The Synthesis and Electrochemical Performance of Si Composite with Hollow Carbon Microtubes by the Carbonization of Milkweed from Nature as Anode Template for Lithium Ion Batteries
Abstract
1. Introduction
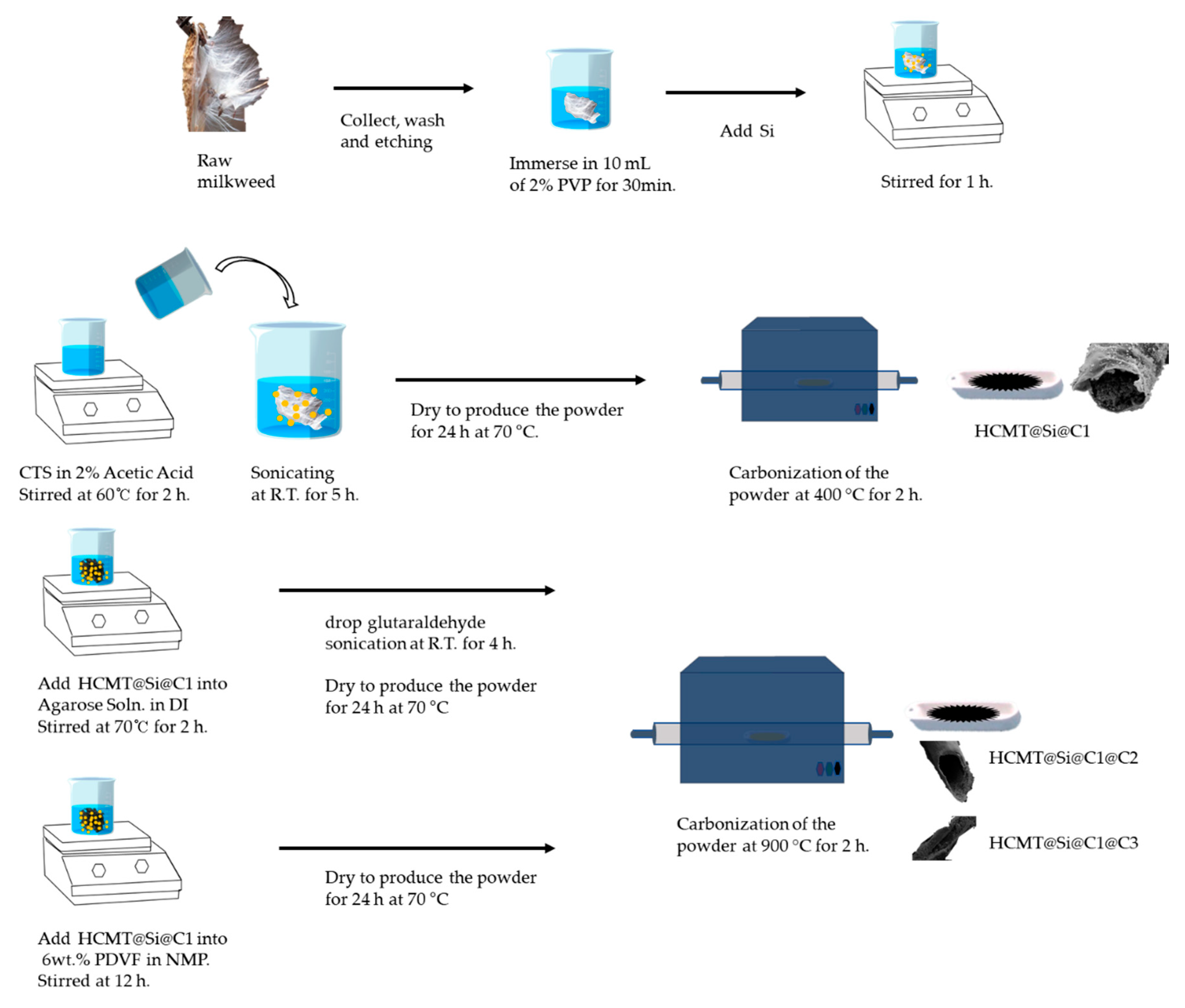
2. Materials and Methods
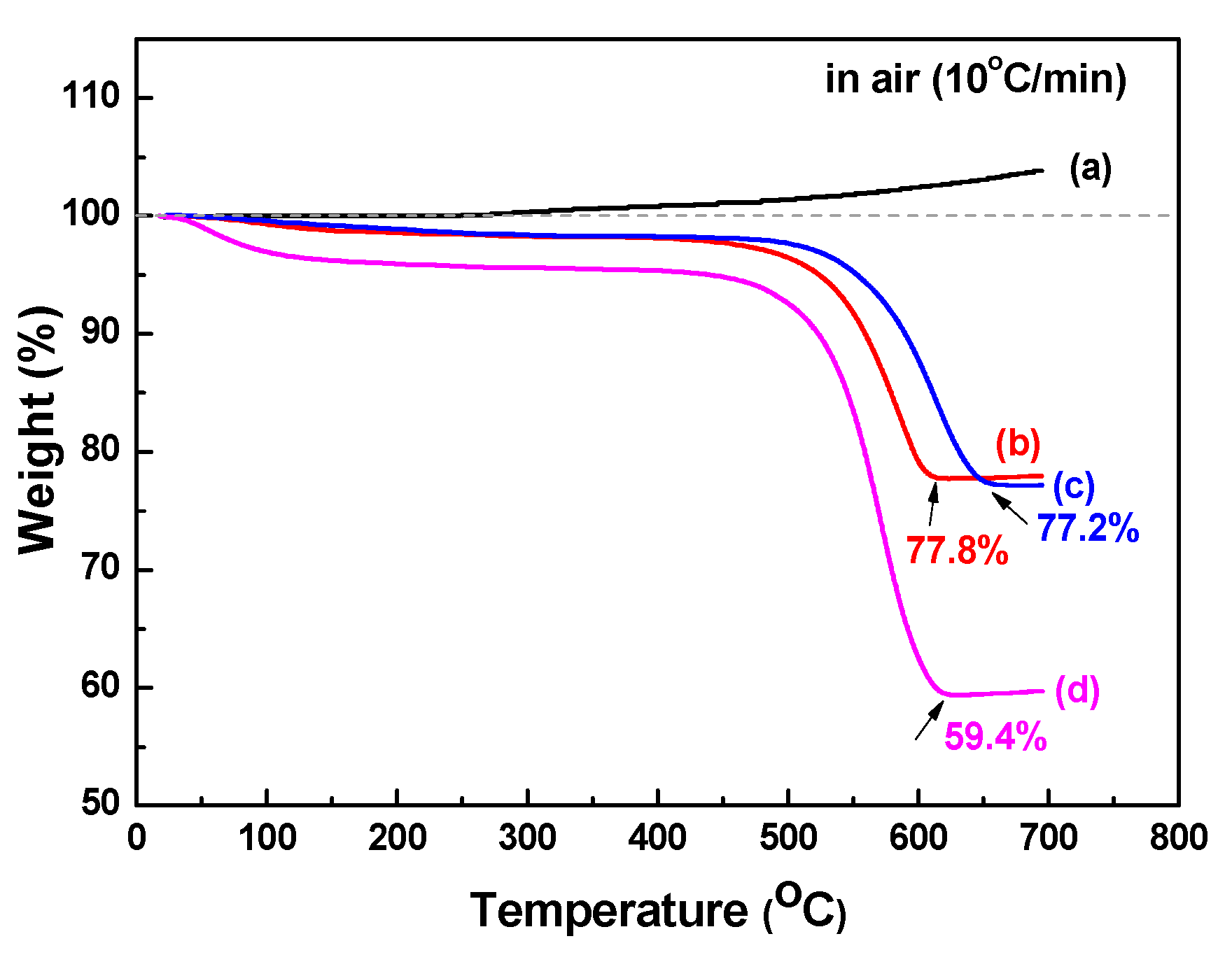
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.; He, S.; Hou, X.; Wang, S.; Chen, F.; Qin, H.; Xia, Y.; Zhou, G. Nano-Si/C microsphere with hollow double spherical interlayer and submicron porous structure to enhance performance for lithium-ion battery anode. Electrochim. Acta 2019, 312, 242–250. [Google Scholar] [CrossRef]
- Zhu, X.; Choi, S.; Tao, R.; Jia, X.; Lu, Y. Building high-rate silicon anodes based on hierarchical Si@C@CNT nanocomposite. J. Alloys Comp. 2019, 791, 1105–1113. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Chen, H.; You, R.; Zheng, G.; Zhang, Q.; Wang, J.; Li, C.; Chen, S.; Yang, Y. Double-shelled microscale porous Si anodes for stable lithium-ion batteries. J. Power Sources 2019, 436, 226794. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, K.H.; Jeon, Y.J.; Lim, S.H.; Lee, S.M. Si/C composite lithium-ion battery anodes synthesized using silicon nanoparticles from porous silicon. Electrochim. Acta 2014, 133, 73–81. [Google Scholar] [CrossRef]
- Mu, G.; Ding, Z.; Mu, D.; Wu, B.; Bi, J.; Zhang, L.; Yang, H.; Wu, H.; Wu, F. Hierarchical void structured Si/PANi/C hybrid anode material for high-performance lithium-ion batteries. Electrochim. Acta 2019, 300, 341–348. [Google Scholar] [CrossRef]
- Yi, X.; Yu, W.J.; Tsiamtsouri, M.A.; Zhang, F.; He, W.; Dai, Q.; Hu, S.; Tong, H.; Zheng, J.; Zhang, B.; et al. Highly conductive C-Si@G nanocomposite as a high-performance anode material for Li-ion batteries. Electrochim. Acta 2019, 295, 719–725. [Google Scholar] [CrossRef]
- Hua, Z.G.; Tana, Z.Y.; Lina, Z.; Chena, J.; Suna, F.; Tanga, X.; Zhenga, R.T.; Chen, Y.C.; Cheng, G.A. Dynamic processes in Si and Si/C anodes in lithium-ion batteries during cycling. J. Electroanal. Chem. 2019, 839, 187–194. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, Y.; Kwon, S.H.; Bae, J.S.; Jeong, E. Cracking resistance and electrochemical performance of silicon anode on binders with different mechanical characteristics. J. Ind. Eng. Chem. 2019, 74, 216–222. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, Y.; Hong, K.S.; Lee, J.; Kim, J.Y.; Bae, J.S.; Jeong, E. Influence of EDTA in poly(acrylic acid) binder for enhancing electrochemical performance and thermal stability of silicon anode. Appl. Surf. Sci. 2018, 447, 442–451. [Google Scholar] [CrossRef]
- Jain, A.; Jayaraman, S.; Ulaganathan, M.; Balasubramanian, R.; Aravindan, V.; Srinivasan, M.P.; Madhavi, S. Highly mesoporous carbon from Teak wood sawdust as prospective electrode for the construction of high energy Li-ion capacitors. Electrochim. Acta 2017, 228, 131–138. [Google Scholar] [CrossRef]
- Han, X.; Chen, H.; Liu, J.; Liu, H.; Wang, P.; Huang, K.; Li, C.; Chen, S.; Yang, Y. A peanut shell inspired scalable synthesis of three-dimensional carbon coated porous silicon particles as an anode for lithium-ion batteries. Electrochim. Acta 2015, 156, 11–19. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Yao, F.; Zamfir, M.R.; Biswas, C.; So, K.P.; Lee, Y.H.; Kim, S.M.; Cha, S.N.; Kim, J.M.; Pribat, D. Highly interconnected Si nanowires for improved stability Li-ion battery anodes. Adv. Energy Mater. 2011, 1, 1154–1161. [Google Scholar] [CrossRef]
- Tian, H.; Tan, X.; Xin, F.; Wang, C.; Han, W. Micro-sized nano-porous Si/C anodes for lithium ion batteries. Nano Energy 2015, 11, 490–499. [Google Scholar] [CrossRef]
- Zuoa, X.; Wang, X.; Xia, Y.; Yin, S.; Ji, Q.; Yang, Z.; Wang, M.; Zheng, X.; Qiu, B.; Liu, Z.; et al. Silicon/carbon lithium-ion battery anode with 3D hierarchical macro-/mesoporous silicon network: Self-templating synthesis via magnesiothermic reduction of silica/carbon composite. J. Power Sources 2019, 412, 93–104. [Google Scholar] [CrossRef]
- Prakash, S.; Zhang, C.; Park, J.D.; Razmjooei, F.; Yu, J.S. Silicon core-mesoporous shell carbon spheres as high stability lithium ion battery anode. J. Colloid Interface Sci. 2019, 534, 47–54. [Google Scholar] [CrossRef]
- Li, W.; Zeng, L.; Wu, Y.; Yu, Y. Nanostructured electrode materials for lithium-ion and sodium-ion batteries via electrospinning. Sci. China Mater. 2016, 59, 287–321. [Google Scholar] [CrossRef]
