The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass
Abstract
1. Introduction
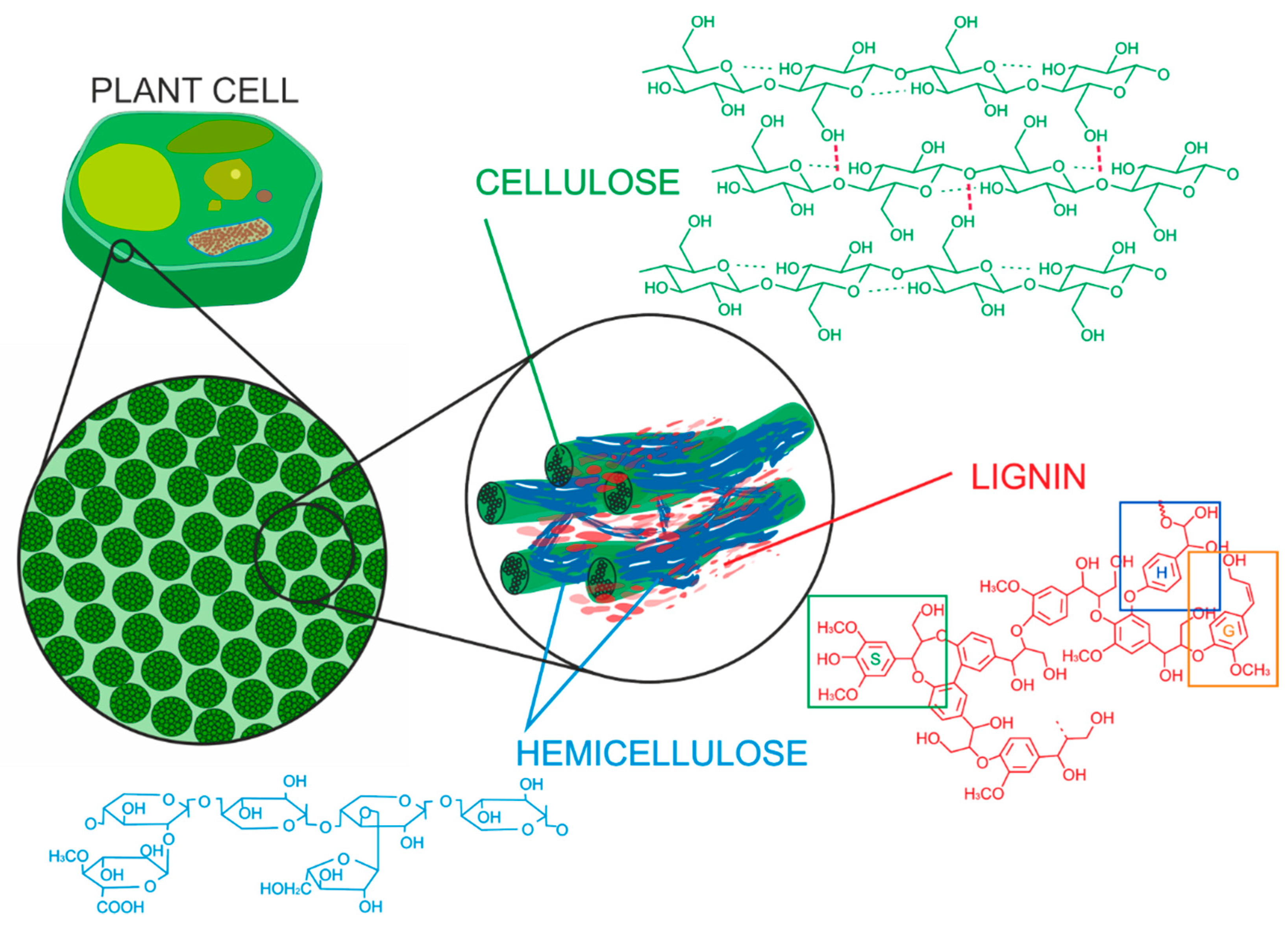
2. The Chemical Composition of Lignocellulosic (LC) Biomass
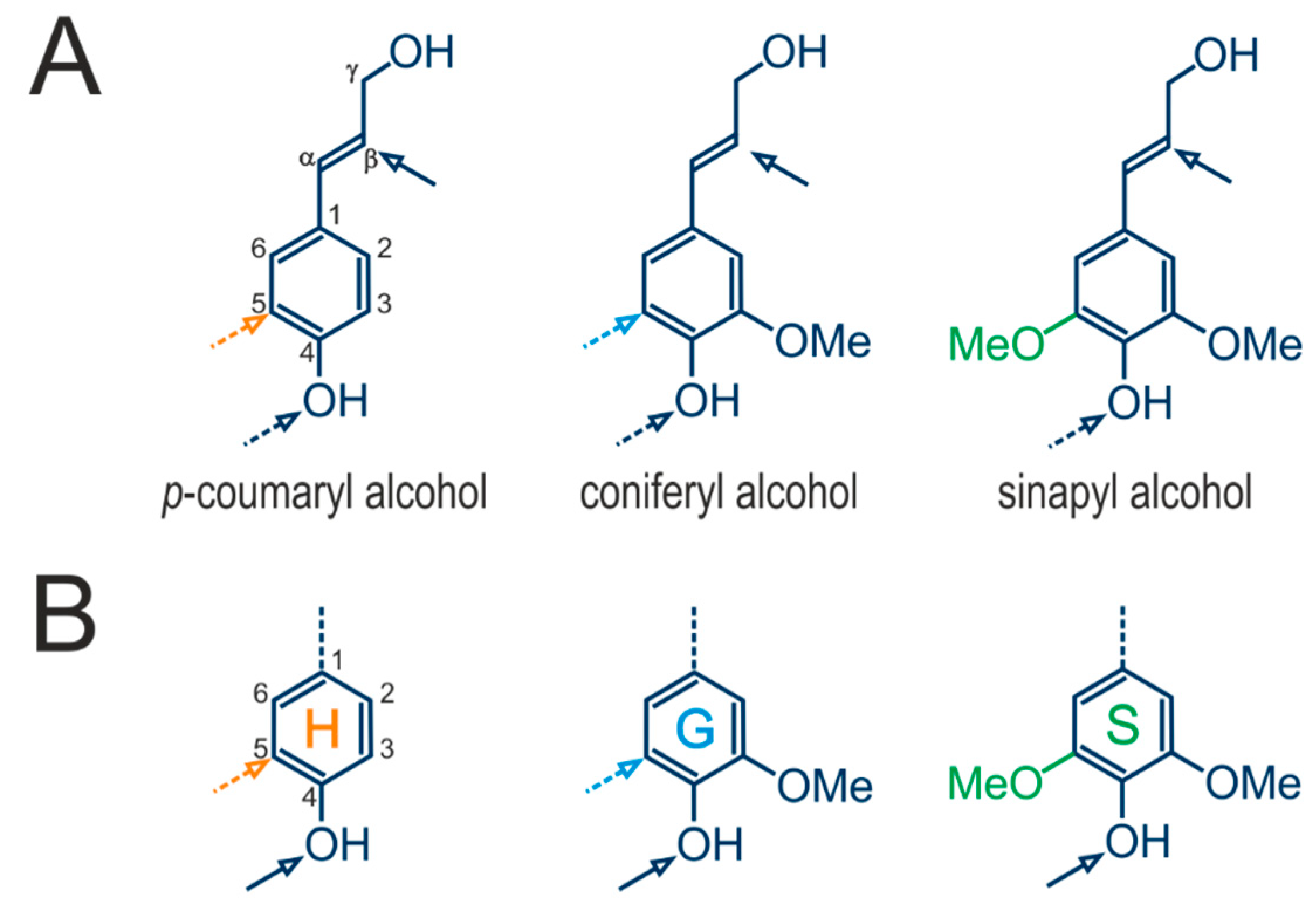
3. Lignin
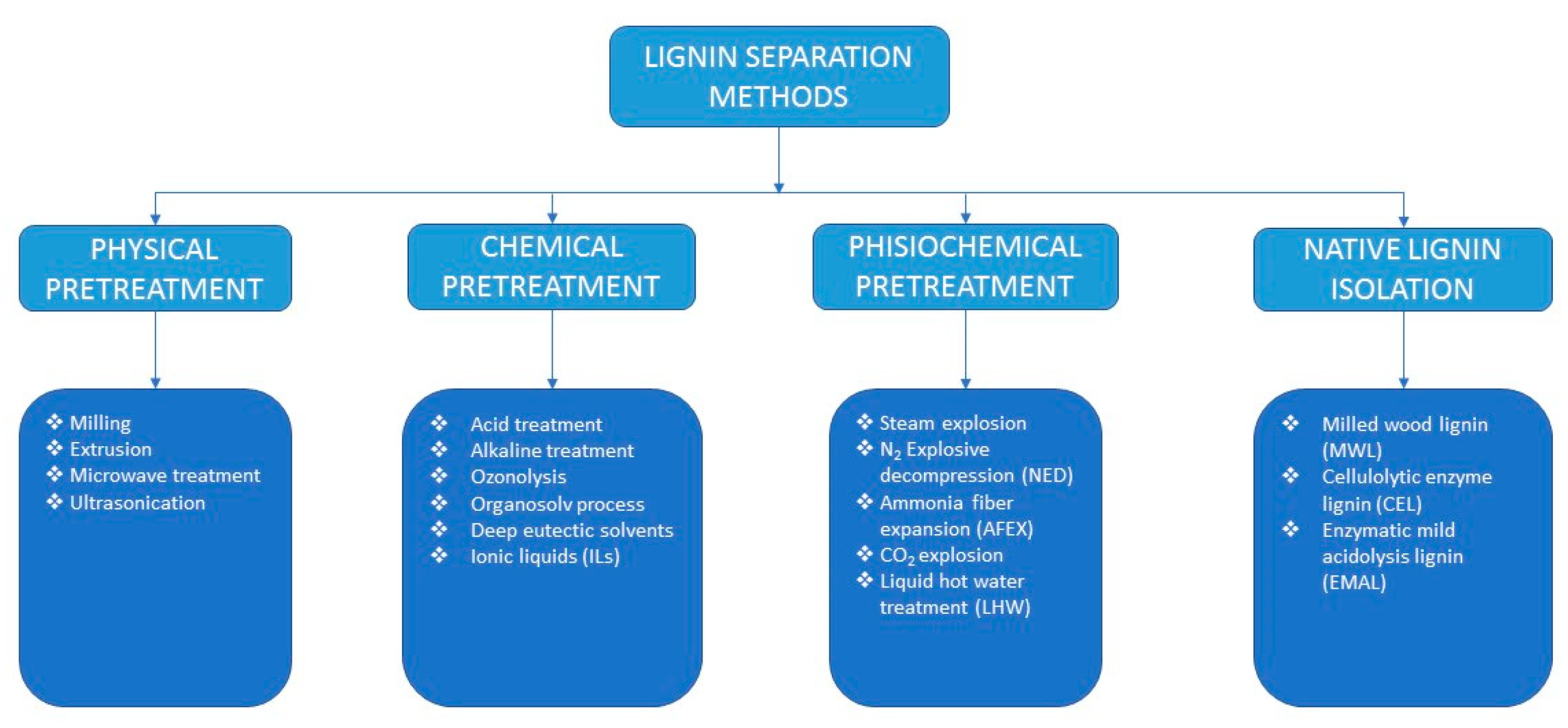
4. LC Pretreatment for Lignin Isolation
4.1. Physical Pretreatment Methods
4.2. Chemical Pretreatment Methods
4.2.1. Kraft Lignin Process
4.2.2. Physicochemical Pretreatment
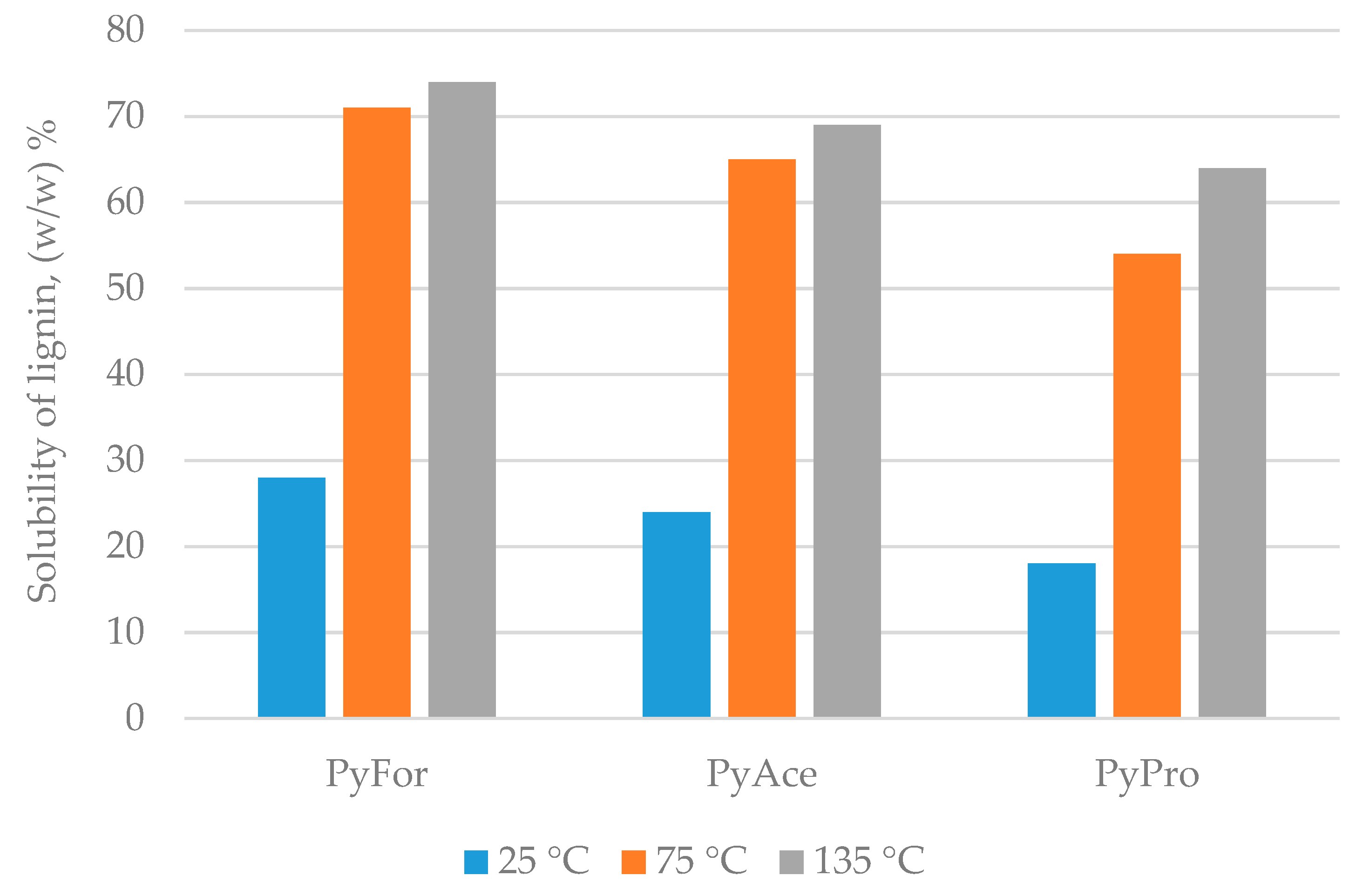
4.3. Lignin Separation with ILs
4.3.1. PILs Targeting
4.3.2. Post Reaction Lignin Properties
4.4. The Recovery and Reuse of ILs
4.5. Lignin Recovery
5. Lignin Valorization
Lignin Valorisation after ILs Treatment
6. Conclusions and Future Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamm, B.; Gruber, P.R.; Kamm, M. Biorefineries—Industrial processes and products. In Ullmann’s Biotechnology and Biochemical Engineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; Volume 2, pp. 785–817. [Google Scholar]
- De Gonzalo, G.; Colpa, D.I.; Habib, M.H.M.; Fraaije, M.W. Bacterial enzymes involved in lignin degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Kikas, T.; Tutt, M.; Raud, M.; Alaru, M.; Lauk, R.; Olt, J. Basis of Energy Crop Selection for Biofuel Production: Cellulose vs. Lignin. Int. J. Green Energy 2016, 13, 49–54. