Repurposing Fly Ash Derived from Biomass Combustion in Fluidized Bed Boilers in Large Energy Power Plants as a Mineral Soil Amendment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Physico-Chemical Properties of Fly Ash from Biomass Combustion in Fluidized Bed Boilers
3.2. Macro and Micronutrient Contents in Biomass Fly Ash in Accordance with Fertilizer Legislation
3.3. Non-Essential Elements and Contaminants
3.4. Bioavailability of Elements from Fly Ash
Speciation of Metals in Fly Ash
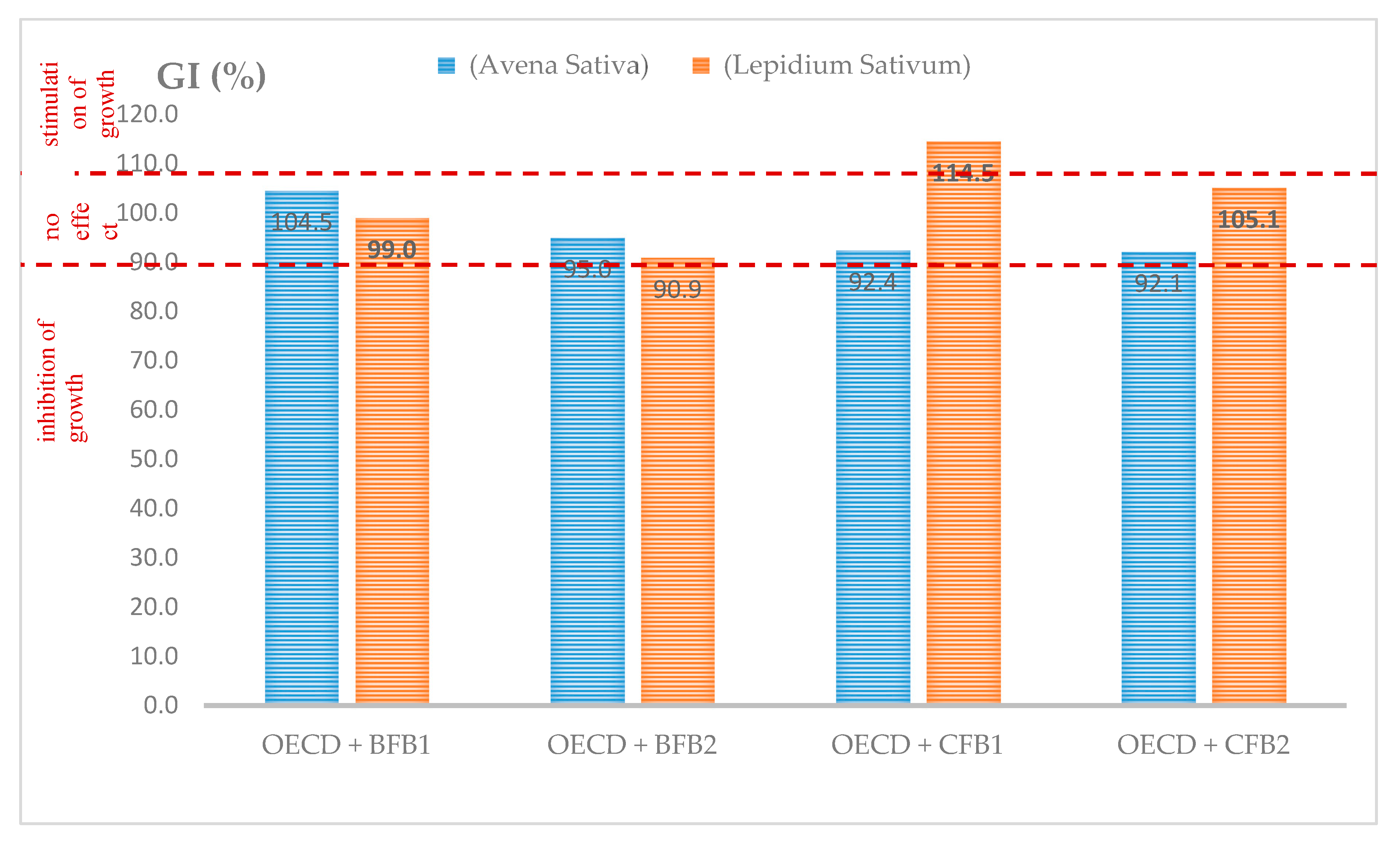
3.5. Acute Toxicity of Fly Ash Amendments to Plants Germination and Growth
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dz.U. 2014 poz. 1923. Regulation of the Minister of the Environment of 9 December 2014 on the Waste Catalog; Journal of Laws of the Republic of PolandJournal of Laws: Warsaw, Poland, 2014. [Google Scholar]
- Pallarès, D.; Johnsson, F. Macroscopic modelling of fluid dynamics in large-scale circulating fluidized beds. Prog. Energy Combust. Sci. 2006, 32, 539–569. [Google Scholar] [CrossRef]
- Leski, K.; Luty, P.; Łucki, A.; Jankowski, D. Application of circulating fluidized bed boilers in the fuel combustion process. Tech. Trans. 2018, 4, 83–96. [Google Scholar]
- Wang, H.; Bolan, N.; Hedley, M.; Horne, D. Potential Uses of Fluidised Bed Boiler Ash (FBA) as a Liming Material, Soil Conditioner and Sulfur Fertilizer. In Coal Combustion Byproducts and Environmental Issues; Sajwan, K.S., Twardowska, I., Punshon, T., Alva, A.K., Eds.; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical characteristics of biomass ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Kochanowska, K. The Influence of biomass ash on the migration of heavy metals in the flooded soil profile—Model experiment. Arch. Environ. Prot. 2014, 40, 3–15. [Google Scholar] [CrossRef][Green Version]
- Ciesielczuk, T.; Kusza, G.; Nemś, A. Nawożenie popiołami z termicznego przekształcania biomasy źródłem pierwiastków śladowych dla gleb. Ochr. Środowiska Zasobów Nat. 2011, 49, 219–227. (In Polish) [Google Scholar]
- Uliasz-Bocheńczyk, A.; Pawluk, A.; Pyzalski, M. Charakterystyka popiołów ze spalania biomasy w kotłach fluidalnych. Miner. Resour. Manag. 2016, 32, 149–162. [Google Scholar] [CrossRef]
- Uliasz-Bocheńczyk, A.; Mokrzycki, E. The elemental composition of biomass ashes as a preliminary assessment of the recovery potential. Miner. Resour. Manag. 2018, 34, 115–132. [Google Scholar] [CrossRef]
- Wilczyńska-Michalik, W.; Gasek, R.; Michalik, M.; Dańko, J.; Plaskota, T. Mineralogy, chemical composition and leachability of ash from biomass combustion and biomass–coal co-combustion. Mineralogia 2018, 49, 67–97. [Google Scholar] [CrossRef]
- Żelazny, S.E.; Jarosiński, A. The evaluation of fertilizer obtained from fly ash derived from biomass. Miner. Resour. Manag. 2019, 35, 139–152. [Google Scholar]
- Olanders, B.; Steenari, B.-M. Characterization of ashes from wood and straw. Biomass Bioenergy 1994, 8, 105–115. [Google Scholar] [CrossRef]
- Schiemenz, K.; Eichler-Löbermann, B. Biomass ashes and their phosphorus fertilizing effect on different crops. Nutr. Cycl. Agroecosyst. 2010, 87, 471–482. [Google Scholar] [CrossRef]
- Patterson, S.J.; Acharya, S.; Thomas, J.; Bertschi, A.B.; Rothwell, R.L. Integrated soil and crop management: Barley biomass and grain yield and canola seed yield response to land application of wood ash. Agron. J. 2004, 96, 971–977. [Google Scholar] [CrossRef]
- Mercl, F.; Tejnecký, V.; Száková, J.; Tlustos, P. Nutrient Dynamics in Soil Solution and Wheat Response after Biomass Ash Amendments. Agron. J. 2016, 108, 2222–2234. [Google Scholar] [CrossRef]
- Nabeela, F.; Murad, W.; Khan, I.; Mian, I.A.; Rehman, H.; Adnan, M.; Azizullah, A. Effect of wood ash application on the morphological, physiological and biochemical parameters of Brassica napus L. Plant Physiol. Biochem. 2015, 95, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ozolinčius, R.; Varnagirytĕ, I.; Armolaitis, K.; Karltun, E. Initial effects of wood ash fertilization on soil, needle and litterfall chemistry in a Scots Pine (Pinus sylvestris L). stand. Balt. For. 2005, 11, 21. [Google Scholar]
