1. Introduction
It is well-known that we are currently living a situation of environmental crisis. This environmental crisis is linked to the need to obtain the necessary energy for society. Over recent decades, most of the energy came from fossil fuels [
1] that generated a large number of greenhouse gas emissions, such as CO
emissions [
2,
3]. For this reason, there has been a change in the energy paradigm, adopting a global transition towards more environmentally friendly activities [
4].
Nowadays, the use of renewable energy sources (RES) to produce energy is growing significantly [
5]. Most of these RES (wind, solar and marine) are usually discontinuous, due to their dependency on the weather conditions. This results in the difficulty, even impossibility, to schedule this type of energy source. In order to satisfy the energy demand at times of low energy production, the need of an energy storage system (ESS) is mandatory [
6,
7]. ESS can store energy, normally the surplus energy from renewable sources, and provide it when the environmental conditions do not make possible to generate all the energy required. Therefore, ESS is seen as an element that can be combined with power generation systems, such as photovoltaic or wind systems [
8,
9]. Another important advantage apart from the load leveling, is its function as a support element for generation and distribution lines of the electricity grid [
10].
There are different types of ESS depending on how energy is obtained. Mainly, it can be differentiated between mechanical, electrical and electrochemical storage systems [
11]. Especially, the search for large-scale energy storage system solutions, in terms of capacity and time, has great importance due to its many benefits. During periods of RES low production, ESS can increase considerably the available power and therefore contribute to guarantee the energy supply.
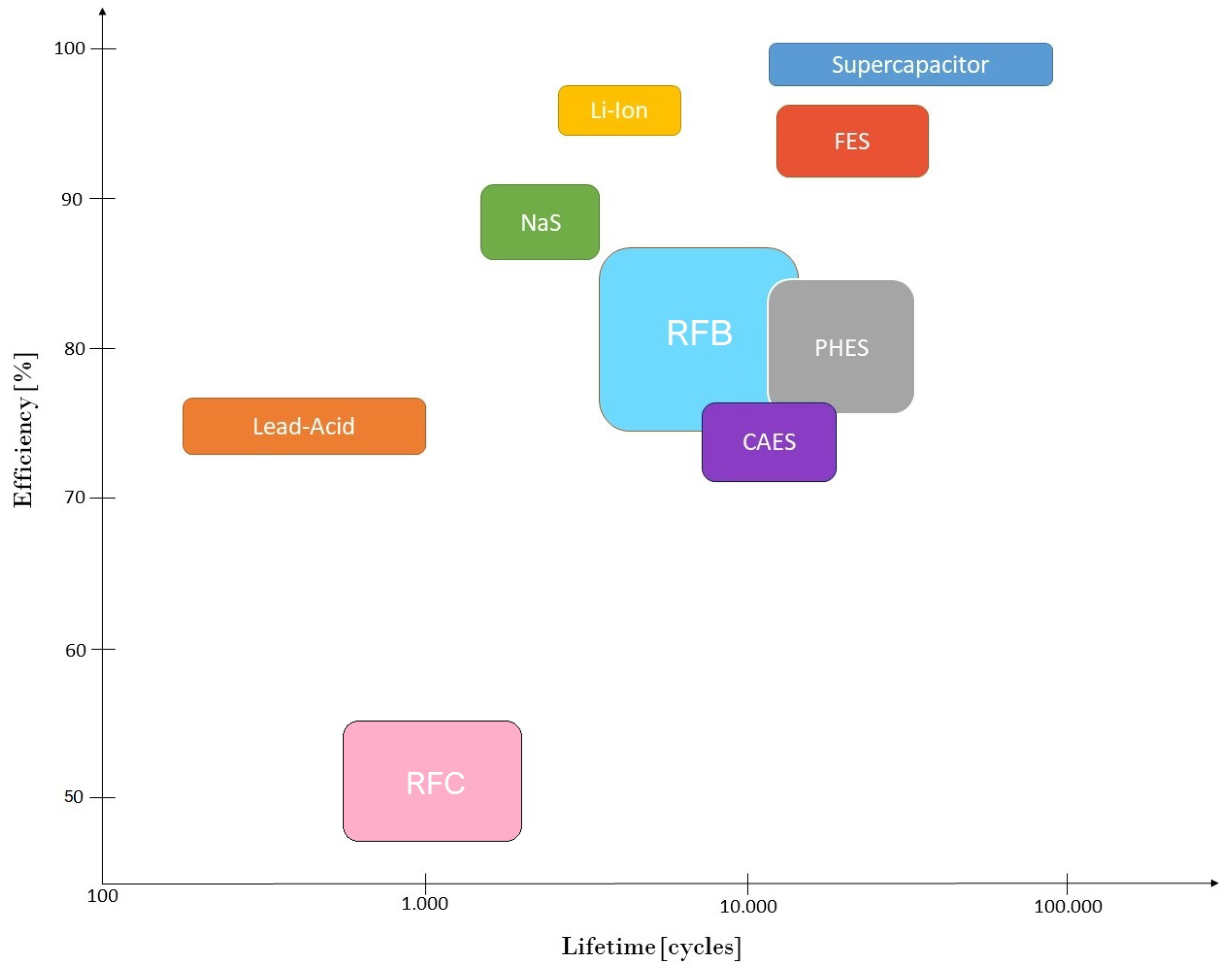
Table 1 summarizes the principal characteristics of the main ESS.