- Kaskhedikar, N.A.; Maier, J. Lithium storage in carbon nanostructures. Adv. Mater. 2009, 21, 2664–2680. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Ge, Y.Q.; Zhu, J.D.; Jiang, H.; Li, Y.Q.; Hu, Y.; Zhang, X. Synthesis of nitrogen-doped electrospun carbon nanofibers as anode material for high-performance sodium-ion batteries. Energy Technol. 2016, 4, 1440–1449. [Google Scholar] [CrossRef]
- Wang, Q.F.; Yu, Y.; Ma, J.; Zhang, N.; Zhang, J.J.; Liu, Z.H.; Cui, G. Electrospun melamine resin-based multifunctional nonwoven membrane for lithium ion batteries at the elevated temperatures. J. Power Sources 2016, 327, 196–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Li, Z.; Chen, P. Effect of mesoporous carbon microtube prepared by carbonizing the poplar catkin on sulfur cathode performance in Li/S batteries. J. Alloy. Comp. 2015, 619, 298–302. [Google Scholar] [CrossRef]
- Liu, L.; Feng, R.; Pan, Y.; Zheng, X.; Bai, L. Nanoporous carbons derived from poplar catkins for high performance supercapacitors with a redox active electrolyte of pphenylenediamine. J. Alloy. Comp. 2018, 748, 473–480. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, N.; Lei, S.; Yan, R.; Tian, X.; Wan, J.; Song, Y.; Xu, D.; Guo, Q.; Liu, L. Promising biomass-based activated carbons derived from willow catkins for high performance supercapacitors. Electrochim. Acta 2015, 166, 1–11. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, J.; Zhang, L.; Zhao, Y.; Fan, Q.; Li, X.; Hu, Z.; Huang, W. The production of carbon microtubes by the carbonization of catkins and their use in the oxygen reduction reaction. Carbon 2011, 49, 5292–5297. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Ren, W.; Tan, Q.; Chen, Y.; Li, H.; Zhong, Z.; Su, F. Scalable synthesis of interconnected porous silicon/carbon composites by the Rochow reaction as high-performance anodes of lithium ion batteries. Angewandte Chem. 2014, 53, 5165–5169. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, Z.Q.; Zhu, S.Y.; Sun, D.Y.; Jin, Y.C. Enteromorpha prolifera-derived carbon as a high-performance cathode material for lithium–sulfur batteries. J. Appl. Electrochem. 2017, 47, 631–639. [Google Scholar] [CrossRef]
