Effect of Nitrogen Doping on the Performance of Mesoporous CMK-8 Carbon Anodes for Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis Process
2.1.1. Silica Template KIT-6
2.1.2. Mesoporous CMK-8 and N-CMK-8 Carbon Materials
2.2. Structural Characterization
2.3. Electrode Preparation
2.4. Electrochemical Analysis and Device Testing
3. Results and Discussion
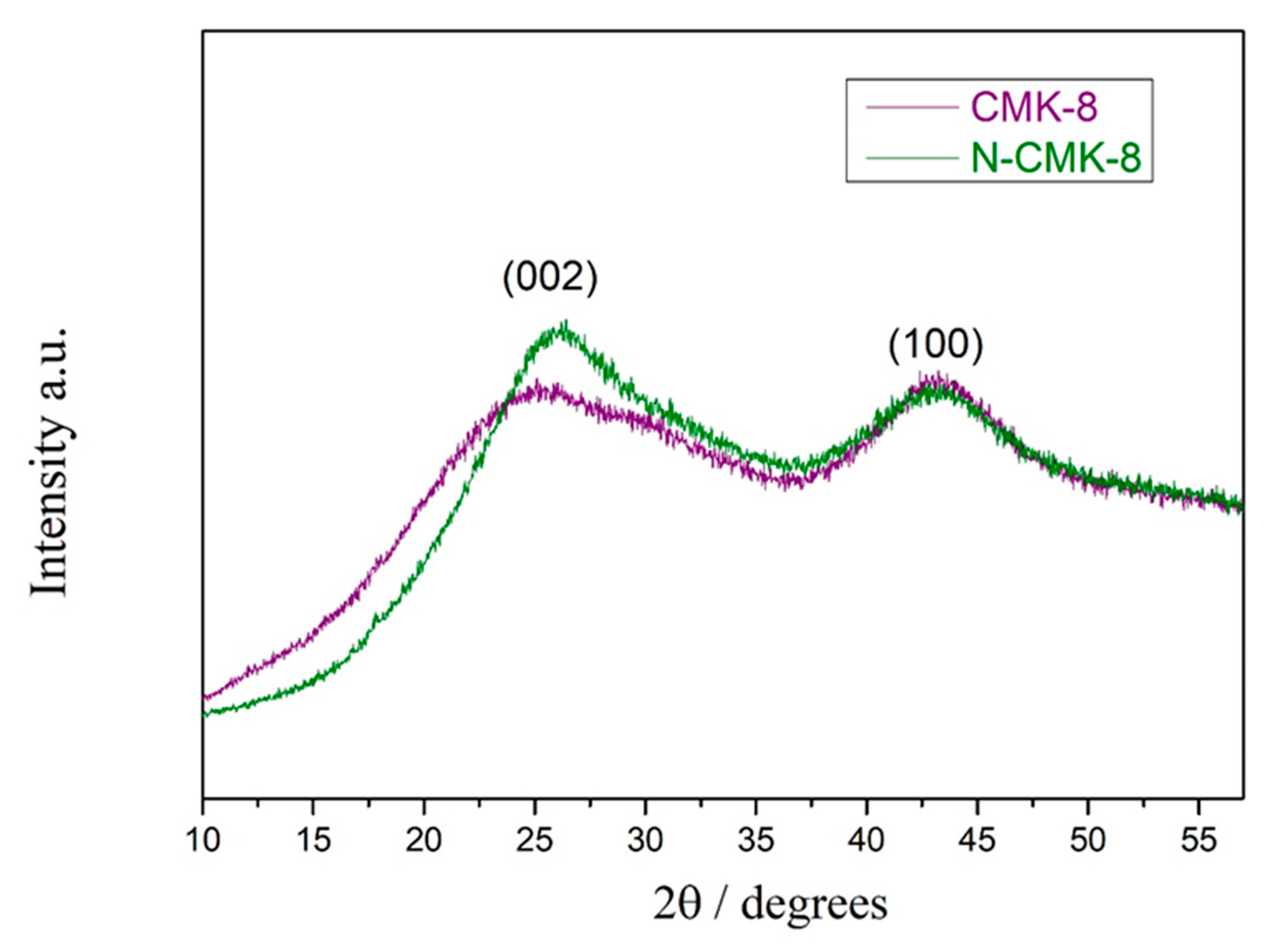
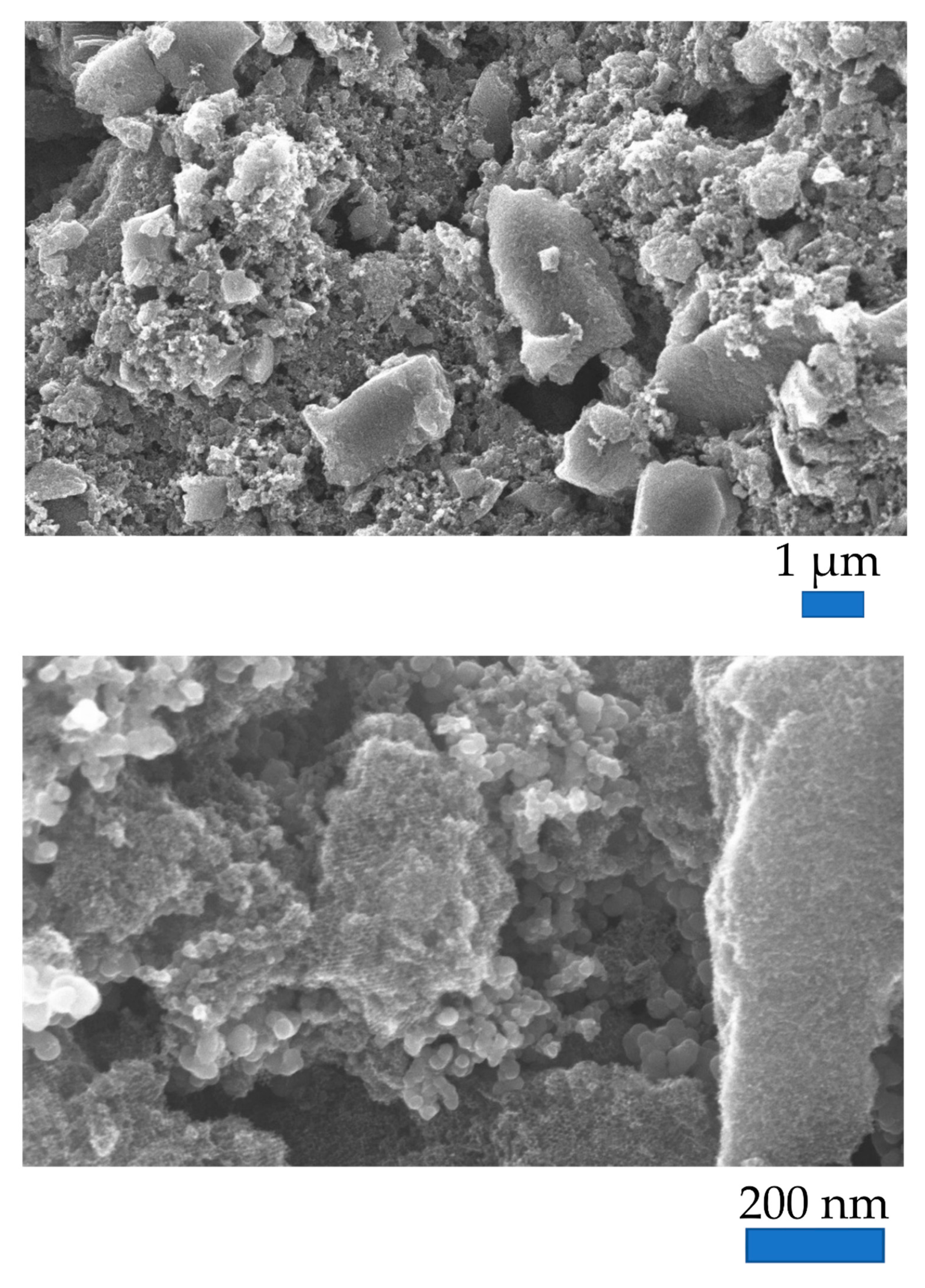
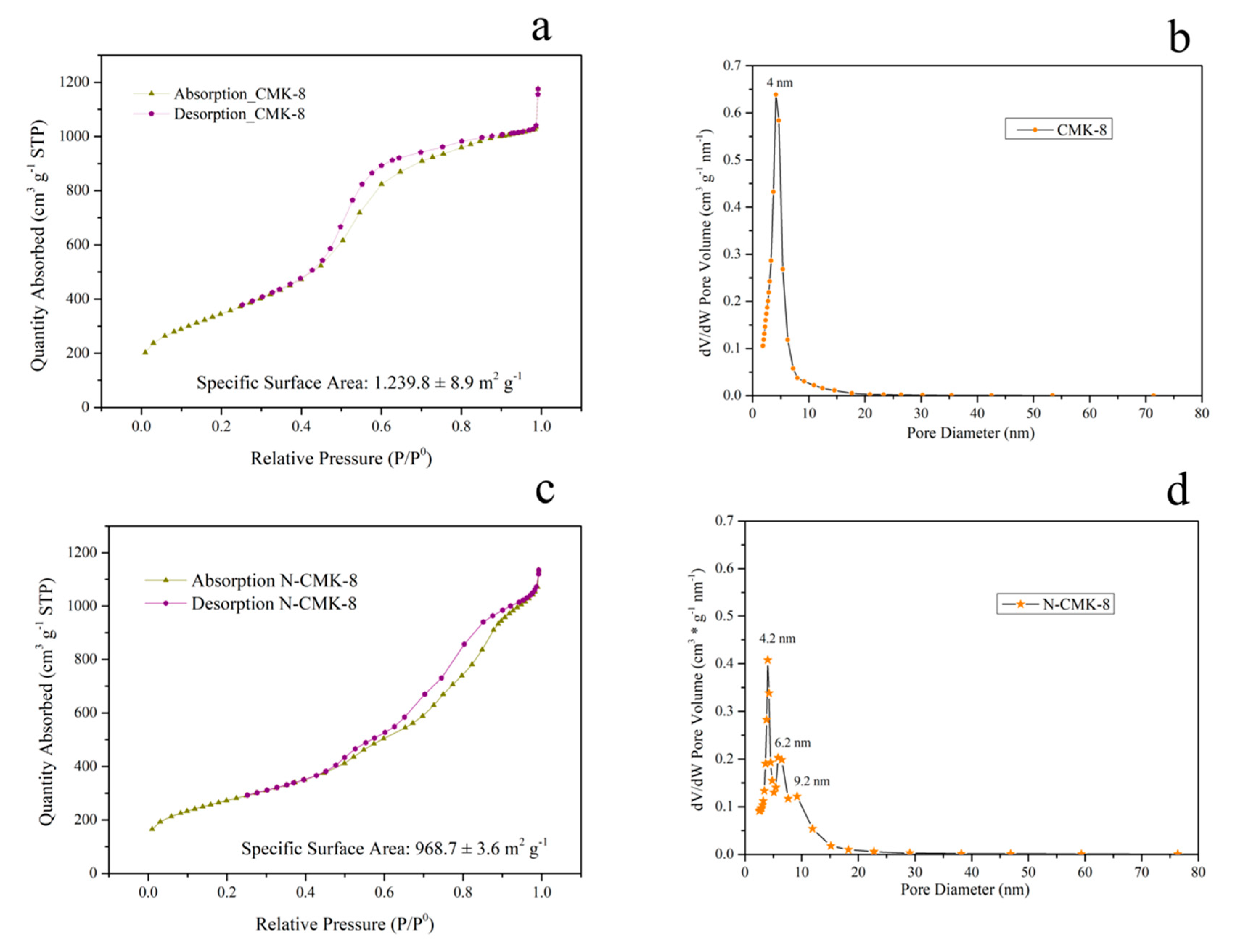
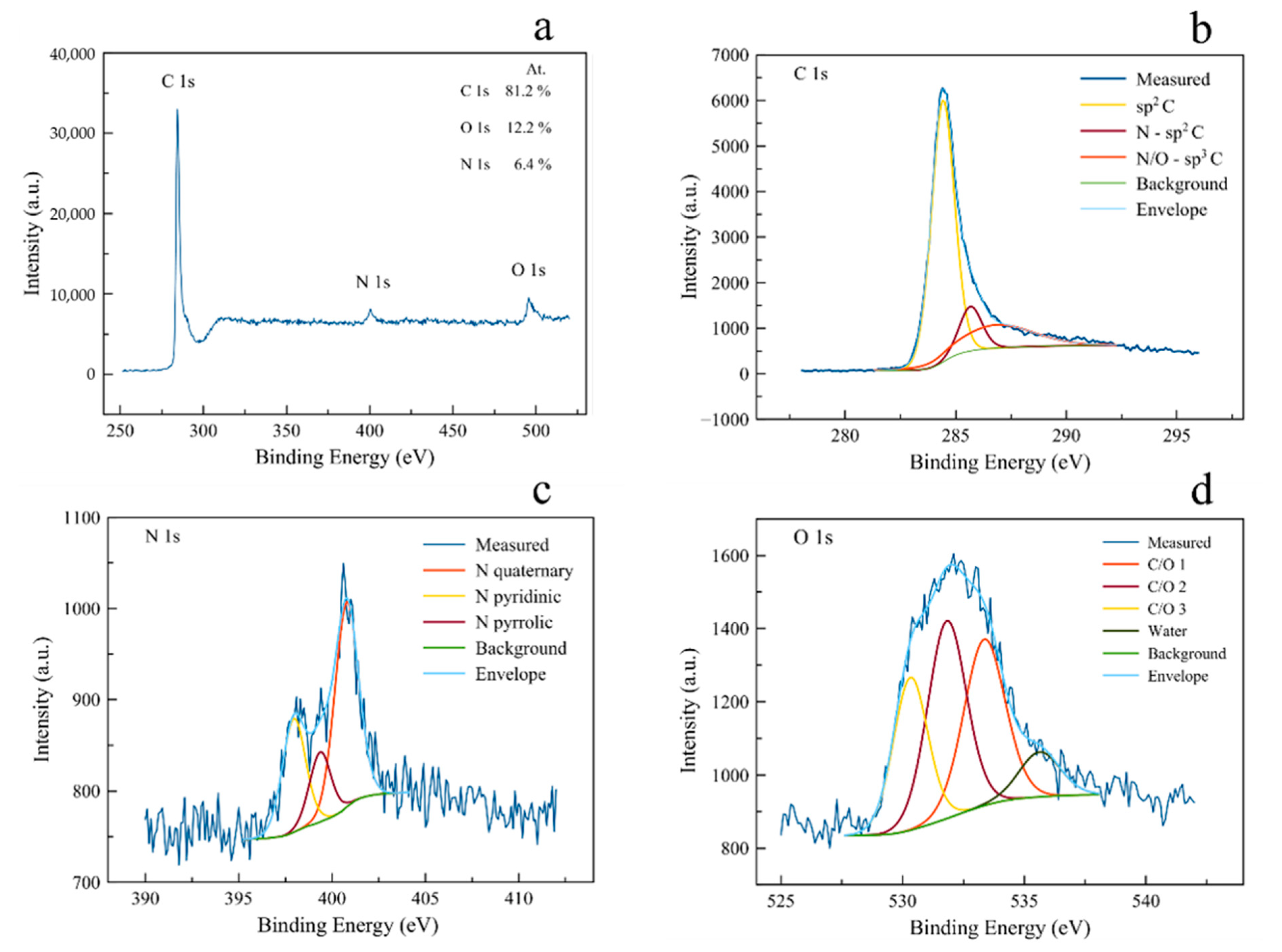
3.1. Structural Characterization: N2 Sorption, XRD, SAXS, and XPS Analysis
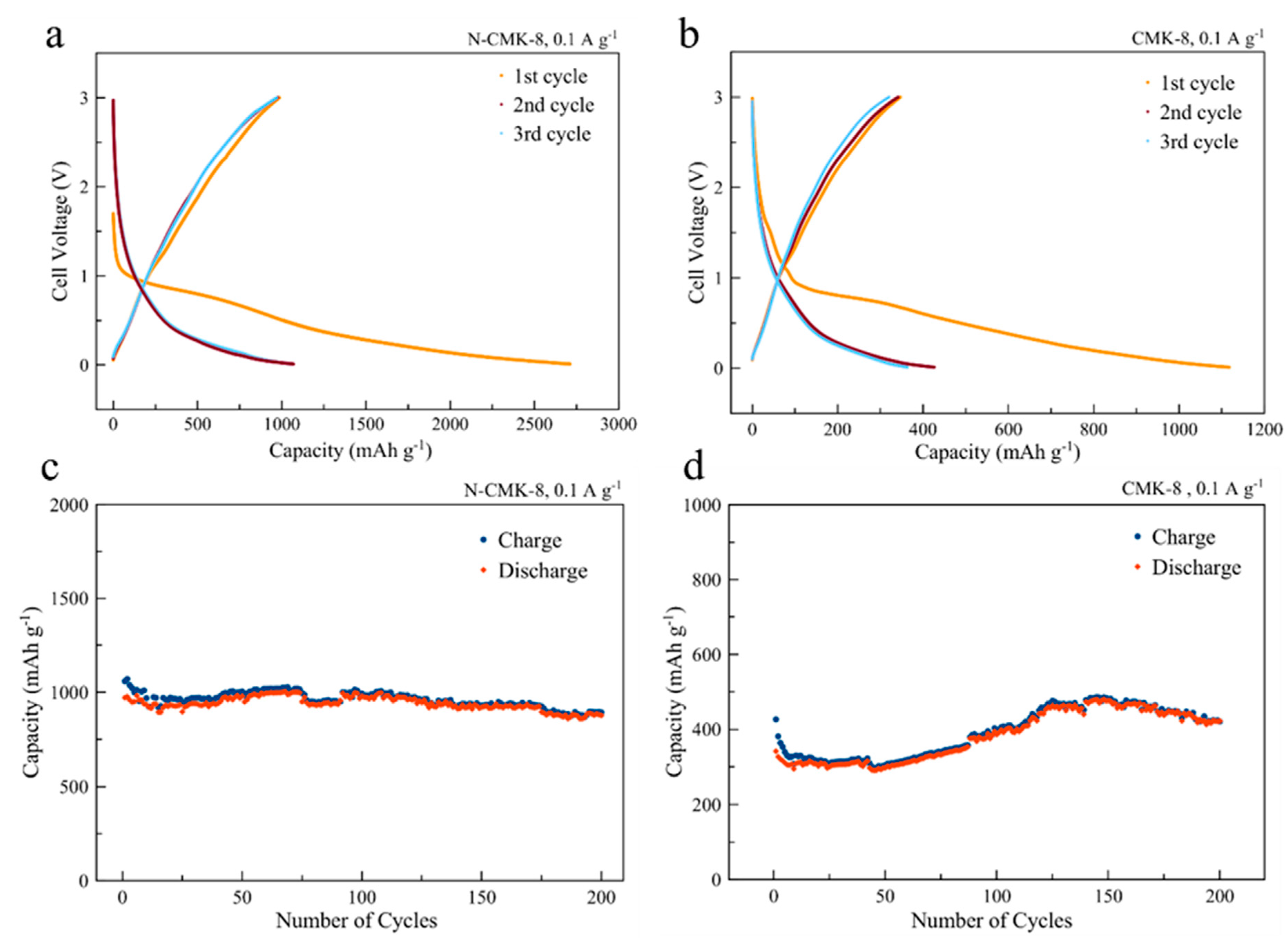
3.2. Electrochemical Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review; Springer: Singapore, 2020; Volume 3, ISBN 0123456789. [Google Scholar]
- Walter, M.; Kovalenko, M.V.; Kravchyk, K.V. Challenges and benefits of post-lithium-ion batteries. New J. Chem. 2020, 44, 1677–1683. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Xu, T.; Lin, N.; Cai, W.; Yi, Z.; Zhou, J.; Han, Y.; Zhu, Y.; Qian, Y. Stabilizing Si/graphite composites with Cu and: In situ synthesized carbon nanotubes for high-performance Li-ion battery anodes. Inorg. Chem. Front. 2018, 5, 1463–1469. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog. Energy Combust. Sci. 2019, 75, 100786. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, C.; Zhu, Y.; He, J.; Wang, H. Reduced Graphene Oxide-Wrapped FeS2 Composite as Anode for High-Performance Sodium-Ion Batteries. Nano-Micro Lett. 2018, 10, 30. [Google Scholar] [CrossRef]