- Nurmesniemi, H.; Manskinen, K.; Poykio, R.; Dahl, O. Forest fertilizer properties of the bottom ash and fly ash from a large-sized (115 MW) industrial power plant incinerating wood-based biomass residues. J. Univ. Chem. Technol. Met. 2012, 47, 43–52. [Google Scholar]
- Ohenoja, K.; Pesonen, J.; Yliniemi, J.; Illikainen, M. Utilization of Fly Ashes from Fluidized Bed Combustion: A Review. Sustainability 2020, 12, 2988. [Google Scholar] [CrossRef]
- Phongpan, S.; Mosier, A. Impact of organic residue management on nitrogen use efficiency in an annual rice cropping sequence of lowland Central Thailand. Nutr. Cycl. Agroecosyst. 2003, 66, 233–240. [Google Scholar] [CrossRef]
- Ikpe, F.N.; Powell, J.M. Nutrient cycling practices and changes in soil properties in the crop-livestock farming systems of western Niger Republic of West Africa. Nutr. Cycl. Agroecosyst. 2002, 62, 37–45. [Google Scholar] [CrossRef]
- Saletnik, B.; Bajcar, M.; Zaguła, G.; Czernick, M.; Puchalski, C. Influence of biochar and biomass ash applied as soil amendment on germination rate of Virginia mallow seeds (Sida hermaphrodita R.). Econtechmod. An Int. Q. J. 2016, 5, 71–76. [Google Scholar]
- Owolabi, O.; Ojeniyi, S.; Amodu, A.; Hazzan, K. Response of cowpea, okra and tomato sawdust ash manure. Moor J. Agric. Res. 2003, 4, 178–182. [Google Scholar] [CrossRef]
- Meller, E.; Bilenda, E. Wpływ popiołów ze spalania biomasy na właściwości fizykochemiczne gleb lekkich. Polityka Energetyczna 2012, 15, 287–292. (In Polish) [Google Scholar]
- Ayeni, L.S.; Oso, O.P.; Ojeniyi, S.O. Effect of sawdust and wood ash application in improving soil chemical properties and growth of cocoa (Theobroma cacao) seedlings in the Nurseries. Agric. J. 2008, 3, 323–326. [Google Scholar]
- Dahl, O.; Nurmesniemi, H.; Poykio, R.; Watkins, G. Heavy metal concentrations in bottom ash and fly ash fractions from a large-sized (246 MW) fluidized bed boiler with respect to their Finnish forest fertilizer limit values. Fuel Process. Technol. 2010, 91, 1634–1639. [Google Scholar] [CrossRef]
- Ohenoja, K.; Tanskanen, P.; Wigren, V.; Kinnunen, P.; Körkkö, M.; Peltosaari, O.; Österbacka, J.; Illikainen, M. Self-hardening of fly ashes from a bubbling fluidized bed combustion of peat, forest industry residuals, and wastes. Fuel 2016, 165, 440–446. [Google Scholar] [CrossRef]
- Dahl, O.; Nurmesniemi, H.; Pöykiö, R.; Watkins, G. Comparison of the characteristics of bottom ash and fly ash from a medium-size (32 MW) municipal district heating plant incinerating forest residues and peat in a fluidized-bed boiler. Fuel Process. Technol. 2009, 90, 871–878. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Mäkelä, M.; Poykio, R.; Manskinen, K.; Dahl, O. Comparison of the forest fertilizer properties of ash fractions from two power plants of pulp and paper mills incinerating biomass-based fuels. Fuel Process. Technol. 2012, 104, 1–6. [Google Scholar] [CrossRef]
- Pöykiö, R.; Nurmesniemi, H.; Keiski, R.L. Total and size fractionated concentrations of metals in combustion ash from forest residues and peat. Proc. Est. Acad. Sci. 2009, 58, 247–255. [Google Scholar] [CrossRef]
- Pöykiö, R.; Nurmesniemi, H.; Perämäki, P.; Kuokkanen, T.; Välimäki, I. Leachability of metals in fly ash from a pulp and paper mill complex and environmental risk characterization for eco-efficient utilization of the fly ash as a fertilizer. Chem. Speciat. Bioavailab. 2005, 17, 1–9. [Google Scholar] [CrossRef]
- FINLEX ®-Viranomaisten Määräyskokoelmat: Maa-ja Metsätalousministeriö-01.09.2011 1784/14/2011. n.d. Available online: http://www.finlex.fi/fi/viranomaiset/normi/400001/37638 (accessed on 1 September 2020).
