photosynthesis, 8 mol quanta of light are in fact required to release 1 mol of O2 [71]. A significant additional energy requirement with respect to such an ideal condition occurred during the stationary growth phase of batch cultures, which suggests that, under these hard environmental conditions, energy was preferentially used for maintenance of existing live cells rather than for growth. This effect was also described by Torre et al. [32] and Bezerra et al. [69] in their bioenergetics studies on A. platensis.
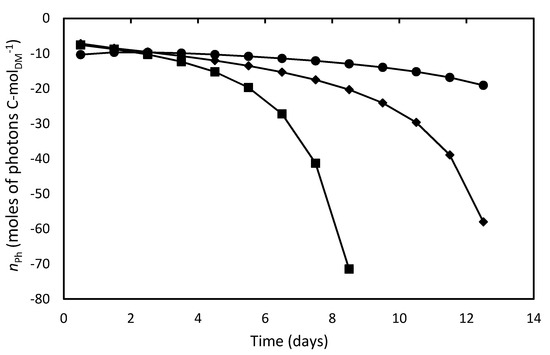
Figure 4.
Moles of photons utilized to produce one C-mol biomass of D. tertiolecta in different culture media. F/2 medium (■), Bold’s Basal medium (●), Alternative medium (◆).
Figure 4 also shows that the culture in F/2 medium needed higher nPh values to sustain autophototrophic growth compared to cultures in Bold’s Basal and alternative media from the fifth day of cultivation onwards, likely due to the previously-supposed nutrient limitation. Consequently, cells reached lower concentration in the stationary growth phase, and most of energy was lost. It has been reported that nitrogen or phosphorus limitation affects the photosynthetic apparatus of D. tertiolecta cells by reducing their chlorophyll and carotenoids contents leading to chlorosis [34]. This effect is consistent with the pigment loss observed in D. tertiolecta cells cultivated in this medium (Figure 2). The balance between energy supplied to PSII by light harvesting and energy requested by photosynthesis and growth appears to be regulated by chlorophyll concentration [34,72]. Although less energy was needed to form 1 C-mol of biomass in the Bold’s basal medium compared to the alternative one, the latter allowed achieving a higher cell concentration at the end of cultivation, which means that a greater fraction of absorbed energy was devoted to cell growth, thus proving a better medium for D. tertiolecta cultivation.
During photosynthesis, the light energy absorbed by the antenna pigments, mainly chlorophylls, xanthophylls and carotenes, is converted into redox energy, whose average value referred to 1 mol of photons (ΔgPh) is 206.2 kJ·mol−1 at wavelength of 580 nm, that drives the formation of high-energy products such as ATP and NADPH [71]. According to the aforementioned photosynthesis model, 8 photons (hν) of light are needed to form 1 mol of O2. Moreover, photorespiration was considered absent, as microalgae suppress photorespiration in the presence of light and inorganic carbon sources such as bicarbonate and CO2, as occurs in C4 plants [73]. Thus, the evolution of O2 can be related to the flux of absorbed photons by the equation:
2 H2O + 8 hν +2 NADP+ → 2 NADPH2 + O2
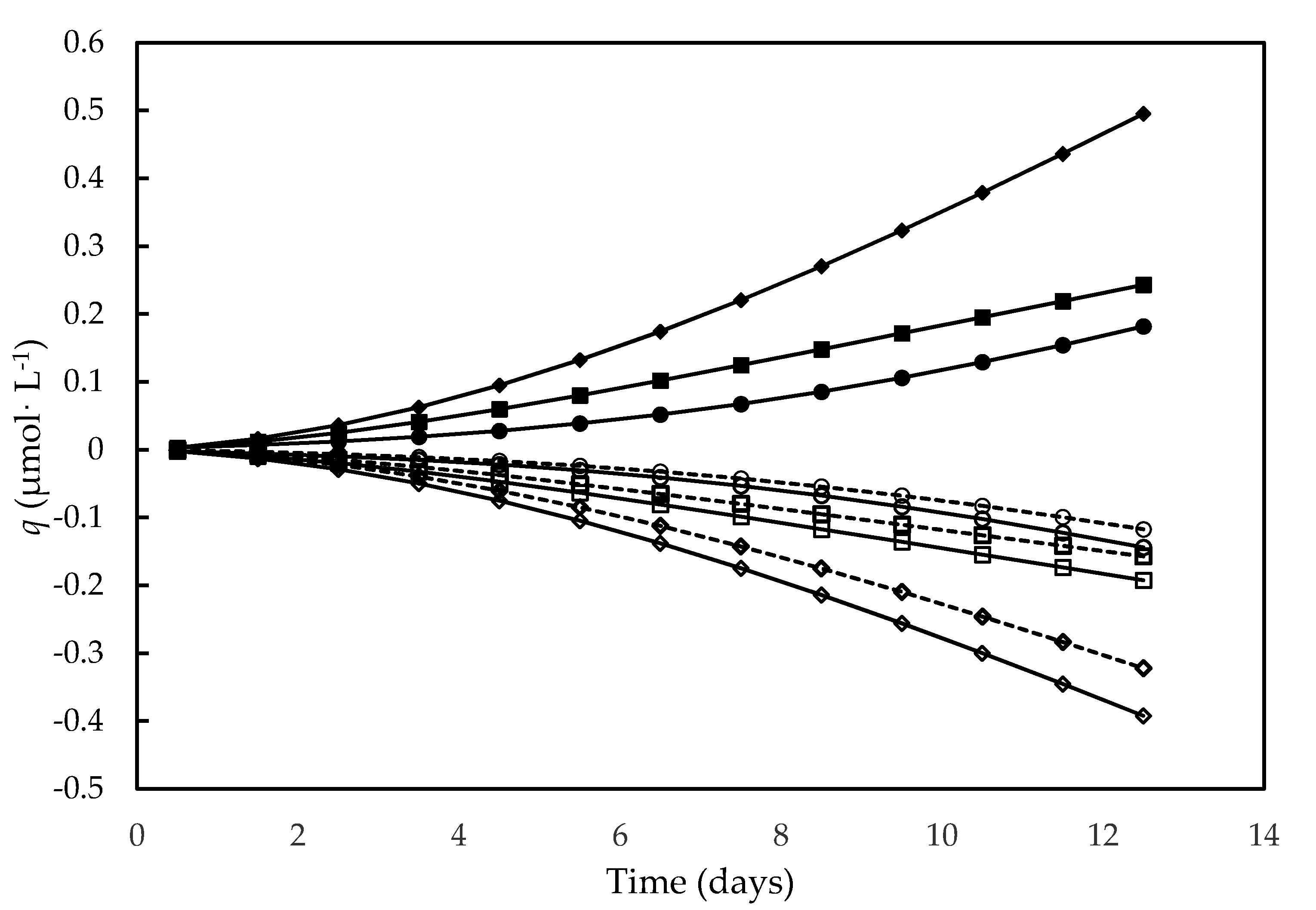
As show in Figure 5, the molar development of O2, consumptions of H+ and H2O variation strictly followed cell growth. In particular, these activities were higher in the culture carried out on the alternative medium compared to the other media, consistently with its higher nutrient contents and higher photosynthesis rate, which resulted in higher cell concentrations.