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Ferreira, J.A.; Mushtaq, M.; Karimi, S.; Orupõld, K.; Kikas, T. Genetic modification of cereal plants: A strategy to enhance bioethanol yields from agricultural waste. Ind. Crops Prod. 2020, 150, 112408. [Google Scholar] [CrossRef]
- Raud, M.; Mitt, M.; Oja, T.; Olt, J.; Orupõld, K.; Kikas, T. The utilisation potential of urban greening waste: Tartu case study. Urban For. Urban Green. 2017, 21, 96–101. [Google Scholar] [CrossRef]
- Xu, A.; Guo, X.; Zhang, Y.; Li, Z.; Wang, J. Efficient and sustainable solvents for lignin dissolution: Aqueous choline carboxylate solutions. Green Chem. 2017, 19, 4067–4073. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.-C.; Sun, R.-C. Fractionational and structural characterization of lignin and its modification as biosorbents for efficient removal of chromium from wastewater: A review. J. Leather Sci. Eng. 2019, 1, 5. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef]
- Ahmad, E.; Pant, K.K. Chapter 14—Lignin Conversion: A Key to the Concept of Lignocellulosic Biomass-Based Integrated Biorefinery. In Waste Biorefinery; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.-J., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 409–444. ISBN 978-0-444-63992-9. [Google Scholar]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Top. Issue Biomater. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Moniruzzaman, M.; Goto, M.; Goto, M. Ionic Liquid Pretreatment of Lignocellulosic Biomass for Enhanced Enzymatic Delignification. Adv. Biochem. Eng. Biotechnol. 2019, 168, 61–77. [Google Scholar]