- Bekendtgørelse om Anvendelse af Bioaske til Jordbrugsformål (Bioaskebekendtgørelsen)-Retsinformation.dk. n.d. Available online: https://www.retsinformation.dk/forms/r0710.aspx?id=116609 (accessed on 1 September 2020).
- Van Dijen, F. Biofficiency. Ash Utilization-Draft for Technical Regulations (D6.3). Ref.Ares(2019)645078518/10/2019. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5c82e4e2f&appId=PPGMS (accessed on 1 September 2020).
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No. 1069/2009 and (EC) No. 1107/2009 and repealing Regulation (EC) No 2003/2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R1009 (accessed on 1 September 2020).
- Regulation (EC) No. 2003/2003 of the European Parliament and of the Council of 13 October 2003 Relating to Fertilisers. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003R2003&from=EN (accessed on 1 September 2020).
- European Committee for Standardization. EN 196-2:2013. Method of Testing Cement. Chemical Analysis of Cement; European Committee for Standardization (CEN) Standards: Brussels, Belgium, 2013. [Google Scholar]
- Polish Committee for Standardization. Standard PN EN 13657: 2006, Characterization of Waste-Digestion for Subsequent Determination of Aqua Regia Soluble Portion of Elements; Polish Committee for Standardization: Warsaw, Poland, 2006. [Google Scholar]
- Polish Committee for Standardization. Standard PN EN ISO 17294-2: 2016-11, Water Quality-Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)-Part 2: Determination of selected elements including Uranium Isotopes (ISO 17294-2:2016); Polish Committee for Standardization: Warsaw, Poland, 2006. [Google Scholar]
- Polish Committee for Standarization. Standard PN-EN 15407:2011, Solid Recovered Fuels-Methods for the Determination of Carbon (C), Hydrogen (H) and Nitrogen (N) Content; Polish Committee for Standardization: Warsaw, Poland, 2011. [Google Scholar]
- Polish Committee for Standarization. Standard PN-EN 15408:2011, Solid Recovered Fuels-Methods for the Determination of Sulphur (S), Chlorine (Cl), Fluorine (F) and Bromine (Br) content; Polish Committee for Standardization: Warsaw, Poland, 2011. [Google Scholar]
- Polish Committee for Standardization. Standard PN-EN 12457:2006. Characterisation of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges. Part 1–4; Polish Committee for Standardization: Warsaw, Poland, 2006. [Google Scholar]
- Polish Committee for Standardization. Standard EN ISO 10304-1:2009/AC:2012 Water Quality—Determination of Dissolved Anions by Liquid Chromatography of Ions—Part 1: Determination of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate—Technical Corrigendum 1 (ISO 10304-1:2007/Cor 1:2010); Polish Committee for Standardization: Warsaw, Poland, 2012. [Google Scholar]