Inside the group of mechanical large-scale ESS, the most popular ones are the pumped hydro energy storage (PHES) and compressed air energy storage (CAES) systems [
12]. The main advantage of PHES and CAES systems is their high energy capacity. PHES systems have also high efficiencies between 70% and 80%, claiming up to 90% in some installations [
13]. PHES systems are also the largest-capacity form of grid energy storage available, being in 2017 the type of active installations with the highest capacity with more than 184 GW worldwide, which represented 95% of all active tracked storage installations [
14]. As disadvantages, both PHES and CAES require stable geological structures, which substantially limit the use of this type of systems [
15]. CAES also has the drawback that its energy efficiency is less than 70% [
16], even reaching values below 45% [
17,
18].
As for electric large-scale ESS, the most common is the superconducting magnetic energy storage (SMES) system [
19], which is based on the use of electro-magnetic energy, and the electric double layer capacitor (EDLC) which directly uses electric energy. The main advantage of SMES is its energy efficiency, about 90% [
20]. The main disadvantage is the high cost of superconducting wire, which, with the refrigeration energy that this system needs, makes this technology more appropriate for short-term applications [
21]. The main advantages of EDLC are that can deliver large amounts of power and its long life cycle (supporting more than one million of charge and discharge cycles). On the other hand, they have low energy density, and they only can store energy for short periods of time due to the self-discharge phenomenon [
22].
In the last group of ESS, which corresponds to the energy storage from chemical reactions, there are different batteries and emerging technologies [
23]. Typical batteries that have been used for many years are lead-acid, sodium-sulphur and lithium-ion. The strengths of lead-acid batteries are their low cost and good efficiencies from 75% to 80% [
24]. In counterpart, they have short life (between 200 and 1800 charge and discharge cycles) and they are extremely toxic [
25]. Sodium-sulphur has higher energy density than lead-acid batteries, with similar efficiencies but with larger lifetime around 2500 cycles [
26,
27]. The main problem of this type of technology is its operating temperature (about 300
C) and a poor safety due to possible leaks of the liquids inside it. Finally, lithium-ion is the best choice compared to the previously named ones, having a larger energy density [
28], and a long life, greater than 2500 cycles [
29]. On the other hand, it is important to remark that some damages, such as overcharge or perforation cause damage to the battery, leading to explosions and fires if is damaged or incorrectly charged.
Nowadays, inside the group of electrochemical ESS, more research is being done, specially in fuel cells and redox flow batteries. Fuel cells [
30,
31] convert chemical energy from a fuel (usually hydrogen) into electric energy through an electrochemical reaction between the fuel with an oxidizing agent (oxygen). Its main distinction from a battery is that requires a continuous source of species. Combining the fuel cell with an electrolyzer it is possible to store the chemical energy, becoming an ESS, which is called regenerative fuel cell (RFC) [
32,
33]. One of the main advantages of its use is that is a clean ESS, being water vapor the residue that comes out. In terms of efficiency, typical values oscillate between 40% and 60% [
34,
35]. Most losses are in the form of heat, so in the case of an application in which it is necessary to capture waste heat in a cogeneration system, efficiencies can reach 85% [
36].
Redox flow batteries (RFB) are composed of an electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids contained within the system and separated by a membrane. They have become one of the most promising options for large-scale energy storage systems [
37,
38]. Depending on the nature of the fuel, there are two modes of operation for a redox flow battery. If the flowing fuel is depleted by the reaction, and cannot be reused, then it is removed from the system and new flow is added. In this situation, it actuates as a fuel cell. The second possibility, is that it works like a rechargeable battery, where there is always fuel flowing in the system. In this second case, it is called RFB.
One of the main characteristics of RFBs and RFCs is the duality between energy capacity and power. Energy capacity is function of the electrolyte volume, which is usually stored in tanks. Depending on their volume, they can provide energy from minutes to several hours [
39]. The power is directly related with the surface of the electrodes, and the number of cells that compose the system [
40]. Other important advantages are their safety, flexible design, long life and that they are specially designed for large-scale storage applications, because self-discharge is practically non-existent, saving or providing energy during long periods of time. In terms of charge/discharge efficiency, RFBs have values between 75% and 85% [
41], while RFCs have lower efficiencies. For that reason, comparing both of them, RFBs are a great proposal for large-scale ESS.
Comparing RFBs with other ESS, taking into account
Table 1 it is possible to make clear that RFBs allow large amounts of energy to be delivered over long periods of time, without the need for a specific geographical location, as in the case of PHES and CAES systems. In terms of safety, they are better than classical batteries such as lead-acid, sodium-sulphur and lithium-ion. If they are compared in terms of efficiency and lifetime, RFBs are also a good choice, as can be noticed in
Figure 1.
In comparison with lithium-ion batteries, which have become one of the most promising options in electrochemical ESS, RFBs have as benefits their greater lifetime, safety and low cost [
43]. However, lithium-ions batteries present higher energy density. In terms of sizing, RFB differs from lithium-ion technology in the duality between power and energy capacity. From an automatic control point of view, lithium-ion batteries contain no active elements consequently do not require any control [
44]. On the other hand, RFBs require a good control strategy to improve its efficiency, required by the pumps which make the electrolyte flow in the system. In both technologies, obtaining good control-oriented models and developing methodologies to estimate most relevant parameters from empirical data are important research topics. Their tuning are of great relevant to estimate the State Of Charge (SOC) and the State Of Health (SOH). In the case of RFB, the SOH study has not yet been developed while for lithium-ion batteries there are already numerous studies. Although there has been a lot research in this topic it is still and open research area [
44,
45,
46,
47].