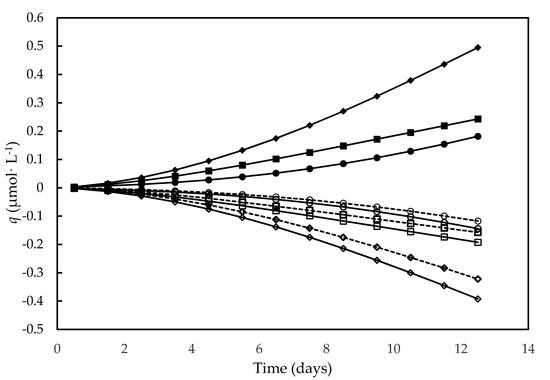
Figure 5.
Development of O2 (full symbols), consumption of H+ (open symbols, continuous line) and H2O variation (open symbols, dashed line) over time in different culture media. F/2 medium (□,■), Bold’s Basal medium (○,●), Alternative medium (◇,◆).
The progressive consumption of H+ described by the qH+ trend in this figure is consistent with the progressive rise in pH detected in D. tertiolecta autotrophic cultures, resulting from the OH− release linked to photosynthetic fixation of CO2 and NO3− uptake [36,74]. Indeed, such a pH increase appears to be a rule in photoautotrophic cultures, having been proposed that the uptake of two HCO3− moles leads to one mole of fixed CO2 and one of CO32− released in the medium [75].
As is well known, ATP is essential for several cell functions including the assembly of biopolymers as well as cell maintenance and division. For instance, in the autotrophic culture of C. pyrenoidosa up to 77% of total ATP produced by cell metabolism is due to the assimilation of CO2 by the Calvin cycle [71]. Therefore, we assumed that a portion of molar Gibbs energy absorbed by the two photosystems (I and II) was used to convert ADP to ATP (ADP + Pi → ATP + H2O, Δg◦ = 30.5 kJ·mol−1) by membrane-bound ATP synthase [76].
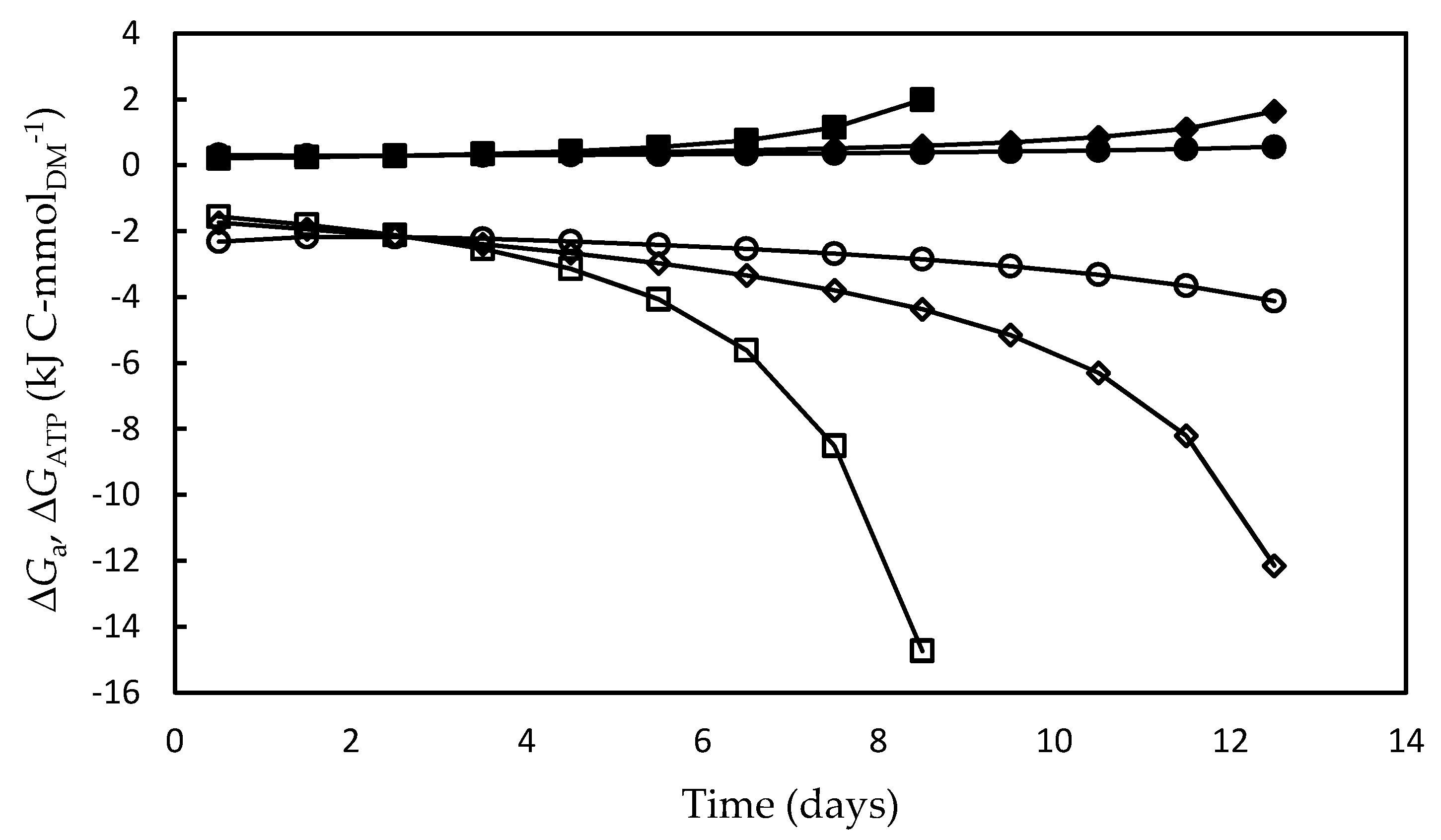
As shown in Figure 6, in all the experiments both the energy absorbed by the system (ΔGa) and the fraction converted into ATP (ΔGATP) increased over time. The highest ΔGa and ΔGATP values were associated to the low growth rates in the stationary growth phase, mainly in the F/2 medium, which can be ascribed to the additional amount of ATP needed to produce biomass from biopolymers, defined elsewhere as “growth-associated maintenance” [77,78]. As previously reported for the cyanobacterium A. platensis [69], unfavorable environmental conditions such as lack of nutrients or release of certain cell metabolites increase the energy needs of cells for transport, translocation, futile cycles of nutrients, and assembly of biopolymers into growing biomass. In addition, microalgae have a high degree of subcellular compartmentation of metabolism, whereby further transport reactions that consume large amounts of energy are involved in the metabolic reactions [71]. Moreover, Kliphuis et al. [79] reported that low cell growth rates are affected by the high energy maintenance requirements that result in low biomass yields.