- Polish Committee for Standardization. Standard PN EN ISO 14911:2002. Water Quality-Determination of Dissolved L[I+], N[A+], NH[4 +], K[+], MN[2+], CA[2+], MG[2+], SR[2+] AND BA[2+] Using Ion Chromatography-Method for Water and Waste Water; Polish Committee for Standardization: Warsaw, Poland, 2002. [Google Scholar]
- Polish Committee for Standardization. Standard PN-R-04023:1996. Chemical and Agricultural Analysis of soil—Determination of Available Phosphorus Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- Polish Committee for Standardization. Standard PN-R-04023:1996/A1:2002. Chemical and Agricultural Analysis of Soil—Determination of Available Potassium in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 2002. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of Heavy Metals in Soils and Sediments. An Account of the Improvement and Harmonization of Extraction Techniques Undertaken Under the Auspices of the BCR of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Polish Committee for Standardization. Standard PN EN ISO 17294-1: 2007-11, Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)-Part 1: General guidelines; Polish Committee for Standardization: Warsaw, Poland, 2007. [Google Scholar]
- Gariglio, N.F.; Buyatti, M.A.; Pilatti, R.A.; Russia, D.E.G.; Acosta, M.R. Use of a germination bioassay to test compost maturity of willow (Salix sp.) sawdust. N. Z. J. Crop. Hortic. Sci. 2002, 30, 135–139. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Poluszyńska, J.; Miłek, D.; Szewczyk, A.; Sławińska, I. Acute Toxicity of Experimental Fertilizers Made of Spent Coffee Grounds. Waste Biomass Valorization 2017, 9, 2157–2164. [Google Scholar] [CrossRef]
- Teacă, C.A.; Bodĭrlău, R. Assessment of toxicity of industrial waste crop plant assays. BioResources 2008, 3, 1130–1145. [Google Scholar]
- Oleszczuk, P.; Hollert, H. Comparison of sewage sludge toxicity to plants and invertebrates in three different soils. Chemosphere 2011, 83, 502–509. [Google Scholar] [CrossRef]
- Baran, A.; Tarnawski, M. Phytotoxkit/Phytotestkit and Microtox® as tools for toxicity assessment of sediments. Ecotoxicol. Environ. Saf. 2013, 98, 19–27. [Google Scholar] [CrossRef]
- Polish Committee for Standardization. Standard PN EN ISO 11269-1: 2013, Soil Quality—Determination of the Effects of Pollutants on Soil Flora–Part 1: Method for Measurement of Inhibition of Root Growth (ISO 11269-1:2012); Polish Committee for Standardization: Warsaw, Poland, 2013. [Google Scholar]
- Uliasz-Bocheńczyk, A.; Pawluk, A.; Sierka, J. Wymywalności zanieczyszczeń z popiołów lotnych ze spalania biomasy. Miner. Resour. Manag. 2015, 31, 145–156. [Google Scholar]