- Dong, Y.; Jiang, X.; Mo, J.; Zhou, Y.; Zhou, J. Hollow CuO nanoparticles in carbon microspheres prepared from cellulose-cuprammonium solution as anode materials for Li-ion batteries. Chem. Eng. J. 2020, 381, 122614. [Google Scholar] [CrossRef]
- Ji, H.; Wang, M.; Liu, S.; Sun, H.; Liu, J.; Qian, T.; Yan, C. Pyridinic and graphitic nitrogen-enriched carbon paper as a highly active bifunctional catalyst for Zn-air batteries. Electrochim. Acta 2020, 334, 135562. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Chen, S.; Yu, X.; Wang, C.; Ma, T. 2D nanoplate assembled nitrogen doped hollow carbon sphere decorated with Fe3O4 as an efficient electrocatalyst for oxygen reduction reaction and Zn-air batteries. Nano Res. 2019, 12, 2774–2780. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Xu, G.; Liu, X.; Zhang, Q.; Yang, L.; Cao, J.; Wei, X. Porous N-doped carbon sheets wrapped MnO in 3D carbon networks as high-performance anode for Li-ion batteries. Electrochim. Acta 2020, 342, 136115. [Google Scholar] [CrossRef]
- Xiao, J.; Wan, L.; Yang, S.; Xiao, F.; Wang, S. Design hierarchical electrodes with highly conductive NiCo 2S4 nanotube arrays grown on carbon fiber paper for high-performance pseudocapacitors. Nano Lett. 2014, 14, 831–838. [Google Scholar] [CrossRef]
- Li, N.; Jin, S.X.; Liao, Q.Y.; Wang, C.X. ZnO anchored on vertically aligned graphene: Binder-free anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 20590–20596. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Choi, S.H.; Kim, N.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 110–135. [Google Scholar] [CrossRef]