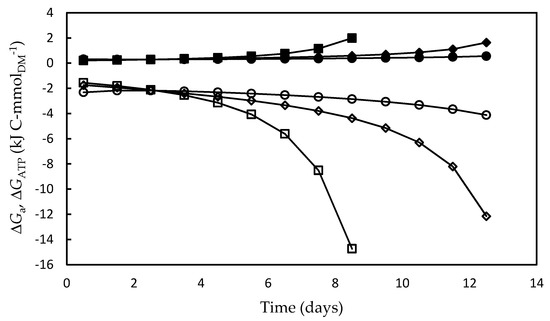
Figure 6.
Total Gibbs energy absorbed by the photosynthetic apparatus (open symbols) (ΔGa) and Gibbs energy transformed into ATP (full symbols) (ΔGATP) during Dunaliella tertiolecta cultivations carried out in different culture media: F/2 medium (□,■), Bold’s Basal medium (○,●), Alternative medium (◇,◆).
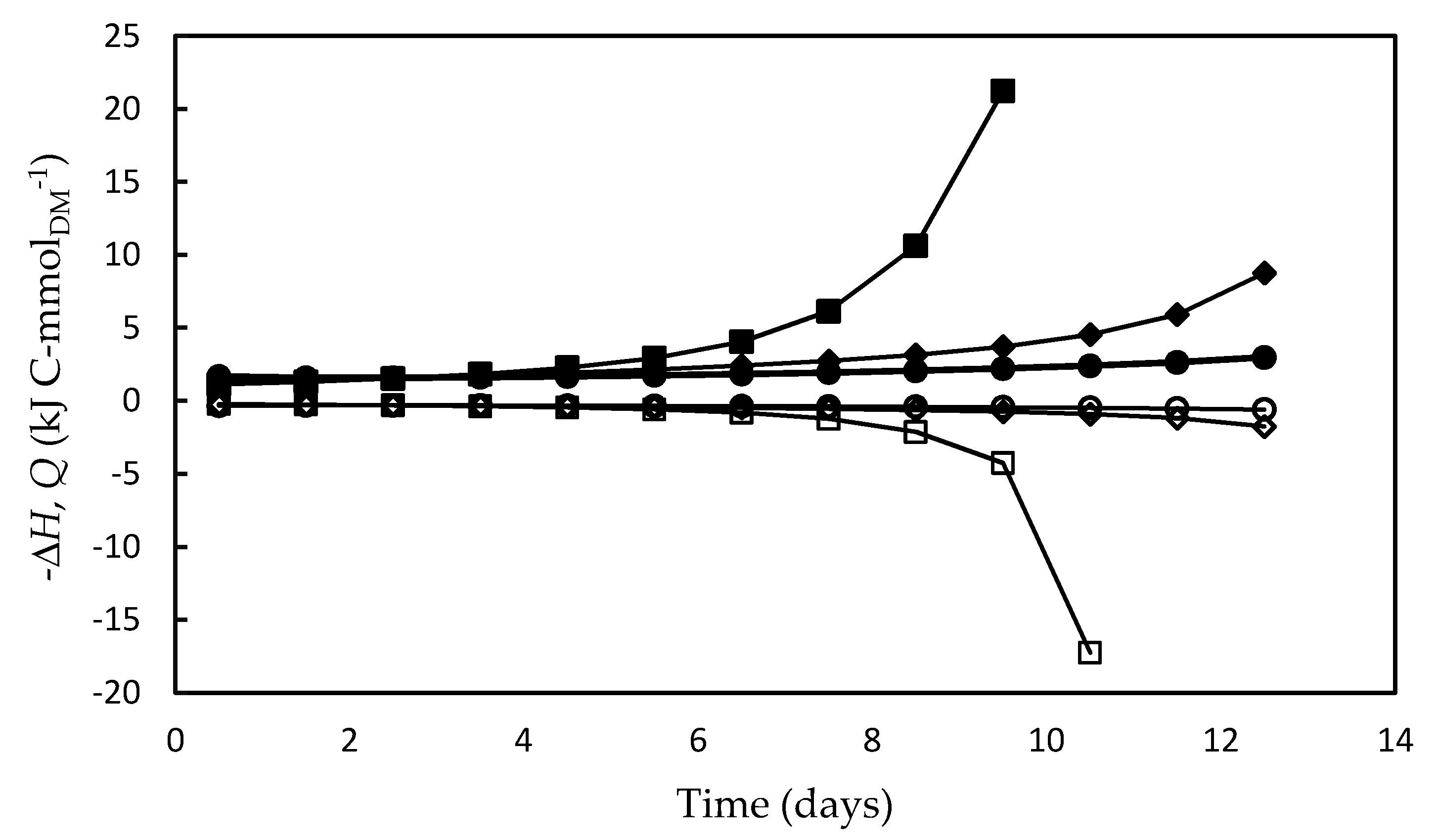
Similarly to what was observed for ΔGa (in absolute values) and ΔGATP, the enthalpy energy component (ΔH) and heat released (Q) also increased over time, and, as expected, the highest values of both occurred in the F/2 medium (Figure 7). This result suggests that, under the nutritional stress conditions occurred in this medium, a significant portion of excess light energy that entered the system (ΔGa) was lost as heat, and, since growth was very poor, most of the energy fraction fixed by the photosynthesis (ΔH) was used for metabolic activities intended for cell maintenance. It is likely that most of the heat was dissipated by nonphotochemical reactions, i.e., by the so-called non-photochemical quenching (NPQ) photoprotection mechanism [73]. This mechanism is essential to remove the excess electrons in the antennae complexes, which would otherwise damage the photosynthetic apparatus by thermal dissipation in the xanthophyll cycle in Dunaliella cell [34,53,80].
