Experimental Investigation of Spontaneous Imbibition of Water into Hydrate Sediments Using Nuclear Magnetic Resonance Method
Abstract
1. Introduction
2. Experimental Equipment and Samples
2.1. Experimental Samples
2.2. Experimental Equipment and Procedures
- Cut six samples into rectangular cuboids of certain shape. Then, it is dried in a drying oven at 75 °C for 24 h until the mass no longer changed. After the samples are taken out, the plastic wrap is kept in a cool place to cool to room temperature.
- Measure the sample size, place the samples in the glass tube, and determine the mass of samples and glass tube.
- Drop the water to the sample surface using a long dropper. When the water droplets are completely imbibed into the sample, the mass and T2 values of samples are measured.
- Repeat step (3) to get the imbibition curves and T2 spectra.The schematic diagram of experimental procedures is presented in Figure 2.
3. Experimental Results
3.1. Observations during Water Imbibition
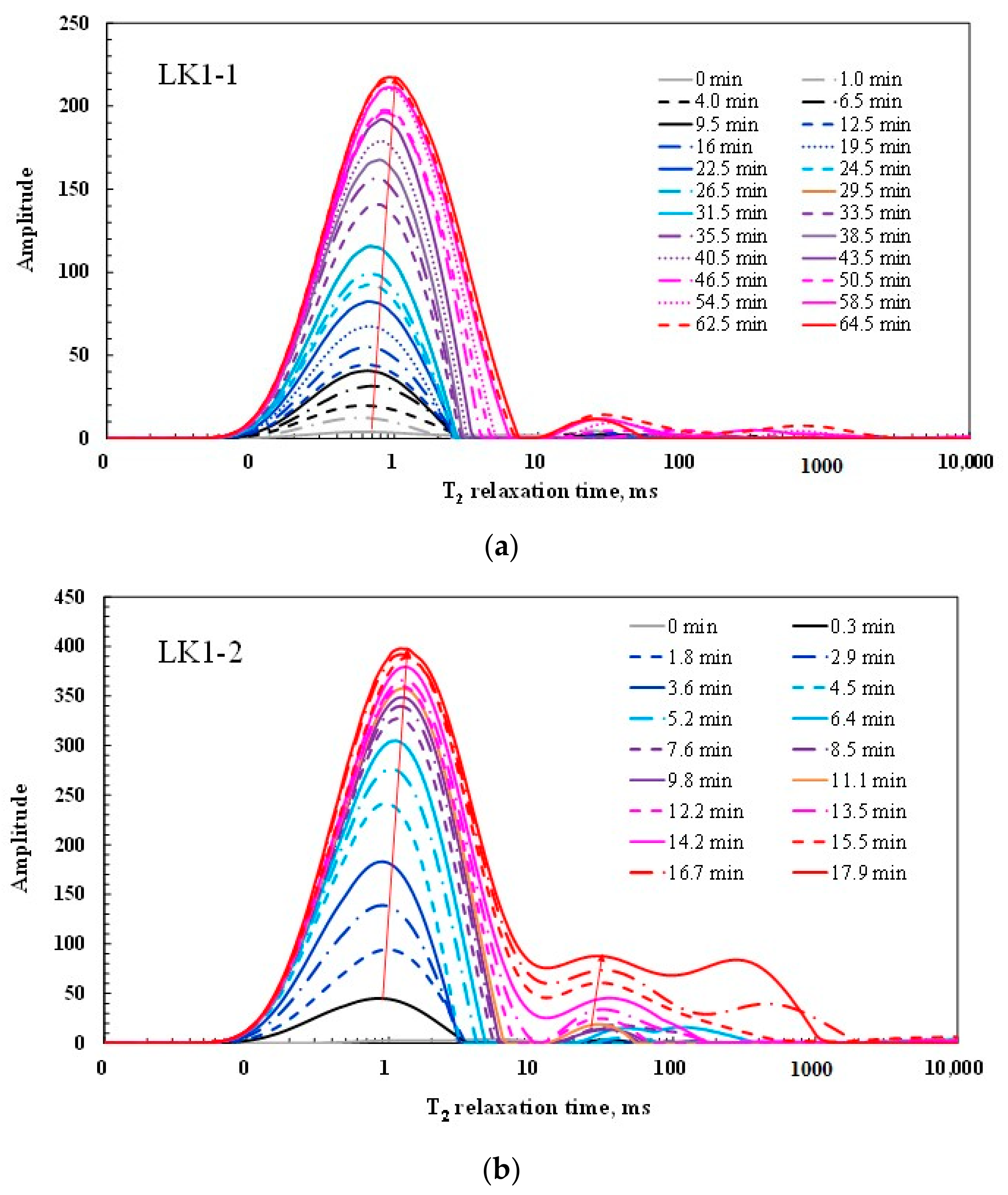
3.2. T2 Spectra Characteristics of Water Imbibition
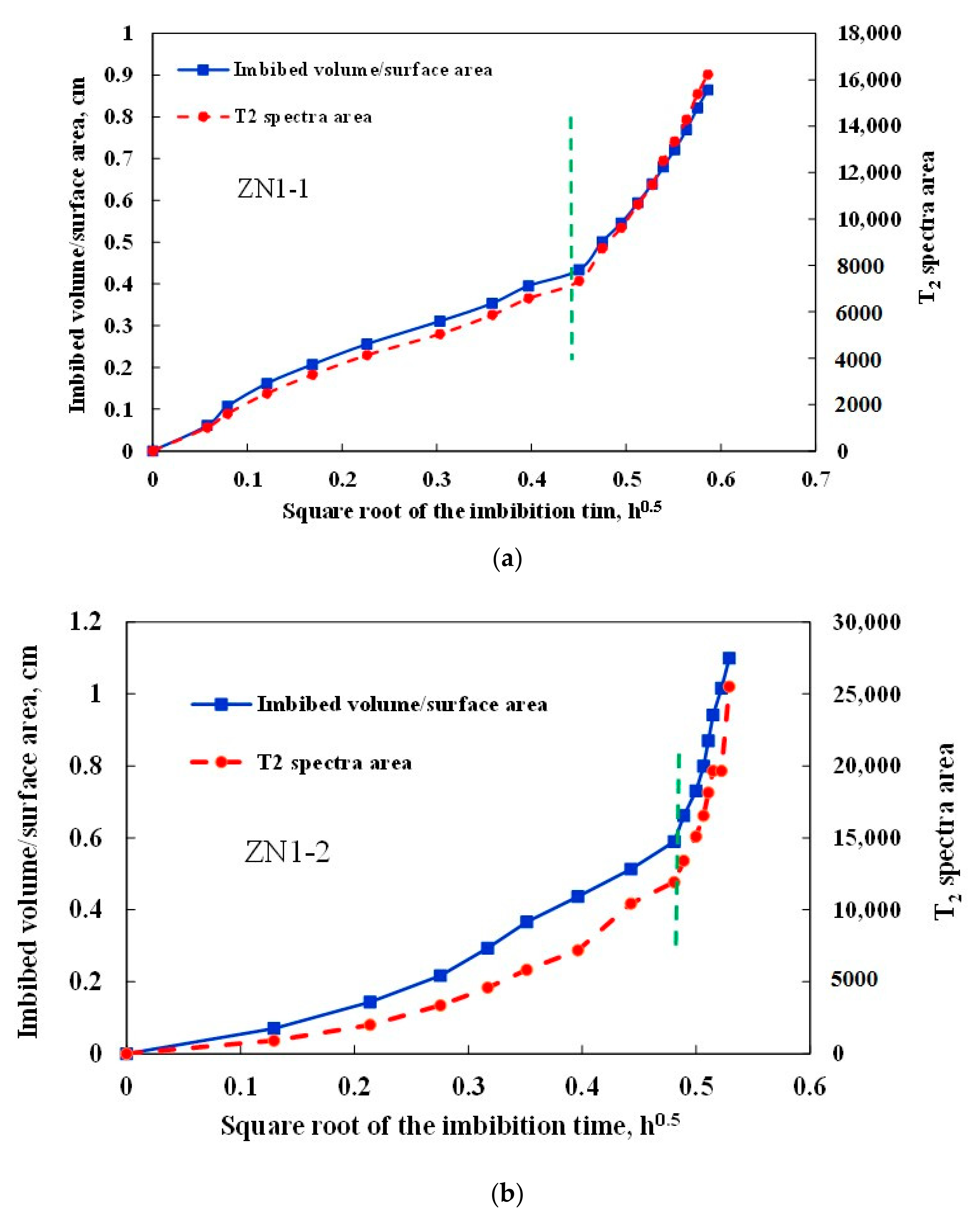
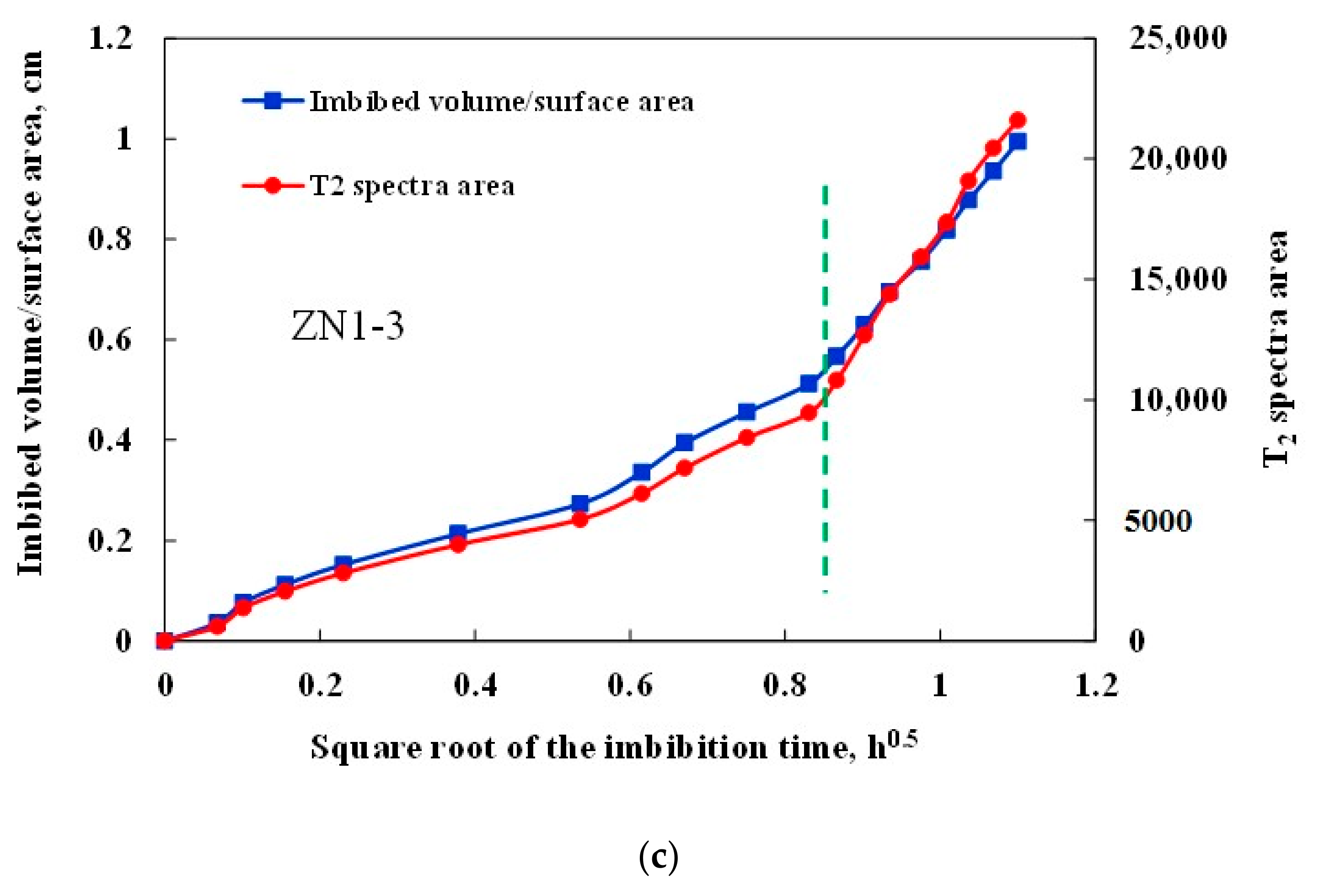
3.3. Imbibition Curve Characteristics
4. Discussion
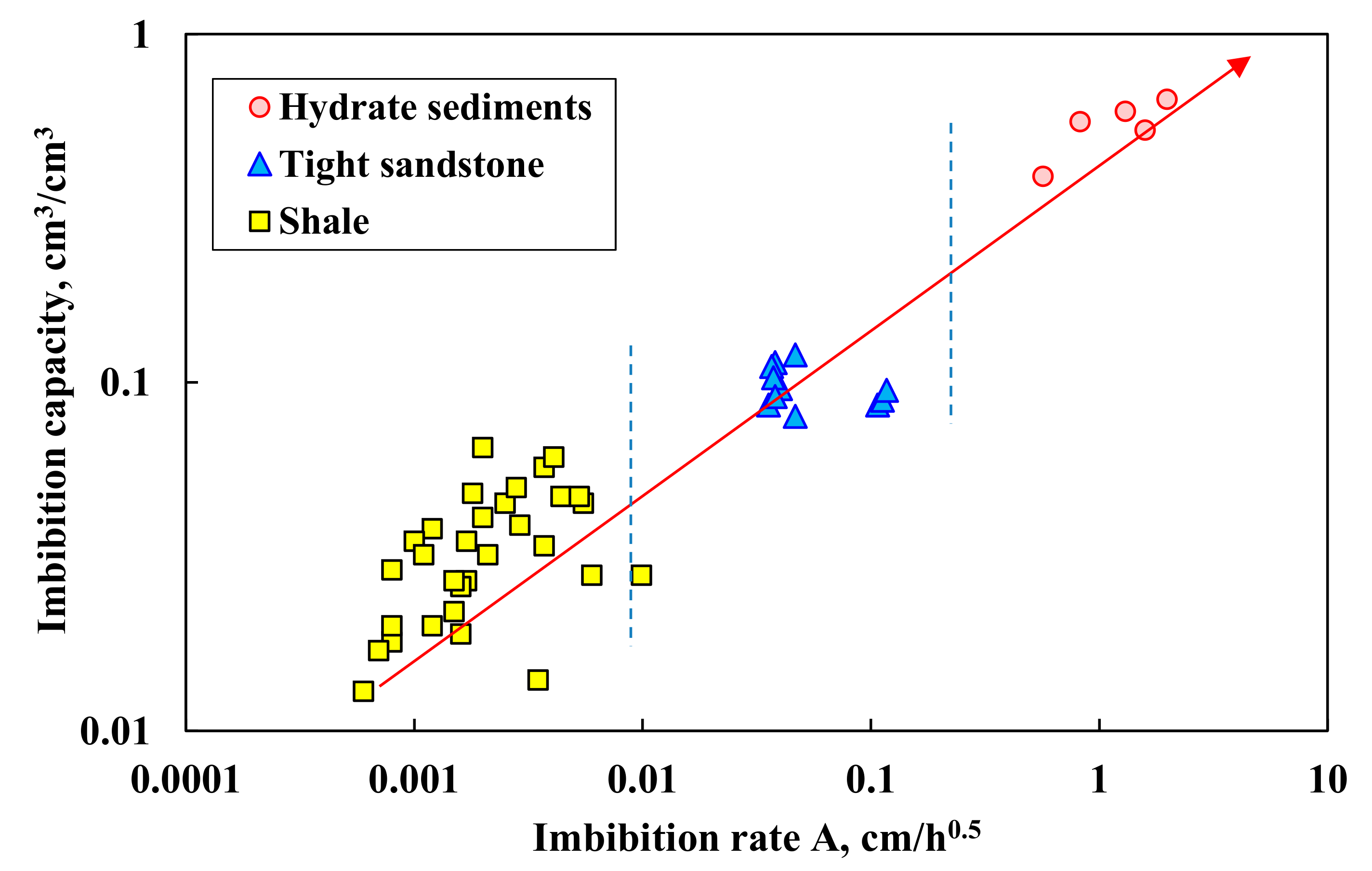
4.1. Imbibition Capacity Comparison of Different Hydrate Formations
