Evaluating the Struvite Recovered from Anaerobic Digestate in a Farm Bio-Refinery as a Slow-Release Fertiliser
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Bio-Refinery
2.2. Pot Experiment
2.3. Analytical Procedures of the Experiment
2.4. Phosphorus- and Nitrogen-Use Efficiency
2.5. Statistical Analysis
3. Results and Discussion
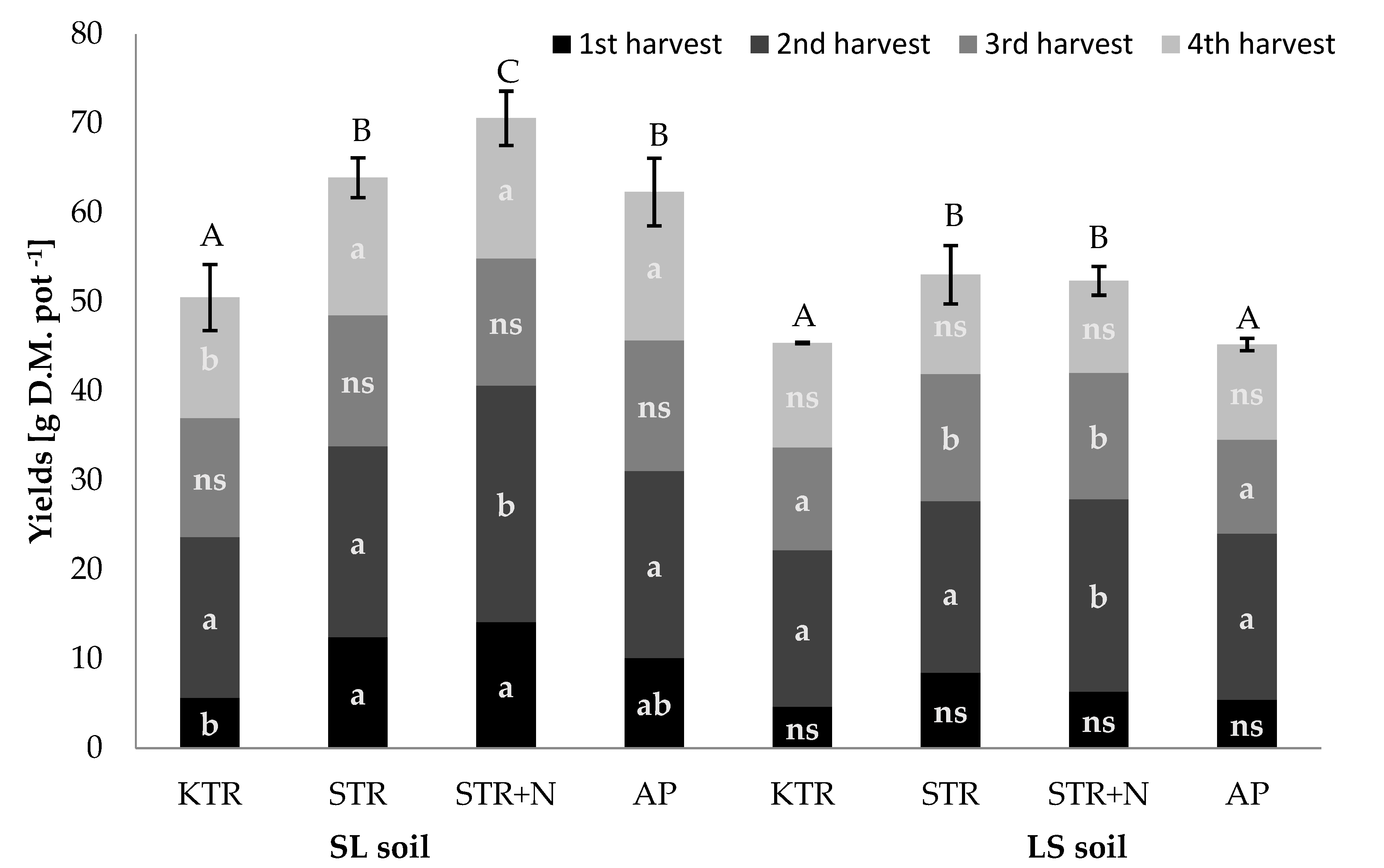
3.1. Crop Yields
3.2. Nutrient Content in Plants
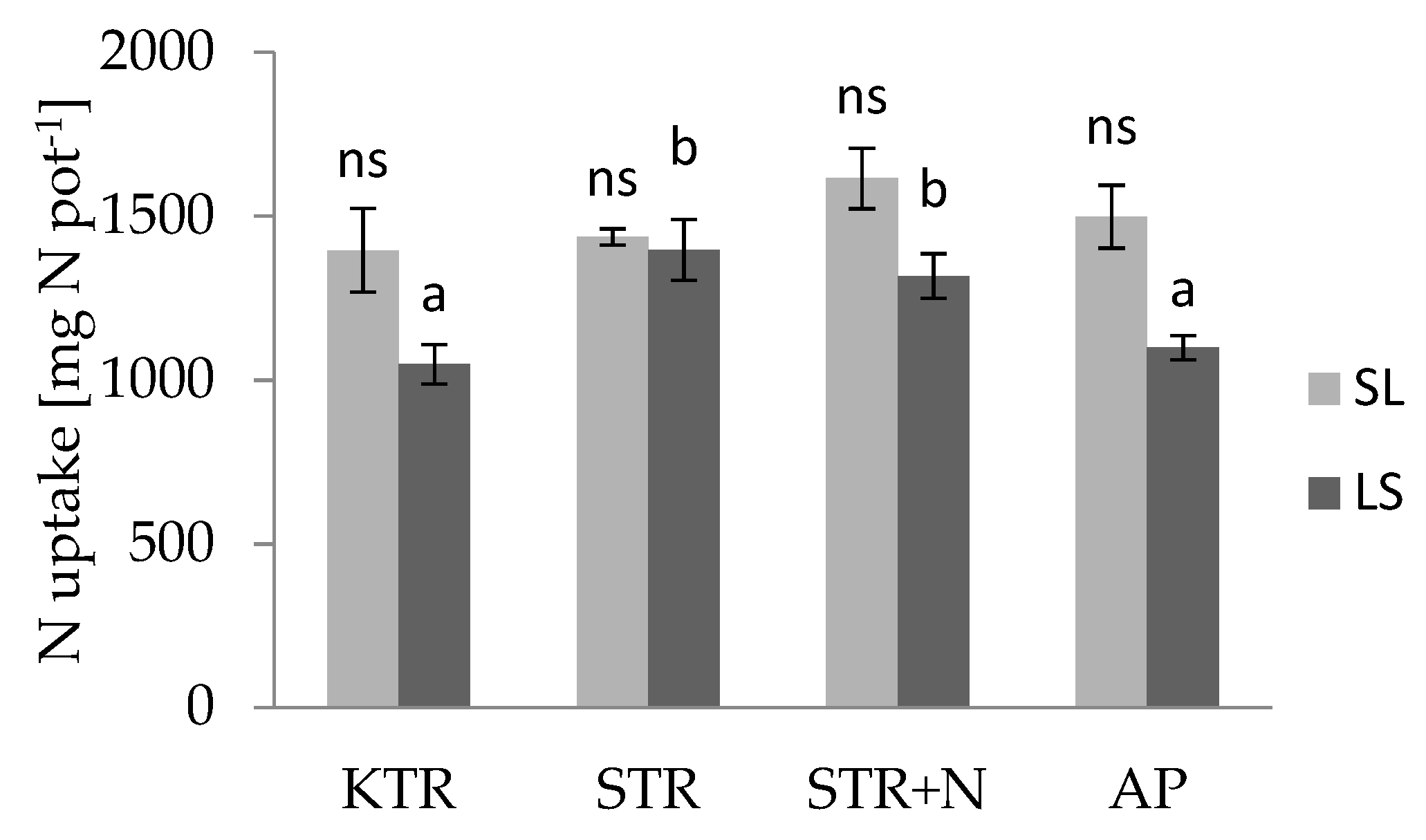
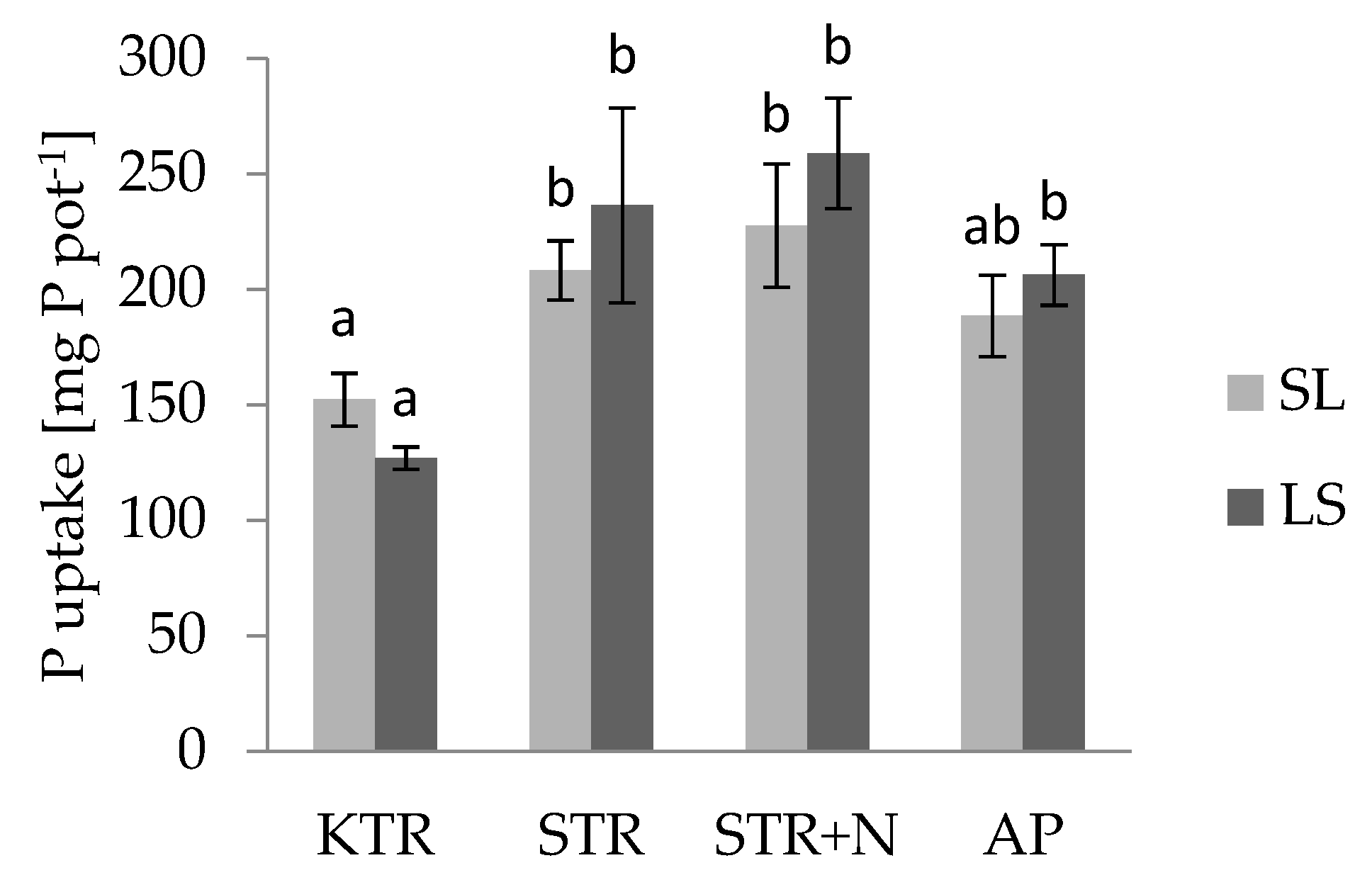
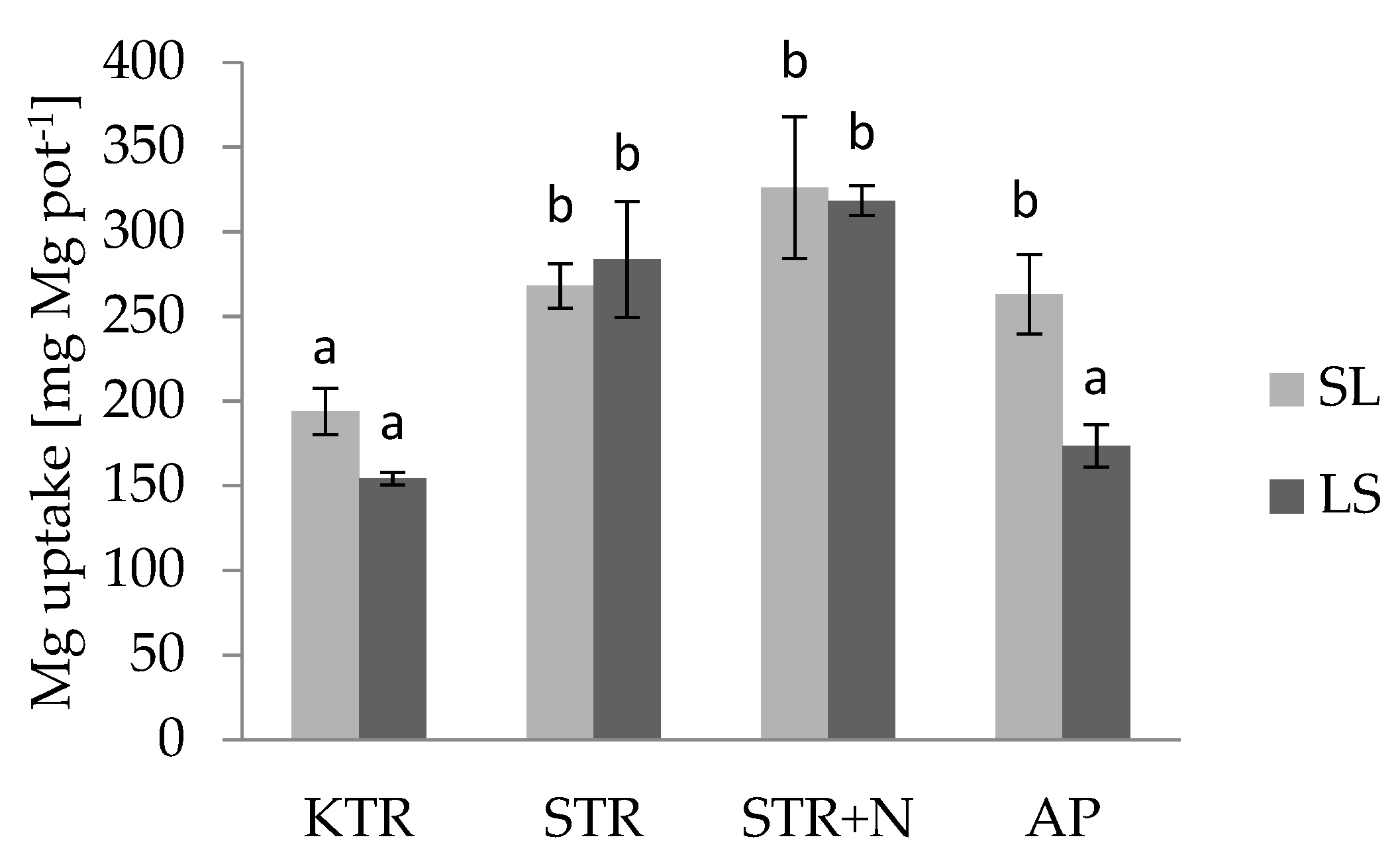
3.3. Nutrient Uptake by Plants
3.4. Phosphorus- and Nitrogen-Use Efficiency
3.5. Effect of Subsequent Struvite Fertilisation on Properties of SL and LS Soil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; He, X.; Deng, Y.; Tsang, D.C.W.; Jiang, R.; Becker, G.C.; Kruse, A. Phosphorus recovered from digestate by hydrothermal processes with struvite crystallization and its potential as a fertilizer. Sci. Total Environ. 2019, 698, 134240. [Google Scholar] [CrossRef] [PubMed]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, P.; Van der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Heffer, P.M.; Prud’homme, M.; Muirheid, B.; Isherwood, K.F. Phosphorus Fertilization Issues and Outlook; International Fertilizer Society: York, UK, 2006; Volume 586, p. 32. [Google Scholar]
- Zalewski, A. The Global Market of Mineral Fertilizers, Taking into Account Changes in the Prices of Direct Energy Carriers and Raw Materials; Instytut Ekonomiki Rolnictwa i Gospodarki Żywnościowej PIB: Warsaw, Poland, 2014; p. 122. [Google Scholar]
- Chrispim, M.C.; Scholz, M.; Nolasco, M.A. Phosphorus recover from municipal wastewater treatment: Critical review of challenges and opportunities for developing countries. J. Environ. Manag. 2019, 248, 109268. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite from farm, municipal and industrial waste: Feedstock suitability, methods and pre-treatments. Waste Manag. 2016, 49, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Iacovidou, E.; Velis, C.A.; Purnell, P.; Zwirner, O.; Brown, A.; Hahladakis, J.; Millward-Hopkins, J.; Williams, P.T. Metrics for optimising the multi-dimensional value of resources recovered from waste in a circular economy: A critical review. J. Clean Prod. 2017, 166, 910–938. [Google Scholar] [CrossRef]