Analyzing the RFB individually, the main benefits of this type of ESS are [
48]:
High duration in terms of energy supply, managing to reach hours. There are several facilities in operation whose supply period is around 5 h, although there are operational cases in which it reaches 10 h [
49].
Modular technology, existing an independence between power and energy density. This allows to have a flexible design depending on the required operation conditions.
Long life, higher than thousands of cycles [
50].
Thermally safe, operating at low temperatures close to ambient ones [
51].
Quickly recharge by replacing the electrolyte or reversing the redox reaction.
Self discharge is not significant, only takes place in the cell where species react. The active species stored in the containers (which are usually much larger than the cell) do not react and self-discharge does not take place [
52].
Its operation could be stopped quickly by cutting off the flow of electrolytes.
Although the advantages listed above, make RFBs a good large-scale storage system, there are some drawbacks to consider. The main one is their low energy density, in comparison with other systems. In order to store large amounts of energy, it is necessary to have larger tanks. Although its charge and discharge efficiencies are high, reaching the order of 80%, is lower than other ESS, due to the energy consumption to allow the flow of electrolytes in the system.
There are different types of RFBs according to the species that react, but all of them present the same operating mechanism. A typical example is the Fe-Cr RFB, which uses Fe(III) and Fe(II) as positive active species, and the species Cr(III) and Cr(II) as negative ones, all of them dissolved in HCl [
53]. Other examples are the iron-chromium (I-Cr) and the zinc-bromide (Zn-Br) batteries [
54,
55].
Specially, vanadium redox flow batteries (VRFB) are of great importance due to their benefits compared to other species, being the object of study in recent years [
56]. The main reason for its use is that since it is made up of only vanadium, there is no possibility that different substances mix and degrade the battery. The VRFB use V(IV) and V(V) oxidation states as positive active species, and V(II) and V(III) as negative species, all of them dissolved in a solution of H