- Raud, M.; Rooni, V.; Kikas, T. Explosive decompression pretreatment: Nitrogen vs. compressed air. Agron. Res. 2016, 14, 569–578. [Google Scholar]
- Earle, M.J.; Seddon, K.R. Ionic Liquids: Green Solvents for the Future. In Clean Solvents; Martin, A.A., Luc, M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; Volume 819, pp. 10–25. ISBN 978-0-8412-3779-7. [Google Scholar]
- Amarasekara, A.S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhao, X.; Hu, Y. Lignocellulosic biomass delignification using aqueous alcohol solutions with the catalysis of acidic ionic liquids: A comparison study of solvents. Bioresour. Technol. 2018, 249, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Aldrich, C.; Lorenzen, L.; Wolfaardt, G. Acidogenic fermentation of lignocellulosic substrate with activated sludge. Chem. Eng. Commun. 2005, 192, 1221–1242. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Mahro, B.; Timm, M. Potential of Biowaste from the Food Industry as a Biomass Resource. Eng. Life Sci. 2007, 7, 457–468. [Google Scholar] [CrossRef]
- U.S. Department of Energy Biomass to Biofuels Workshop Report. Available online: https://genomicscience.energy.gov/biofuels/b2bworkshop.shtml (accessed on 17 February 2020).