- Yu, K.; Wang, J.; Wang, X.; Liang, J.; Liang, C. Sustainable application of biomass by-products: Corn straw-derived porous carbon nanospheres using as anode materials for lithium ion batteries. Mater. Chem. Phys. 2020, 243, 122644. [Google Scholar] [CrossRef]
- Zhong, S.; Liu, H.; Wei, D.; Hu, J.; Zhang, H.; Hou, H.; Peng, M.; Zhang, G.; Duan, H. Long-aspect-ratio N-rich carbon nanotubes as anode material for sodium and lithium ion batteries. Chem. Eng. J. 2020, 395, 125054. [Google Scholar] [CrossRef]
- Tang, Y.; Deng, S.; Shi, S.; Wu, L.; Wang, G.; Pan, G.; Lin, S.; Xia, X. Ultrafast and durable lithium ion storage enabled by intertwined carbon nanofiber/Ti2Nb10O29 core-shell arrays. Electrochim. Acta 2020, 332, 135433. [Google Scholar] [CrossRef]
- Sonia, F.J.; Aslam, M.; Mukhopadhyay, A. Understanding the processing-structure-performance relationship of graphene and its variants as anode material for Li-ion batteries: A critical review. Carbon N. Y. 2020, 156, 130–165. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, B.; Cao, L.; Wu, X.; Lin, Z.; Yu, X.; Zhang, X.; Zeng, D.; Xie, F.; Zhang, W.; et al. Iodine doped graphene as anode material for lithium ion battery. Carbon N. Y. 2015, 94, 1–8. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Maegawa, K.; Tan, W.K.; Kawamura, G.; Kar, K.K.; Matsuda, A. Heteroatom doped graphene engineering for energy storage and conversion. Mater. Today 2020. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, M.; Chen, W.; Zeng, L.; Wei, M. An in situ formed Se/CMK-3 composite for rechargeable lithium-ion batteries with long-term cycling performance. J. Mater. Chem. A 2016, 4, 13646–13651. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Shen, P.K. Simultaneous formation of ultrahigh surface area and three-dimensional hierarchical porous graphene-like networks for fast and highly stable supercapacitors. Adv. Mater. 2013, 25, 2474–2480. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, D. Synthesis of replica mesostructures by the nanocasting strategy. J. Mater. Chem. 2005, 15, 1217–1231. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous carbon materials: Synthesis and modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef]
- Saikia, D.; Wang, T.H.; Chou, C.J.; Fang, J.; Tsai, L.D.; Kao, H.M. A comparative study of ordered mesoporous carbons with different pore structures as anode materials for lithium-ion batteries. RSC Adv. 2015, 5, 42922–42930. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H. Nanostructured carbon materials synthesized from mesoporous silica crystals by replication. In Studies in Surface Science and Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2004; Volume 148, pp. 241–260. [Google Scholar]
- Bhattacharjya, D.; Park, H.Y.; Kim, M.S.; Choi, H.S.; Inamdar, S.N.; Yu, J.S. Nitrogen-doped carbon nanoparticles by flame synthesis as anode material for rechargeable lithium-ion batteries. Langmuir 2014, 30, 318–324. [Google Scholar] [CrossRef]
- Kesavan, T.; Partheeban, T.; Vivekanantha, M.; Prabu, N.; Kundu, M.; Selvarajan, P.; Umapathy, S.; Vinu, A.; Sasidharan, M. Design of P-Doped Mesoporous Carbon Nitrides as High-Performance Anode Materials for Li-Ion Battery. ACS Appl. Mater. Interfaces 2020, 12, 24007–24018. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Q.; Xiang, H.; Han, F.; Tang, W.; Yuan, G.; Cong, Y.; Fan, C.; Westwood, A.; Li, X. Enhanced active sulfur in soft carbon via synergistic doping effect for ultra–stable lithium–ion batteries. Energy Storage Mater. 2020, 24, 450–457. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.; Shen, W.; Xu, Q.; Liu, H.; Wang, Y. B-doped carbon coating improves the electrochemical performance of electrode materials for Li-ion batteries. Adv. Funct. Mater. 2014, 24, 5511–5521. [Google Scholar] [CrossRef]
- Mao, Y.; Duan, H.; Xu, B.; Zhang, L.; Hu, Y.; Zhao, C.; Wang, Z.; Chen, L.; Yang, Y. Lithium storage in nitrogen-rich mesoporous carbon materials. Energy Environ. Sci. 2012, 5, 7950–7955. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. IUPAC Technical Report Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Biswal, M.; Banerjee, A.; Deo, M.; Ogale, S. From dead leaves to high energy density supercapacitors. Energy Environ. Sci. 2013, 6, 1249–1259. [Google Scholar] [CrossRef]
- Dombrovskis, J.K.; Jeong, H.Y.; Fossum, K.; Terasaki, O.; Palmqvist, A.E.C. Transition metal ion-chelating ordered mesoporous carbons as noble metal-free fuel cell catalysts. Chem. Mater. 2013, 25, 856–861. [Google Scholar] [CrossRef]