- Dz.U. 119.765. Regulation of the Minister of Agriculture and Rural Development of June 18, 2008 on the Implementation of Certain Provisions of the Act on Fertilizers and Fertilization; Journal of Laws of the Republic of Poland: Warsaw, Poland, 2008. [Google Scholar]
- Saletnik, B.; Zaguła, G.; Bajcar, M.; Czernicka, M.; Puchalski, C. Biochar and biomass ash as a soil ameliorant: The effect on selected soil properties and yield of Giant Miscanthus (Miscanthus × giganteus). Energies 2018, 11, 2535. [Google Scholar] [CrossRef]
- Zapałowska, A.; Puchalski, C.; Hury, G.; Makarewicz, A. Influence of fertilization with the use of biomass ash and sewage sludge on the chemical coposition of Jerusalem Artichole used for energy related purposes. J. Ecol. Eng. 2017, 18, 235–245. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Cobreros, C.; Reyes-Araiza, J.L.; Manzano-Ramírez, A.; Nava, R.; Rodríguez, M.; Mondragón-Figueroa, M.; Apátiga, L.M.; Rivera-Muñoz, E.M. Barley straw ash: Pozzolanic activity and comparison with other natural and artificial pozzolans from Mexico. BioResources 2015, 10, 3757–3774. [Google Scholar] [CrossRef]
- Jaworek, A.; Czech, T.; Sobczyk, A.T.; Krupa, A. Properties of biomass vs. coal fly ashes deposited in electrostatic precipitator. J. Electrost. 2013, 71, 165–175. [Google Scholar] [CrossRef]
- García-Jiménez, A.; Trejo-Téllez, L.I.; Guillén-Sánchez, D.; Gómez-Merino, F.C. Vanadium stimulates pepper plant growth and flowering, increases concentrations of amino acids, sugars and chlorophylls, and modifies nutrient concentrations. PLoS ONE 2018, 13, e0201908. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Liu, Z. Effect of vanadium on the growth of soybean seedlings. Plant Soil 1999, 216, 47–51. [Google Scholar] [CrossRef]
- Shanker, A.; Djanaguiraman, M.; Venkateswarlu, B. Chromium interactions in plants: Current status and future strategies. Metallomics 2009, 1, 375–383. [Google Scholar] [CrossRef]
- Dz.U. 2016 poz. 1395. Regulation of the Minister of the Environment of September 1, 2016 on the Method of Assessing the Pollution of the Earth’s Surface; Journal of Laws of the Republic of Poland: Warsaw, Poland, 2016. [Google Scholar]
- Badura, L. Metale w glebach-stan zagrożenia na przykładzie województwa katowickiego. In Ekologiczne problemy Górnego Śląska, red J. Śliwiok. Wyd; Wszechnica Górnośląskiego Towarzystwa Przyjaciół Nauk im. W. Roździeńskiego: Katowice, Poland, 1995. (In Polish) [Google Scholar]
- Wang, L.K.; Chen, J.P.; Hung, Y.; Shammas, N.K. Heavy Metals in the Environment; CRP Press Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Abbasi, S.A.; Abbasi, N.; Soni, R. Heavy Metals in the Environment; Mittal Publications: New Delhi, India, 2019. [Google Scholar]