- Li, M.; Pu, Y.; Ragauskas, A.J. Current Understanding of the Correlation of Lignin Structure with Biomass Recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef]
- Amore, A.; Knott, B.C.; Supekar, N.T.; Shajahan, A.; Azadi, P.; Zhao, P.; Wells, L.; Linger, J.G.; Hobdey, S.E.; Vander Wall, T.A.; et al. Distinct roles of N- and O-glycans in cellulase activity and stability. Proc. Natl. Acad. Sci. USA 2017, 114, 13667. [Google Scholar] [CrossRef]
- Zeng, Y.; Himmel, M.E.; Ding, S.-Y. Visualizing chemical functionality in plant cell walls. Biotechnol. Biofuels 2017, 10, 263. [Google Scholar] [CrossRef]
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Rocha Meneses, L.; Raud, M.; Orupõld, K.; Kikas, T. Second-generation bioethanol production: A review of strategies for waste valorisation. Agron. Res. 2017, 15, 830–847. [Google Scholar]
- Chen, Y.; Knappe, D.R.U.; Barlaz, M.A. Effect of cellulose/hemicellulose and lignin on the bioavailability of toluene sorbed to waste paper. Environ. Sci. Technol. 2004, 38, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schmauder, H.-P.; Heinze, T. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. In Biopolymers Online; Wiley-VCH Verlag: Oklahoma City, OK, USA, 2005; ISBN 978-3-527-60003-8. [Google Scholar]
- Zhou, L.; Santomauro, F.; Fan, J.; Macquarrie, D.; Clark, J.; Chuck, C.J.; Budarin, V. Fast microwave-assisted acidolysis: A new biorefinery approach for the zero-waste utilisation of lignocellulosic biomass to produce high quality lignin and fermentable saccharides. Faraday Discuss. 2017, 202, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Chapter 3—Lignocellulose biorefinery feedstock engineering. In Lignocellulose Biorefinery Engineering; Chen, H., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 37–86. ISBN 978-0-08-100135-6. [Google Scholar]
- Deshavath, N.N.; Veeranki, V.D.; Goud, V.V. Chapter 1—Lignocellulosic feedstocks for the production of bioethanol: Availability, structure, and composition. In Sustainable Bioenergy; Rai, M., Ingle, A.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. ISBN 978-0-12-817654-2. [Google Scholar]
- Rashid, T.; Kait, C.F.; Regupathi, I.; Murugesan, T. Dissolution of kraft lignin using Protic Ionic Liquids and characterization. Ind. Crops Prod. 2016, 84, 284–293. [Google Scholar] [CrossRef]
- Yoo, C.G.; Li, M.; Meng, X.; Pu, Y.; Ragauskas, A.J. Effects of organosolv and ammonia pretreatments on lignin properties and its inhibition for enzymatic hydrolysis. Green Chem. 2017, 19, 2006–2016. [Google Scholar] [CrossRef]
- Zhu, X.; Peng, C.; Chen, H.; Chen, Q.; Zhao, Z.; Zheng, Q.; Xie, H. Opportunities of Ionic Liquids for Lignin Utilization from Biorefinery. ChemistrySelect 2018, 3, 7945–7962. [Google Scholar] [CrossRef]
- Yinghuai, Z.; Yuanting, K.T.; Hosmane, N.S. Applications of Ionic Liquids in Lignin Chemistry. In Ionic Liquids—New Aspects for the Future, Jun-ichi Kadokawa; IntechOpen: London, UK, 2013. [Google Scholar]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895. [Google Scholar] [CrossRef]
- Barker, T. Applied Immunology and Biochemistry; ED-Tech Press: Essex, UK, 2019; ISBN 978-1-83947-170-4. [Google Scholar]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Li, J.; Carlson, B.E.; Lacis, A.A. Application of spectral analysis techniques to the intercomparison of aerosol data—Part 4: Synthesized analysis of multisensor satellite and ground-based AOD measurements using combined maximum covariance analysis. Atmos. Meas. Tech. 2014, 7, 2531–2549. [Google Scholar] [CrossRef]
- Luo, J.; Fang, Z.; Smith, R.L., Jr. Ultrasound-enhanced conversion of biomass to biofuels. Prog. Energy Combust. Sci. 2013, 41. [Google Scholar] [CrossRef]
- Marita, J.; Ralph, J.; Catherine, L.; Jouanin, L.; Boerjan, W. NMR characterization of lignins from transgenic poplars with suppressed caffeic acid O-methyltransferase activity. J. Chem. Soc. Perkin 1 2001, 2001. [Google Scholar] [CrossRef]
- Singh, S.K. Solubility of lignin and chitin in ionic liquids and their biomedical applications. Int. J. Biol. Macromol. 2019, 132, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Junying, Z. Clean production technology of integrated pretreatment for Lignocellulose. Afr. J. Agric. Res. 2013, 8, 339–348. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Achyuthan, K.E.; Achyuthan, A.M.; Adams, P.D.; Dirk, S.M.; Harper, J.C.; Simmons, B.A.; Singh, A.K. Supramolecular self-assembled chaos: Polyphenolic lignin’s barrier to cost-effective lignocellulosic biofuels. Mol. Basel Switz. 2010, 15, 8641–8688. [Google Scholar] [CrossRef]
- Erdtman, H. Lignins: Occurrence, formation, structure and reactions, K.V. Sarkanen and C. H. Ludwig, Eds., John Wiley & Sons, Inc., New York, 1971. 916 pp. $35.00. J. Polym. Sci. B 1972, 10, 228–230. [Google Scholar] [CrossRef]
- Chang, H.; Cowling, E.B.; Brown, W. Comparative Studies on Cellulolytic Enzyme Lignin and Milled Wood Lignin of Sweetgum and Spruce. J. Biol. Chem. Phys. Technol. Wood 1975, 29, 153–159. [Google Scholar] [CrossRef]
- Guerra, A.; Filpponen, I.; Lucia, L.A.; Saquing, C.; Baumberger, S.; Argyropoulos, D.S. Toward a better understanding of the lignin isolation process from wood. J. Agric. Food Chem. 2006, 54, 5939–5947. [Google Scholar] [CrossRef]
