Study on the Coupling Effect of a Solar-Coal Unit Thermodynamic System with Carbon Capture
Abstract
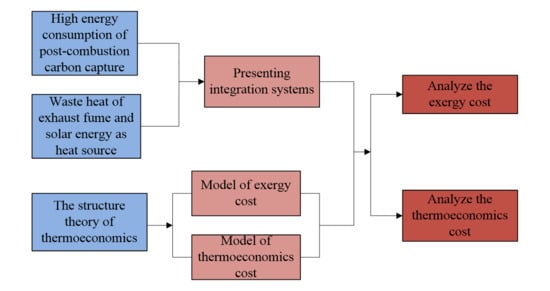
1. Introduction
2. Methods and Models
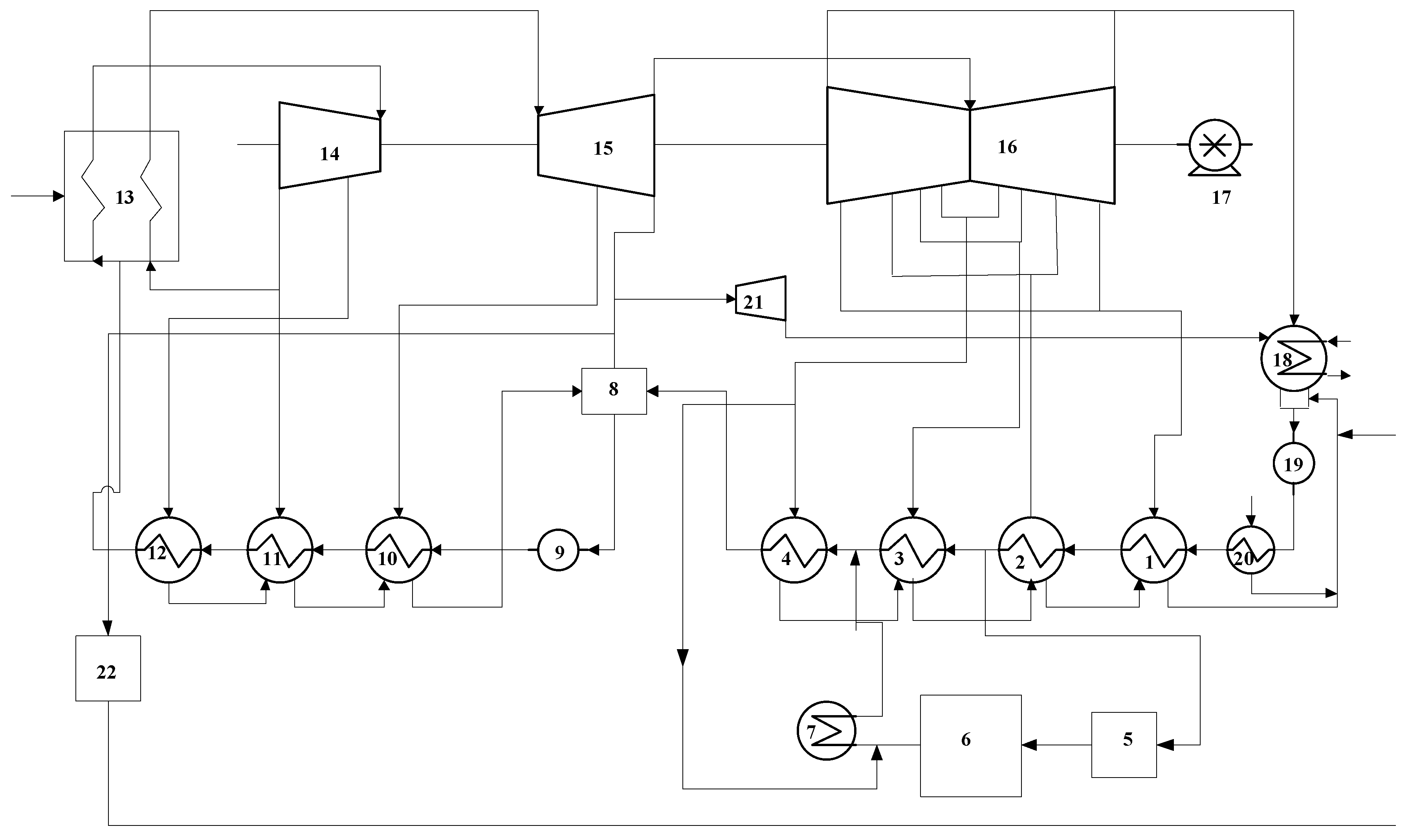
2.1. Pollutant Emission Reduction Method of a Solar Auxiliary Coal-Fired Unit
2.2. The Model of Structural Theory for the Integration System
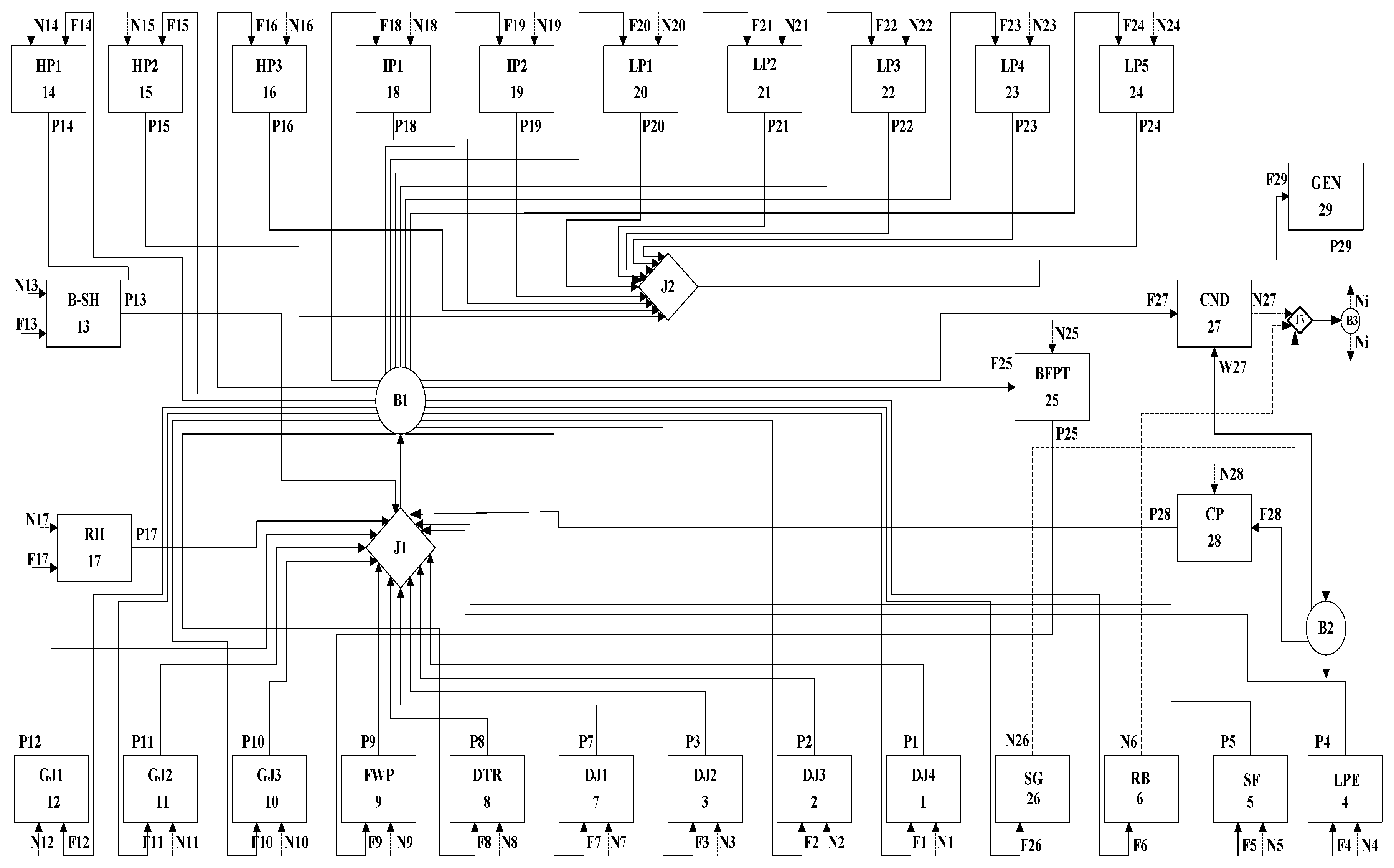
2.2.1. Physical Structure
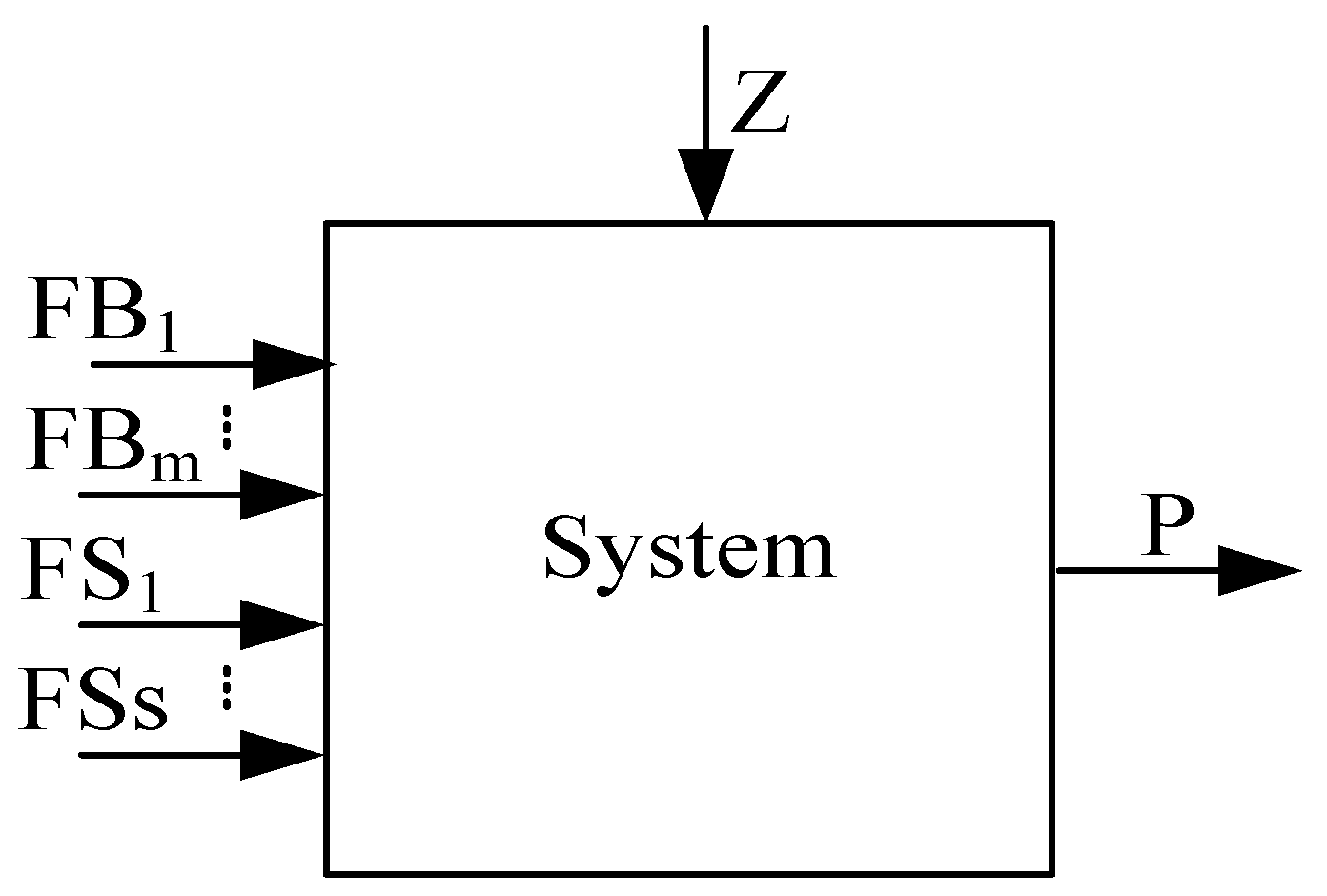
2.2.2. Production Structure
2.2.3. Characteristic Equation
2.2.4. Exergy Cost Equation
3. The Results and Discussion
4. Conclusions
- (1)
- The insufficiency of the traditional thermodynamics analysis is compensated for by the structural theory of thermo-economics. The information of the cost structure and energy transformation of the relevant equipment can be analyzed based on this theory;
- (2)
- The unit exergy cost and its distribution law in every component in the integration system and the original system are calculated based on the structural theory. The high exergy cost of the components in the integration system is mainly due to the increase of the irreversible exergy cost. The unit exergy cost of the component is impacted by the exergy efficiency and fuel exergy cost of the component. The unit exergy cost can be reduced by increasing the exergy efficiency of the boiler and solar collector field;
- (3)
- The composition and distribution law of the unit product thermo-economic cost of the integration system and the original system is analyzed based on the equation of the thermo-economic cost. The influence of the fuel price and equipment investment on the product thermo-economic cost of every component is studied.
