Experimental Investigation of the Hydrate-Based Gas Separation of Synthetic Flue Gas with 5A Zeolite
Abstract
1. Introduction
2. Experimental
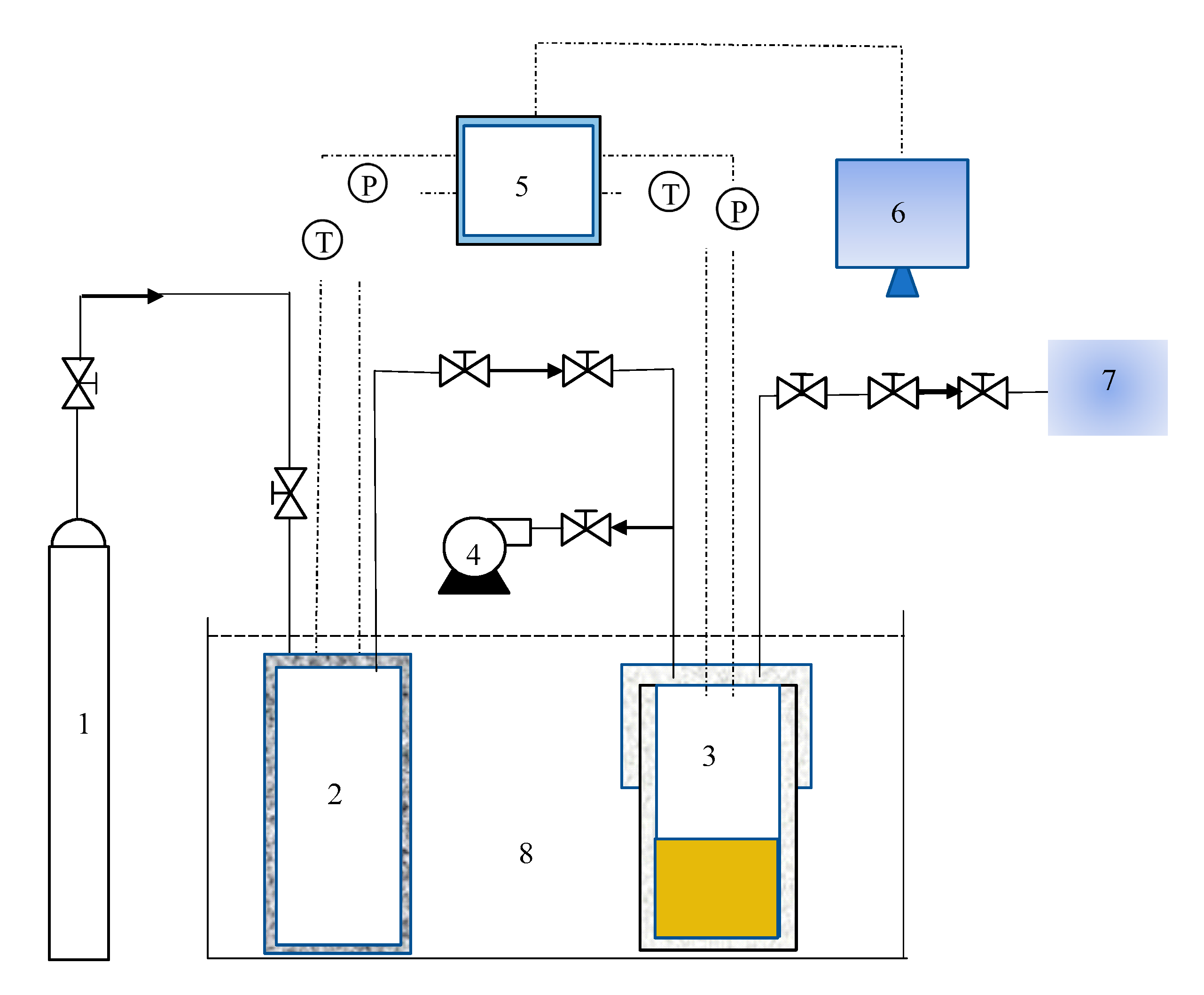
2.1. Experimental Apparatus and Materials
2.2. Experimental Procedures
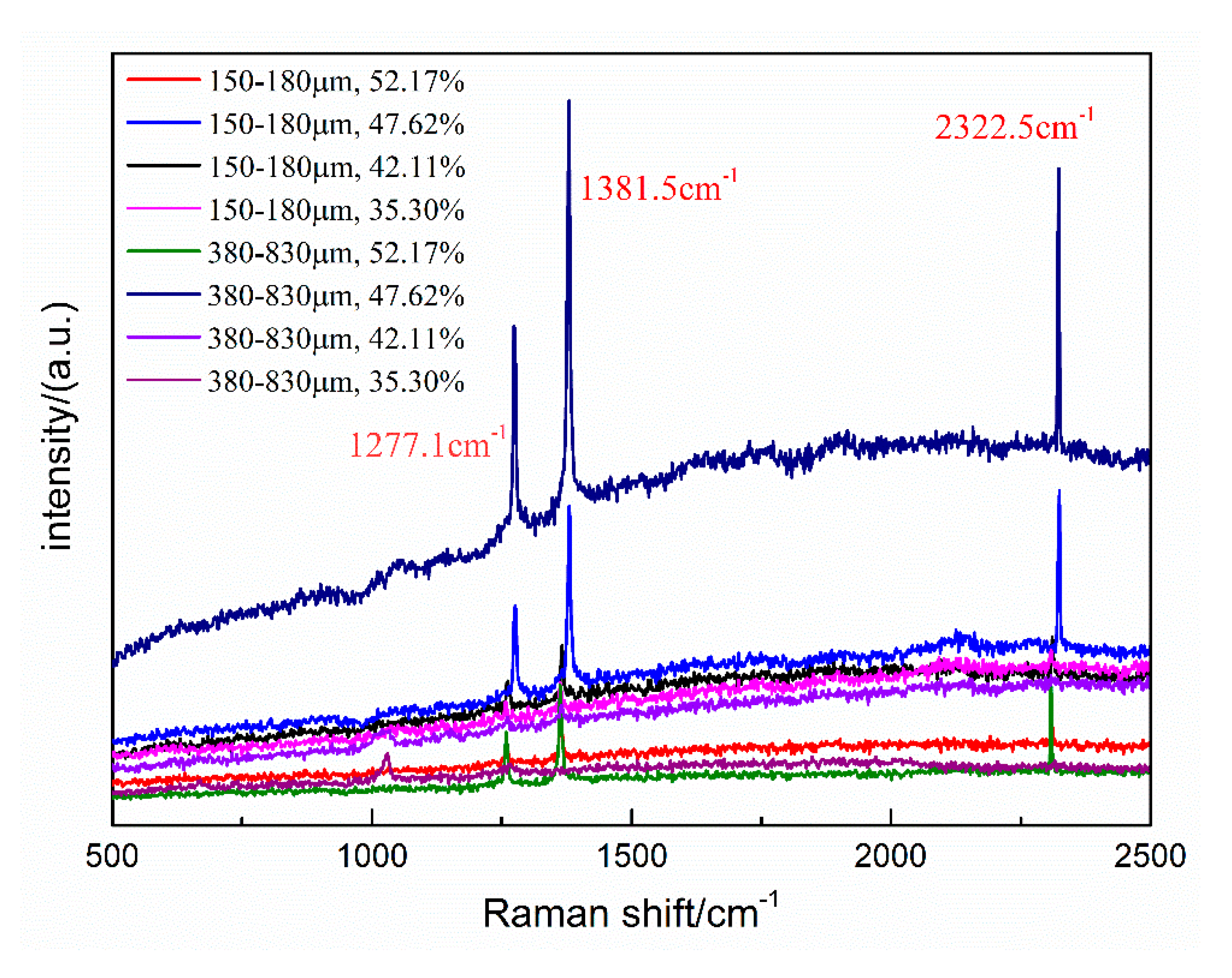
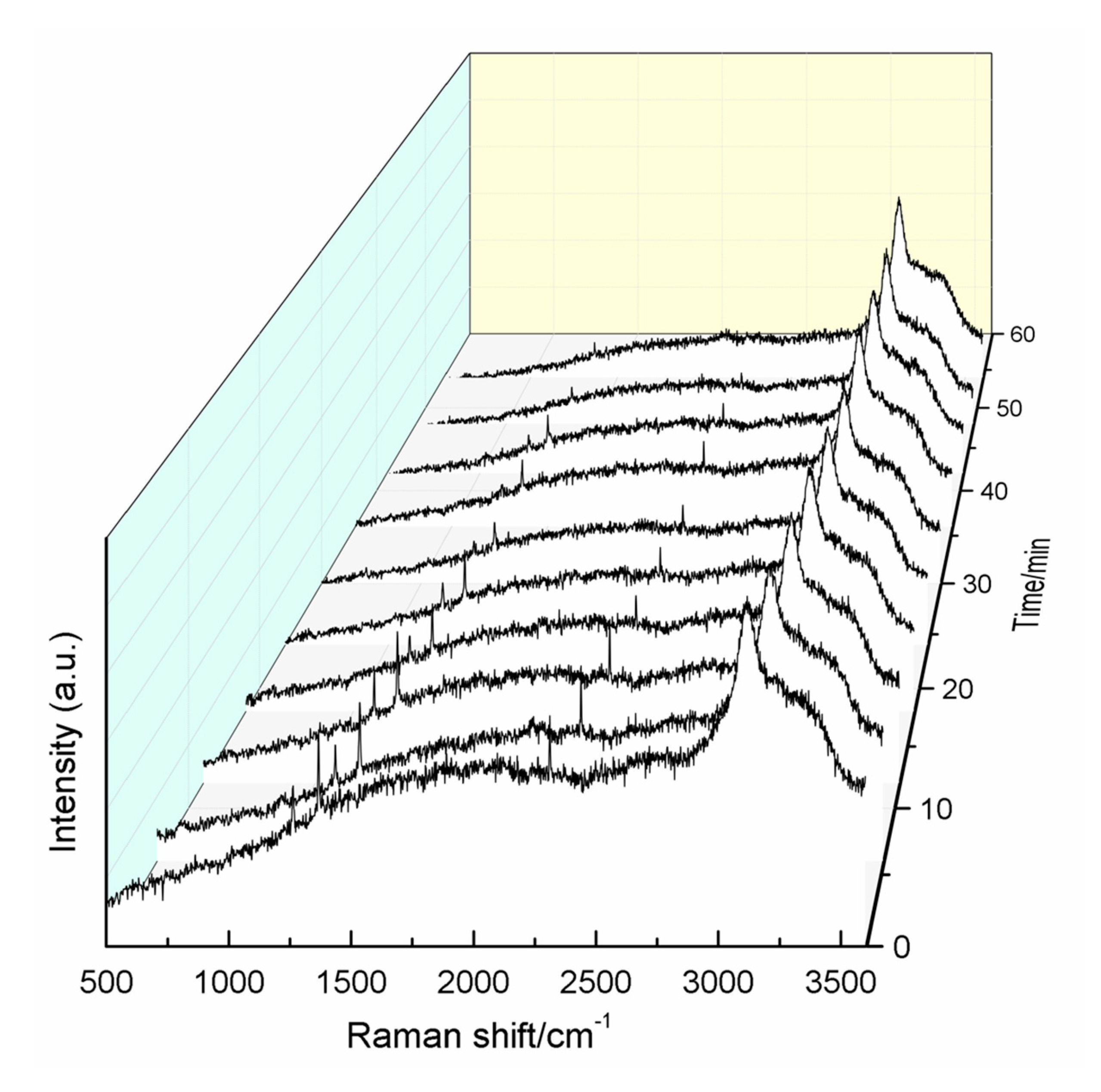
2.3. Microscopic Analysis of Hydrate Samples
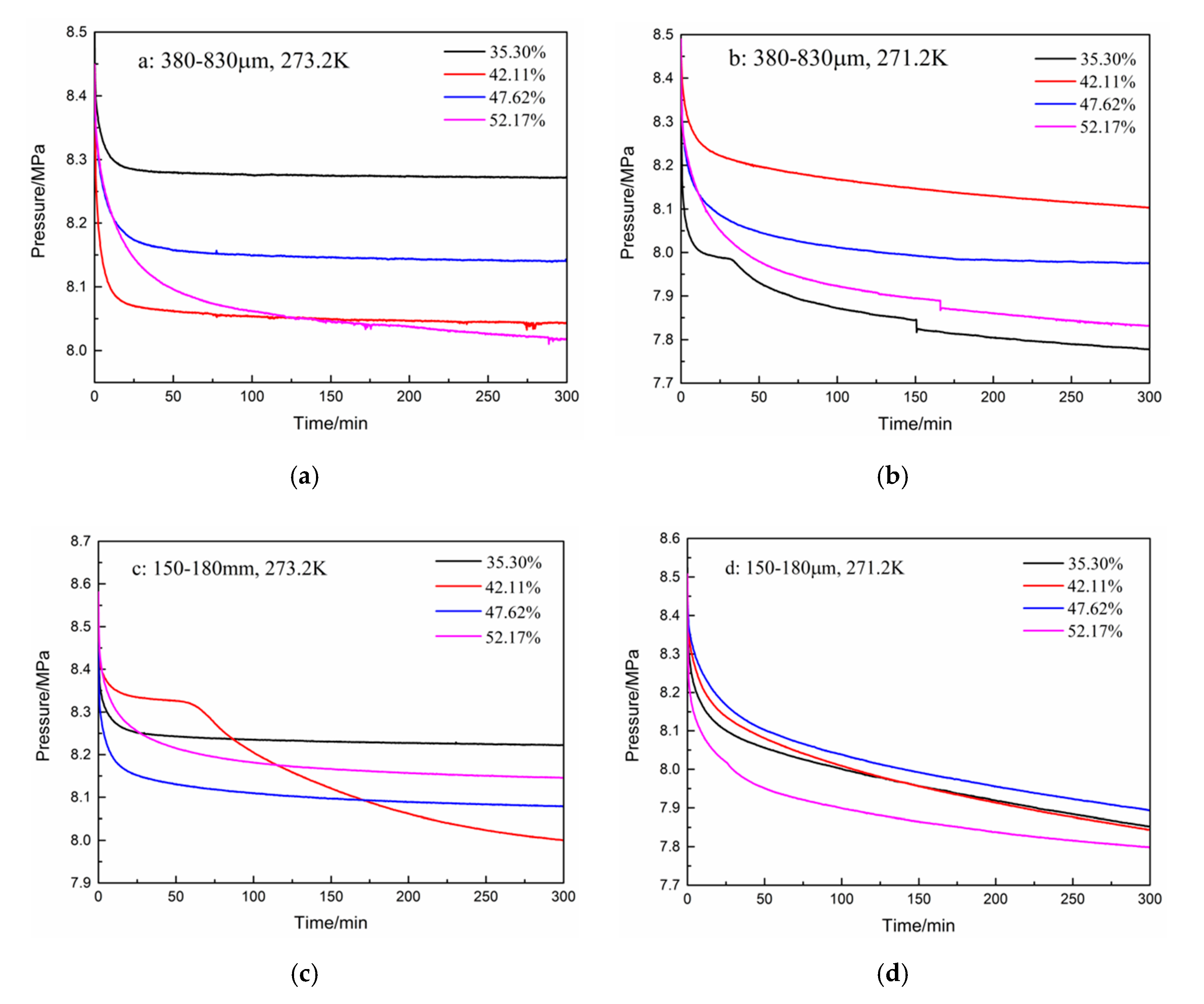
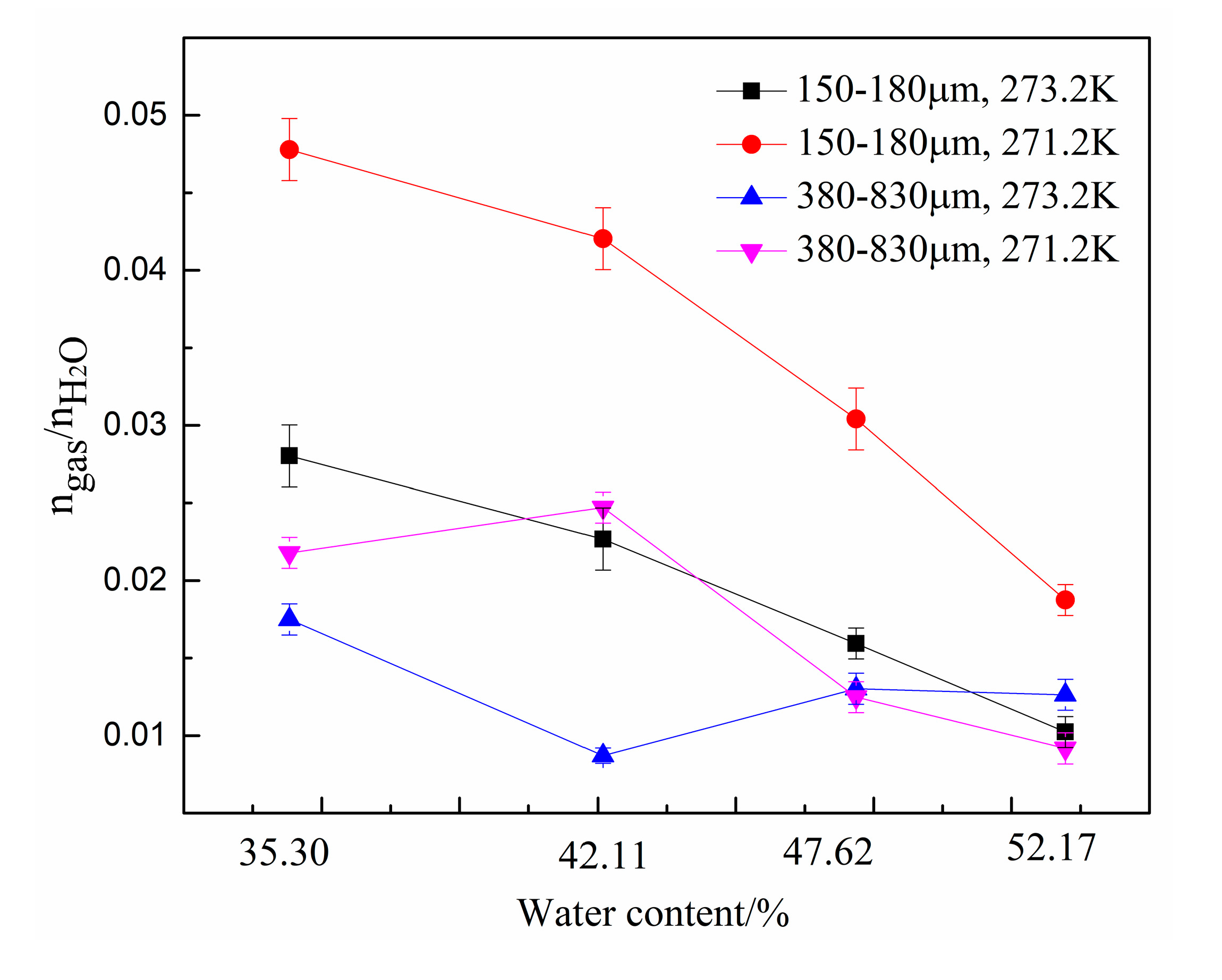
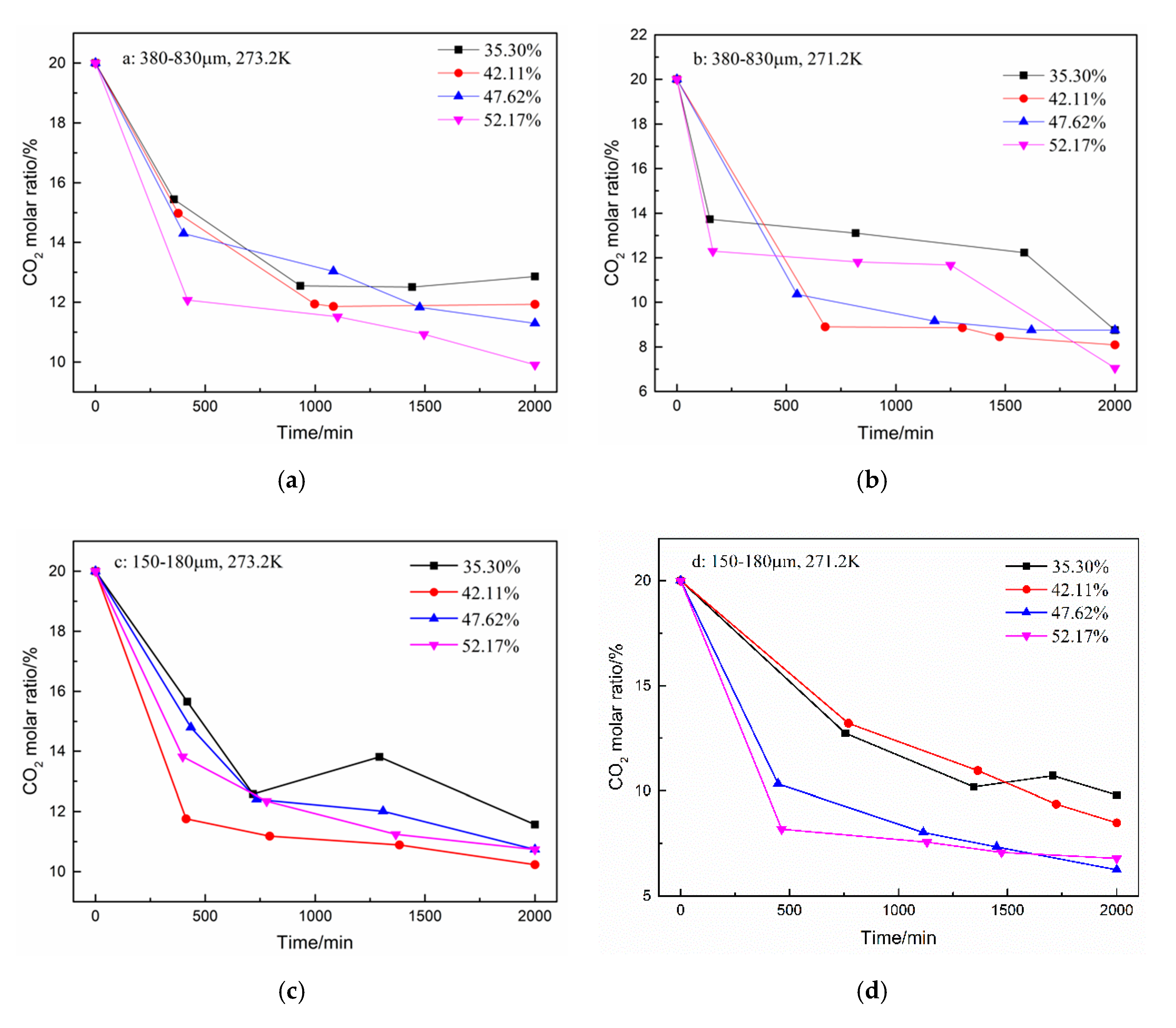
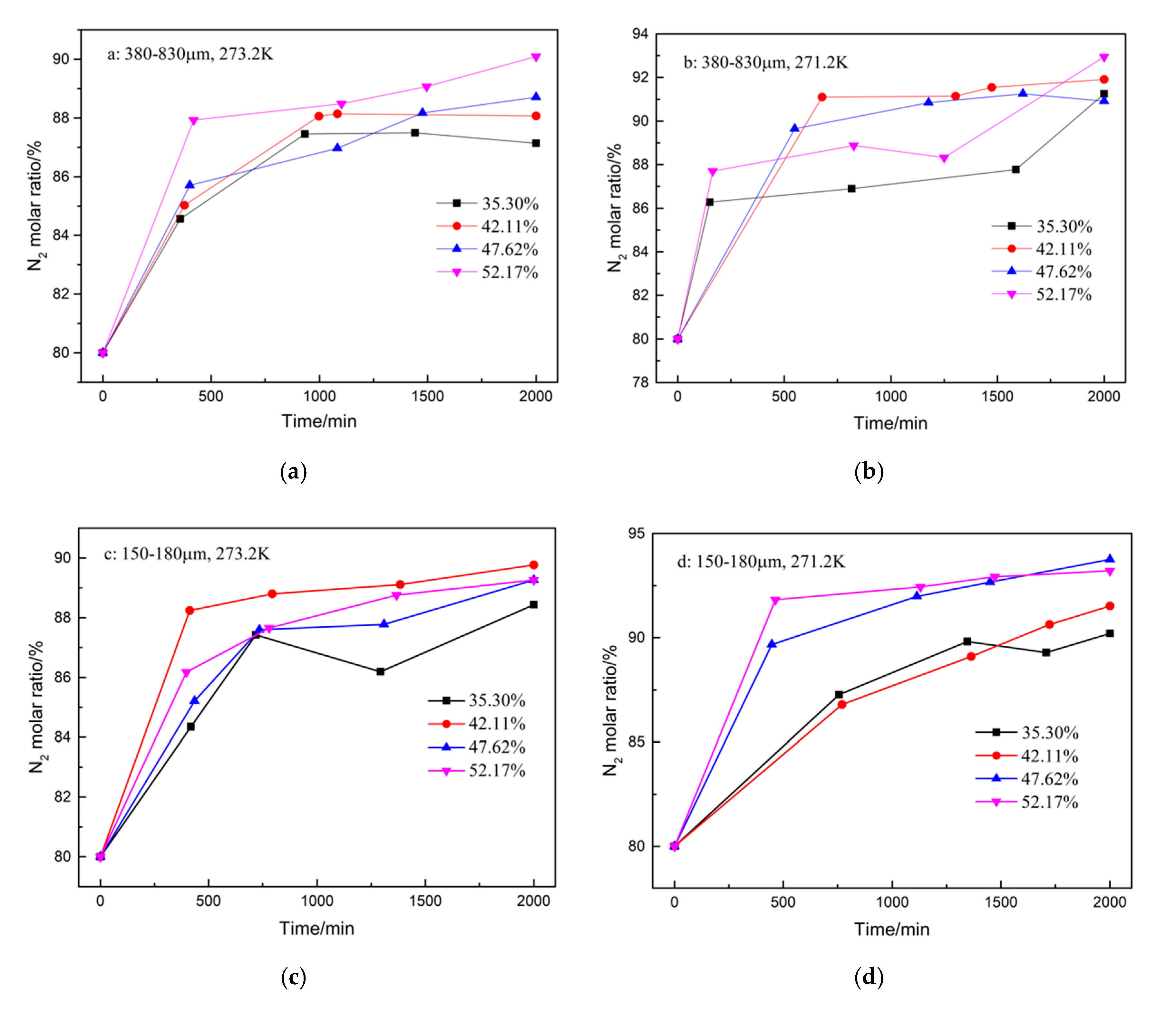
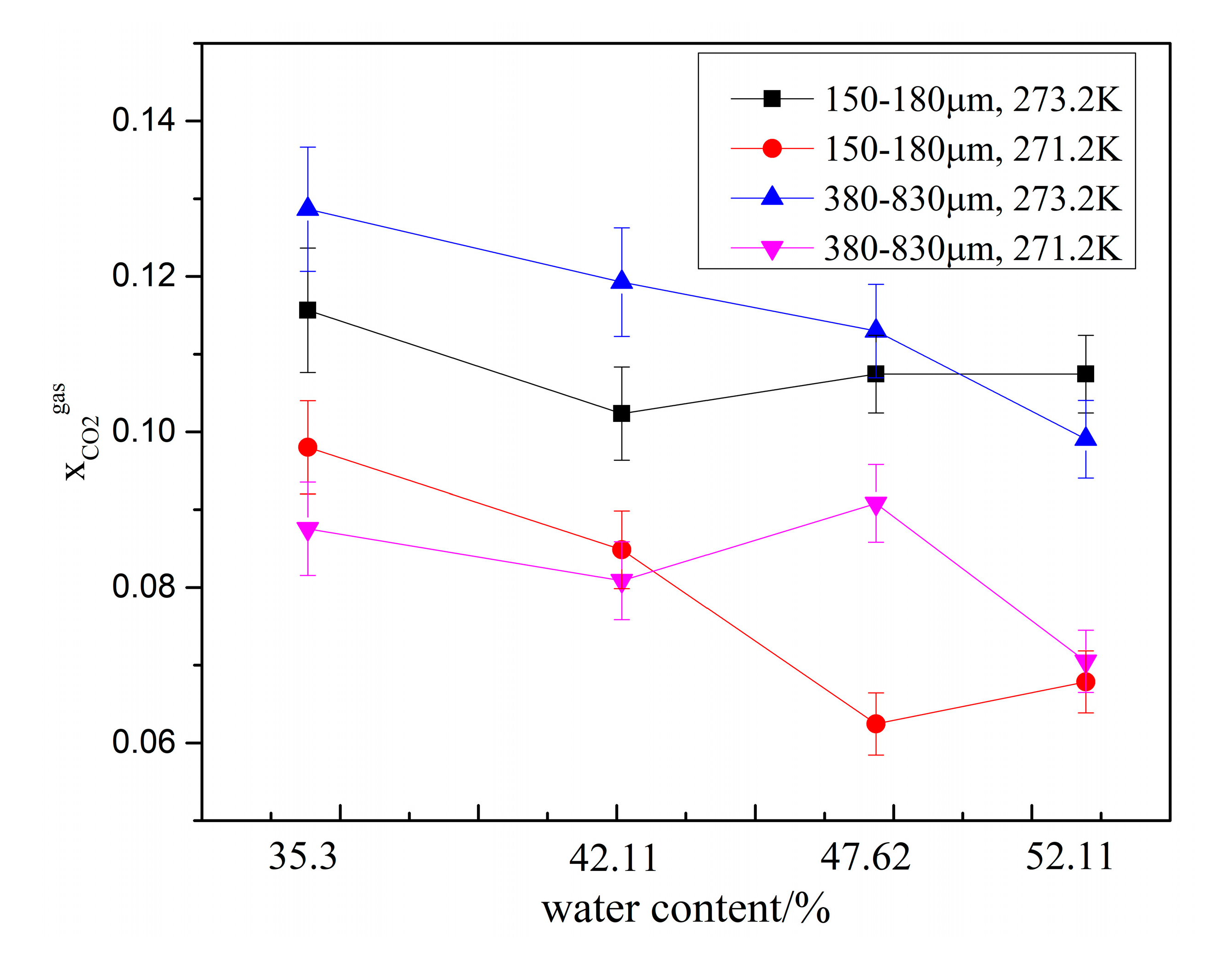
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, M. Introduction to Modern Climate Change. Sci. Total. Environ. 2020, 734, 139397. [Google Scholar] [CrossRef]
- Bachmann, T.M. Considering environmental costs of greenhouse gas emissions for setting a CO2 tax: A review. Sci. Total. Environ. 2020, 720, 137524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Pudasainee, D.; Duan, Y.; Gupta, R.; Liu, M.; Lu, J. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Prog. Energy Combust. Sci. 2019, 73, 26–64. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total. Environ. 2020, 714, 136712. [Google Scholar] [CrossRef] [PubMed]
- Patron, G.D.; Ricardez-Sandoval, L. A robust nonlinear model predictive controller for a post-combustion CO2 capture absorber unit. Fuel 2020, 265, 116932. [Google Scholar] [CrossRef]
- Anantharaman, R.; Peters, T.; Xing, W.; Fontaine, M.-L.; Bredesen, R. Dual phase high-temperature membranes for CO2 separation—Performance assessment in post- and pre-combustion processes. Faraday Discuss. 2016, 192, 251–269. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Ravanchi, M.T.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 1–34. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, X.; Chen, Y.; Huang, J.; Luo, H.; Deng, S. Adsorption of CO2, CH4, and N2 on Ordered Mesoporous Carbon: Approach for Greenhouse Gases Capture and Biogas Upgrading. Environ. Sci. Technol. 2013, 47, 5474–5480. [Google Scholar] [CrossRef]
- Ding, S.; Liu, Y. Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 2020, 260, 116382. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Inamuddin; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.H.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and Zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Pour, A.A.; Sharifnia, S.; NeishaboriSalehi, R.; Ghodrati, M. Performance evaluation of clinoptilolite and 13X zeolites in CO2 separation from CO2/CH4 mixture. J. Nat. Gas Sci. Eng. 2015, 26, 1246–1253. [Google Scholar] [CrossRef]
- Liu, J.; Ding, J.X.; Liang, D.Q. Experimental study on hydrate-based gas separation of mixed CH4/CO2 using unstable ice in a silica gel bed. Energy 2018, 157, 54–64. [Google Scholar] [CrossRef]
- Mofarahi, M.; Gholipour, F. Gas adsorption separation of CO2/CH4 system using zeolite 5A. Microporous Mesoporous Mater. 2014, 200, 1–10. [Google Scholar] [CrossRef]