- Pinkert, A.; Goeke, D.F.; Marsh, K.N.; Pang, S. Extracting wood lignin without dissolving or degrading cellulose: Investigations on the use of food additive-derived ionic liquids. Green Chem. 2011, 13, 3124–3136. [Google Scholar] [CrossRef]
- Nasrullah, A.; Bhat, A.H.; Sada Khan, A.; Ajab, H. Chapter 9—Comprehensive approach on the structure, production, processing, and application of lignin. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Jawaid, M., Md Tahir, P., Saba, N., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 165–178. ISBN 978-0-08-100959-8. [Google Scholar]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Hydrolytic depolymerization of hydrolysis lignin: Effects of catalysts and solvents. Bioresour. Technol. 2015, 190, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef]
- Krigstin, S.; Sameni, J.; Sain, M. Solubility of lignin and acetylated lignin in organic solvents. BioResources 2017, 12, 1548–1565. [Google Scholar]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzyme Res. 2011, 2011, 787532. [Google Scholar] [CrossRef] [PubMed]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—A review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Cellulose based grafted biosorbents—Journey from lignocellulose biomass to toxic metal ions sorption applications—A review. J. Mol. Liq. 2017, 232, 62–93. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Tayyab, M.; Noman, A.; Islam, W.; Waheed, S.; Arafat, Y.; Ali, F.; Zaynab, M.; Lin, S.; Zhang, H.; Khan, D. Bioethanol production from lignocellulosic biomass by environment-friendly pretreatment methods: A review. Appl. Ecol. Environ. Res. 2017, 16. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Ivetić, D.Ž.; Omorjan, R.P.; Đorđević, T.R.; Antov, M.G. The impact of ultrasound pretreatment on the enzymatic hydrolysis of cellulose from sugar beet shreds: Modeling of the experimental results. Environ. Prog. Sustain. Energy 2017, 36, 1164–1172. [Google Scholar] [CrossRef]
- Bensah, E.; Mensah, M. Emerging Physico-Chemical Methods for Biomass Pretreatment; IntechOpen: London, UK, 2019; ISBN 978-1-78984-937-0. [Google Scholar]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Aftab, M.N.; Iqbal, I.; Riaz, F.; Karadag, A.; Tabatabaei, M. Different Pretreatment Methods of Lignocellulosic Biomass for Use in Biofuel Production. In Biomass for Bioenergy; Irfana, I., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-988-1. [Google Scholar]
- Smith, E.; Abbott, A.; Ryder, K. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114. [Google Scholar] [CrossRef]
- Melro, E.; Alves, L.; Antunes, F.; Medronho, B. A brief overview on lignin dissolution. J. Mol. Liq. 2018, 265. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Bonhivers, J.-C.; Stuart, P.R. Chapter 25—Applications of Process Integration Methodologies in the Pulp and Paper Industry. In Handbook of Process Integration (PI); Klemeš, J.J., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 765–798. ISBN 978-0-85709-593-0. [Google Scholar]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Norgren, M.; Lindström, B. Dissociation of Phenolic Groups in Kraft Lignin at Elevated Temperatures. Holzforschung 2000, 54, 519–527. [Google Scholar] [CrossRef]
- Li, T.; Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuels Bioprod. Biorefining Biofpr 2018, 12, 756–787. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Ishola, M.M.; Richards, T.; Taherzadeh, M.J. Chapter 1—An Overview of Existing Individual Unit Operations. In Biorefineries; Qureshi, N., Hodge, D.B., Vertès, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–36. ISBN 978-0-444-59498-3. [Google Scholar]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N.J. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Oliva, J.M.; Saez, F.; Ballesteros, I.; Gonzalez, A.; Negro, M.J.; Manzanares, P.; Ballesteros, M. Effect of lignocellulosic degradation compounds from steam explosion pretreatment on ethanol fermentation by thermotolerant yeast Kluyveromyces marxianus. Appl. Biochem. Biotechnol. 2003, 105–108, 141–153. [Google Scholar] [CrossRef]
- Bals, B.; Wedding, C.; Balan, V.; Sendich, E.; Dale, B. Evaluating the impact of ammonia fiber expansion (AFEX) pretreatment conditions on the cost of ethanol production. Bioresour. Technol. 2011, 102, 1277–1283. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Valorizing Recalcitrant Cellulolytic Enzyme Lignin via Lignin Nanoparticles Fabrication in an Integrated Biorefinery. ACS Sustain. Chem. Eng. 2017, 5, 2702–2710. [Google Scholar] [CrossRef]
- Merino, O.; Fundora-Galano, G.; Luque, R.; Martínez-Palou, R. Understanding Microwave-Assisted Lignin Solubilization in Protic Ionic Liquids with Multiaromatic Imidazolium Cations. ACS Sustain. Chem. Eng. 2018, 6, 4122–4129. [Google Scholar] [CrossRef]
- Xu, A.; Li, W.; Zhang, Y.; Xu, H. Eco-friendly polysorbate aqueous solvents for efficient dissolution of lignin. RSC Adv. 2016, 6, 8377–8379. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Mai, N.L.; Ahn, K.; Koo, Y.-M. Methods for recovery of ionic liquids—A review. Process Biochem. 2014, 49, 872–881. [Google Scholar] [CrossRef]
- Da Costa Lopes, A.M.; João, K.G.; Morais, A.R.C.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquids as a tool for lignocellulosic biomass fractionation. Sustain. Chem. Process. 2013, 1, 3. [Google Scholar] [CrossRef]