4.2. Imbibition Capacity Comparison of Hydrate Sediments and Other Formations
5. Conclusions
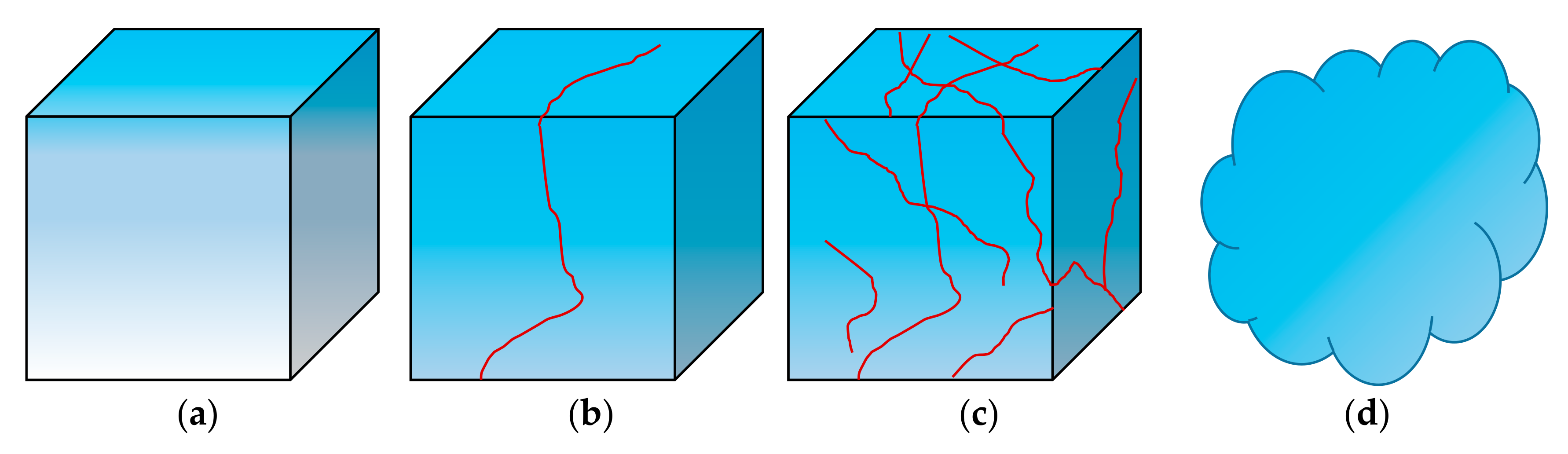
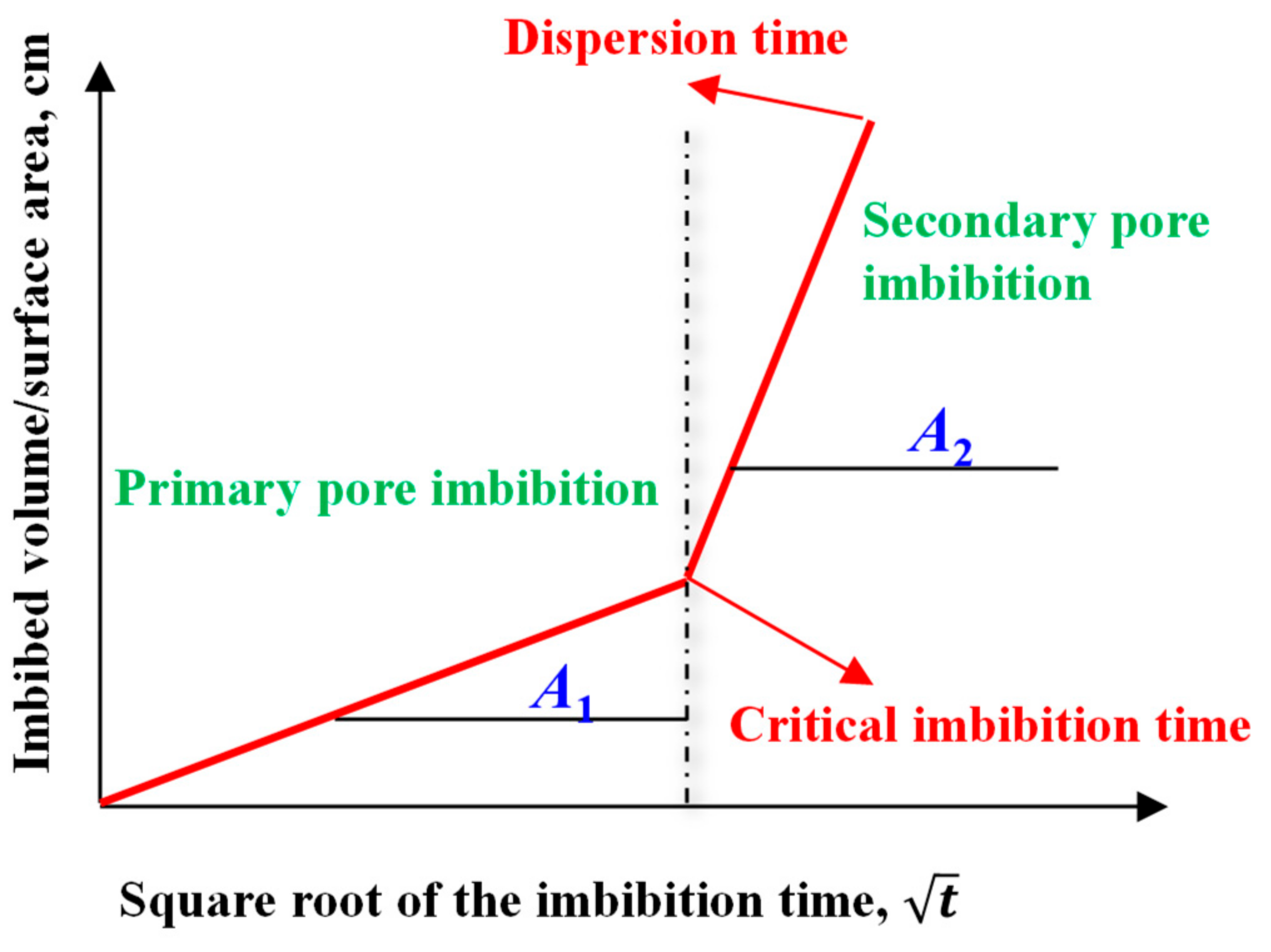
- As the imbibition time increases, the water is gradually imbibed into matrix pores. Water imbibition can cause dramatic changes in pore structure, such as microfractures initiation, fracture network generation, and dispersion.