Author Contributions
Funding
Conflicts of Interest
References
- EU. Global and European Sea-Level Rise. Available online: http://www.eea.europa.eu/data-and-maps/indicators/sea-level-rise-2/assessment (accessed on 18 May 2018).
- IEA. Electricity Information 2017; IEA Publications: Paris, France, 2017; Available online: https://euagenda.eu/publications/electricity-information-2017 (accessed on 18 May 2018).
- Global CCS Institute. The Global Status of CCS: 2018, GCCSI, Oct. 2018. Available online: https://www.globalccsinstitute.com/resources/global-status-report/ (accessed on 18 May 2018).
- UNFCCC. Historic Paris Agreement on Climate Change. Available online: http://newsroom.unfccc.int/unfccc-newsroom/finale-cop21/ (accessed on 18 May 2018).
- Zhao, R.; Deng, S.; Zhao, L.; Liu, Y.; Tan, Y. Energy-saving pathway exploration of CCS integrated with solar energy: Literature research and comparative analysis. Energy Convers. Manag. 2015, 102, 66–80. [Google Scholar] [CrossRef]
- Petrescu, L.; Bonalumi, D.; Valenti, G.; Cormos, A.-M.; Cormos, C.-C. Life Cycle Assessment for supercritical pulverized coal power plants with post-combustion carbon capture and storage. J. Clean. Prod. 2017, 157, 10–21. [Google Scholar] [CrossRef]
- Lindqvist, K.; Jordal, K.; Haugen, G.; Hoff, K.A.; Anantharaman, R. Integration aspects of reactive absorption for post-combustion CO 2 capture from NGCC (natural gas combined cycle) power plants. Energy 2014, 78, 758–767. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, Q.; Lei, J.; Jin, H. Investigation on the mid-temperature solar thermochemical power generation system with methanol decomposition. Appl. Energy 2018, 217, 56–65. [Google Scholar] [CrossRef]
- Wu, X.; Wang, M.; Liao, P.; Shen, J.; Li, Y. Solvent-based post-combustion COpost>2 capture for power plants: A critical review and perspective on dynamic modelling, system identification, process control and flexible operation. Appl. Energy 2020, 257, 113941. [Google Scholar] [CrossRef]
- Castellani, B.; Gambelli, A.M.; Morini, E.; Nastasi, B.; Presciutti, A.; Filipponi, M.; Nicolini, A.; Rossi, F. Experimental investigation on CO2 methanation process for solar energy storage compared to co2-based methanol synthesis. Energies 2017, 10, 855. [Google Scholar] [CrossRef]
- Castellani, B.; Rinaldi, S.; Morini, E.; Nastasi, B.; Rossi, F. Flue gas treatment by power-to-gas integration for methane and ammonia synthesis–Energy and environmental analysis. Energy Convers. Manag. 2018, 171, 626–634. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, L.; Zhao, L.; Deng, S.; Li, S.; Zhang, Y.; Li, H. Techno-economic analysis of carbon capture from a coal-fired power plant integrating solar-assisted pressure-temperature swing adsorption (PTSA). J. Clean. Prod. 2019, 214, 440–451. [Google Scholar] [CrossRef]
- Li, C.; Zhai, R.; Zhang, B.; Chen, W. Thermodynamic performance of a novel solar tower aided coal-fired power system. Appl. Therm. Eng. 2020, 171, 115–127. [Google Scholar] [CrossRef]