SO
[
57].
Due to the complexity of a RFB system, that depends on different variables such as the electrolyte concentration, current density, temperature or flow management, more research will be done to understand better the RFB operation that will be directly translate in well optimized RFB systems. Moreover, due to the presence of harmful phenomena such as leakage currents, electrolyte escape and the possible corrosion and degradation of membrane, electrodes and other stack components, a research in mitigation strategies of these phenomena will be necessary [
58].
Another important aspect to consider is the cost of the RFB system. Inside this economical scenario, more research is being done in order to design low-cost RFB systems that are capable to store large quantities of energy maximizing the performance of the battery. In order to accomplish it, the correct choice of the main components such as electrodes, membranes, bipolar plates and cells is critical. Selecting a good electrode structure allows to have a higher electrocatalytic activity, and if the surface area is higher enough, it will be possible to increase the volume production of new species. For the membrane, there are different studies that analyze and compare different materials in order to find the one that best suits with the designed battery. Thus, taking into account the species that react, as well the design of the other components, further investigations will be conducted to find an equilibrium between a better selectivity and stability, as also a lower cost. In terms of bipolar plates, more research is necessary to scale-up the battery, increasing both power and energy density.
Considering the materials with which the RFB are built, the search for organic materials in terms of active materials, electrolytes and membranes is one of the research hot topics. In this scenario, there is recent progress in the search for organic RFB [
59], which would allow to have a more environmentally attractive technology.