- Bååth, E. Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut. 1989, 47, 335–379. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Tausz, M.; De Kok, L.J. Role of Sulfur for Plant Production in Agricultural and Natural Ecosystems. In Sulfur Metabolism in Phototrophic Organisms; Hell, R., Dahl, C., Knaff, D., Leustek, T., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 27, pp. 417–435. [Google Scholar]
- Hawkesford, M.J. Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. J. Exp. Bot. 2000, 51, 131–138. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Ohno, T. Erich MS Effect of wood ash application on soil pH and soil test nutrient levels. Agric. Ecosyst. Environ. 1990, 32, 223–239. [Google Scholar] [CrossRef]
- Pels, J.R.; De Nie, D.S.; Kiel, J.H.A. Utilization of ashes from biomass combustion and gasification. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005. ECN-RX-05-182. [Google Scholar]
- Sarkar, S.K.; Favas, P.J.C.; Rakshit, D.; Satpathy, K.K. Geochemical speciation and risk assessment of heavymetals in soils and sediments. In Environmental Risk Assessment of Soil Contamination; Hernandez-Soriano, M.C., Ed.; InTech: London, UK, 2014; p. 918. [Google Scholar]
- Agrawal, R.; Kumar, B.; Priyanka, K.; Narayan, C.; Shukla, K.; Sarkar, J. Anshumali Micronutrient Fractionation in Coal Mine-Affected Agricultural Soils, India. Bull. Environ. Contam. Toxicol. 2016, 96, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Dong, D. Adsorption of Pb and Cd onto metal oxides and organic material in natural surface coatings as determined by selective extractions: New evidence for the importance of Mn and Fe oxides. Water Res. 2000, 34, 427–436. [Google Scholar] [CrossRef]
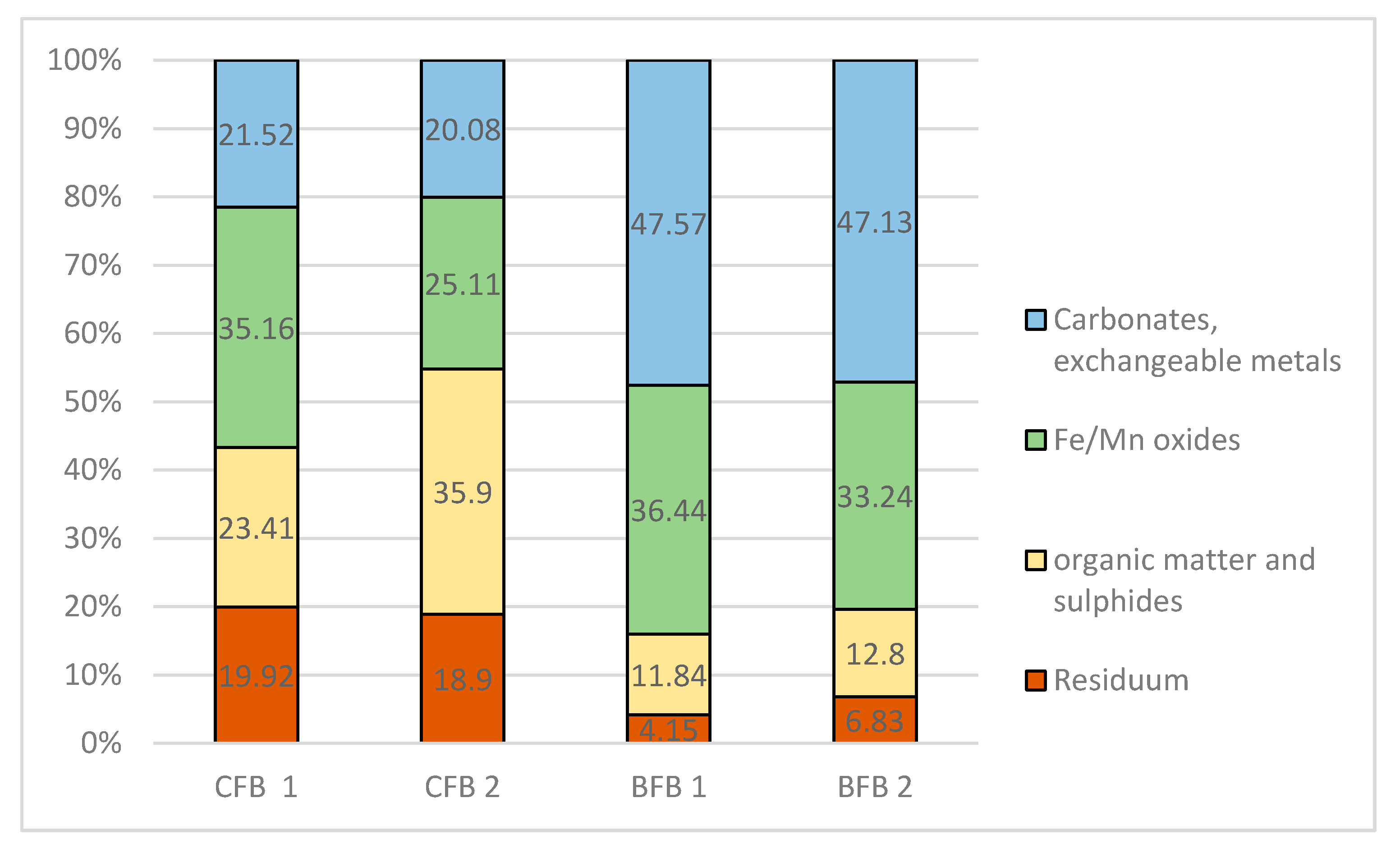
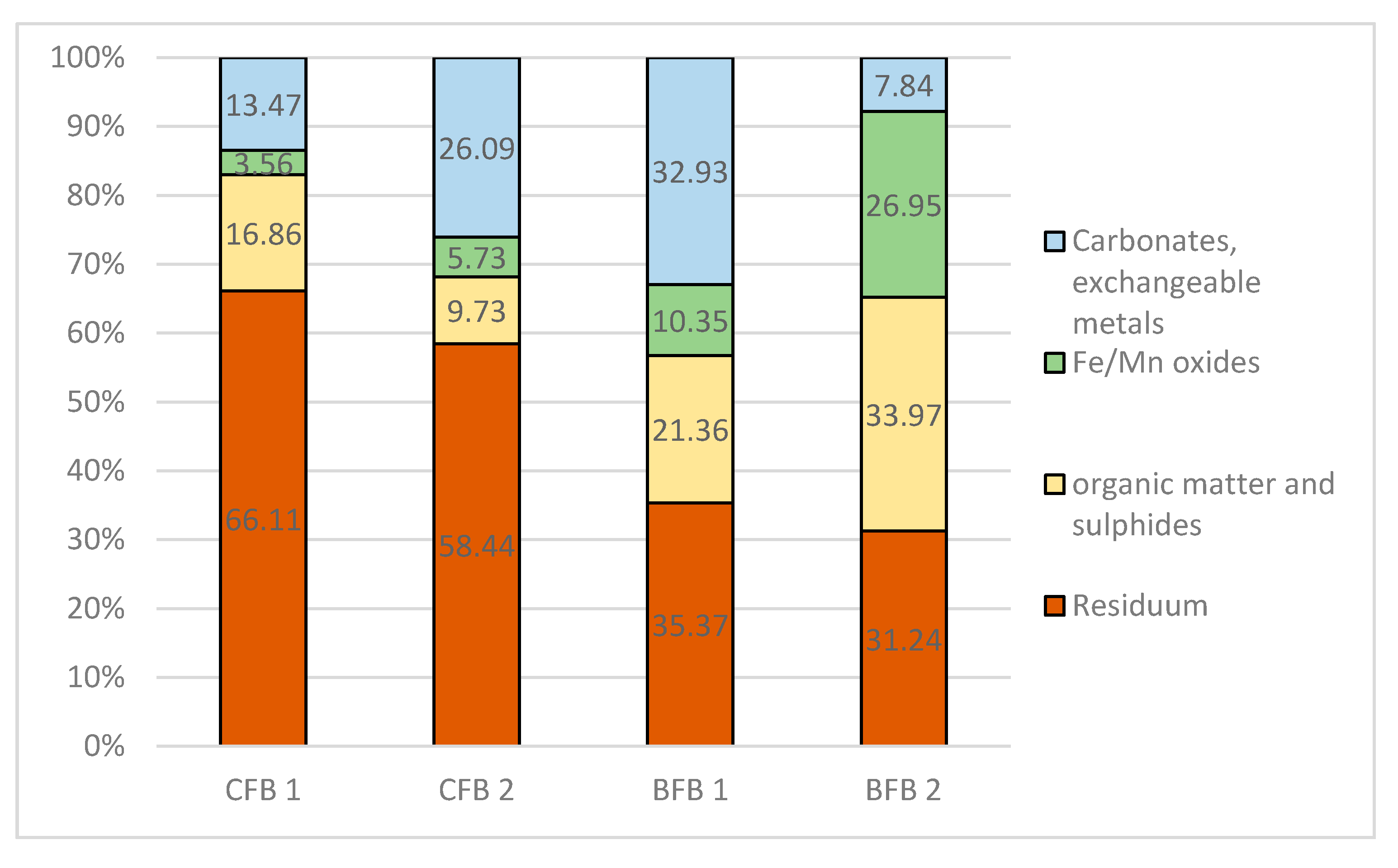
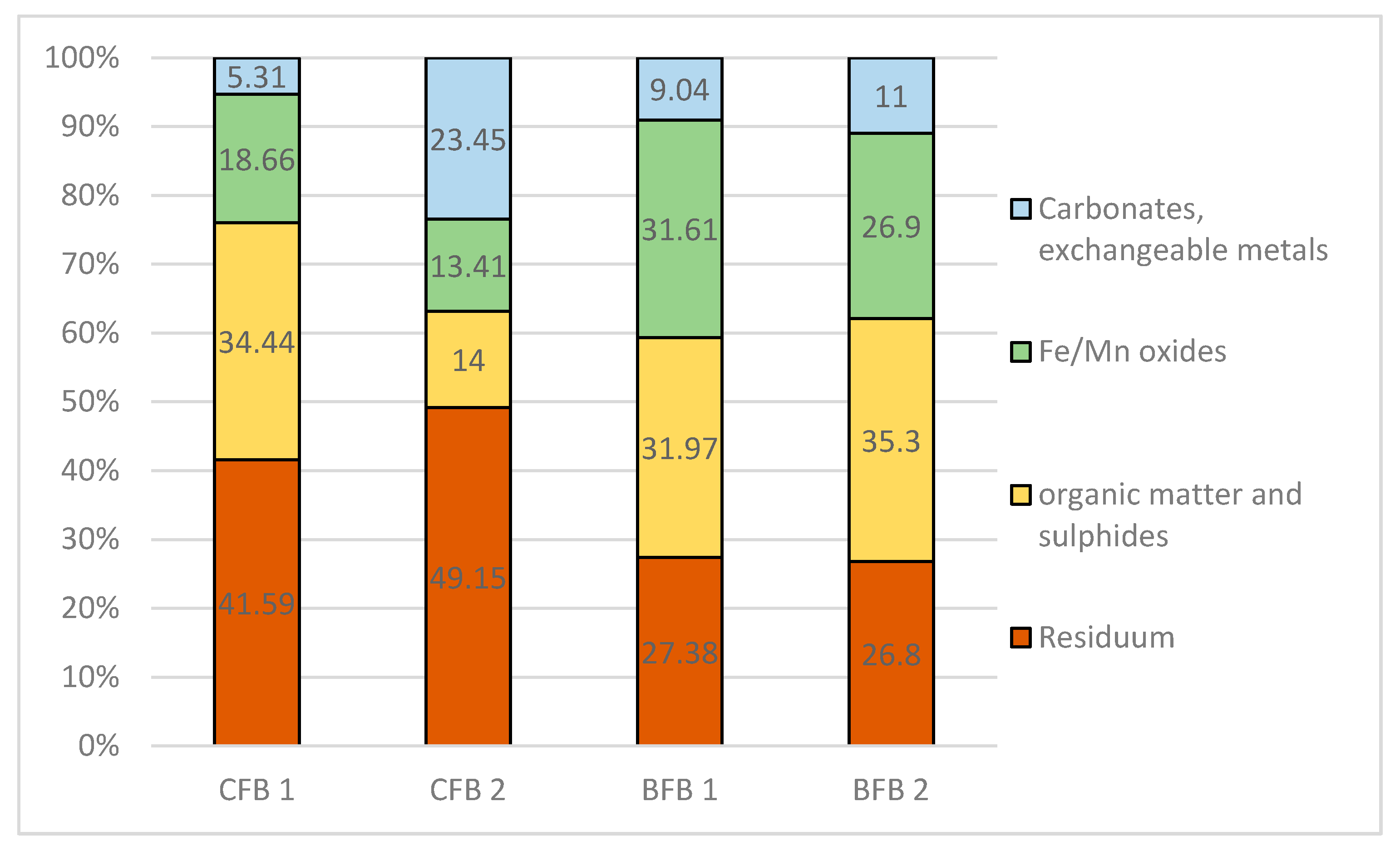
| Extraction Step | Fraction | Extractant |
|---|---|---|
| I | Acid soluble: exchangeable metals bound with carbonates. | 0.11 M CH3COOH S/L = 1:40 16 h shaking 30 rotation/min |
| II | Reducible: metals bound to Fe and Mn oxyhydroxides. | 0.1 M NH2OH·HCl, pH 2 (HNO3) S/L = 1:40 16 h shaking 30 rotation/min |
| III | Oxidisable: metals bound to organic matter and sulfides. | 30% H2O2 per 1 h, then 1M CH3COONH4, pH 2 (HNO3), S/L = 1:50 16 h shaking 30 rotation/min |
| IV * | Residual: lithogenous, non-silicate bound metals. | 65% HNO3 + H2O2 |
| Parameter | Fly Ash from CFB 1 | Fly Ash from CFB 2 | Fly Ash from BFB 1 | Fly Ash from BFB 2 | Threshold Values for Mineral Fertilizers (mg/kg) * | Minimum Nutrient Content for Mineral Fertilizers (wt%) * | |
|---|---|---|---|---|---|---|---|