- Velenturf, A.P.M.; Purnell, P. Resource Recovery from Waste: Restoring the Balance between Resource Scarcity and Waste Overload. Sustainability 2017, 9, 1603. [Google Scholar] [CrossRef]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef]
- Bernas, J.; Moudrý, J., Jr.; Jelinkova, Z.U.; Kopecky, M.A. Greenhouse gasses emissions during maize growing for energy purposes. In MendelNet 2014; Mendelu: Brno, Czech Republic, 2014; pp. 219–223. ISBN 978-80-7509-174-1. [Google Scholar]
- Bernas, J.; Moudrý, J., Jr.; Kopecký, M.; Konvalina, P.; Štěrba, Z. Szarvasi-1 and Its Potential to Become a Substitute for Maize Which Is Grown for the Purposes of Biogas Plants in the Czech Republic. Agronomy 2019, 9, 98. [Google Scholar] [CrossRef]
- Szymańska, M.; Nowaczewska, D.; Świerżewska, E.; Wrzosek-Jakubowska, J.; Gworek, B. An attempt to assess physicochemical properties of soil fertilized with fresh and treated digestate from biogas plant. Przem. Chem. 2016, 95, 572–576. [Google Scholar]
- Rehl, T.; Müller, J. Life cycle assessment of biogas digestate processing technologies. Res. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- Szymańska, M.; Sosulski, T.; Szara, E.; Pilarski, K. Conversion and properties of anaerobic digestates from biogas production. Przem. Chem. 2015, 94, 1419–1423. [Google Scholar]
- Tao, W.; Fattah, K.P.; Huchzermeier, M.P. Struvite recovery from anaerobically digested dairy manure: A review of application potential and hindrances. J. Environ. Manag. 2016, 169, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, J.; Dong, R.; Ahring, B.K.; Zhang, W. Properties of plant nutrient: Comparison of two nutrient recovery techniques using liquid fraction of digestate from anaerobic digester treating pig manure. Sci. Total Environ. 2016, 544, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Murphy, J.D. Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Res. Conserv. Recycl. 2016, 107, 142–156. [Google Scholar] [CrossRef]