- Álvarez, V.H.; Dosil, N.; Gonzalez-Cabaleiro, R.; Mattedi, S.; Martin-Pastor, M.; Iglesias, M.; Navaza, J.M. Brønsted Ionic Liquids for Sustainable Processes: Synthesis and Physical Properties. J. Chem. Eng. Data 2010, 55, 625–632. [Google Scholar] [CrossRef]
- Anugwom, I.; Eta, V.; Virtanen, P.; Mäki-Arvela, P.; Hedenström, M.; Hummel, M.; Sixta, H.; Mikkola, J.-P. Switchable Ionic Liquids as Delignification Solvents for Lignocellulosic Materials. ChemSusChem 2014, 7, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Moyer, P.; Smith, M.D.; Abdoulmoumine, N.; Chmely, S.C.; Smith, J.C.; Petridis, L.; Labbé, N. Relationship between lignocellulosic biomass dissolution and physicochemical properties of ionic liquids composed of 3-methylimidazolium cations and carboxylate anions. Phys. Chem. Chem. Phys. 2018, 20, 2508–2516. [Google Scholar] [CrossRef]
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of Lignocellulosic Biomass with Ionic Liquids and Ionic Liquid-Based Solvent Systems. Molecules 2017, 22, 490. [Google Scholar] [CrossRef]
- Achinivu, E. Protic Ionic Liquids for Lignin Extraction—A Lignin Characterization Study. Int. J. Mol. Sci. 2018, 19, 428. [Google Scholar] [CrossRef]
- Miranda, R.d.C.M.; Neta, J.V.; Ferreira, L.F.R.; Gomes, W.A.J.; do Nascimento, C.S.; Gomes, E.d.B.; Mattedi, S.; Soares, C.M.F.; Lima, A.S. Pineapple crown delignification using low-cost ionic liquid based on ethanolamine and organic acids. Carbohydr. Polym. 2019, 206, 302–308. [Google Scholar] [CrossRef]
- Scriven, E.F.V.; Toomey, J.E., Jr.; Murugan, R. Pyridine and Pyridine Derivatives. In Kirk-Othmer Encyclopedia of Chemical Technology; American Cancer Society: Oklahoma City, OK, USA, 2000; ISBN 978-0-471-23896-6. [Google Scholar]
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.S.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef]
- Tolesa, L.; Gupta, B.; Lee, M.-J. Degradation of Lignin with Aqueous Ammonium-Based Ionic Liquid Solutions under Milder Condition. New J. Chem. 2019, 43. [Google Scholar] [CrossRef]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2009, 102, 1368–1376. [Google Scholar] [CrossRef]
- Achinivu, E.C.; Howard, R.M.; Li, G.; Gracz, H.; Henderson, W.A. Lignin extraction from biomass with protic ionic liquids. Green Chem. 2014, 16, 1114–1119. [Google Scholar] [CrossRef]
- Pu, Y.; Jiang, N.; Ragauskas, A.J. Ionic Liquid as a Green Solvent for Lignin. J. Wood Chem. Technol. 2007, 27, 23–33. [Google Scholar] [CrossRef]
- Xue, Z.; Zhao, X.; Sun, R.; Mu, T. Biomass-Derived γ-Valerolactone-Based Solvent Systems for Highly Efficient Dissolution of Various Lignins: Dissolution Behavior and Mechanism Study. ACS Sustain. Chem. Eng. 2016, 4, 3864–3870. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L.; Li, K.; Ma, Y.; Ma, N.; Ding, S.; Wang, L.; Zhao, D.; Yan, B.; Wan, W.; et al. Lignin dissolution in dialkylimidazolium-based ionic liquid-water mixtures. Bioresour. Technol. 2014, 170C, 499–505. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Material 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Shin, E.-J.; Eom, I.-Y.; Won, K.; Kim, Y.H.; Choi, D.; Choi, I.-G.; Choi, J.W. Structural features of lignin macromolecules extracted with ionic liquid from poplar wood. Bioresour. Technol. 2011, 102, 9020–9025. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.Y.; MacFarlane, D.R.; Upfal, J.; Edye, L.A.; Doherty, W.O.S.; Patti, A.F.; Pringle, J.M.; Scott, J.L. Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid. Green Chem. 2009, 11, 339–345. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.; Bustam, M.A.; Mutalib, M.I.A.; Wilfred, C.D.; Rafiq, S. Dissolution and delignification of bamboo biomass using amino acid-based ionic liquid. Appl. Biochem. Biotechnol. 2011, 165, 998–1009. [Google Scholar] [CrossRef]
- Xu, J.-K.; Sun, Y.-C.; Sun, R.-C. Structural and Hydrolysis Characteristics of Cypress Pretreated by Ionic Liquids in a Microwave Irradiation Environment. BioEnergy Res. 2014, 7, 1305–1316. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Kang, S.U.-M.; Zhang, X.; Yu-Ying, W.U.; Sun, R.-C. Isolation and physico-chemical characterization of lignin from hybrid poplar in dmso/licl system induced by microwave-assisted irradiation. Cellul. Chem. Technol. 2012, 46, 409–418. [Google Scholar]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodríguez, H.; Rogers, R.D. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- Sun, N.; Parthasarathi, R.; Socha, A.M.; Shi, J.; Zhang, S.; Stavila, V.; Sale, K.L.; Simmons, B.A.; Singh, S. Understanding pretreatment efficacy of four cholinium and imidazolium ionic liquids by chemistry and computation. Green Chem. 2014, 16, 2546–2557. [Google Scholar] [CrossRef]
- Dutta, T.; Papa, G.; Wang, E.; Sun, J.; Isern, N.G.; Cort, J.R.; Simmons, B.A.; Singh, S. Characterization of Lignin Streams during Bionic Liquid-Based Pretreatment from Grass, Hardwood, and Softwood. ACS Sustain. Chem. Eng. 2018, 6, 3079–3090. [Google Scholar] [CrossRef]