| V | (mg/kg) | 22.6 ± 8.9 | 24.8 ± 9.7 | 14.3 ± 5.6 | 18.9 ± 7.4 | - | - |
| Cr | 50.0 ± 20.8 | 44.5 ± 18.5 | 48.5 ± 20.1 | 53.6 ± 22.2 | - | - | |
| Mn | 2315 ± 801 | 2299 ± 795 | 5698 ± 1972 | 7157 ± 2476 | - | - | |
| Co | 6.17 ± 2.42 | 5.31 ± 2.09 | 4.31 ± 1.69 | 4.72 ± 1.85 | - | - | |
| Ni | 33.8 ± 10.9 | 27.2 ± 8.8 | 17.3 ± 5.6 | 21.0 ± 6.8 | - | - | |
| Cu | 112 ± 38 | 92.9 ± 31,7 | 146 ± 50 | 86.8 ± 29.6 | - | - | |
| Zn | 325 ± 131 | 337 ± 135 | 583 ± 234 | 593 ± 238 | - | - | |
| As | 15.9 ± 4.9 | 6.41 ± 1.99 | 6.82 ± 2.12 | 7.85 ± 2.44 | 50 | - | |
| Cd | 6.12 ± 2.01 | 6.10 ± 2.00 | 8.14 ± 2.67 | 8.15 ± 2.67 | 50 */8 **/5 *** | - | |
| Sn | 7.50 ± 2.85 | 3.63 ± 1.38 | 1.02 ± 0.39 | b.d.l. | - | - | |
| Sb | 2.25 ± 0.68 | 2.67 ± 0.81 | 0.050 ± 0.015 | 0.800 ± 0.242 | - | - | |
| Tl | 1.15 ± 0.42 | 0.945 ± 0.347 | 2.11 ± 0.77 | 2.90 ± 1.06 | - | - | |
| Pb | 129 ± 45 | 71.3 ± 24.7 | 61.7 ± 21.4 | 51.4 ± 17.8 | 140 */200 **/600 *** | - | |
| Hg | 0.086 ± 0.023 | 0.064 ± 0.017 | 0.220 ± 0.059 | 0.240 ± 0.064 | 2 | - | |
| P2O5 | (% mass) | 2.00 ± 0.40 | 2.38 ± 0.48 | 3.57 ± 0.74 | 4.63 ± 0.93 | - | 2 |
| P | 0.880 | 1.04 | 1.57 | 2.04 | - | - | |
| K2O | 6.20 ± 0.37 | 6.88 ± 0.41 | 6.62 ± 0.40 | 8.24 ± 0.49 | - | 2 | |
| K | 5.14 | 5.71 | 5.49 | 6.84 | - | - | |
| CaO | 12.9 ± 2.3 | 14.1 ± 2.5 | 26.5 ± 4.8 | 24.8 ± 4.5 | - | - | |
| Ca | 9.19 | 10.06 | 18.94 | 17.73 | - | - | |
| MgO | 3.77 ± 0.45 | 4.06 ± 0.49 | 2.97 ± 0.36 | 3.31 ± 0.40 | - | - | |
| Mg | 2.27 | 2.45 | 1.79 | 1.99 | - | - | |
| SO3 | 4.59 ± 0.92 | 3.97 ± 0.79 | 4.16 ± 0.83 | 4.53 ± 0.91 | - | - | |
| S | 1.84 ± 0.18 | 1.59 ± 0.16 | 1.66 ± 0.16 | 1.81 ± 0.18 | - | 2 | |
| N | 0.040 ± 0.004 | 0.030 ± 0.003 | 0.020 ± 0.002 | 0.020 ± 0.002 | - | - | |
| Cl | 1.54 ± 0.16 | 1.16 ± 0.12 | 1.42 ± 0.15 | 1.32 ± 0.14 | - | - | |
| pH | - | -- | |||||
| PEW | (mS/m) | 12.07 | 10.5 | 20.4 | 18.77 | - | - |
| Leachable Concentration of Elements | Fly Ash from CFB 1 | Fly Ash from CFB 2 | Fly Ash from BFB 1 | Fly Ash from BFB 2 | |
|---|---|---|---|---|---|
| V | (mg/kg) | b.d.l. | b.d.l | 0.0035 ± 0.0013 | 0.0022 ± 0.0008 |
| Cr | 5.5 ± 1.46 | 5.2 ± 1.37 | 4.46 ± 1.19 | 4.99 ± 1.33 | |
| Mn | 0.01 ± 0.002 | 0.032 ± 0.0064 | 0.037 ± 0.0079 | 0.043 ± 0.0092 | |
| Co | b.d.l. | b.d.l. | 0.0022 ± 0.00053 | 0.0015 ± | |
| Ni | 0.5 ± 0.086 | 0.4 ± 0.068 | 0.066 ± 0.0114 | 0.0136 ± 0.0023 | |
| Cu | b.d.l. | b.d.l. | 0.385 ± 0.073 | 0.074 ± 0.0141 | |
| Zn | 0.21 ± 0.076 | 0.36 ± 0.13 | 0.59 ± 0.135 | 0.75 ± 0.169 | |
| As | 0.09 ± 0.033 | 0.05 ± 0.018 | 0.036 ± 0.00131 | <0.001 | |
| Cd | b.d.l. | b.d.l. | <0.001 | 0.00079 ± 0.00021 | |
| Sn | 0.12 ± 0.0316 | b.d.l. | <0.001 | <0.001 | |
| Tl | b.d.l. | b.d.l. | 0.0148 ± 0.0049 | 0.0361 ± 0.0118 | |