- Wang, C.; He, B.; Sun, S.; Wu, Y.; Yan, N.; Yan, L.; Pei, X. Application of a low pressure economizer for waste heat recovery from the exhaust flue gas in a 600 MW power plant. Energy 2012, 48, 196–202. [Google Scholar] [CrossRef]
- Madhlopa, A. Thermodynamic Cycles of Solar Gas Turbines; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Mokhtar, M.; Ali, M.T.; Khalilpour, R.; Abbas, A.; Shah, N.; Hajaj, A.A.; Armstrong, P.; Chiesa, M.; Sgouridis, S. Solar-assisted post-combustion carbon capturefeasibility study. Appl. Energy 2012, 92, 668–676. [Google Scholar] [CrossRef]
- Wang, W.; Fu, Y.W.; Fan, Q.W.; Huang, J.S.; Chang, D.F. Thermodynamic analysis of a solar aided coal-fired power plant. IOP Conf. Ser. Earth Environ. Sci. 2018, 153, 042020. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Q.; Ren, M.; Cheng, L. Combustion optimization for coal fired power plant boilers based on improved distributed ELM and distributed PSO. Energies 2019, 12, 1036. [Google Scholar] [CrossRef]
- Zhao, W.; Bai, R.; Wang, J.; Bai, R. Analysis thermodynamic performances and techno-economic of solar coal-fired units based on carbon capture. Chem. Ind. Eng. Prog. 2014, 34, 724–733. [Google Scholar]
- Hou, H.; Gao, S.; Yang, Y. Thermodynamics analysis of coal-fired power generation system aided by parabolic trough collective fields. Acta Energ. Sol. Sin. 2012, 32, 1772–1776. [Google Scholar]
- Zhang, C.; Liu, L.-M.; Chen, S.; Zheng, C.-G. Performance evaluation of thermal power system based on the structure theory of thermo-economic. Proc. Chin. Soc. Electr. Eng. 2005, 25, 108–113. [Google Scholar]
- Frangopoulos, C.A. Application of the thermo-economic functional approach to the CGAM problem. Energy 1994, 19, 323–342. [Google Scholar] [CrossRef]
- Von Spakovsky, M.R. Application of engineering functional analysis to the analysis and optimization of the CGAM problem. Energy 1994, 19, 343–364. [Google Scholar] [CrossRef]
- Valero, A.; Lerch, F.; Serra, L.; Royo, J. Structural theory and thermo-economic diagnosis: Part II: Application to an actual power plant. Energy Convers. Manag. 2002, 43, 1519–1535. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Han, Z.; Liu, X.; Qian, J. Calculation and analysis of the chemical exergy based on high heating values of fuel. J. Chin. Soc. Power Eng. 2012, 32, 804–808. [Google Scholar]
- Saidur, R.; Boroumandjazi, G.; Mekhlif, S.; Jameel, M. Exergy analysis of solar energy applications. Renew. Sustain. Energy Rev. 2012, 16, 350–356. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Zhan, D.; Zhuang, J. Analysis of exergy in thermal power generation system using steam directly produced from the parabolic trough type solar energy facility. Therm. Power Gener. 2008, 37, 39–43. [Google Scholar]
- Badescu, V. How much work can be extracted from diluted solar radiation? Sol. Energy 2018, 170, 1095–1100. [Google Scholar] [CrossRef]
- Chena, S.; Lib, Y.; Qin, Y. The health costs of the industrial leap forward in China: Evidence from the sulfur dioxide emissions of coal-fired power stations. China Econ. Rev. 2018, 49, 68–83. [Google Scholar] [CrossRef]