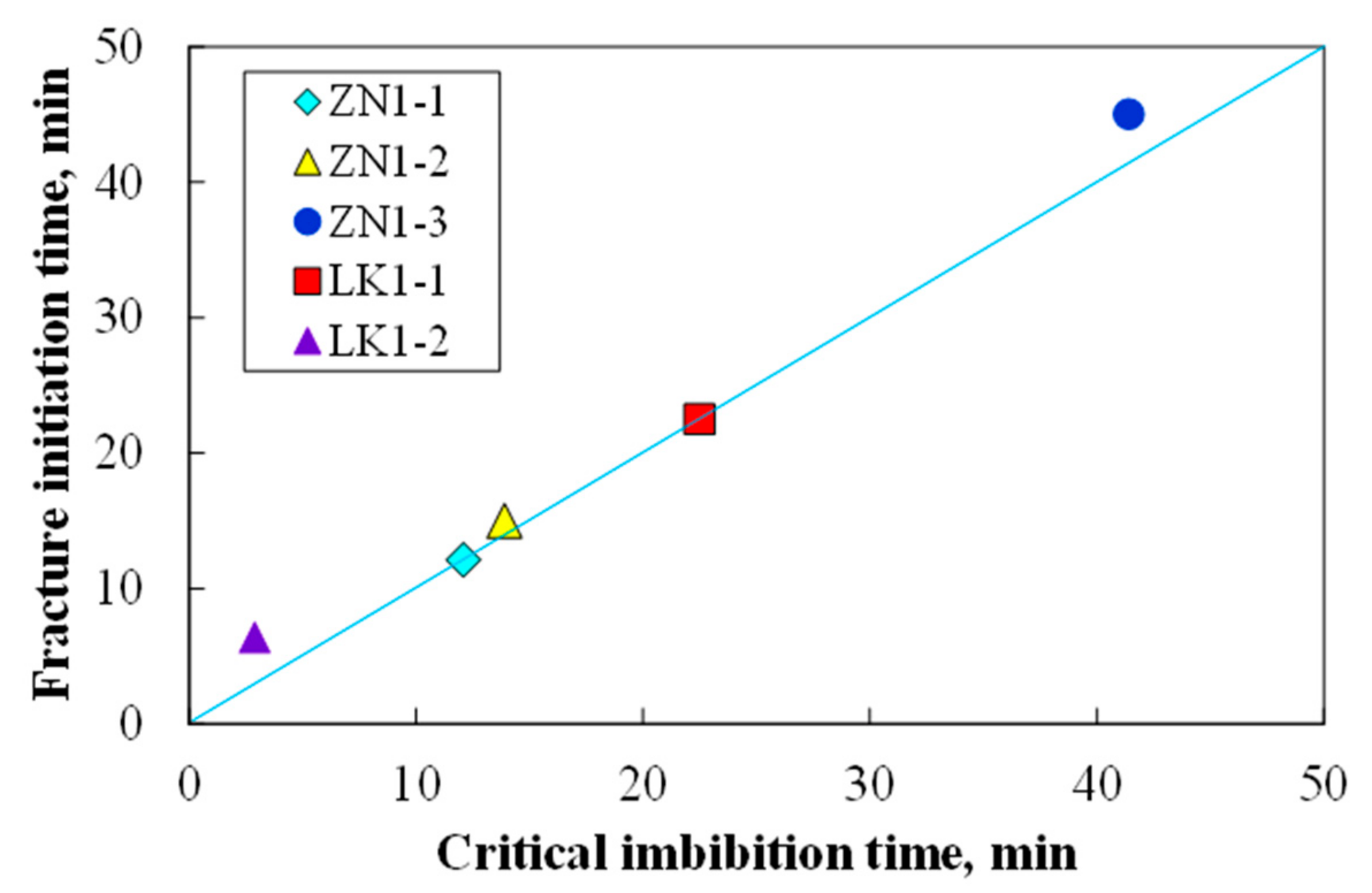
- When the imbibition time exceeds the critical imbibition time, a large number of secondary pores (new large pores and micro-fractures) start to appear. When the imbibition time exceeds the dispersion time, the fracture networks are generated, eventually leading to dispersion of the sediment samples.
- The imbibition curves of hydrate sediments can be divided into two linear stages, which corresponds to water imbibition of primary pores and secondary pores, respectively. The imbibition rate of secondary pores is significantly larger than that of primary pores, indicating that the generation of new fractures can greatly accelerate the imbibition rate.