- Aroyo, M.I. (Ed.) International Tables for Crystallography; International Union of Crystallography: Chester, UK, 2016; Volume A, ISBN 978-0-470-97423-0. [Google Scholar]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 3, 2136–2137. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, X.; Solovyov, L.A.; Liu, Z.; Yang, H.; Zhang, Z.; Xie, S.; Zhang, F.; Tu, B.; Yu, C.; et al. Facile Synthesis and Characterization of Novel Mesoporous and Mesorelief Oxides with Gyroidal Structures. J. Am. Chem. Soc. 2004, 126, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.; Tsubakiyama, T.; Carlsson, A.; Sakamoto, Y.; Ohsuna, T.; Terasaki, O.; Joo, S.H.; Ryoo, R. Structural study of mesoporous MCM-48 and carbon networks synthesized in the spaces of MCM-48 by electron crystallography. J. Phys. Chem. B 2002, 106, 1256–1266. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Kim, T.W.; Solovyov, L.A. Synthesis and characterization of large-pore ordered mesoporous carbons using gyroidal silica template. J. Mater. Chem. 2006, 16, 1445–1455. [Google Scholar] [CrossRef]
- Kapteijn, F.; Moulijn, J.A.; Matzner, S.; Boehm, H.P. Development of nitrogen functionality in model chars during gasification in CO2 and O2. Carbon N. Y. 1999, 37, 1143–1150. [Google Scholar] [CrossRef]
- Czerw, R.; Terrones, M.; Charlier, J.C.; Blase, X.; Foley, B.; Kamalakaran, R.; Grobert, N.; Terrones, H.; Tekleab, D.; Ajayan, P.M.; et al. Identification of Electron Donor States in N-Doped Carbon Nanotubes. Nano Lett. 2001, 1, 457–460. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Chang, P.Y.; Bindumadhavan, K.; Doong, R.A. Size effect of ordered mesoporous carbon nanospheres for anodes in Li-ion battery. Nanomaterials 2015, 5, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Liu, Z.; Wang, L.; Han, P.; Xu, H.; Zhang, K.; Dong, S.; Yao, J.; Cui, G. Nitrogen-doped graphene nanosheets with excellent lithium storage properties. J. Mater. Chem. 2011, 21, 5430–5434. [Google Scholar] [CrossRef]