| Pb | 0.04 ± 0.010 | 0.0352 ± 0.009 | 0.602 ± 0.155 | 0.715 ± 0.184 | |
| Cl− | (mg/kg) | 13,168 ± 1027 | 10,439 ± 814 | 12,230 ± 954 | 14,850 ± 1158 |
| SO42− | 18,200 ± 3585 | 27,720 ± 5461 | 23,320 ± 4594 | 27,170 ± 5352 | |
| PO43− | b.d.l. | b.d.l. | b.d.l. | b.d.l. | |
| NO3− | 147 ± 11 | 152 ± 11 | 140 ± 10 | 146 ± 11 | |
| Ca2+ | 6209 ± 1130 | 4411 ± 803 | 6940 ± 1263 | 6135 ± 1117 | |
| Mg2+ | 339 ± 40 | 88.8 ± 10.4 | 0.200 ± 0.023 | 0.200 ± 0.023 | |
| Na+ | 97.4 ± 11.3 | 39.7 ± 4.6 | 87.6 ± 10.16 | 44.7 ± 5.18 | |
| K+ | 38,842 ± 2447 | 23,036 ± 1451 | 29,864 ± 1881 | 32,989 ± 2078 | |
| Kbioavailable(K2O) | (mg/100 g) | 4043 | 3520 | 3750 | 3000 |
| Pbioavailable(P2O5) | 2.5 | 1.8 | 2.1 | 2.2 | |
| Mixtures | An Average Number of Germinated Seeds | The Percentage Inhibition of Seed Germination IG (%) | ||
|---|---|---|---|---|
| Avena sativa | Lepidium sativum | Avena sativa | Lepidium sativum | |
| OECD control soil | 10.00 | 10.00 | - | - |
| OECD + BFB1 | 9.67 | 9.67 | 3.30 | 3.30 |
| OECD + BFB2 | 9.67 | 10.00 | 3.30 | 0.00 |
| OECD + CFB1 | 9.33 | 10.00 | 6.70 | 0.00 |
| OECD + CFB2 | 10.00 | 10.00 | 0.00 | 0.00 |
| Mixtures | An Average Root Length of Germinated Seeds (mm) | Root Growth Inhibition (%) | ||
|---|---|---|---|---|
| Avena sativa | Lepidium sativum | Avena sativa | Lepidium sativum | |
| OECD control soil | 95.0 | 55.0 | - | - |
| OECD + BFB1 | 102.7 | 56.3 | −8.10 * (8.10% stimulation) | −2.36 (2.36% stimulation |
| OECD + BFB2 | 93.3 | 50.0 | 1.79 | 9.09 |
| OECD + CFB1 | 94.1 | 63.0 | 0.95 | −14.55 (14.55% stimulation) |
| OECD + CFB2 | 87.5 | 57.8 | 7.89 | −5.09 (5.09% stimulation) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz-Krzemińska, E.; Poluszyńska, J. Repurposing Fly Ash Derived from Biomass Combustion in Fluidized Bed Boilers in Large Energy Power Plants as a Mineral Soil Amendment. Energies 2020, 13, 4805. https://doi.org/10.3390/en13184805
Jarosz-Krzemińska E, Poluszyńska J. Repurposing Fly Ash Derived from Biomass Combustion in Fluidized Bed Boilers in Large Energy Power Plants as a Mineral Soil Amendment. Energies. 2020; 13(18):4805. https://doi.org/10.3390/en13184805
Chicago/Turabian StyleJarosz-Krzemińska, Elżbieta, and Joanna Poluszyńska. 2020. "Repurposing Fly Ash Derived from Biomass Combustion in Fluidized Bed Boilers in Large Energy Power Plants as a Mineral Soil Amendment" Energies 13, no. 18: 4805. https://doi.org/10.3390/en13184805
APA StyleJarosz-Krzemińska, E., & Poluszyńska, J. (2020). Repurposing Fly Ash Derived from Biomass Combustion in Fluidized Bed Boilers in Large Energy Power Plants as a Mineral Soil Amendment. Energies, 13(18), 4805. https://doi.org/10.3390/en13184805