Author Contributions
Funding
Conflicts of Interest
References
- Mu, L.; von Solms, N. Hydrate thermal dissociation behavior and dissociation enthalpies in methane-carbon dioxide swapping process. J. Chem. Thermodyn. 2018, 117, 33–42. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; von Solms, N. Insights into kinetics of methane hydrate formation in the presence of surfactants. Processes 2019, 7, 598. [Google Scholar] [CrossRef]
- Omelchenko, R.Y.; Makogon, Y.F. Commercial gas production from Messoyakha deposit in hydrate conditions. J. Nat. Gas Sci. Eng. 2013, 11, 1–6. [Google Scholar]
- Englezos, P. Extraction of methane hydrate energy by carbon dioxide injection-key challenges and a paradigm shift. Chin. J. Chem. 2019, 27, 2044–2048. [Google Scholar] [CrossRef]
- Linga, P.; Clarke, M. A review of reactor designs and materials employed for increasing the rate of gas hydrate formation. Energy Fuel 2017, 31, 1–13. [Google Scholar] [CrossRef]
- Liang, S.; Liang, D.; Wu, N.; Yi, L.; Hu, G. Transient translational and rotational water defects in gas hydrates. J. Phys. Chem. C 2017, 121, 17595–17602. [Google Scholar] [CrossRef]
- Safronov, A.F.; Shits, E.Y.; Grigor’ev, M.N.; Semenov, M.E. Formation of gas hydrate deposits in the Siberian Arctic shelf. Russ. Geol. Geophys. 2009, 51, 83–87. [Google Scholar] [CrossRef]
- Dearman, J.L. Gas Hydrate Formation in Gulf of Mexico Sediments. Master’s Thesis, Mississippi State University, Starkville, MS, USA, 9 April 2007. [Google Scholar]
- Radich, J.; Rogers, R.E.; French, W.T.; Zhang, G. Biochemical reaction and diffusion in seafloor gas hydrate capillaries: Implications for gas hydrate stability. Chem. Eng. Sci. 2009, 64, 4278–4285. [Google Scholar] [CrossRef]
- Sun, Q.; Du, M.; Li, X.; Guo, X.; Liu, A.; Chen, G.; Yang, L. Study on ethane hydrate formation/dissociation in a sub-millimeter sized capillary. Chem. Eng. Sci. 2019, 206, 1–9. [Google Scholar] [CrossRef]
- Sujith, K.S.; Ramachandran, C.N. Effect of surface roughness on adsorption and distribution of methane at the water-methane interface. J. Mol. Liq. 2018, 266, 856–863. [Google Scholar] [CrossRef]
- Kang, D.H.; Yun, T.S.; Kim, K.Y. Effect of hydrate nucleation mechanisms and capillarity on permeability reduction in granular media. Geophys. Res. Lett. 2016, 43, 9018–9025. [Google Scholar] [CrossRef]
- Crutchley, G.J.; Fraser, D.R.A.; Pecher, I.A.; Gorman, A.R.; Maslen, G.; Henrys, S.A. Gas migration into gas hydrate-bearing sediments on the southern Hikurangi margin of New Zealand. J. Geophys. Res. 2015, 120, 725–743. [Google Scholar] [CrossRef]