- Yue, H.; Li, F.; Yang, Z.; Tang, J.; Li, X.; He, D. Nitrogen-doped carbon nanofibers as anode material for high-capacity and binder-free lithium ion battery. Mater. Lett. 2014, 120, 39–42. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.M.; Wang, Z.H.; Shao, Q.G.; Li, X.; Yuan, L.X.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Niu, D.; Zheng, N.; Yu, H.; He, J.; Li, Y. Facile Synthesis of Nitrogen-Doped Double-Shelled Hollow Mesoporous Carbon Nanospheres as High-Performance Anode Materials for Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 5999–6007. [Google Scholar] [CrossRef]
- Zhang, W.J. Structure and performance of LiFePO4 cathode materials: A review. J. Power Sources 2011, 196, 2962–2970. [Google Scholar] [CrossRef]
- Schneier, D.; Harpak, N.; Menkin, S.; Davidi, G.; Goor, M.; Mados, E.; Peled, E.; Ardel, G.; Patolsky, F.; Golodnitsky, D.; et al. Analysis of Scale-up Parameters in 3D Silicon-Nanowire Lithium-Battery Anodes. J. Electrochem. Soc. 2020, 167, 050511. [Google Scholar] [CrossRef]
- Chen, L.; Fan, X.; Hu, E.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Li, J.; Su, D.; Yang, X.; et al. Achieving High Energy Density through Increasing the Output Voltage: A Highly Reversible 5.3 V Battery. Chem 2019, 5, 896–912. [Google Scholar] [CrossRef]


| Material | Potential Window (V) | Specific Capacity at 0.1 A·g−1 (mAh·g−1) | Capacity Retention (Number of Cycles) | Ref. |