- Casas, A.; Palomar, J.; Alonso, M.; Oliet, M.; Omar, S. Comparison of lignin and cellulose solubilities in ionic liquids by COSMO-RS analysis and experimental validation. Ind. Crops Prod. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Lee, J.-M. Recovery of lignin and ionic liquid by using organic solvents. J. Ind. Eng. Chem. 2017, 49, 122–132. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, Y.; Ma, F.; Zhang, X.; Yu, H. Effect of biopretreatment on thermogravimetric and chemical characteristics of corn stover by different white-rot fungi. Bioresour. Technol. 2010, 101, 5475–5479. [Google Scholar] [CrossRef]
- Wen, J.-L.; Yuan, T.-Q.; Sun, S.-L.; Xu, F.; Sun, R.-C. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Factors governing dissolution process of lignocellulosic biomass in ionic liquid: Current status, overview and challenges. Bioresour. Technol. 2015, 178, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and purification of ionic liquids from solutions: A review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef]
- Krzysztoforski, J.; Henczka, M.; Krasinski, A.; Piątkiewicz, W. Enhancement of Supercritical Fluid Extraction in Membrane Cleaning Process by Addition of Organic Solvents. Inzynieria Chem. Proces. 2013, 43, 403–414. [Google Scholar] [CrossRef]
- Su, L.; Li, M.; Zhu, X.; Wang, Z.; Chen, Z.; Li, F.; Zhou, Q.; Hong, S. In situ crystallization of low-melting ionic liquid [BMIM][PF6] under high pressure up to 2 GPa. J. Phys. Chem. B 2010, 114, 5061–5065. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, J.S.; Zaworotko, M.J. Air and water sTable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun. 1992, 965–967. [Google Scholar] [CrossRef]
- Ulrich, J.; Jones, M.J. Industrial Crystallization: Developments in Research and Technology. 50th Anniv. Eur. Fed. Chem. Eng. 2004, 82, 1567–1570. [Google Scholar] [CrossRef]
- Solà Cervera, J.L.; Keil, P.; König, A. Determination of Distribution Coefficients in 1-Ethyl-3-Methyl Imidazolium Chloride-Methylimidazole Mixtures by Zone Melting. Chem. Eng. Technol. 2010, 33, 821–826. [Google Scholar] [CrossRef]
- Choudhury, A.R.; Winterton, N.; Steiner, A.; Cooper, A.I.; Johnson, K.A. In situ Crystallization of Low-Melting Ionic Liquids. J. Am. Chem. Soc. 2005, 127, 16792–16793. [Google Scholar] [CrossRef]
- Guardani, R.; Neiro, S.M.S.; Bülau, H.; Ulrich, J. Experimental comparison and simulation of static and dynamic solid layer melt crystallization. Ind. Cryst. 2001, 56, 2371–2379. [Google Scholar] [CrossRef]
- König, A.; Stepanski, M.; Kuszlik, A.; Keil, P.; Weller, C. Ultra-purification of ionic liquids by melt crystallization. ECCE-6 2008, 86, 775–780. [Google Scholar] [CrossRef]
- Birdwell, J.F.; McFarlane, J.; Hunt, R.D.; Luo, H.; DePaoli, D.W.; Schuh, D.L.; Dai, S. Separation of Ionic Liquid Dispersions in Centrifugal Solvent Extraction Contactors. Sep. Sci. Technol. 2006, 41, 2205–2223. [Google Scholar] [CrossRef]
- Santos, E.; Albo, J.; Irabien, A. Magnetic ionic liquids: Synthesis, properties and applications. RSC Adv. 2014, 4, 40008–40018. [Google Scholar] [CrossRef]
- Hayashi, S.; Hamaguchi, H. Discovery of a Magnetic Ionic Liquid [bmim]FeCl4. Chem. Lett. 2004, 33, 1590–1591. [Google Scholar] [CrossRef]
- Freire, M.G.; Ventura, S.P.M.; Santos, L.M.N.B.F.; Marrucho, I.M.; Coutinho, J.A.P. Evaluation of COSMO-RS for the prediction of LLE and VLE of water and ionic liquids binary systems. Fluid Phase Equilibria 2008, 268, 74–84. [Google Scholar] [CrossRef]
- Freire, M.G.; Santos, L.M.N.B.F.; Fernandes, A.M.; Coutinho, J.A.P.; Marrucho, I.M. An overview of the mutual solubilities of water–imidazolium-based ionic liquids systems. Prop. Phase Equilibria Prod. Process Des. 2007, 261, 449–454. [Google Scholar] [CrossRef]
- Williams, C.L.; Li, C.; Hu, H.; Allen, J.C.; Thomas, B.J. Three Way Comparison of Hydrophilic Ionic Liquid, Hydrophobic Ionic Liquid, and Dilute Acid for the Pretreatment of Herbaceous and Woody Biomass. Front. Energy Res. 2018, 6, 67. [Google Scholar] [CrossRef]
- Feng, D.; Li, L.; Yang, F.; Tan, W.; Zhao, G.; Zou, H.; Xian, M.; Zhang, Y. Separation of ionic liquid [Mmim][DMP] and glucose from enzymatic hydrolysis mixture of cellulose using alumina column chromatography. Appl. Microbiol. Biotechnol. 2011, 91, 399–405. [Google Scholar] [CrossRef]
- Shill, K.; Padmanabhan, S.; Xin, Q.; Prausnitz, J.M.; Clark, D.S.; Blanch, H.W. Ionic liquid pretreatment of cellulosic biomass: Enzymatic hydrolysis and ionic liquid recycle. Biotechnol. Bioeng. 2011, 108, 511–520. [Google Scholar] [CrossRef]
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Canongia Lopes, J.N.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef]
- Øye, H.A.; Jagtoyen, M.; Oksefjell, T.; Wilkes, J.S. Vapour Pressure and Thermodynamics of the System 1-Methyl-3-Ethyl-Imidazolium Chloride—Aluminium Chloride. Mater. Sci. Forum 1991, 73–75, 183–190. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Meli, L.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnol. Bioeng. 2011, 108, 1229–1245. [Google Scholar] [CrossRef]
- Glas, D.; Van Doorslaer, C.; Depuydt, D.; Liebner, F.; Rosenau, T.; Binnemans, K.; De Vos, D.E. Lignin solubility in non-imidazolium ionic liquids. J. Chem. Technol. Biotechnol. 2015, 90, 1821–1826. [Google Scholar] [CrossRef]
- Lignin Recovery. Available online: https://www.andritz.com/products-en/group/pulp-and-paper/pulp-production/kraft-pulp/lignin-recovery (accessed on 16 December 2019).