- Ge, H.; Yang, L.; Shen, Y.; Ren, K.; Meng, F.; Ji, W.; Wu, S. Experimental investigation of shale imbibition capacity and the factors influencing loss of hydraulic fracturing fluids. Pet. Sci. 2018, 12, 636–650. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Lan, Q.; Saeed, Y.; Fei, H.; Qi, Z. Spontaneous imbibition of brine and oil in gas shales: Effect of water adsorption and resulting micro fractures. Energy Fuel 2013, 27, 3039–3049. [Google Scholar] [CrossRef]
- Yang, L.; Ge, H.; Shi, X.; Cheng, Y.; Zhang, K.; Chen, H.; Shen, Y. The effect of microstructure and rock mineralogy on water imbibition characteristics in tight reservoirs. J. Nat. Gas Sci. Eng. 2016, 33, 1461–1471. [Google Scholar] [CrossRef]
- Shagapov, V.; Tazetdinov, B.I. Formation and dissociation of gas hydrate inclusions during migration in water. Thermophys. Aeromech. 2014, 21, 337–345. [Google Scholar] [CrossRef]
- Ge, X.; Liu, J.; Fan, Y.; Xing, D.; Deng, S.; Cai, J. Laboratory investigation into the formation and dissociation process of gas hydrate by low-field NMR technique. J. Geophys. Res. 2018, 123, 3339–3346. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, C.; Wu, M.; Peng, Y.; Yao, Y.; Luo, W. Review of exploration and production technology of natural gas hydrate. Adv. Geo-Energy Res. 2018, 2, 53–62. [Google Scholar] [CrossRef]
- Liu, L.; Dai, S.; Ning, F.; Cai, J.; Liu, C.; Wu, N. Fractal characteristics of unsaturated sands−implications to relative permeability in hydrate-bearing sediments. J. Nat. Gas Sci. Eng. 2019, 66, 11–17. [Google Scholar] [CrossRef]
- Handy, L.L. Determination of effective capillary pressures for porous media from imbibition data. Petroleum Trans. AIME 1960, 219, 75–80. [Google Scholar]
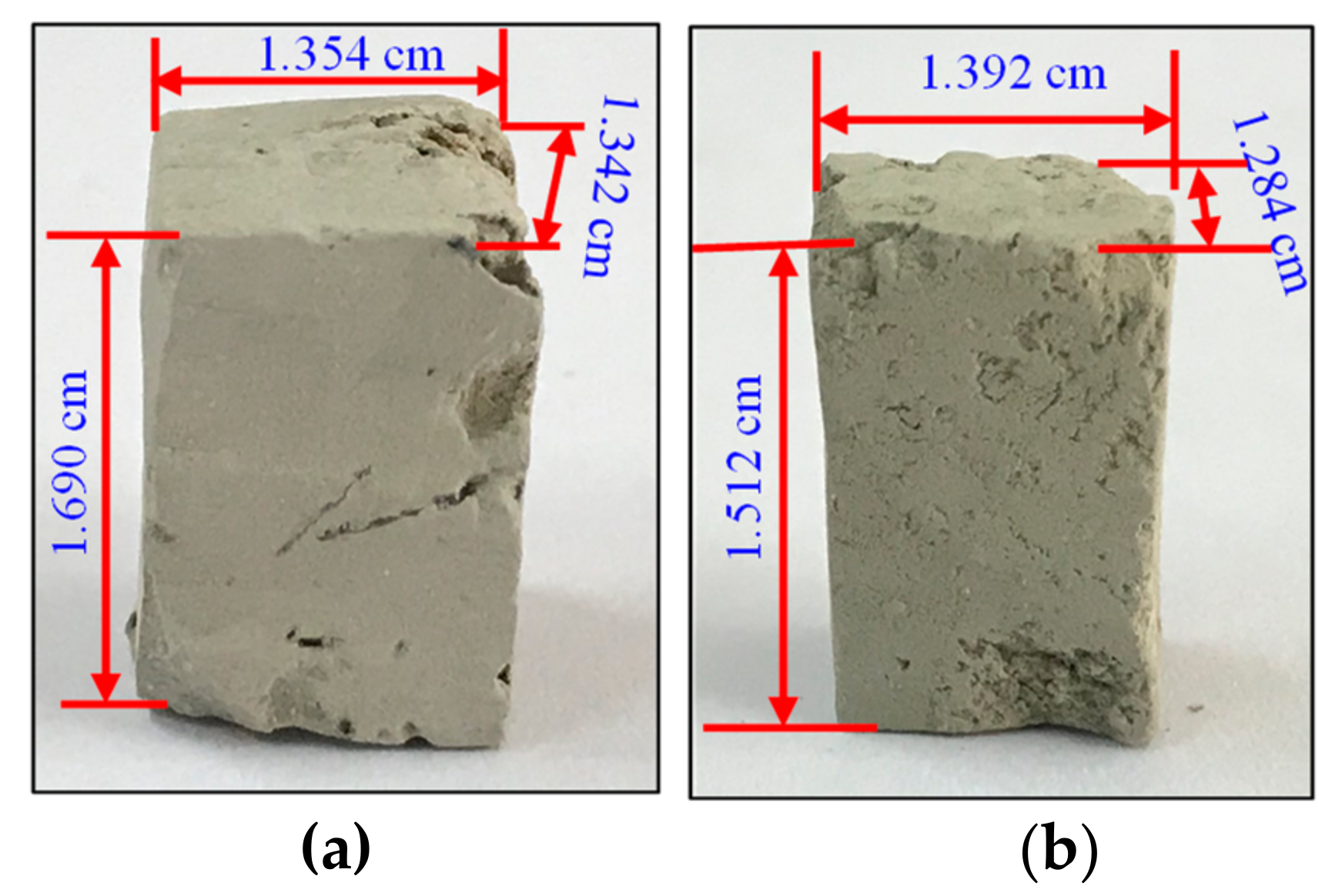



| Samples | Porosity, % | Permeability, mD | Length, cm | Width, cm | Height, cm | Sectional Area, cm2 | Dry Mass, g |
|---|---|---|---|---|---|---|---|
| ZN1-1 | 32.5 | 7.8 | 1.472 | 1.183 | 1.446 | 1.741 | 3.5483 |
| ZN1-2 | 21.5 | 1.0 | 1.354 | 1.342 | 1.690 | 1.817 | 4.5585 |
| ZN1-3 | 31.4 | 6.6 | 1.647 | 1.335 | 1.780 | 2.199 | 5.9983 |
| LK1-1 | 35.4 | 5.1 | 1.356 | 1.341 | 1.137 | 1.818 | 2.9334 |
| LK1-2 | 29.5 | 0.3 | 1.392 | 1.284 | 1.512 | 1.787 | 3.4324 |
| Label | Total Mineral Composition, wt.% | Relative Clay Abundance, wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