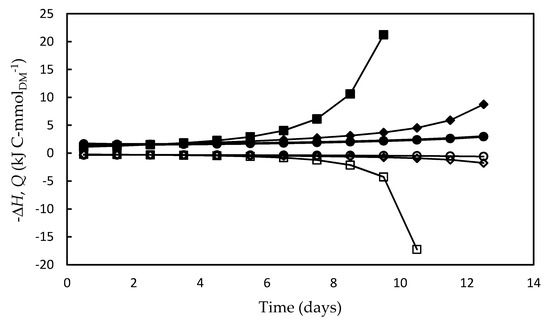
Figure 7.
Energy fixed by the photosynthetic apparatus (open symbols) (ΔH) and energy released as heat (full symbols) (Q) during Dunaliella tertiolecta cultivations carried out in different culture media: F/2 medium (□,■), Bold’s Basal medium (○,●), Alternative medium (◇,◆). The choice of illustrating -Q instead of Q was only due to the need to avoid overlapping with the ΔH curves.
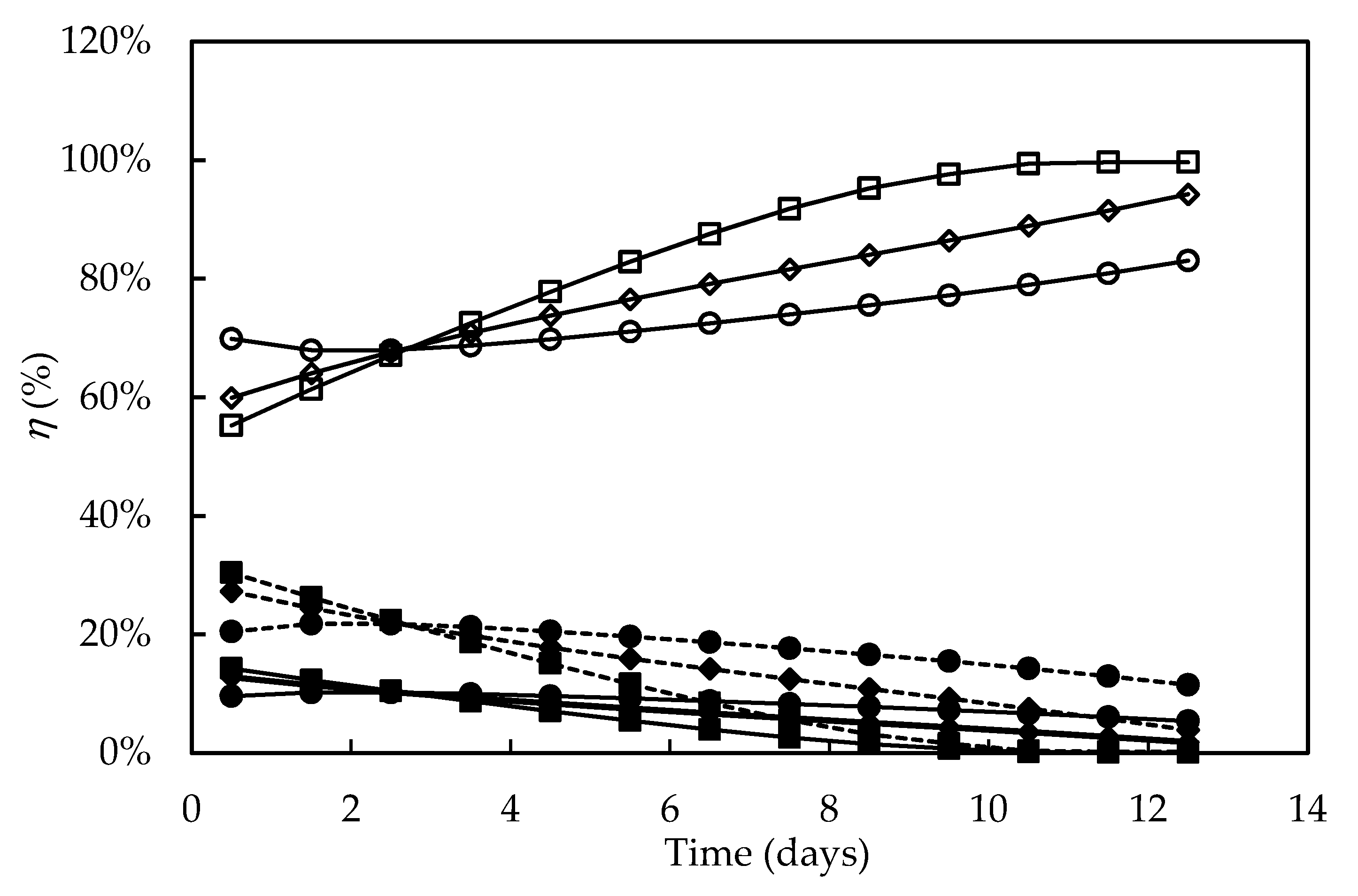
Figure 8 shows the percentage distribution of the light energy absorbed versus time in the different culture media. In all the experiments, both the energy fraction stored in the phosphoanhydride bonds of ATP (ηATP) and the enthalpic one fixed by the systems (ηF) decreased over time, whereas that released as heat (ηQ) increased. ηF and ηATP values were higher at the beginning of cultivations in the media constituted by seawater (F/2 and alternative medium) and decreased more sharply when compared to Bold’s Basal medium. The highest ηATP values (9.6–14.2%) were close to that found for the autotrophic cultivation of C. pyrenoidosa (ηATP = 10%) [71], and the slight decrease in ηF and ηATP values in the Bold’s Basal medium is consistent with the higher cell concentration observed compared to the F/2 medium. Furthermore, while in the F/2 medium the ηF values were practically null from the ninth day onwards, in the alternative one they remained positive, even if decreasing, during the entire course of the culture, suggesting that significant percentages of the energy fixed by the photosynthetic apparatus and of that transformed into ATP were directed to cell growth. It has been reported that the availability of nutrients, mainly nitrogen and phosphorus sources, is a main environmental factor capable of influencing the composition of the photosynthetic apparatus of D. tertiolecta [34]; therefore, the declining values of ηF could also have been the result of depletion of some nutrient especially at the end of cultivations. Bezerra et al. [69] also reported that high cell concentrations reduce the light availability to the cell through the so-called shading effect, thus reducing ηF more sharply. However, in the present work, the sharpest decrease in ηF occurred in the culture with the lowest cell concentration, indicating that light intensity was not the limiting factor.