- Bolzonella, D.; Fatone, F.; Gottardo, M.; Frison, N. Nutrients recovery from anaerobic digestate of agro-waste: Techno-economic assessment of full scale applications. J. Environ. Manag. 2018, 216, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikova, E.; Kruse, A.; Becker, G.C. Feedstock-Dependent Phosphate Recovery in a Pilot-Scale Hydrothermal Liquefaction Bio-Crude Production. Energies 2020, 13, 379. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Huang, H.M.; Munir, T.; Wang, G.Q.; Young, B.R. Phosphorus recovery through struvite crystallization: Challenges for future design. Sci. Total Environ. 2019, 648, 1244–1256. [Google Scholar] [CrossRef]
- Degryse, F.; Baird, R.; da Silva, R.C.; McLaughlin, M.J. Dissolution rate and agronomic effectiveness of struvite fertilizers – effect of soil pH, granulation and base excess. Plant Soil. 2017, 410, 139–152. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Sylvester-Bradley, R.; Jones, D.L.; Healey, J.R.; Talboys, P.J. Feed the crop not the soil: Rethinking phosphorus management in the food chain. Environ. Sci. Technol. 2014, 48, 6523–6530. [Google Scholar] [CrossRef]
- Bonvin, C.; Etter, B.; Udert, K.M.; Frossard, E.; Nanzer, S.; Tamburini, F.; Oberson, A. Plant uptake of phosphorus and nitrogen recycled from synthetic source-separated urine. Ambio 2015, 44 (Suppl. 2), 217–227. [Google Scholar] [CrossRef] [PubMed]
- Kahiluoto, H.; Kuisma, M.; Ketoja, E.; Salo, T.; Heikkinen, J. Phosphorus in manure and sewage sludge more recyclable than in soluble inorganic fertilizer. Environ. Sci. Technol. 2015, 49, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Steingrobe, B.; Römer, W.; Claassen, N. Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr. Cycl. Agroecosyst. 2011, 91, 173–184. [Google Scholar] [CrossRef]