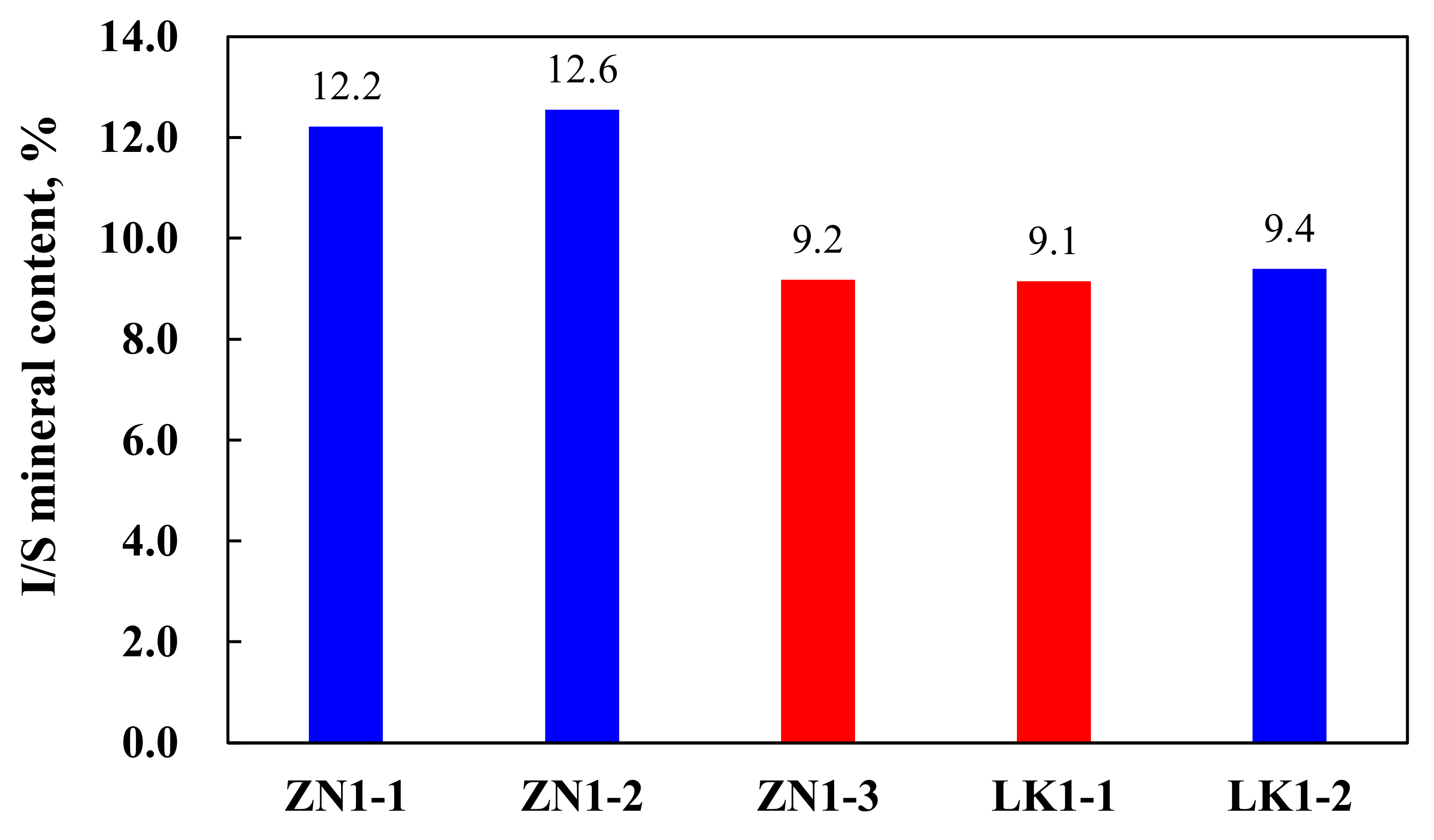
| Quartz | Feldspar | Calcite | Dolomite | Pyrite | Clay | Illite | I/S a | Chlorite | Kaolinite | |
| ZN1-1 | 36.7 | 8.7 | 13.7 | 3.5 | 7.6 | 29.8 | 36 | 41 | 14 | 9 |
| ZN1-2 | 36.0 | 5.9 | 23.0 | 3.6 | 2.3 | 29.2 | 32 | 43 | 16 | 9 |
| ZN1-3 | 45.7 | 10.4 | 12.6 | 3.0 | 0.5 | 27.8 | 38 | 33 | 19 | 10 |
| LK1-1 | 47.9 | 8.7 | 10.8 | 4.6 | 1.1 | 26.9 | 37 | 34 | 18 | 11 |
| LK1-2 | 44.3 | 7.8 | 12.4 | 4.1 | 1.1 | 30.3 | 38 | 31 | 18 | 13 |
| Sample | Wetting | Microfracture Initiation | Fracture Network | Dispersion |
|---|---|---|---|---|
| ZN1-2 |  | 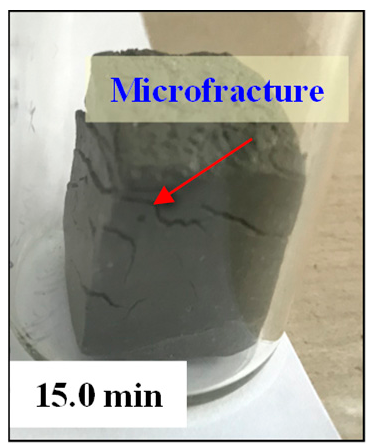 | 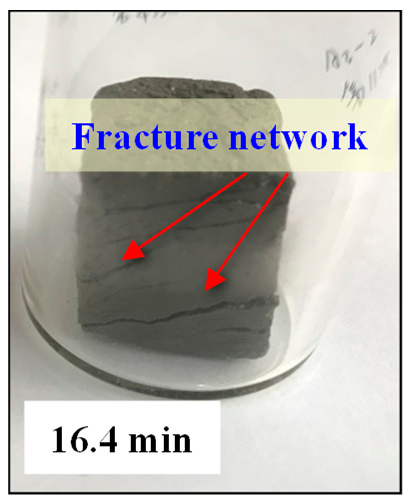 |  |
| LK1-2 |  |  |  |  |
| Sample | Critical Imbibition Time, min | Fracture Initiation Time, min | Dispersion Time, min |
|---|---|---|---|
| ZN1-1 | 12.1 | 12.1 | 21.2 |
| ZN1-2 | 13.9 | 15.0 | 17.7 |
| ZN1-3 | 41.4 | 45.0 | 80.9 |
| LK1-1 | 22.5 | 22.5 | 64.5 |
| LK1-2 | 2.9 | 6.4 | 17.9 |
| No. | Sample Volume, cm3 | Water Imbibition Volume, cm3 | Imbibition Capacity, cm3/cm3 | Imbibition Rate, cm/h0.5 | Average Imbibition Rate, cm/h0.5 | |
|---|---|---|---|---|---|---|
| A1 | A2 | A | ||||
| ZN1-1 | 2.518 | 1.505 | 0.60 | 1.0192 | 3.1443 | 2.0818 |
| ZN1-2 | 3.071 | 1.997 | 0.65 | 1.2698 | 10.699 | 5.9844 |
| ZN1-3 | 3.914 | 2.186 | 0.56 | 0.5610 | 1.6339 | 1.0975 |
| LK1-1 | 2.068 | 0.797 | 0.39 | 0.2360 | 0.5673 | 0.4017 |
| LK1-2 | 2.702 | 1.435 | 0.53 | 0.9230 | 1.7002 | 1.3116 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, C.; Cai, J.; Lu, H. Experimental Investigation of Spontaneous Imbibition of Water into Hydrate Sediments Using Nuclear Magnetic Resonance Method. Energies 2020, 13, 445. https://doi.org/10.3390/en13020445
Yang L, Zhang C, Cai J, Lu H. Experimental Investigation of Spontaneous Imbibition of Water into Hydrate Sediments Using Nuclear Magnetic Resonance Method. Energies. 2020; 13(2):445. https://doi.org/10.3390/en13020445
Chicago/Turabian StyleYang, Liu, Chuanqing Zhang, Jianchao Cai, and Hongfeng Lu. 2020. "Experimental Investigation of Spontaneous Imbibition of Water into Hydrate Sediments Using Nuclear Magnetic Resonance Method" Energies 13, no. 2: 445. https://doi.org/10.3390/en13020445
APA StyleYang, L., Zhang, C., Cai, J., & Lu, H. (2020). Experimental Investigation of Spontaneous Imbibition of Water into Hydrate Sediments Using Nuclear Magnetic Resonance Method. Energies, 13(2), 445. https://doi.org/10.3390/en13020445