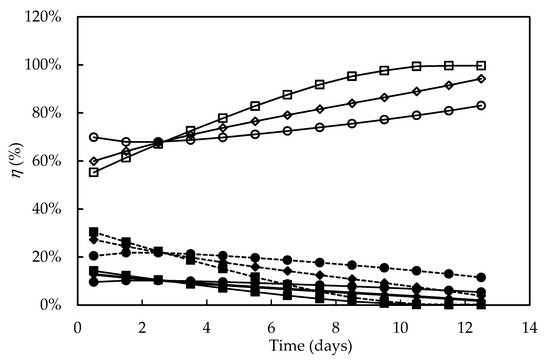
Figure 8.
Percentage distribution of the light energy absorbed during Dunaliella tertiolecta cultivations in different culture media. Energy fixed by the photosystems (dashed line), energy transformed into ATP (continuous line, full symbols), energy released as heat (open symbols). F/2 medium (□,■), Bold’s Basal medium (○,●), Alternative medium (◇,◆).
4. Conclusions
This work demonstrated that Dunaliella tertiolecta grown in Bold’s Basal medium had high contents of carotenoids (52.0 ± 7.2 mg·g−1) and chlorophyll (162.6 ± 15.7 mg·g−1). However, to obtain high concentrations of biomass to be used for energy purposes, after the extraction and recovery of these precious components, an alternative medium based on seawater has proven to be an efficient alternative for the cultivation of this microalga, allowing a high cell productivity (102 mgDM·L−1·d−1) and photosynthesis rate. In fact, it was able to ensure, at the end of batch cultivation, more than twice the cell concentration obtained in the Bold’s Basal medium. As regard the bioenergetic study, the optimal conditions for D. tertiolecta growth in the alternative medium were highlighted by high values of biomass yield on Gibbs energy dissipation (YGX), high molar development of O2 and consumption of H+ as well as a high energy fraction stored as ATP (ηATP) during cultivation.
Author Contributions
Conceptualization: R.P.B. and D.d.A.V.-M.; methodology: R.P.B. and Y.A.S.M.; validation: all authors; formal analysis: Y.A.S.M., R.P.B., D.d.A.V.-M., and A.C.; investigation: Y.A.S.M., R.P.B., and D.d.A.V.-M.; resources: A.L.F.P.; data curation: Y.A.S.M., R.P.B., D.d.A.V.-M., and A.C.; writing—original draft preparation: Y.A.S.M., R.P.B., D.d.A.V.-M., and A.C.; writing—review and editing: A.C.; visualization, Y.A.S.M., R.P.B., and A.C.; supervision: A.C.; project administration: R.P.B., D.d.A.V.-M., and A.C.; funding acquisition: A.L.F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation for Science and Technology of the State of Pernambuco, Brazil (FACEPE), grant number APQ-0252-5.07/14.
Acknowledgments
The authors acknowledge with thanks the Research Support Center (CENAPESQ, Recife, Brazil) and Laboratory of Technology of Bioactives (LABTECBIO, Recife, Brazil).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, X.J.; Wu, M.J.; Jiang, Y.; Yang, Y.; Yan, Y.B. Dunaliella salina Hsp90 Is Halotolerant. Int. J. Biol. Macromol. 2015, 75, 418–425. [Google Scholar] [CrossRef]
- Madkour, F.F.; Abdel-Daim, M.M. Hepatoprotective and Antioxidant Activity of Dunaliella salina in Paracetamol-Induced Acute Toxicity in Rats. Indian J. Pharm. Sci. 2013, 75, 642–648. [Google Scholar]
- Srinivasan, R.; Chaitanyakumar, A.; Mageswari, A.; Gomathi, A.; Pavan Kumar, J.G.S.; Jayasindu, M.; Bharath, G.; Shravan, J.S.; Gothandam, K.M. Oral Administration of Lyophilized Dunaliella salina, a Carotenoid-Rich Marine Alga, Reduces Tumor Progression in Mammary Cancer Induced Rats. Food Funct. 2017, 8, 4517–4527. [Google Scholar] [CrossRef]
- Caroprese, M.; Albenzio, M.; Ciliberti, M.G.; Francavilla, M.; Sevi, A. A Mixture of Phytosterols from Dunaliella tertiolecta Affects Proliferation of Peripheral Blood Mononuclear Cells and Cytokine Production in Sheep. Vet. Immunol. Immunopathol. 2012, 150, 27–35. [Google Scholar] [CrossRef]
- Talebi, A.F.; Tohidfar, M.; Mousavi Derazmahalleh, S.M.; Sulaiman, A.; Baharuddin, A.S.; Tabatabaei, M. Biochemical Modulation of Lipid Pathway in Microalgae Dunaliella sp. for Biodiesel Production. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative Natural Functional Ingredients from Microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Khozingoldberg, I.; Cohen, Z. The Effect of Phosphate Starvation on the Lipid and Fatty Acid Composition of the Fresh Water Eustigmatophyte Monodus subterraneus. Phytochemistry 2006, 67, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Prathima Devi, M.; Venkata Mohan, S. CO2 Supplementation to Domestic Wastewater Enhances Microalgae Lipid Accumulation under Mixotrophic Microenvironment: Effect of Sparging Period and Interval. Bioresour. Technol. 2012, 112, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tanaka, M.; Shinkawa, H.; Nakada, T.; Ano, Y.; Kurano, N.; Soga, T.; Tomita, M. Metabolic and Morphological Changes of an Oil Accumulating Trebouxiophycean Alga in Nitrogen-Deficient Conditions. Metabolomics 2013, 9, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, K.; Rohit, M.V.; Venkata Mohan, S. Microalgae-Based Carotenoids Production. Algal Green Chem. 2017, 139–147. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chew, K.W.; Yew, G.Y.; Leong, W.H.; Chai, Y.H.; Show, P.L.; Chen, W.-H. Recent Advances in Downstream Processing of Microalgae Lipid Recovery for Biofuel Production. Bioresour. Technol. 2020, 304, 122996. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from Microalgae: Technologies, Challenges and Opportunities. Renew. Sust. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Sun, J.; Xiong, X.; Wang, M.; Du, H.; Li, J.; Zhou, D.; Zuo, J. Microalgae Biodiesel Production in China: A Preliminary Economic Analysis. Renew. Sust. Energy Rev. 2019, 104, 296–306. [Google Scholar] [CrossRef]