Taking into account the great possibilities that RFBs present as large-scale storage systems, as well as the numerous studies that are being carried out on them, in this work a review of most relevant concepts in RFB, from an automatic control point of view, will be presented. As previously stated, other topics such as materials will be very important in the near future but are outside the scope of this work.
The work has the following organization:
Section 2 shows the composition of a RFB;
Section 3 presents the main expressions needed to determine the battery sizing according to specific conditions of power and energy;
Section 4 shows the main characteristics and applications of RFB;
Section 5 presents a review on the literature of RFB models;
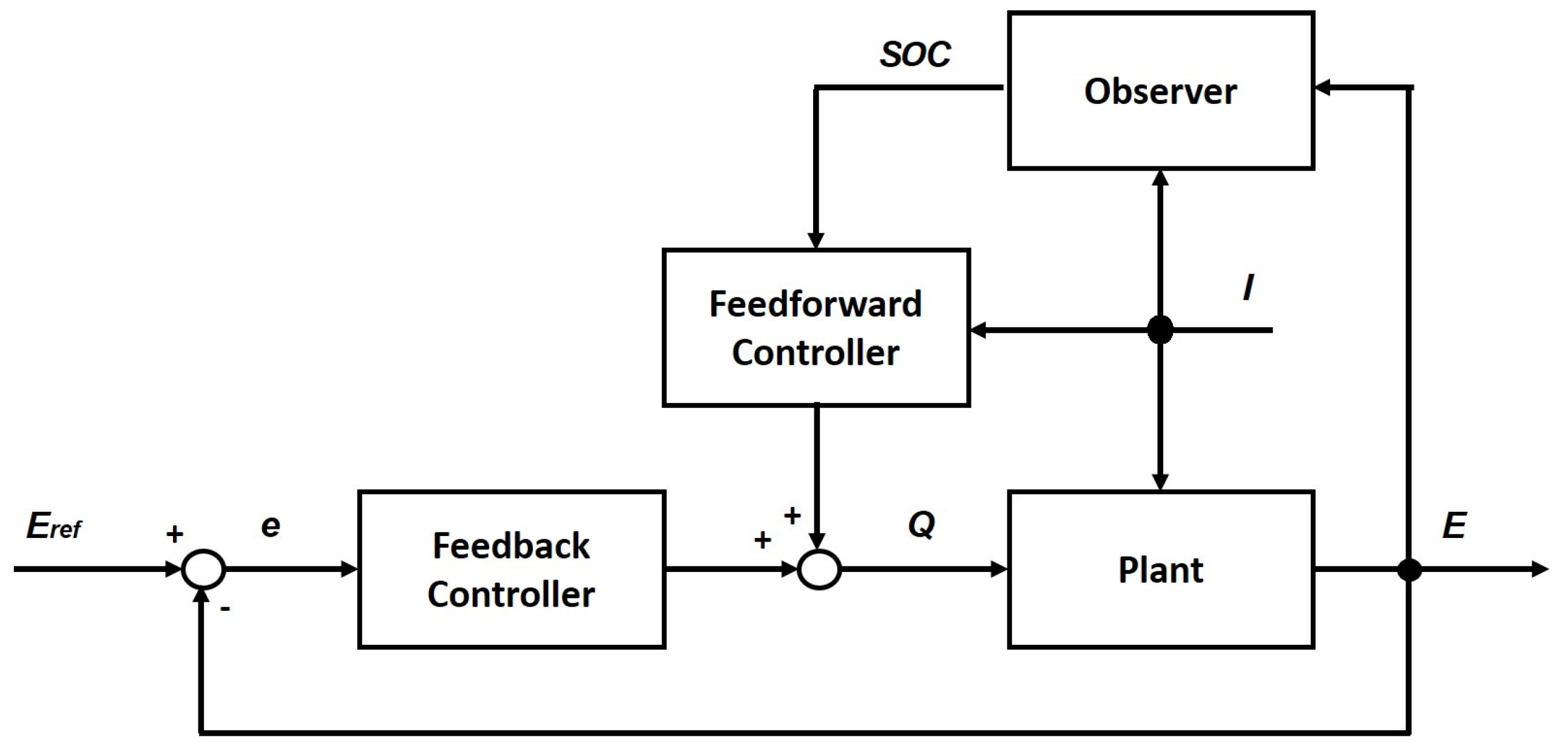
Section 6.1 presents the main control strategies of RFB systems;
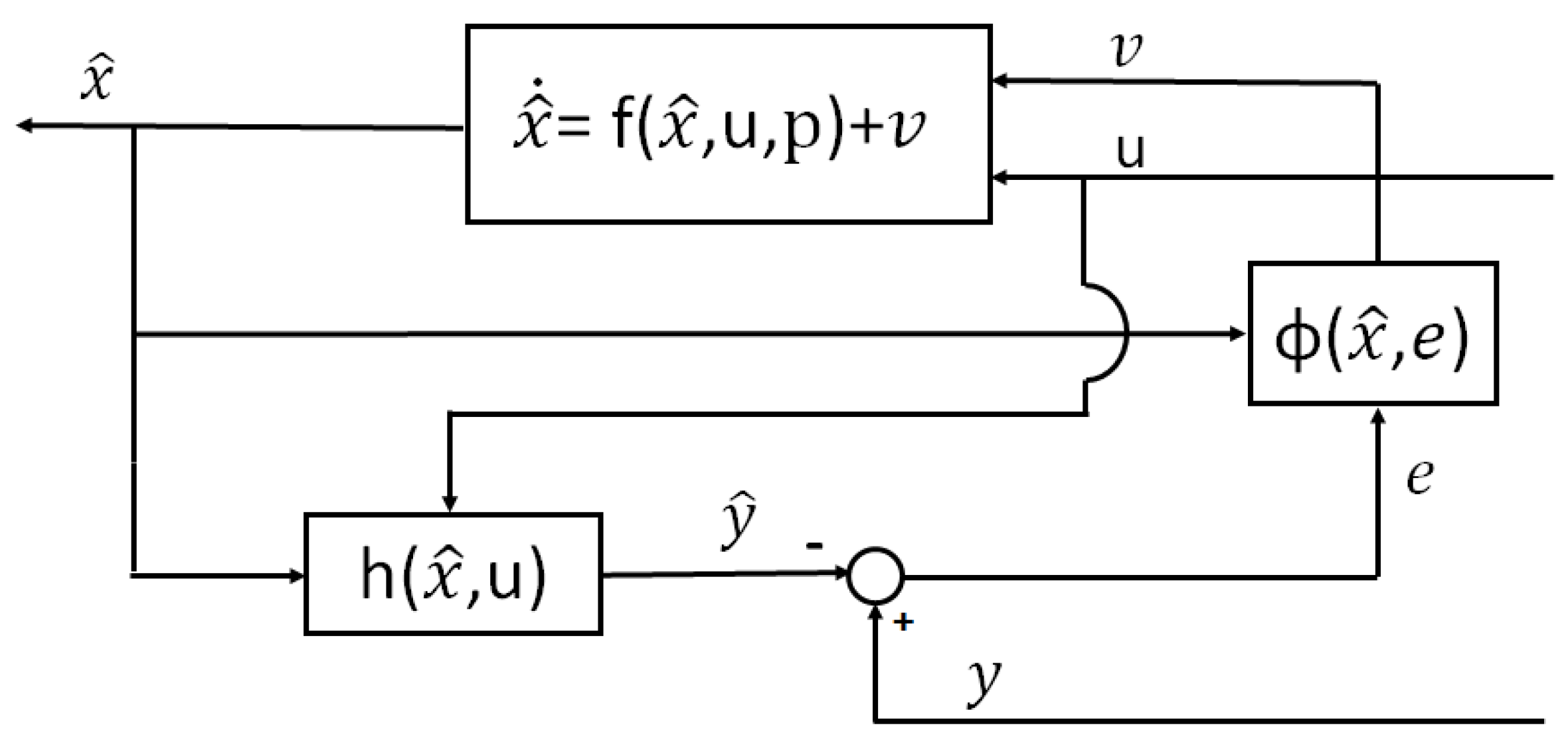
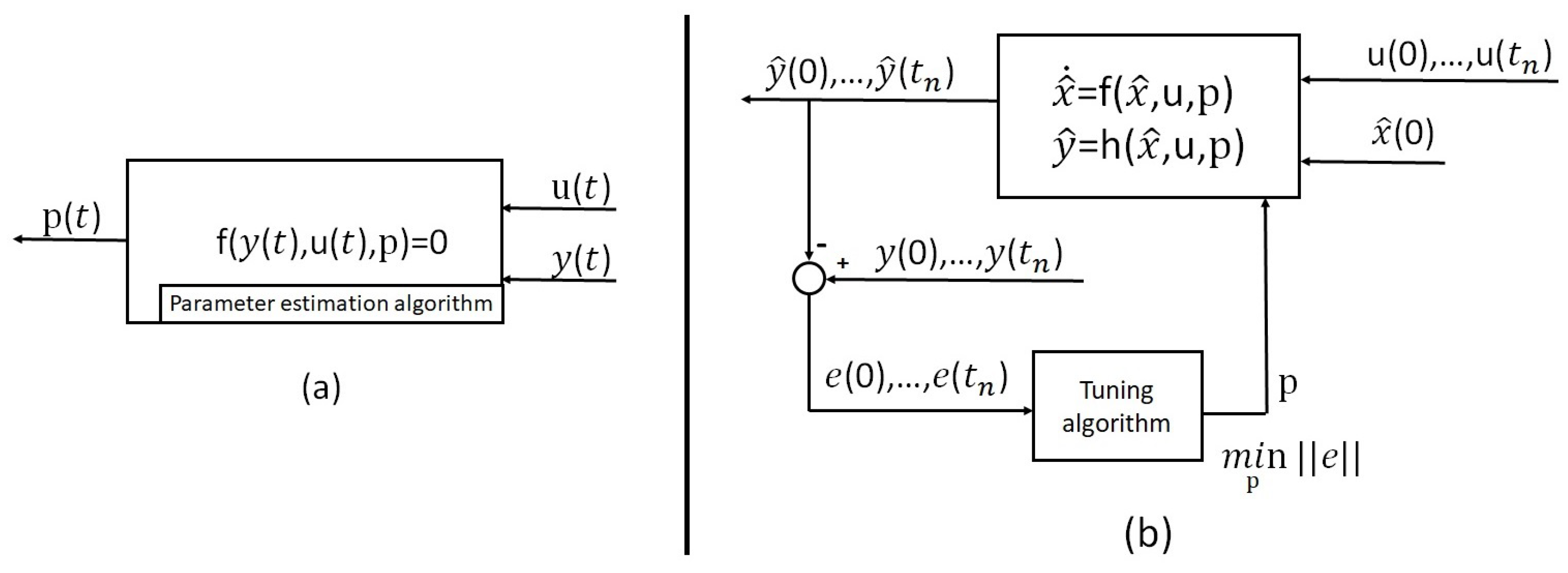
Section 6.2 presents the main techniques to estimate the state of charge, and other parameters and variables. Finally,
Section 7 contains some conclusions and future investigations.
2. Composition and Operation of VRFB
RFB are secondary batteries, which means they are rechargeable. Its principle of energy conversion is based on electrochemical reactions of two redox couples [
60]. As its name suggests, they are based on the principle of redox reactions. A redox reaction can be defined as a phenomenon in which there exists an exchange of electrons between two different species [
61]. On the one hand, species that losse electrons are called oxidized species. On the other hand, species that win electrons are the reduced ones. The gain or loss of an electron is equivalent to a change in the oxidation state of the element. Then, both process of oxidation and reduction can be defined as:
Oxidation: increased oxidation state of a specie, caused by the loss of one or more electrons.
Reduction: decreased oxidation state of a specie, caused by the gain of one or more electrons.
Once the principles of redox reaction have been explained, it is possible to understand the operation of a redox flow battery, and their main components.