- Thompson, L.U.; Rickard, S.E.; Orcheson, L.J.; Seidl, M.M. Flaxseed and its lignan and oil components reduce mammary tumor growth at a late stage of carcinogenesis. Carcinogenesis 1996, 17, 1373–1376. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Li, C.; Chen, C.; Wu, X.; Tsang, C.-W.; Mou, J.; Yan, J.; Liu, Y.; Lin, C.S.K. Recent advancement in lignin biorefinery: With special focus on enzymatic degradation and valorization. Bioresour. Technol. 2019, 291, 121898. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lei, P.; Zhai, R.; Wen, Z.; Jin, M. Recent advances in lignin valorization with bacterial cultures: Microorganisms, metabolic pathways, and bio-products. Biotechnol. Biofuels 2019, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yu, I.K.M.; Liu, Y.; Ruan, X.; Tsang, D.C.W.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Lignin valorization for the production of renewable chemicals: State-of-the-art review and future prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Suhas, D.; Carrott, P.; Ribeiro Carrott, M.M. Lignin–From natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Ji, H.; Lv, P. Mechanistic insights into the lignin dissolution behaviors of a recyclable acid hydrotrope, deep eutectic solvent (DES), and ionic liquid (IL). Green Chem. 2020, 22, 1378–1387. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Dong, K.; Fan, M.; Zhang, S.; Zhang, Y.; He, H.; Dong, K.; Fan, M.; Zhang, S. A DFT study on lignin dissolution in imidazolium-based ionic liquids. RSC (R. Soc. Chem.) Adv. 2017, 7, 12670–12681. [Google Scholar] [CrossRef]
| Compound | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Reference |
|---|---|---|---|---|
| Hardwood | 40–55 | 24–40 | 18–25 | [23] |
| Softwood | 45–50 | 25–35 | 25–35 | [23] |
| Sugarcane Straw | 33–40 | 21–32 | 19–32 | [23] |
| Sugarcane Bagasse | 36–45 | 25–35 | 19–32 | [24] |
| Wheat Straw | 33–40 | 20–25 | 15–20 | [25] |
| Rice Straw | 28–36 | 23–28 | 12–14 | [26] |
| Waste Paper | 60–65 | 4–13 | 1–2 | [27] |
| Sample | Solvent | Temp (°C) | Time (h) | Lignin (%) | Cellulose (%) | References |
|---|---|---|---|---|---|---|
| Kraft lignin | Py | 90 | 24 | >50 | 0.10 ± 0.00 | [90] |
| Kraft lignin | Mim | 90 | 24 | >50 | 0.24 ± 0.02 | [90] |
| Kraft lignin | Pyrr | 90 | 24 | 7.98 ± 0.10 | 0.63 ± 0.00 | [90] |
| Kraft lignin | HAc | 90 | 24 | 0.72 ± 0.04 | 0.07 ± 0.01 | [90] |
| Kraft lignin | [Py] [For] | 75 | 1 | 70 | <1 | [91] |
| Kraft lignin | [Py] [Pro] | 75 | 1 | 55 | [32] | |
| Kraft lignin | [Py] [Ac] | 75 | 1 | 64 | [92] | |
| Kraft lignin | [Mmim] [MeSO4] | 80 | 24 | 50 | [92] | |
| Kraft lignin | [Bmim] [CF3SO3] | 80 | 24 | 50 | [90] | |
| Kraft lignin | [Py] [Ac] | 90 | 24 | >50 | 0.12 ± 0.03 | [90] |
| Kraft lignin | [Mim] [Ac] | 90 | 24 | >50 | 0.20 ± 0.05 | [90] |
| Kraft lignin | [Pyrr] [Ac] | 90 | 24 | >50 | 0.79 ± 0.04 | [93] |
| Kraft lignin | GVL/[Bmim]Ac | 30 | 20.9 | [93] | ||
| Kraft lignin | GVL/[Bmim]Ac | 60 | 28.0 | [93] | ||
| Kraft lignin | GVL/[Amim]Cl | 30 | 13.4 | [93] | ||
| Kraft lignin | GVL/[Amim]Cl | 60 | 43 | [94] | ||
| Kraft lignin | [Emim]Ac/water | 60 | 38 | [50] | ||
| Pinus radiata | [C2mim] Ace | 100 | 2 | 58 | [50] | |
| Pinus radiata | [C4mim] Ace | 100 | 2 | 63 | [50] | |
| Pinus radiata | [C4mim]Ace/DMSO | 100 | 2 | 51 | [95] | |
| Maple | [Emim]Ac | 110 | 1.5 | 9 | [95] | |
| Maple | [Emim]Ac | 130 | 24 | 6 | [95] | |
| Maple | [Mmim] [MeSO4] | 80 | 24 | 43.4a | [95] | |
| Maple | [Bmim] [CF3SO3] | 80 | 24 | 5.8b | [32] | |
| Birch | [Emim] [OAc] | 110 | 16 | 97c | [96] | |
| Polar wood | [Emim] [OAc] | 110 | 16 | 85.3c | [97] | |
| Bagasse | [Emim] [ABS] | 190 | 1–1.5 | 60.0c | [98] | |
| Bamboo | [Emim] [Gly] | 120 | 8 | 13.7a | [34] | |
| Corn stover | [Emim] [OAc]/NMP | 140 | 1 | 10.51a | [34] | |
| Cotton stalk | [Amim] [Cl]/DMSO | 130 | 4 | 74.4c | [34] | |
| Bagasse | [Bmim] [Cl] + NaOH | 110 | 12 | 12.73b | [34] | |
| Polar wood | [Emim] [OAc] + NaOH | 110 | 12 | 8.72b | ||
| Bamboo | [Amim] [Cl] + NaOH | 100 | 5 | 4.04b | ||
| Corncob | [Emim] [OAc]/H2O + NaOH | 110 | 9.78b | |||
| Corncob | [Emim] [OAc]/DMF + NaOH | 7.24b | ||||
| Corncob | [Emim] [OAc]/DMSO + NaOH | 19.5a | [99] | |||
| Corncob | [Emim] [OAc]/DMAc + NaOH | 32a | ||||
| Eucalyptus | [Bmim] [Ace] + NaOH | 120 | 3 | 37.3a | ||
| Eucalyptus | [Bmim] [Ace]/DMAc + NaOH | 25.8a | ||||
| Eucalyptus | [Bmim] [Ace]/Dioxane + NaOH | 29.9a | ||||
| Eucalyptus | [Bmim] [Ace]/Ethylacetate + NaOH | 70 | [34] | |||
| Eucalyptus | [Bmim] [Ace]/Toluene + NaOH | 74 | [34] | |||
| Eucalyptus | [Ch] [Lys] | 21 | [34] | |||
| Switchgrass | [Ch] [Lys] | 140 | 1 | |||
| Pine | [Ch] [Lys] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanov, I.; Raud, M.; Kikas, T. The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass. Energies 2020, 13, 4864. https://doi.org/10.3390/en13184864
Hasanov I, Raud M, Kikas T. The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass. Energies. 2020; 13(18):4864. https://doi.org/10.3390/en13184864
Chicago/Turabian StyleHasanov, Isa, Merlin Raud, and Timo Kikas. 2020. "The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass" Energies 13, no. 18: 4864. https://doi.org/10.3390/en13184864
APA StyleHasanov, I., Raud, M., & Kikas, T. (2020). The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass. Energies, 13(18), 4864. https://doi.org/10.3390/en13184864