- Liang, M.-H.; Qv, X.-Y.; Chen, H.; Wang, Q.; Jiang, J.-G. Effects of Salt Concentrations and Nitrogen and Phosphorus Starvations on Neutral Lipid Contents in the Green Microalga Dunaliella tertiolecta. J. Agric. Food Chem. 2017, 65, 3190–3197. [Google Scholar] [CrossRef]
- Rodríguez, M.B.R. Simulation of an Assisted Culture Medium for Production of Dunaliella tertiolecta. Algal Res. 2020, 47, 101838. [Google Scholar] [CrossRef]
- García Morales, J.; López Elías, J.A.; Medina Félix, D.; García Lagunas, N.; Fimbres Olivarría, D. Efecto Del Estrés Por Nitrógeno y Salinidad En El Contenido de β-Caroteno de La Microalga Dunaliella tertiolecta//Effect of Nitrogen and Salinity Stress on the β-Carotene Content of the Microalgae Dunaliella tertiolecta. Biotecnia 2020, 22, 13–19. [Google Scholar] [CrossRef]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- McWilliams, A. The Global Market for Carotenoids; BCC Research: Wellesley, MA, USA, 2018. [Google Scholar]
- Guedes, A.C.; Amaro, H.M.; Sousa-Pinto, I.; Malcata, F.X. Algal Spent Biomass—A Pool of Applications. Biofuels Algae 2019, 397–433. [Google Scholar] [CrossRef]
- Sedjati, S.; Santosa, G.; Yudiati, E.; Supriyantini, E.; Ridlo, A.; Kimberly, F. Chlorophyll and Carotenoid Content of Dunaliella salina at Various Salinity Stress and Harvesting Time. IOP Conf. Ser. Earth Environ. Sci. 2019, 246, 012025. [Google Scholar] [CrossRef]
- Tredici, M.R. Photobiology of Microalgae Mass Cultures: Understanding the Tools for the next Green Revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Küçük, K.; Tevatia, R.; Sorgüven, E.; Demirel, Y.; Özilgen, M. Bioenergetics of Growth and Lipid Production in Chlamydomonas reinhardtii. Energy 2015, 83, 503–510. [Google Scholar] [CrossRef]
- Demirel, Y.; Sieniutycz, S. Nonequilibrium Thermodynamics: Transport and Rate Processes in Physical and Biological Systems, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies on Marine Planktonic Diatoms I. Cyclotella Nana Hustedt and Detonula Confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Bischoff, H.W.; Bold, H.C. Some Soil Algae from Enchanted Rock and Related Algal Species. Phycol. Stud. IV 1963, 6318, 1–95. [Google Scholar]
- Jesus, S.S.; Maciel Filho, R. Modeling Growth of Microalgae Dunaliella salina under Different Nutritional Conditions. Am. J. Biochem. Biotechnol. 2010, 6, 279–283. [Google Scholar] [CrossRef]
- Lichtenthaler, H. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzym. 1987, 148, 350–382. [Google Scholar]
- Soletto, D.; Binaghi, L.; Ferrari, L.; Lodi, A.; Carvalho, J.C.M.; Zilli, M.; Converti, A. Effects of Carbon Dioxide Feeding Rate and Light Intensity on the Fed-Batch Pulse-Feeding Cultivation of Spirulina platensis in Helical Photobioreactor. Biochem. Eng. J. 2008, 39, 369–375. [Google Scholar] [CrossRef]
- Heijnen, J.J. Stoichiometry and Kinetics of Microbial Growth from a Thermodynamic Perspective. In Basic Biotechnology; Cambridge University Press: Cambridge, UK, 2001; pp. 45–58. [Google Scholar] [CrossRef]
- Torre, P.; Sassano, C.E.; Sato, S.; Converti, A.; Gioielli, L.A.; Carvalho, J.C. Fed-Batch Addition of Urea for Spirulina platensis Cultivation. Enzym. Microb. Technol. 2003, 33, 698–707. [Google Scholar] [CrossRef]
- Kim, S.-S.; Ly, H.V.; Kim, J.; Lee, E.Y.; Woo, H.C. Pyrolysis of Microalgae Residual Biomass Derived from Dunaliella tertiolecta after Lipid Extraction and Carbohydrate Saccharification. Chem. Eng. J. 2015, 263, 194–199. [Google Scholar] [CrossRef]
- Geider, R.; Macintyre; Graziano, L.; McKay, R.M. Responses of the Photosynthetic Apparatus of Dunaliella tertiolecta (Chlorophyceae) to Nitrogen and Phosphorus Limitation. Eur. J. Phycol. 1998, 33, 315–332. [Google Scholar] [CrossRef]
- Wongsnansilp, T.; Juntawong, N.; Wu, Z. Effects of Phosphorus on the Growth and Chlorophyll Fluorescence of a Dunaliella salina Strain Isolated from Saline Soil under Nitrate Limitation. J. Biol. Res. Boll. Soc. Ital. Biol. Sper. 2016, 89. [Google Scholar] [CrossRef]
- Kumar, A.; Guria, C.; Pathak, A.K. Optimal Cultivation towards Enhanced Algae-Biomass and Lipid Production Using Dunaliella tertiolecta for Biofuel Application and Potential CO2 Bio-Fixation: Effect of Nitrogen Deficient Fertilizer, Light Intensity, Salinity and Carbon Supply Strategy. Energy 2018. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Tran, D.; Ben-Amotz, A. History, Distribution, and Habitats of Algae of the Genus Dunaliella Teodoresco (Chlorophyceae). In The Alga Dunaliella: Biodiversity, Physiology, Genomics and Biotechnology; Ben-Amotz, A., Polle, J.E.W., Rao, D.V.S., Eds.; Science Publishers Inc.: Enfield, NH, USA, 2009; pp. 1–13. [Google Scholar]
- Kulshreshtha, J.; Singh, G.P. Evaluation of Various Inorganic Media for Growth and Biopigment of Dunaliella salina. Int. J. Pharm. Bio. Sci. 2013, 4, 1083–1089. [Google Scholar]