- Ehmann, A.; Bach, I.M.; Laopeamthong, S.; Bilbao, J.; Lewandowski, I. Can phosphate salts recovered from manure replace conventional phosphate fertilizer? Agric 2017, 7, 1. [Google Scholar] [CrossRef]
- Uysal, A.; Yılmazel, Y.D.; Demirer, G.N. The determination of fertilizer quality of the formed struvite from effluent of a sewage sludge anaerobic digester. J. Hazard. Mater. 2010, 181, 248–254. [Google Scholar] [CrossRef]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.J.; Withers, P.J.A. Struvite: A slow-release fertilizer for sustainable phosphorus management? Plant Soil. 2016, 401, 109–123. [Google Scholar] [CrossRef]
- Everaert, M.; Da, R.S.; Degryse, F.; Mclaughlin, M.J.; Smolders, E. Limited dissolved phosphorus runoff losses from layered double hydroxide and struvite fertilizers in a rainfall simulation study. J. Environ. Qual. 2018, 47, 371–377. [Google Scholar] [CrossRef]
- Plaza, C.; Sanz, R.; Clemente, C.; Fernandez, J.M.; Gonzalez, R.; Polo, A.; Colmenarejo, M.F. Greenhouse evaluation of struvite and sludges from municipal wastewater treatment works as phosphorus sources for plants. J. Agric. Food Chem. 2007, 55, 8206–8212. [Google Scholar] [CrossRef]
- Liu, Y.H.; Rahman, M.M.; Kwag, J.H.; Kim, J.H.; Ra, C.S. Eco-friendly production of maize using struvite recovered from swine wastewater as a sustainable fertilizer source. Asian Aust. J. Anim. Sci. 2011, 24, 1699–1705. [Google Scholar] [CrossRef]
- Antonini, S.; Arias, M.A.; Eichert, T.; Clemens, J. Greenhouse evaluation and environmental impact assessment of different urine-derived struvite fertilizers as phosphorus sources for plants. Chemosphere 2012, 89, 1202–1210. [Google Scholar] [CrossRef]
- Gonzalez-Ponce, R.; Lopez-de-Sa, E.G.; Plaza, C. Lettuce response to phosphorus fertilization with struvite recovered from municipal wastewater. HortScience 2009, 44, 426–430. [Google Scholar] [CrossRef]
- Szymańska, M.; Szara, E.; Wąs, A.; Sosulski, T.; van Pruissen, G.W.; Cornelissen, R.L. Struvite—An Innovative Fertilizer from Anaerobic Digestate Produced in a Bio-Refinery. Energies 2019, 12, 296. [Google Scholar] [CrossRef]