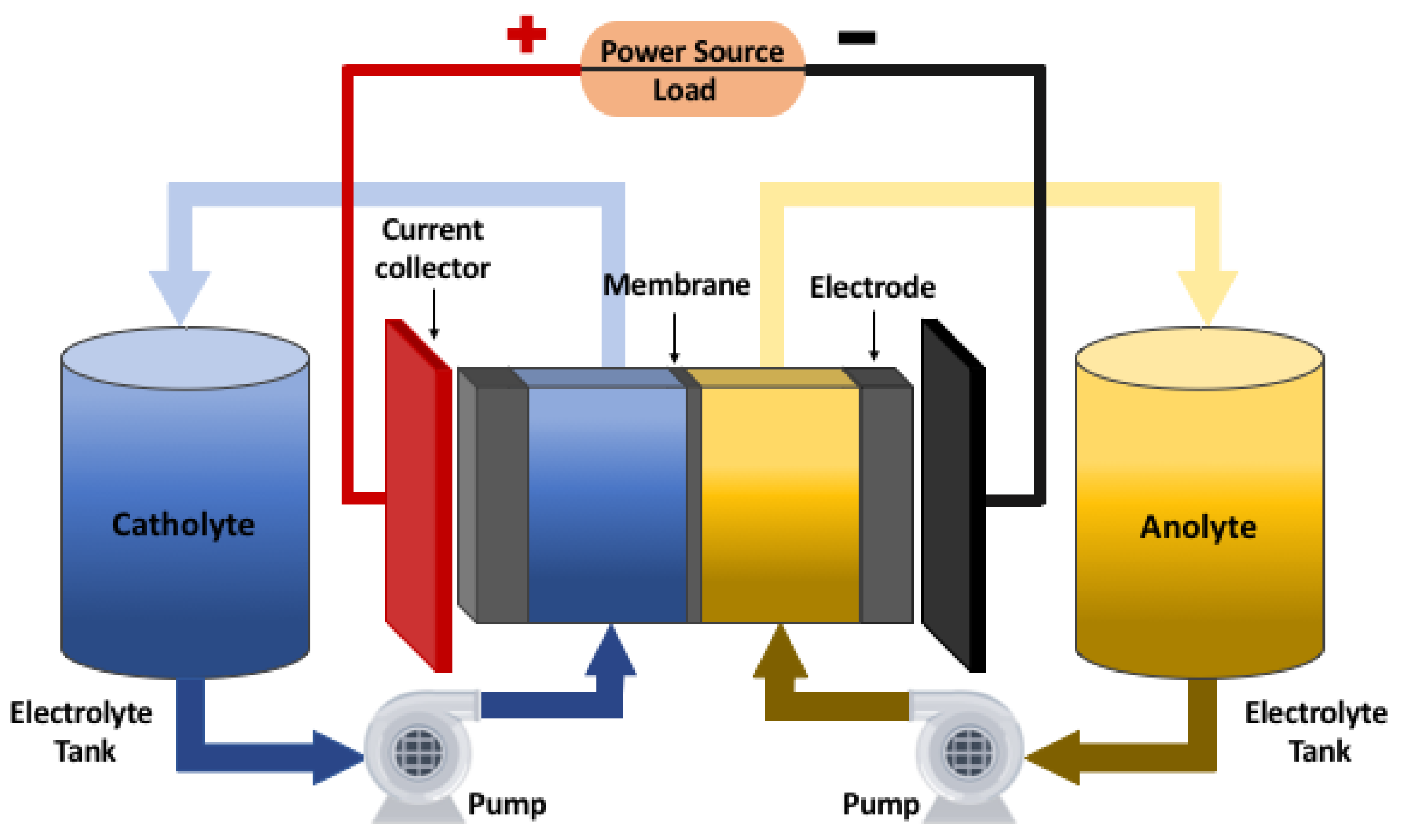
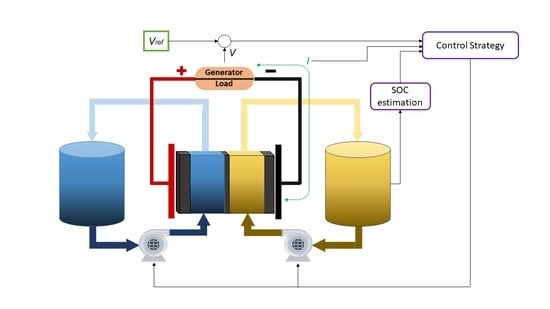
Figure 2 shows the scheme of a redox flow battery with its main components.
A redox flow battery is composed by two electrodes whose function is to be the surface where oxidation-reduction reactions take place. The potential difference that is generated between the electrodes is determined by the electrochemical reaction that occurs, which depends on the chemical elements that make up the reaction. Lot of research has been done to find the best materials and dimensions for the electrodes. In terms of electrode composition, first investigations analyzed carbon and non-carbon materials. For the case of non-carbon materials as metals, it was found that were unsuitable for use in both half-cells [
62]. Carbon has been identified as the best material for use in both electrodes of a VRFB, and different studies have been done that analyze its use and advantages [
63,
64,
65,
66].
The electrons that appear on the redox reaction can be defined as a current that passes through the cell continuously. Current flow exists due to the presence of two collector plates located at the end of the cell. The direction of current flow depends on whether the battery is in the process of charging or discharging. The number of electrons will depend on the current density and the electrode surface area. Typical values of current densities vary between 10 mA/cm
to 800 mA/cm
, depending on the design of the VRFB [
67]. Using a Nafion membrane, usual values of current densities must vary only in a window between 80 mA/cm
to 140 mA/cm
obtaining efficiencies between 80% and 90%, because increasing the current density above the 150 mA/cm
lower efficiency is observed due to the higher ohmic resistance of Nafion membrane [
68]. Different studies have analyzed the limitation of the maximum current density for VRFB obtaining 750 mA/cm
for three layers of carbon fiber paper [
69]. Taking into account that typical current values vary from 20 A to 150 A it is possible to have an idea about the surface of the electrode, taking values between 100 cm
and 2000 cm
.