- Ohse, S.; Derner, R.B.; Ozório, R.Á.; Cunha, P.C.R.; Lamarca, C.P.; Santos, M.E.; Mender, L.B.B. Revisão: Sequestro de Carbono Realizado Por Microalgas e Florestas e a Capacidade de Produção de Lipídios Pelas Microalgas. Insula 2007, 36, 39–74. [Google Scholar]
- Millero, F.J.; Feistel, R.; Wright, D.G.; McDougall, T.J. The Composition of Standard Seawater and the Definition of the Reference-Composition Salinity Scale. Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, H.; Zhou, Z.; Li, K.; Hou, G.; Xu, Q.; Chuai, W.; Zhang, C.; Han, D.; Hu, Q. Ultrahigh-cell-density Heterotrophic Cultivation of the Unicellular Green Microalga Scenedesmus acuminatus and Application of the Cells to Photoautotrophic Culture Enhance Biomass and Lipid Production. Biotechnol. Bioeng. 2020, 117, 96–108. [Google Scholar] [CrossRef]
- Xinyi, E.; Crofcheck, C.; Crocker, M. Application of Recycled Media and Algae-Based Anaerobic Digestate in Scenedesmus Cultivation. J. Renew. Sust. Energy 2016, 8, 1–14. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Chang, J.-S.; Chen, W. Effect of Light Supply and Carbon Source on Cell Growth and Cellular Composition of a Newly Isolated Microalga Chlorella vulgaris ESP-31. Eng. Life Sci. 2010, 10, 201–208. [Google Scholar] [CrossRef]
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.Y.S.; Salley, S.O. Effect of Nutrients on Growth and Lipid Accumulation in the Green Algae Dunaliella tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655. [Google Scholar] [CrossRef]
- Hexin, L.; Xianggan, C.; Zhilei, T.; Shiru, J. Analysis of Metabolic Responses of Dunaliella salina to Phosphorus Deprivation. J. Appl. Phycol. 2017, 29, 1251–1260. [Google Scholar] [CrossRef]
- Tammam, A.A.; Fakhry, E.M.; El-Sheekh, M. Effect of Salt Stress on Antioxidant System and the Metabolism of the Reactive Oxygen Species in Dunaliella salina and Dunaliella tertiolecta. Afr. J. Biotechnol. 2011, 10, 3795–3808. [Google Scholar]
- Katz, A.; Pick, U. Plasma Membrane Electron Transport Coupled to Na+ Extrusion in the Halotolerant Alga Dunaliella. Biochim. Biophys. Acta Bioenerg. 2001, 1504, 423–431. [Google Scholar] [CrossRef]
- Oren, A. A Hundred Years of Dunaliella Research: 1905–2005. Saline Syst. 2005, 1, 2. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.-G.; Wu, G.-H. Effects of Salinity Changes on the Growth of Dunaliella salina and Its Isozyme Activities of Glycerol-3-Phosphate Dehydrogenase. J. Agric. Food Chem. 2009, 57, 6178–6182. [Google Scholar] [CrossRef]
- Jiang, Y.; Yoshida, T.; Quigg, A. Photosynthetic Performance, Lipid Production and Biomass Composition in Response to Nitrogen Limitation in Marine Microalgae. Plant Physiol. Biochem. 2012, 54, 70–77. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of Nitrogen Sources on Cell Growth and Lipid Accumulation of Green Alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Wykoff, D.D.; Davies, J.P.; Melis, A.; Grossman, A.R. The Regulation of Photosynthetic Electron Transport during Nutrient Deprivation in Chlamydomonas reinhardtii. Plant Physiol. 1998, 117, 129–139. [Google Scholar] [CrossRef]
- Zhang, L.; Happe, T.; Melis, A. Biochemical and Morphological Characterization of Sulfur-Deprived and H2-Producing Chlamydomonas reinhardtii (Green Alga). Planta 2002, 214, 552–561. [Google Scholar] [CrossRef]
- Volgusheva, A.A.; Zagidullin, V.E.; Antal, T.K.; Korvatovsky, B.N.; Krendeleva, T.E.; Paschenko, V.Z.; Rubin, A.B. Examination of Chlorophyll Fluorescence Decay Kinetics in Sulfur Deprived Algae Chlamydomonas reinhardtii. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 559–564. [Google Scholar] [CrossRef][Green Version]
- Srinivasan, R.; Mageswari, A.; Subramanian, P.; Suganthi, C.; Chaitanyakumar, A.; Aswini, V.; Gothandam, K.M. Bicarbonate Supplementation Enhances Growth and Biochemical Composition of Dunaliella salina V-101 by Reducing Oxidative Stress Induced during Macronutrient Deficit Conditions. Sci. Rep. 2018, 8, 6972. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, M.; Wang, C. Nutrient Deprivation Enhances Lipid Content in Marine Microalgae. Bioresour. Technol. 2013, 147, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Watanabe, Y.; Saiki, H. Photosynthetic Productivity of Conical Helical Tubular Photobioreactor Incorporatin Chlorella sorokiniana under Field Conditions. Biotechnol. Bioeng. 2002, 77, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-C.; Karam, A.L.; Sederoff, H.W.; Ducoste, J.J.; de los Reyes, F.L. Relating Nitrogen Concentration and Light Intensity to the Growth and Lipid Accumulation of Dunaliella viridis in a Photobioreactor. J. Appl. Phycol. 2019, 31, 3397–3409. [Google Scholar] [CrossRef]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P.A. Look Back at the U.S. Department of Energy’s Aquatic Species Program Biodiesel from Algae. Nat. Renew. Energy Lab. 1998, 328, 1–294. [Google Scholar]