- Ackerman, J.N.; Zvomuya, F.; Cicek, N.; Flaten, D. Evaluation of manure-derived struvite as a phosphorus source for canola. Can. J. Plant Sci. 2013, 93, 419–424. [Google Scholar] [CrossRef]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]
- Szymańska, M.; Szara, E.; Sosulski, T.; Wąs, A.; Van Pruissen, G.W.P.; Cornelissen, R.L.; Borowik, M.; Konkol, M. A Bio-Refinery Concept for N and P Recovery—A Chance for Biogas Plant Development. Energies 2019, 12, 155. [Google Scholar] [CrossRef]
- Reza, A.; Shim, S.; Kim, S.; Ahmed, N.; Won, S.; Ra, C. Nutrient Leaching Loss of Pre-Treated Struvite and Its Application in Sudan Grass Cultivation as an Eco-Friendly and Sustainable Fertilizer Source. Sustainability 2019, 11, 4204. [Google Scholar] [CrossRef]
- Johnston, A.E.; Richards, I.R. Effectiveness of different precipitated phosphates as phosphorus sources for plants. Soil Use Manag. 2003, 19, 45–49. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Turkdogan, F.I.; Gunay, A.; Yilmaz, T.; Kaleli, M. Medicinal plants grown in soil amended with struvite recovered from anaerobically pretreated poultry manure wastewater. J. Anim. Plant Sci. 2013, 23, 261–270. [Google Scholar]
- Uysal, A.; Kuru, B. Magnesium ammonium phosphate production from wastewater through Box–Behnken design and its effect on nutrient element uptake in plants. Clean Soil Air Water 2013, 41, 447–454. [Google Scholar] [CrossRef]
- Uysal, A.; Demir, S.; Sayilgan, E.; Eraslan, F.; Kucukyumuk, Z. Optimization of struvite fertilizer formation from baker’s yeast wastewater: Growth and nutrition of maize and tomato plants. Environ. Sci. Pollut. Res. 2014, 21, 3264–3274. [Google Scholar] [CrossRef]
- Prabhu, M.; Mutnuri, S. Cow urine as a potential source for struvite production. Int. J. Recycl. Org. Waste Agric. 2014, 3, 1–12. [Google Scholar] [CrossRef]
- Gell, K.; Ruijter, F.J.; Kuntke, P.; Graaff, M.; Smit, A.L. Safety and effectiveness of struvite from black water and urine as a phosphorus fertilizer. J. Agric. Sci. 2011, 3, 67–80. [Google Scholar] [CrossRef]