- Mendes, P.A.P.; Ribeiro, A.M.; Gleichmann, K.; Ferreira, A.F.P.; Rodrigues, A.E. Separation of CO2/N2 on binderless 5A zeolite. J. CO2 Util. 2017, 20, 224–233. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Mofarahi, M. Pure and Binary Adsorption Equilibria of Methane and Nitrogen on Zeolite 5A. J. Chem. Eng. Data 2014, 59, 626–639. [Google Scholar] [CrossRef]
- Li, S.; Fan, S.; Wang, J.; Lang, X.; Wang, Y. Clathrate Hydrate Capture of CO2 from Simulated Flue Gas with Cyclopentane/Water Emulsion. Chin. J. Chem. Eng. 2010, 18, 202–206. [Google Scholar] [CrossRef]
- Li, L.; Fan, S.; Chen, Q.; Yang, G.; Zhao, J.; Wei, N.; Wen, Y. Simulation of post-combustion CO2 capture process by non-equilibrium stage hydrate-based gas separation technology. Int. J. Greenh. Gas Control. 2018, 79, 25–33. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.-D.; Seo, Y. CO2 capture from flue gas using clathrate formation in the presence of thermodynamic promoters. Energy 2017, 118, 950–956. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Wang, X.; Zhang, F.; Lipiński, W. Research progress and challenges in hydrate-based carbon dioxide capture applications. Appl. Energy 2020, 269, 114928. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; Von Solms, N. Screening of Amino Acids and Surfactant as Hydrate Promoter for CO2 Capture from Flue Gas. Processes 2020, 8, 124. [Google Scholar] [CrossRef]
- Abu Hassan, M.H.; Sher, F.; Zarren, G.; Suleiman, N.; Tahir, A.A.; Snape, C.E. Kinetic and thermodynamic evaluation of effective combined promoters for CO2 hydrate formation. J. Nat. Gas Sci. Eng. 2020, 78, 103313. [Google Scholar] [CrossRef]
- Nasir, Q.; Suleman, H.; Elsheikh, Y.A. A review on the role and impact of various additives as promoters/inhibitors for gas hydrate formation. J. Nat. Gas Sci. Eng. 2020, 76, 103211. [Google Scholar] [CrossRef]
- Khurana, M.; Yin, Z.; Linga, P. A Review of Clathrate Hydrate Nucleation. ACS Sustain. Chem. Eng. 2017, 5, 11176–11203. [Google Scholar] [CrossRef]