- Warman, E.; Nasution, F.S.; Fahmi, F. Energy cost unit of street and park lighting system with solar technology for a more friendly city. IOP Conf. Ser. Earth Environ. Sci. 2018, 126, 012033. [Google Scholar] [CrossRef]
- Wegeng, R.S.; Humble, P.H.; Krishnan, S.; Leith, S.D.; Palo, D.R.; Dagle, R.A. Solar Thermochemical Processing System and Method; Battelle Memorial Institute Inc: Columbus, OH, USA, 2018. [Google Scholar]


| Car | Har | Oar | Nar | Sar |
|---|---|---|---|---|
| 60.40 | 2.89 | 3.07 | 0.87 | 0.68 |
| Serial Number | Component | Product Exergy Cost | Fuel Exergy Cost | Specific Irreversible Exergy Cost | Specific Negentropy Exergy Cost | ||||
|---|---|---|---|---|---|---|---|---|---|
| Original System | Integration System | Original System | Integration System | Original System | Integration System | Original System | Integration System | ||
| 1 | 4DJ | 3.196 | 3.931 | 2.086 | 2.524 | 1.026 | 1.153 | 0.084 | 0.254 |
| 2 | 3DJ | 2.477 | 2.976 | 2.086 | 2.524 | 0.373 | 0.402 | 0.017 | 0.049 |
| 3 | 2DJ | 2.381 | 2.717 | 2.086 | 2.524 | 0.282 | 0.079 | 0.013 | 0.113 |
| 4 | LPE | - | 5.795 | - | 1 | - | 3.596 | - | 1.199 |
| 5 | SF | - | 4.538 | - | 1 | - | 2.651 | - | 0.886 |
| 6 | RB | - | 0.952 | - | 2.524 | - | 0.952 | - | - |
| 7 | 1DJ | 2.354 | 2.865 | 2.086 | 2.524 | 0.256 | 0.23 | 0.012 | 0.11 |
| 8 | DTR | 2.284 | 3.073 | 2.086 | 2.524 | 0.151 | 0.486 | 0.047 | 0.062 |
| 9 | FWP | 2.943 | 3.712 | 2.728 | 3.626 | 0.2 | 0.075 | 0.015 | 0.011 |
| 10 | 3GJ | 2.329 | 2.791 | 2.086 | 2.524 | 0.231 | 0.236 | 0.011 | 0.031 |
| 11 | 2GJ | 2.214 | 2.679 | 2.086 | 2.524 | 0.122 | 0.137 | 0.006 | 0.018 |
| 12 | 1GJ | 2.178 | 2.631 | 2.086 | 2.524 | 0.087 | 0.094 | 0.004 | 0.012 |
| 13 | B-SH | 2.022 | 2.204 | 1 | 1 | 0.941 | 0.94 | 0.081 | 0.264 |
| 14 | HP1 | 2.535 | 2.984 | 2.086 | 2.524 | 0.427 | 0.406 | 0.021 | 0.054 |
| 15 | HP2 | 2.185 | 2.665 | 2.086 | 2.524 | 0.094 | 0.124 | 0.005 | 0.016 |
| 16 | HP3 | 2.195 | 2.681 | 2.086 | 2.524 | 0.104 | 0.139 | 0.005 | 0.018 |
| 17 | RH | 2.015 | 2.109 | 1 | 1 | 0.934 | 0.868 | 0.081 | 0.242 |
| 18 | IP1 | 2.185 | 2.678 | 2.086 | 2.524 | 0.095 | 0.135 | 0.005 | 0.018 |
| 19 | IP2 | 2.143 | 2.606 | 2.086 | 2.524 | 0.054 | 0.072 | 0.003 | 0.01 |
| 20 | LP1 | 2.176 | 2.648 | 2.086 | 2.524 | 0.086 | 0.109 | 0.004 | 0.014 |
| 21 | LP2 | 2.145 | 2.6 | 2.086 | 2.524 | 0.056 | 0.067 | 0.003 | 0.009 |
| 22 | LP3 | 2.144 | 2.615 | 2.086 | 2.524 | 0.055 | 0.08 | 0.003 | 0.011 |
| 23 | LP4 | 2.174 | 2.633 | 2.086 | 2.524 | 0.084 | 0.096 | 0.004 | 0.013 |
| 24 | LP5 | 2.445 | 2.994 | 2.086 | 2.524 | 0.342 | 0.414 | 0.017 | 0.055 |
| 25 | BFPT | 2.728 | 3.626 | 2.086 | 2.524 | 0.611 | 0.973 | 0.03 | 0.129 |
| 26 | SG | - | 1.263 | - | 2.524 | - | 1.263 | - | - |
| 27 | CND | 0.103 | 0.108 | 2.086 | 2.129 | 0.102 | 0.104 | 0 | 0 |
| 28 | CP | 2.892 | 3.577 | 2.27 | 2.764 | 0.595 | 0.725 | 0.027 | 0.088 |
| 29 | GEN | 2.27 | 2.764 | 2.225 | 2.709 | 0.045 | 0.055 | 0 | 0 |
| Serial Number | Component | Product Unit Thermo-Economic Cost | Fuel Unit Thermo-Economic Cost’ | Increase of the Thermo-Economic Cost Caused by Irreversibilities | Increase of the Thermo-Economic Cost Cased by Negentropy | ||||