- Antal, T.K.; Volgusheva, A.A.; Kukarskikh, G.P.; Krendeleva, T.E.; Tusov, V.B.; Rubin, A.B. Examination of Chlorophyll Fluorescence in Sulfur-Deprived Cells of Chlamydomonas reinhardtii. Biophysics 2006, 51, 251–257. [Google Scholar] [CrossRef]
- Shaker, S.; Morowvat, M.H.; Ghasemi, Y. Effects of Sulfur, Iron and Manganese Starvation on Growth, β-Carotene Production and Lipid Profile of Dunaliella salina. J. Young Pharm. 2017, 9, 43–46. [Google Scholar] [CrossRef]
- Johnson, M.K.; Johnson, E.J.; Macelroy, R.D.; Speer, H.L.; Bruff, B.S. Effects of Salts on the Halophilic Alga Dunaliella viridis. J. Bacteriol. Res. 1968, 95, 1461–1468. [Google Scholar] [CrossRef]
- Fazeli, M.R.; Tofighi, H.; Samadi, N.; Jamalifar, H. Carotenoids Accumulation by Dunaliella tertiolecta (Lake Urmia Isolate) and Dunaliella salina (CCAP 19/18 & Wt) under Stress Conditions. DARU J. Pharm. Sci. 2012, 14, 146–150. [Google Scholar]
- Ye, Z.-W.; Jiang, J.-G.; Wu, G.-H. Biosynthesis and Regulation of Carotenoids in Dunaliella: Progresses and Prospects. Biotechnol. Adv. 2008, 26, 352–360. [Google Scholar] [CrossRef]
- Ahmed, R.A.; He, M.; Aftab, R.A.; Zheng, S.; Nagi, M.; Bakri, R.; Wang, C. Bioenergy Application of Dunaliella salina SA 134 Grown at Various Salinity Levels for Lipid Production. Sci. Rep. 2017, 7, 8118. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Casazza, A.A.; Ferrari, P.F.; Perego, P.; Bezerra, R.P.; Converti, A.; Porto, A.L.F. A New Bioenergetic and Thermodynamic Approach to Batch Photoautotrophic Growth of Arthrospira (Spirulina) Platensis in Different Photobioreactors and under Different Light Conditions. Bioresour. Technol. 2016, 207, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Sassano, C.E.N.; Carvalho, J.C.M.; Gioielli, L.A.; Sato, S.; Torre, P.; Converti, A. Kinetics and Bioenergetics of Spirulina platensis Cultivation by Fed-Batch Addition of Urea as Nitrogen Source. Appl. Biochem. Biotechnol. 2004, 112, 143–150. [Google Scholar] [CrossRef]
- Stephanopoulos, G.; Aristidou, A.A.; Nielsen, J. Metabolic Engineering: Principles and Methodologies, 1st ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Bezerra, R.P.; Matsudo, M.C.; Sato, S.; Perego, P.; Converti, A.; de Carvalho, J.C.M. Effects of Photobioreactor Configuration, Nitrogen Source and Light Intensity on the Fed-Batch Cultivation of Arthrospira (Spirulina) Platensis. Bioenergetic Aspects. Biomass Bioenerg 2012, 37, 309–317. [Google Scholar] [CrossRef]
- Richmond, A. Phototrophic Microalgae. In Biotechnology; Rehm, H.J., Reed, G., Dellweg, H., Eds.; Verlag Chemie: Weinheim, Germany, 1983; Volume 3, pp. 109–143. [Google Scholar]
- Yang, C.; Hua, Q.; Shimizu, K. Energetics and Carbon Metabolism during Growth of Microalgal Cells under Photoautotrophic, Mixotrophic and Cyclic Light-Autotrophic/Dark-Heterotrophic Conditions. Biochem. Eng. J. 2000, 6, 87–102. [Google Scholar] [CrossRef]
- Escoubas, J.M.; Lomas, M.; LaRoche, J.; Falkowski, P.G. Light Intensity Regulation of Cab Gene Transcription Is Signaled by the Redox State of the Plastoquinone Pool. Proc. Natl. Acad. Sci. USA 1995, 92, 10237–10241. [Google Scholar] [CrossRef]
- Mukhanov, V.S.; Kemp, R.B. Simultaneous Photocalorimetric and Oxygen Polarographic Measurements on Dunaliella Maritima Cells Reveal a Thermal Discrepancy That Could Be Due to Nonphotochemical Quenching. Thermochim. Acta 2006, 446, 11–19. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. The Wavelength Dependence Of Massive Carotene Synthesis In Dunaliella Bardawil (Chlorophyceae)1. J. Phycol. 1989, 25, 175–178. [Google Scholar] [CrossRef]
- Binaghi, L.; Del Borghi, A.; Lodi, A.; Converti, A.; Del Borghi, M. Batch and Fed-Batch Uptake of Carbon Dioxide by Spirulina platensis. Process Biochem. 2003, 38, 1341–1346. [Google Scholar] [CrossRef]
- Sakurai, H.; Masukawa, H.; Kitashima, M.; Inoue, K. Photobiological Hydrogen Production: Bioenergetics and Challenges for Its Practical Application. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 1–25. [Google Scholar] [CrossRef]
- Kayser, A.; Weber, J.; Hecht, V.; Rinas, U. Metabolic Flux Analysis of Escherichia coli in Glucose-Limited Continuous Culture. I. Growth-Rate-Dependent Metabolic Efficiency at Steady State. Microbiology 2005, 151, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Taymaz-Nikerel, H.; Borujeni, A.E.; Verheijen, P.J.T.; Heijnen, J.J.; van Gulik, W.M. Genome-Derived Minimal Metabolic Models for Escherichia coli MG1655 with Estimated in Vivo Respiratory ATP Stoichiometry. Biotechnol. Bioeng. 2010, 107, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Kliphuis, A.M.J.; Klok, A.J.; Martens, D.E.; Lamers, P.P.; Janssen, M.; Wijffels, R.H. Metabolic Modeling of Chlamydomonas reinhardtii: Energy Requirements for Photoautotrophic Growth and Maintenance. J. Appl. Phycol. 2012, 24, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Liu, J.; Qin, R. Light Absorption and Growth Response of Dunaliella under Different Light Qualities. J. Appl. Phycol. 2020, 32, 1041–1052. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).