- Zhou, X.; Long, Z.; Liang, S.; He, Y.; Yi, L.; Li, D.-L.; Liang, D. In SituRaman Analysis on the Dissociation Behavior of Mixed CH4–CO2Hydrates. Energy Fuels 2016, 30, 1279–1286. [Google Scholar] [CrossRef]
- Zhong, D.-L.; Li, Z.; Lu, Y.-Y.; Wang, J.-L.; Yan, J.; Qing, S.-L. Investigation of CO2 Capture from a CO2 + CH4 Gas Mixture by Gas Hydrate Formation in the Fixed Bed of a Molecular Sieve. Ind. Eng. Chem. Res. 2016, 55, 7973–7980. [Google Scholar] [CrossRef]
- Chazallon, B.; Pirim, C. Selectivity and CO2 capture efficiency in CO2-N2 clathrate hydrates investigated by in-situ Raman spectroscopy. Chem. Eng. J. 2018, 342, 171–183. [Google Scholar] [CrossRef]

| Items | Specification | Uncertainties |
|---|---|---|
| Temperature sensors | PT-100/Beijing West Air Technology Co., Ltd., Beijing, China | ±0.1 K |
| Pressure sensor | CYB-20S/Beijing West Air Technology Co., Ltd., Beijing, China | ±0.1 MPa |
| Data acquisition system | Agilent 34970A/Agilent Technologies Co., Ltd., Santa Clara, CA, USA | - |