|---|---|---|---|---|
| This work | 0.01–3 | ~950 | ~95% (200) | |
| CMK3 | 0.01–3 | ~400 | ~88% (100) | [21] |
| N doped carbon nanotubes | 0.01–3 | ~600 | ~93% (10) | [16] |
| N doped carbon nanofibers | 0.01–3 | ~850 | >95% (150) | [46] |
| N doped graphene nanosheets | 0.01–3 | ~720 | ~100% (10) | [45] |
| N doped porous carbon nanofiber webs | 0.01–3 | ~1280 | >100% (600) (2 A·g−1) | [47] |
| N doped double shelled hollow nanospheres | 0.01–3 | ~850 | ~100% (100) | [48] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcagno, G.; Agostini, M.; Xiong, S.; Matic, A.; Palmqvist, A.E.C.; Cavallo, C. Effect of Nitrogen Doping on the Performance of Mesoporous CMK-8 Carbon Anodes for Li-Ion Batteries. Energies 2020, 13, 4998. https://doi.org/10.3390/en13194998
Calcagno G, Agostini M, Xiong S, Matic A, Palmqvist AEC, Cavallo C. Effect of Nitrogen Doping on the Performance of Mesoporous CMK-8 Carbon Anodes for Li-Ion Batteries. Energies. 2020; 13(19):4998. https://doi.org/10.3390/en13194998
Chicago/Turabian StyleCalcagno, G., M. Agostini, S. Xiong, A. Matic, A. E. C. Palmqvist, and C. Cavallo. 2020. "Effect of Nitrogen Doping on the Performance of Mesoporous CMK-8 Carbon Anodes for Li-Ion Batteries" Energies 13, no. 19: 4998. https://doi.org/10.3390/en13194998
APA StyleCalcagno, G., Agostini, M., Xiong, S., Matic, A., Palmqvist, A. E. C., & Cavallo, C. (2020). Effect of Nitrogen Doping on the Performance of Mesoporous CMK-8 Carbon Anodes for Li-Ion Batteries. Energies, 13(19), 4998. https://doi.org/10.3390/en13194998