- Kern, B.; Heinzmann, B.; Markus, B.; Kaufmann, A.C.; Soethe, N.; Engels, C. Recycling and assessment of struvite phosphorus from sewage sludge. Agric. Eng. Int. 2018, 10, 1–13. [Google Scholar]
- Ryu, H.D.; Lim, C.S.; Kang, M.K.; Lee, S. Evaluation of struvite obtained from semiconductor wastewater as a fertilizer in cultivating Chinese cabbage. J. Hazard. Mater. 2012, 221, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Diwani, G.E.; Rafie, S.E.; Ibiari, N.N.; El-Aila, H.I. Recovery of ammonia nitrogen from industrial wastewater treatment as struvite slow releasing fertilizer. Desalination 2007, 214, 200–214. [Google Scholar] [CrossRef]
- Rahman, M.d.D.; Liu, Y.; Kwag, J.-H.; Ra, C. Recovery of struvite from animal wastewater and its nutrient leaching loss in soil. J. Hazard. Mater. 2011, 186, 2026–2030. [Google Scholar] [CrossRef]
- Latifian, M.; Liu, J.; Mattiasson, B. Struvite-based fertilizer and its physical and chemical properties. Environ. Technol. 2012, 33, 2691–2697. [Google Scholar] [CrossRef]
- Kalkhoran, S.S.; Pannell, D.J.; Thamo, T.; White, B.; Polyakov, M. Soil acidity, lime application, nitrogen fertility, and greenhouse gas emissions: Optimizing their joint economic management. Agric. Syst. 2019, 176, 102684. [Google Scholar] [CrossRef]
- Mercik, S. Agricultural Chemistry—Theoretical and Practical Basics; SGGW: Warsaw, Poland, 2002; p. 287. [Google Scholar]
- Johnston, A.E.; Poulton, P.R. Phosphorus in Agriculture: A Review of Results from 175 Years of Research at Rothamsted, UK. J. Environ. Q. 2019. [Google Scholar] [CrossRef]
- Rech, I.; Withers, P.J.A.; Jones, D.L.; Pavinato, P.S. Solubility, Diffusion and Crop Uptake of Phosphorus in Three Different Struvites. Sustainability 2019, 11, 134. [Google Scholar] [CrossRef]