- Li, Y.; Wangn, G.; Wei, T.; Fann, Z.; Yan, P. Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 2016, 19, 165–175. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Liu, J. Biomass-derived porous carbon materials for advanced lithium sulfur batteries. J. Energy Chem. 2019, 34, 171–185. [Google Scholar] [CrossRef]
- Wanga, K.; Songa, Y.; Yana, R.; Zhaoa, N.; Tiana, X.; Lia, X.; Guoa, Q.; Liu, Z. High capacitive performance of hollow activated carbon fibers derived from willow catkins. Appl. Surf. Sci. 2017, 394, 569–577. [Google Scholar] [CrossRef]
- Wang, K.; Yan, R.; Zhao, N.; Tian, X.; Li, X.; Lei, S.; Song, Y.; Guo, Q.; Liu, L. Bio-inspired hollow activated carbon microtubes derived from willow catkins for supercapacitors with high volumetric performance. Mater. Lett. 2016, 174, 249–252. [Google Scholar] [CrossRef]
- Su, X.L.; Cheng, M.Y.; Fu, L.; Yang, J.H.; Zheng, X.C.; Guan, X.X. Superior supercapacitive performance of hollow activated carbon nanomesh with hierarchical structure derived from poplar catkins. J. Power Sources 2017, 362, 27–38. [Google Scholar] [CrossRef]
- Tao, L.; Huang, Y.; Zheng, Y.; Yang, X.; Liu, C.; Di, M.; Larpkiattaworn, S.; Nimlos, M.R.; Zheng, Z. Porous carbon nanofiber derived from a waste biomass as anode material in lithium-ion batteries. J. Taiwan Ins. Chem. Eng. 2019, 95, 217–226. [Google Scholar] [CrossRef]
- Fromm, O.; Heckmann, A.; Rodehorst, U.C.; Frerichs, J.; Becker, D.; Winter, M.; Placke, T. Carbons from biomass precursors as anode materials for lithium ion batteries: New insights into carbonization and graphitization behavior and into their correlation to electrochemical performance. Carbon 2018, 128, 147–163. [Google Scholar] [CrossRef]
- Teixeira, E.M.; Correˆa, A.C.; Manzoli, A.; Leite, F.L.; Oliveira, C.R. Cellulose nanofibers from white and naturally colored cotton fibers. Cellulose 2010, 17, 595–606. [Google Scholar] [CrossRef]
- Mishra, K.; George, K.; Zhou, X.D. Submicron silicon anode stabilized by single-step carbon and germanium coatings for high capacity lithium-ion batteries. Carbon 2018, 138, 419–426. [Google Scholar] [CrossRef]