| Gas chromatography | FL9790/Zhejiang Fuli Analytical Instrument Co., Ltd., Beijing, China | ±0.1% |
| Constant temperature water bath | THD-2030/Ningbo Tianheng Instrument Factory, Ningbo, China | ±0.1 K |
| Experiments No. | Zeolite | Temperature/K | Water Content/(wt.%) | /% | ||
|---|---|---|---|---|---|---|
| 1 | Type A: 380–830 μm | 273.2 | 35.30 | 0.017 | 0.129 | 9.65 |
| 2 | 42.11 | 0.009 | 0.119 | 10.59 | ||
| 3 | 47.62 | 0.013 | 0.113 | 11.77 | ||
| 4 | 52.17 | 0.013 | 0.099 | 13.60 | ||
| 5 | 271.2 | 35.30 | 0.022 | 0.088 | 14.71 | |
| 6 | 42.11 | 0.025 | 0.081 | 15.80 | ||
| 7 | 47.62 | 0.010 | 0.091 | 13.72 | ||
| 8 | 52.17 | 0.009 | 0.070 | 16.70 | ||
| 9 | Type B: 150–180 μm | 273.2 | 35.30 | 0.028 | 0.116 | 11.60 |
| 10 | 42.11 | 0.023 | 0.102 | 13.27 | ||
| 11 | 47.62 | 0.016 | 0.107 | 12.62 | ||
| 12 | 52.17 | 0.010 | 0.107 | 12.43 | ||
| 13 | 271.2 | 35.30 | 0.048 | 0.098 | 14.30 | |
| 14 | 42.11 | 0.042 | 0.085 | 16.02 | ||
| 15 | 47.62 | 0.030 | 0.062 | 18.34 | ||
| 16 | 52.17 | 0.019 | 0.068 | 17.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, X.; Zhang, N.; Zhou, X.; Wan, L.; Liang, D. Experimental Investigation of the Hydrate-Based Gas Separation of Synthetic Flue Gas with 5A Zeolite. Energies 2020, 13, 4556. https://doi.org/10.3390/en13174556
Zang X, Zhang N, Zhou X, Wan L, Liang D. Experimental Investigation of the Hydrate-Based Gas Separation of Synthetic Flue Gas with 5A Zeolite. Energies. 2020; 13(17):4556. https://doi.org/10.3390/en13174556
Chicago/Turabian StyleZang, Xiaoya, Na Zhang, Xuebing Zhou, Lihua Wan, and Deqing Liang. 2020. "Experimental Investigation of the Hydrate-Based Gas Separation of Synthetic Flue Gas with 5A Zeolite" Energies 13, no. 17: 4556. https://doi.org/10.3390/en13174556
APA StyleZang, X., Zhang, N., Zhou, X., Wan, L., & Liang, D. (2020). Experimental Investigation of the Hydrate-Based Gas Separation of Synthetic Flue Gas with 5A Zeolite. Energies, 13(17), 4556. https://doi.org/10.3390/en13174556