- Vogel, T.; Kruse, J.; Siebers, N.; Nelles, M.; Eichler-Lobermann, B. Recycled products from municipal wastewater: Composition and effects on phosphorus mobility in a sandy soil. J. Environ. Q. 2017, 46, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, M.; Szara, E.; Wąs, A.; Korc, M.; Borowik, M.; Zdunek, A.; Rusek, P.; Schab, S. Agronomic value of powder and granulated struvite. Przem. Chem. 2018, 97, 277–281. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Treatment | P (g) | N (g) | Mg (g) |
|---|---|---|---|
| Struvite (STR) | 2.0 | 1.17 | 1.6 |
| Struvite with ammonium sulphate (STR + N) | 2.0 | 1.8 (1.17 as STR and 0.63 as AS) | 1.6 |
| Commercial ammonium phosphate fertiliser (AP) | 2.0 | 1.8 | 0 |
| Control treatment (KTR) | 0 | 0 | 0 |
| Fertilisation | N | P | Mg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Harvest | 2nd Harvest | 3rd Harvest | 4th Harvest | 1st Harvest | 2nd Harvest | 3rd Harvest | 4th Harvest | 1st Harvest | 2nd Harvest | 3rd Harvest | 4th Harvest | |
| SL soil | ||||||||||||
| KTR | 23.5 c | 34.1 ns | 26.5 b | 21.7 b | 4.7 c | 3.0 ns | 2.8 ns | 2.5 a | 4.1 b | 3.4 a | 3.6 ns | 4.6 ns |
| STR | 17.4 b | 27.8 ns | 22.0 a | 19.6 ab | 3.3 ab | 2.9 ns | 3.5 ns | 3.6 b | 3.7 ab | 4.3 ab | 3.6 ns | 4.9 ns |
| STR + N | 14.0 ab | 31.3 ns | 22.0 a | 17.5 a | 2.9 a | 3.3 ns | 2.9 ns | 3.7 b | 3.6 a | 5.1 b | 4.3 ns | 5.0 ns |
| AP | 13.6 a | 31.7 ns | 22.9 ab | 21.5 b | 4.2 bc | 3.3 ns | 2.8 ns | 2.3 a | 3.3 a | 4.4 ab | 3.8 ns | 5.0 ns |
| LS soil | ||||||||||||
| KTR | 15.7 bc | 29.8 ns | 24.4 a | 14.9 a | 5.3 ns | 2.5 a | 3.6 b | 3.2 a | 3.6 ab | 3.2 a | 2.9 a | 4.2 a |
| STR | 16.1 c | 33.7 ns | 27.7 b | 19.8 b | 6.4 ns | 4.3 b | 1.8 a | 4.5 ab | 5.1 b | 5.8 b | 4.9 b | 5.4 b |
| STR + N | 11.8 a | 34.7 ns | 22.7 ab | 17.1 ab | 6.2 ns | 3.7 ab | 3.6 b | 7.2 c | 8.4 c | 5.3 b | 6.4 c | 5.9 b |
| AP | 13.5 ab | 31.0 ns | 25.3 ab | 17.4 ab | 5.1 ns | 4.6 b | 4.6 c | 5.3 b | 3.1 a | 4.1 ab | 3.4 a | 4.3 a |
| Fertiliser | SL | LS | ||
|---|---|---|---|---|
| REFN | REFP | REFN | REFP | |
| STR | 39.7 a | 154.5 a | 526.8 a | 138.0 a |
| STR + N | 214.3 b | 207.8 a | 681.2 a | 166.2 a |
| AP | 100.0 | 100.0 | 100.0 | 100.0 |
| Treatment | pH | Ntot | PCaCl2 | PM3 | MgCaCl2 | MgM3 |