- Lin, D.; Lu, Z.; Hsu, P.C.; Lee, H.R.; Liu, N.; Zhao, J.; Wang, H.; Liu, C.; Cui, Y. A high tap density secondary silicon particle anode fabricated by scalable mechanical pressing for lithium-ion batteries. Energy Environ. Sci. 2015, 8, 2371–2376. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Shen, Z.; Chen, R.; He, X.; Zhang, X.; Zhang, Y.; Wu, K. Sandwich structure of graphene-protected silicon/carbon nanofibers for lithium-ion battery anodes. Electrochim. Acta 2016, 210, 53–60. [Google Scholar] [CrossRef]
- Deng, L.; Cui, Y.; Chen, J.; Wu, J.; Baker, A.P.; Li, Z.; Zhang, X. A Core-shell Si@NiSi2/Ni/C nanocomposite as an anode material for lithium-ion batteries. Electrochim. Acta 2016, 192, 303–309. [Google Scholar] [CrossRef]
- Xu, Z.L.; Zhang, B.; Kim, J.K. Electrospun carbon nanofiber anodes containing monodispersed Si nanoparticles and graphene oxide with exceptional high rate capacities. Nano Energy 2014, 6, 27–35. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, E.H.; Kim, J.P.; Kim, H.G.; Chung, J.-M.; Lee, S.-J.; Bae, J.-S.; Jeong, E.D. The Synthesis and Electrochemical Performance of Si Composite with Hollow Carbon Microtubes by the Carbonization of Milkweed from Nature as Anode Template for Lithium Ion Batteries. Energies 2020, 13, 5124. https://doi.org/10.3390/en13195124
Chung EH, Kim JP, Kim HG, Chung J-M, Lee S-J, Bae J-S, Jeong ED. The Synthesis and Electrochemical Performance of Si Composite with Hollow Carbon Microtubes by the Carbonization of Milkweed from Nature as Anode Template for Lithium Ion Batteries. Energies. 2020; 13(19):5124. https://doi.org/10.3390/en13195124
Chicago/Turabian StyleChung, Eun Hyuk, Jong Pil Kim, Hyun Gyu Kim, Jae-Min Chung, Sei-Jin Lee, Jong-Seong Bae, and Euh Duck Jeong. 2020. "The Synthesis and Electrochemical Performance of Si Composite with Hollow Carbon Microtubes by the Carbonization of Milkweed from Nature as Anode Template for Lithium Ion Batteries" Energies 13, no. 19: 5124. https://doi.org/10.3390/en13195124
APA StyleChung, E. H., Kim, J. P., Kim, H. G., Chung, J.-M., Lee, S.-J., Bae, J.-S., & Jeong, E. D. (2020). The Synthesis and Electrochemical Performance of Si Composite with Hollow Carbon Microtubes by the Carbonization of Milkweed from Nature as Anode Template for Lithium Ion Batteries. Energies, 13(19), 5124. https://doi.org/10.3390/en13195124