|---|---|---|---|---|---|---|---|---|---|
| Original System | Integration System | Original System | Integration System | Original System | Integration System | Original System | Integration System | ||
| 1 | 4DJ | 97.179 | 96.435 | 63.428 | 61.931 | 31.197 | 28.275 | 2.554 | 6.23 |
| 2 | 3DJ | 75.305 | 72.998 | 63.428 | 61.931 | 11.351 | 9.855 | 0.526 | 1.212 |
| 3 | 2DJ | 72.38 | 66.66 | 63.428 | 61.931 | 8.559 | 1.947 | 0.394 | 2.782 |
| 4 | LPE | - | 29.404 | - | 0 | - | 0 | - | 29.404 |
| 5 | SF | - | 21.746 | - | 0 | - | 0 | - | 21.746 |
| 6 | RB | - | 85.282 | - | 61.931 | - | 23.351 | - | - |
| 7 | 1DJ | 71.576 | 70.28 | 63.428 | 61.931 | 7.787 | 5.642 | 0.361 | 2.707 |
| 8 | DTR | 69.441 | 75.383 | 63.428 | 61.931 | 4.587 | 11.927 | 1.426 | 1.525 |
| 9 | FWP | 89.487 | 91.066 | 82.938 | 88.959 | 6.092 | 1.842 | 0.457 | 0.265 |
| 10 | 3GJ | 70.813 | 68.478 | 63.428 | 61.931 | 7.038 | 5.781 | 0.347 | 0.766 |
| 11 | 2GJ | 67.32 | 65.735 | 63.428 | 61.931 | 3.71 | 3.359 | 0.183 | 0.445 |
| 12 | 1GJ | 66.207 | 64.552 | 63.428 | 61.931 | 2.649 | 2.315 | 0.131 | 0.307 |
| 13 | B-SH | 61.49 | 65.447 | 30.404 | 30.404 | 28.61 | 28.572 | 2.475 | 6.471 |
| 14 | HP1 | 77.061 | 73.214 | 63.428 | 61.931 | 12.992 | 9.964 | 0.642 | 1.32 |
| 15 | HP2 | 66.421 | 65.37 | 63.428 | 61.931 | 2.852 | 3.037 | 0.141 | 0.402 |
| 16 | HP3 | 66.743 | 65.78 | 63.428 | 61.931 | 3.159 | 3.399 | 0.156 | 0.45 |
| 17 | RH | 61.264 | 62.712 | 30.404 | 30.404 | 28.404 | 26.381 | 2.456 | 5.926 |
| 18 | IP1 | 66.445 | 65.687 | 63.428 | 61.931 | 2.876 | 3.317 | 0.142 | 0.439 |
| 19 | IP2 | 65.148 | 63.924 | 63.428 | 61.931 | 1.64 | 1.76 | 0.081 | 0.233 |
| 20 | LP1 | 66.172 | 64.952 | 63.428 | 61.931 | 2.615 | 2.668 | 0.129 | 0.353 |
| 21 | LP2 | 65.203 | 63.781 | 63.428 | 61.931 | 1.692 | 1.634 | 0.084 | 0.216 |
| 22 | LP3 | 65.184 | 64.156 | 63.428 | 61.931 | 1.674 | 1.965 | 0.083 | 0.26 |
| 23 | LP4 | 66.113 | 64.589 | 63.428 | 61.931 | 2.559 | 2.347 | 0.126 | 0.311 |
| 24 | LP5 | 74.343 | 73.444 | 63.428 | 61.931 | 10.402 | 10.167 | 0.514 | 1.346 |
| 25 | BFPT | 82.938 | 88.959 | 63.428 | 61.931 | 18.592 | 23.867 | 0.918 | 3.161 |
| 26 | SG | - | 30.973 | - | 61.931 | - | 30.973 | - | 0 |
| 27 | CND | 3.132 | 2.646 | 63.428 | 61.931 | 3.098 | 3.025 | - | 0 |
| 28 | CP | 87.938 | 87.73 | 69.023 | 67.806 | 18.093 | 17.774 | 0.821 | 2.15 |
| 29 | GEN | 69.023 | 67.806 | 67.65 | 66.456 | 1.374 | 1.349 | 0 | 0 |
| Serial Number | Component | Product Unit Thermo-Economic Cost | Fuel Unit Thermo-Economic Cost’ | Increase of the Thermo-Economic Cost Caused by Irreversibilities | Increase of the Thermo-Economic Cost Cased by Negentropy | ||||
|---|---|---|---|---|---|---|---|---|---|
| Original System | Integration System | Original System | Integration System | Original System | Integration System | Original System | Integration System | ||
| 1 | 4DJ | 211.383 | 278.481 | 101.198 | 136.918 | 49.775 | 62.512 | 5.999 | 27.927 |
| 2 | 3DJ | 148.653 | 191.59 | 101.198 | 136.918 | 18.111 | 21.789 | 1.236 | 5.435 |
| 3 | 2DJ | 140.66 | 193.79 | 101.198 | 136.918 | 13.656 | 4.305 | 0.925 | 12.472 |