The main feature of this type of system is that the electrolytes are not only contained within the cell as in conventional batteries, but are stored in two independent tanks. The electrolytes are composed by the active species in an specific concentration, and are dissolved in a strong acid solution that typically is hydrochloric acid (HCl) or sulfuric acid (HSO). One tank contains the positive electrolyte (catholyte) and the other contains the negative electrolyte (anolyte). Fluids from the tanks containing the electrolytes are pumped into two closed circuits through the battery stack (consisting of several flow cells stacked together) and the redox reaction occurs. The new species after the reaction are recirculated to their corresponding tank due to the closed circuit.
The electrolytes tanks always contain the same amount of liquid, but the oxidation state of each specie will depend on the reaction that takes place in the cell during the operation of the system. As long as there are species that can be transformed, the battery can continue the process of charging or discharging.
The membrane has the function of separating the electrolytes inside the cell, preventing them from mixing with the redox species. At the same time, it has to allow the transfer of ions to maintain the electroneutrality of the system [
68]. For that reason, it is necessary to choose an special ion-selective membrane depending on the species [
70]. Different types of membranes have been explored and investigated as Nafion cation exchange membranes (CEMs), tungstophosphoric acid (TPA), sulfonated polymer membranes and others [
71,
72]. Some studies have shown that over time, the membrane degrades and begins to appear the crossing of ions from one electrolyte to the other, polluting both electrolytes [
73]. This fact causes a reduction of the efficiency and lifetime of the complete system, making it necessary to change the cell and electrolytes. Taking into account this common problem in redox flow batteries, the best solution that has been implemented in the recent years is the use of vanadium species as electrolytes [
58,
72]. The first VRFBs explorations were done in the 1930s, by Pissoort [
74]. In the 1970s, Pellegri and Spaziante patent the VRFB [
75], but they could not demonstrate the correct operation of this technology. Skyllas-Kazacos in the 1980s was the first person to succesfully demonstrate the all-vanadium RFB [
76].
In a VRFB, the electrolytes contain vanadium salts dissolved in concentrated solutions of sulfuric acid. Their redox reactions involve four oxidation states of vanadium (+II, +III, +IV, +V). They are the same one with different oxidation states, allowing the possible mixing of electrolytes without becoming a problem for the system [
77].
The negative electrolyte (anolyte) is composed by
and
vanadium species. The positive electrolyte (catholyte) is composed by
and
. The maximum vanadium ion concentration that can be employed for wide temperature range operation is typically 2 M or less [
57,
78]. This means that for each liter of solution is composed of vanadium ions and H
SO
, there are 2 mols of vanadium species dissolved on it. This concentration is equivalent to an energy density of around 25 Wh/kg. This value ensures the solubility of vanadium ions even in temperatures below 5
C. At the same time ensures the stability of vanadium ions at possible temperatures above 40
C [
79].
During the charging process, the oxidizes and becomes releasing an electron. This electron is transferred from the anode to the cathode through external circuit, and reduces to on the other electrode.
During the discharging process, the oxidation of to takes place in the negative electrolyte (anode) and the released electron goes to cathode reducing to . The vanadium species and exist as oxides, which are respectively, and .
The chemical reactions that take place in the cell are the following ones:
where → represents the charge process, and ← the discharge process.
The electrochemical cell, can be seen from a chemical point of view, as a two-part system according to the presented redox equations. The cell potential has a contribution from both anode and cathode reactions. In the anode as a measure of its ability to lose electrons (oxidation potential). The cathode has a contribution based on its ability to gain electrons (reduction potential). The standard cell potential
can then be written as:
The standard potential for each half-reaction process in standard conditions of pressure, concentration and temperature are defined in chemistry reaction tables [
80,
81]. For the case of the VRFB, the standard potential is:
Taking into account (
1) and (
3), it is possible to write the global reaction obtained from both reactions together, with his cell standard potential:
The cell standard potential gives only information about the chemical part of the reaction that takes place inside the cell. The electrode potential
E depends on the concentrations of vanadium species
that exist in the cell. Its value can be obtained using (
5) which is the Nerst equation:
where
R and
F are, respectively, the gas and Faraday constant, and
is the concentration of each vanadium species, which is found in the catholyte or anolyte electrolyte (see
Table 2).
Taking into account Equation (
5) it is possible to obtain the theoretical value of the cell voltage. As it can be noticed, it only depends on the vanadium concentrations, without any external load connected. For that reason, it is called open-circuit voltage (OCV). It is important to take into account that the theoretical OCV of the VRFB has two extreme values which correspond to a state of complete or null charge of the cell. In both cases, these extreme values are far from those measured experimentally (theoretically tend to infinity). For this reason, the extreme values of the OCV are considered experimentally. Many studies agree that the maximum value of the cell voltage during the charge is between 1.6 and 1.7 V, and drops to 1.1 V in the discharge case [
82,
83].
From this experimental value it is possible to see that the power that the cell can generate is not very high. As it has been explained, VRFBs are especially useful to work with high values of power and energy. In order to increase the power, a number of cells have to be connected in series, obtaining what is commonly named stack. The total voltage of the stack can be calculated as the sum of all cells voltage connected in series.
5. Mathematical Models
To study the behaviour of the VRFBs from a theoretical point of view, it is necessary to model the real system taking into account the variables that make it up. Most of the systems use mathematical models, that can take different forms using dynamic formulation, differential equations, statistical models or logical formulation, among others. The mathematical model of a system can be based on one or more parts of study.