|---|---|---|---|---|---|---|
| SL soil | ||||||
| KTR | 6.3 a | 2.3 ns | 1.44 a | 16.54 a | 142.51 a | 314.71 a |
| STR | 6.5 a | 2.5 ns | 6.37 c | 192.78 c | 244.13 b | 594.09 b |
| STR + N | 6.4 a | 2.4 ns | 4.40 b | 214.28 c | 341.40 c | 621.83 b |
| AP | 5.8 b | 2.6 ns | 2.09 a | 79.40 b | 144.33 a | 344.83 a |
| LS soil | ||||||
| KTR | 5.5 a | 0.84 ns | 0.63 a | 62.63 a | 22.61 a | 41.25 a |
| STR | 6.7 a | 0.69 ns | 38.87 d | 320.07 d | 175.85 c | 366.18 c |
| STR + N | 5.7 a | 0.67 ns | 25.15 c | 234.38 c | 111.07 b | 186.86 b |
| AP | 5.1 b | 0.70 ns | 7.19 b | 207.42 b | 24.76 a | 51.89 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, M.; Sosulski, T.; Bożętka, A.; Dawidowicz, U.; Wąs, A.; Szara, E.; Malak-Rawlikowska, A.; Sulewski, P.; van Pruissen, G.W.P.; Cornelissen, R.L. Evaluating the Struvite Recovered from Anaerobic Digestate in a Farm Bio-Refinery as a Slow-Release Fertiliser. Energies 2020, 13, 5342. https://doi.org/10.3390/en13205342
Szymańska M, Sosulski T, Bożętka A, Dawidowicz U, Wąs A, Szara E, Malak-Rawlikowska A, Sulewski P, van Pruissen GWP, Cornelissen RL. Evaluating the Struvite Recovered from Anaerobic Digestate in a Farm Bio-Refinery as a Slow-Release Fertiliser. Energies. 2020; 13(20):5342. https://doi.org/10.3390/en13205342
Chicago/Turabian StyleSzymańska, Magdalena, Tomasz Sosulski, Adriana Bożętka, Urszula Dawidowicz, Adam Wąs, Ewa Szara, Agata Malak-Rawlikowska, Piotr Sulewski, Gijs W. P. van Pruissen, and René L. Cornelissen. 2020. "Evaluating the Struvite Recovered from Anaerobic Digestate in a Farm Bio-Refinery as a Slow-Release Fertiliser" Energies 13, no. 20: 5342. https://doi.org/10.3390/en13205342
APA StyleSzymańska, M., Sosulski, T., Bożętka, A., Dawidowicz, U., Wąs, A., Szara, E., Malak-Rawlikowska, A., Sulewski, P., van Pruissen, G. W. P., & Cornelissen, R. L. (2020). Evaluating the Struvite Recovered from Anaerobic Digestate in a Farm Bio-Refinery as a Slow-Release Fertiliser. Energies, 13(20), 5342. https://doi.org/10.3390/en13205342