| 4 | LPE | - | 155.11 | - | 0 | - | 0 | - | 131.814 |
| 5 | SF | - | 232.249 | - | 0 | - | 0 | - | 97.485 |
| 6 | RB | - | 105.131 | - | 136.918 | - | 51.625 | - | 0 |
| 7 | 1DJ | 135.977 | 185.402 | 101.198 | 136.918 | 12.424 | 12.473 | 0.848 | 12.137 |
| 8 | DTR | 124.902 | 183.184 | 101.198 | 136.918 | 7.318 | 26.369 | 3.349 | 6.838 |
| 9 | FWP | 188.473 | 256.887 | 156.599 | 230.329 | 11.503 | 4.769 | 1.074 | 1.189 |
| 10 | 3GJ | 126.1 | 165.552 | 101.198 | 136.918 | 11.229 | 12.782 | 0.816 | 3.432 |
| 11 | 2GJ | 119.104 | 157.721 | 101.198 | 136.918 | 5.919 | 7.427 | 0.43 | 1.994 |
| 12 | 1GJ | 117.333 | 154.818 | 101.198 | 136.918 | 4.226 | 5.117 | 0.307 | 1.374 |
| 13 | B-SH | 96.34 | 119.613 | 30.404 | 30.404 | 28.61 | 28.572 | 5.813 | 29.01 |
| 14 | HP1 | 142.51 | 182.477 | 101.198 | 136.918 | 20.729 | 22.028 | 1.507 | 5.915 |
| 15 | HP2 | 119.981 | 159.486 | 101.198 | 136.918 | 4.551 | 6.715 | 0.331 | 1.803 |
| 16 | HP3 | 125.221 | 165.277 | 101.198 | 136.918 | 5.04 | 7.514 | 0.366 | 2.018 |
| 17 | RH | 82.673 | 100.883 | 30.404 | 30.404 | 28.404 | 26.381 | 5.767 | 26.567 |
| 18 | IP1 | 121.429 | 157.37 | 101.198 | 136.918 | 4.588 | 7.333 | 0.334 | 1.969 |
| 19 | IP2 | 119.431 | 158.416 | 101.198 | 136.918 | 2.616 | 3.891 | 0.19 | 1.045 |
| 20 | LP1 | 120.629 | 160.697 | 101.198 | 136.918 | 4.172 | 5.899 | 0.303 | 1.584 |
| 21 | LP2 | 122.872 | 162.492 | 101.198 | 136.918 | 2.699 | 3.613 | 0.196 | 0.97 |
| 22 | LP3 | 123.2 | 162.482 | 101.198 | 136.918 | 2.67 | 4.345 | 0.194 | 1.167 |
| 23 | LP4 | 125.013 | 162.98 | 101.198 | 136.918 | 4.084 | 5.189 | 0.297 | 1.394 |
| 24 | LP5 | 134.825 | 181.248 | 101.198 | 136.918 | 16.596 | 22.477 | 1.207 | 6.036 |
| 25 | BFPT | 156.599 | 230.329 | 101.198 | 136.918 | 29.663 | 52.766 | 2.156 | 14.17 |
| 26 | SG | - | 16180.037 | - | 136.918 | - | 68.477 | - | 0 |
| 27 | CND | 7.357 | 8.12 | 101.198 | 136.918 | 4.943 | 6.688 | 0 | 0 |
| 28 | CP | 178.603 | 237.517 | 129.17 | 169.641 | 33.86 | 44.468 | 1.929 | 9.638 |
| 29 | GEN | 129.17 | 169.641 | 124.323 | 164.035 | 2.524 | 3.331 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, W.; Meng, X.; Liu, X.; Gao, Y.; Yu, Z.; Bai, Y.; Yang, X. Study on the Coupling Effect of a Solar-Coal Unit Thermodynamic System with Carbon Capture. Energies 2020, 13, 4779. https://doi.org/10.3390/en13184779
Wang J, Liu W, Meng X, Liu X, Gao Y, Yu Z, Bai Y, Yang X. Study on the Coupling Effect of a Solar-Coal Unit Thermodynamic System with Carbon Capture. Energies. 2020; 13(18):4779. https://doi.org/10.3390/en13184779
Chicago/Turabian StyleWang, Jixuan, Wensheng Liu, Xin Meng, Xiaozhen Liu, Yanfeng Gao, Zuodong Yu, Yakai Bai, and Xin Yang. 2020. "Study on the Coupling Effect of a Solar-Coal Unit Thermodynamic System with Carbon Capture" Energies 13, no. 18: 4779. https://doi.org/10.3390/en13184779
APA StyleWang, J., Liu, W., Meng, X., Liu, X., Gao, Y., Yu, Z., Bai, Y., & Yang, X. (2020). Study on the Coupling Effect of a Solar-Coal Unit Thermodynamic System with Carbon Capture. Energies, 13(18), 4779. https://doi.org/10.3390/en13184779