The first distinction that exists between models is how the behaviour of the system is described as a function of time. That is to say, whether the effect of time is taken into account or not. If time is taken into account, the model is dynamic whereas if the system is not time dependent, it is called static [
98]. In terms of model VRFBs, it is obvious that due to the characteristics of both flow and current, it must be modelled taking into account the behaviour along the time.
The second distinction for modelling is taking into account, or not, the space dimension. Models that take into account the space dimension are usually named Distributed parameter models and use partial derivative equations. In RFBs there exist many phenomena that can only be described through distributed models, such as the distribution of the vanadium species or temperature along the tanks and cells, or the flow rate in the pipes. There are different studies that have modelled some aspects of the RFB using this type of model [
99]. These models make possible to analyse how the flow is distributed along the cells, obtaining a realistic approximation about the distribution of vanadium concentration inside them [
100]. Non-uniform distribution of flow field is analysed using a parameter distributed model in [
101,
102]. One of the main computational tools that exist to obtain 3D models, is COMSOL [
103], which is a finite element analysis, that allows to solve multiphysics systems. This software has been used to analyse the VRFB performance taking into account the electrode and flow field [
104].
The disadvantage of Distributed parameter models, is that they are very complex and they can not be used for analytical analysis. Their solution requires relevant computational resources and can not be solved in short time.
For this reason, RFB automatic control problems are addressed using reduced order models, or Lumped parameter models. In this type of models most relevant variables, such as current or flow, do not depend on space. These assumptions allows to simplify the model. These models allow to perform analytical analysis and obtain their solution with a reduced computational cost. Despite its simplicity these models closely resemble the actual behaviour of the system [
105]. In this scenario, multiple and different models have been proposed.
Table 6 contains a classification of most relevant RFB models.
A VRFB system, can be mainly separated in three different physical models, which are the electrochemical, the thermal and the hydraulic. All of them are interconnected. In the electrochemical part, the current and flow rate play a key role, but the temperature has also importance. In the thermal model, the concentration of species from the electrochemical part, together with the flow rate and current are the important factors to take into account. Finally, the hydraulic part is related to the other models by the flow rate, which is the variable that plays the key role. In the following, a review of the characteristics of each of these parts is presented.
5.1. Electrochemical Model
One of the main parts of interest of the VRFBs is the behaviour of the vanadium concentrations. As have been explained, it has a direct effect on the voltage and therefore, in the power and energy of the system. There are different approaches to model the electrochemical part.
Some studies use differential equations that determine the behaviour of concentration of vanadium species through the redox reactions that take place [
106,
107]. Another ones, take into account the Nerst equation, and by measuring the voltage are able to model the behaviour of vanadium concentration species inside the system [
108]. This second approach, has the drawback that it only takes into account the cell voltage, and not the input variables such as the flow rate or current. For this reason, it cannot be used for control purposes, since input variables such as current or flow rate, that are the only ones that the user can manage, have not effect in the model presented. Finally, there are some studies which use equivalent circuit models, to describe the behaviour of the electrochemical part. An electric circuit model is used in [
118,
119] to model the state of charge. Another study that uses this type of model to monitor the capacity decay of the VRFB is [
120].
Using directly the redox reactions that take place, it is possible to obtain a model of the vanadium concentration variation along the time. A realistic electrochemical model was develop by Maria Kazacos [
107], which is based on the determination of the vanadium concentrations behaviour with respect to the current and the flow rate of electrolytes. This model has been widely used in different studies [
109,
110,
111].
The model assumes an homogeneous concentration in the cell. The behaviour of the concentrations changes in a cell (
) for RFB of any species is described by the following differential equation:
where
is the volume of the cell,
is the concentration of vanadium in the tank and
F the Faraday’s constant. As can be noticed in Equation (
14) the concentration of each vanadium species,
i, depends on three different factors, which are the flow rate, the current and the diffusion:
Finally, it is possible to model the concentration of vanadium specie in a cell using the state-space representation for the case of a charging process (positive current):
In the case of a discharging process, the unique difference will be the sign of the current vector. The tanks, can be modelled taking into account the number of cells that compose the stack:
where
is the volume of each electrolyte tank and
N is the number of cells that compose the stack.
The states of the system are the vanadium concentrations of both tanks and cells, while the other parameters are constants or variables that can depend on the operating conditions as the temperature.
It is important to remark, that this mathematical model is a realistic approach of a VRFB, and it allows to understand what factors change the vanadium concentrations inside the cell and in the tanks. This is of great importance, since the concentrations in the cell determine the voltage, which is calculated by Nerst expression (
5), and the concentrations inside the tank determine the state of charge (SOC) of the battery:
All parameters that appear are summarized in
Table 7.
5.2. Thermal Model
Usually, the effect of temperature on VRFBs is not detrimental to the system efficiency, since it is normally found close to environmental values [
109]. However, it has been seen that under certain operational conditions, and depending on the membrane, there can be an increase in the temperature above the environmental one [
110]. In this situation, a heat exchanger mechanism could be necessary to improve the behaviour of the battery. Furthermore, as the temperature is an easy variable to measure in any physical system, obtaining a thermal model for VRFBs can help to monitor its correct operation, or even its possible automatic control [
111].
The correct determination of the temperature inside the stack is necessary for two main factors. On the one hand, it can determine how much heat is absorbed or generated during the reaction [
112]. On the other hand, it is directly related with the diffusion coefficients which play an important role inside the electrochemical model.
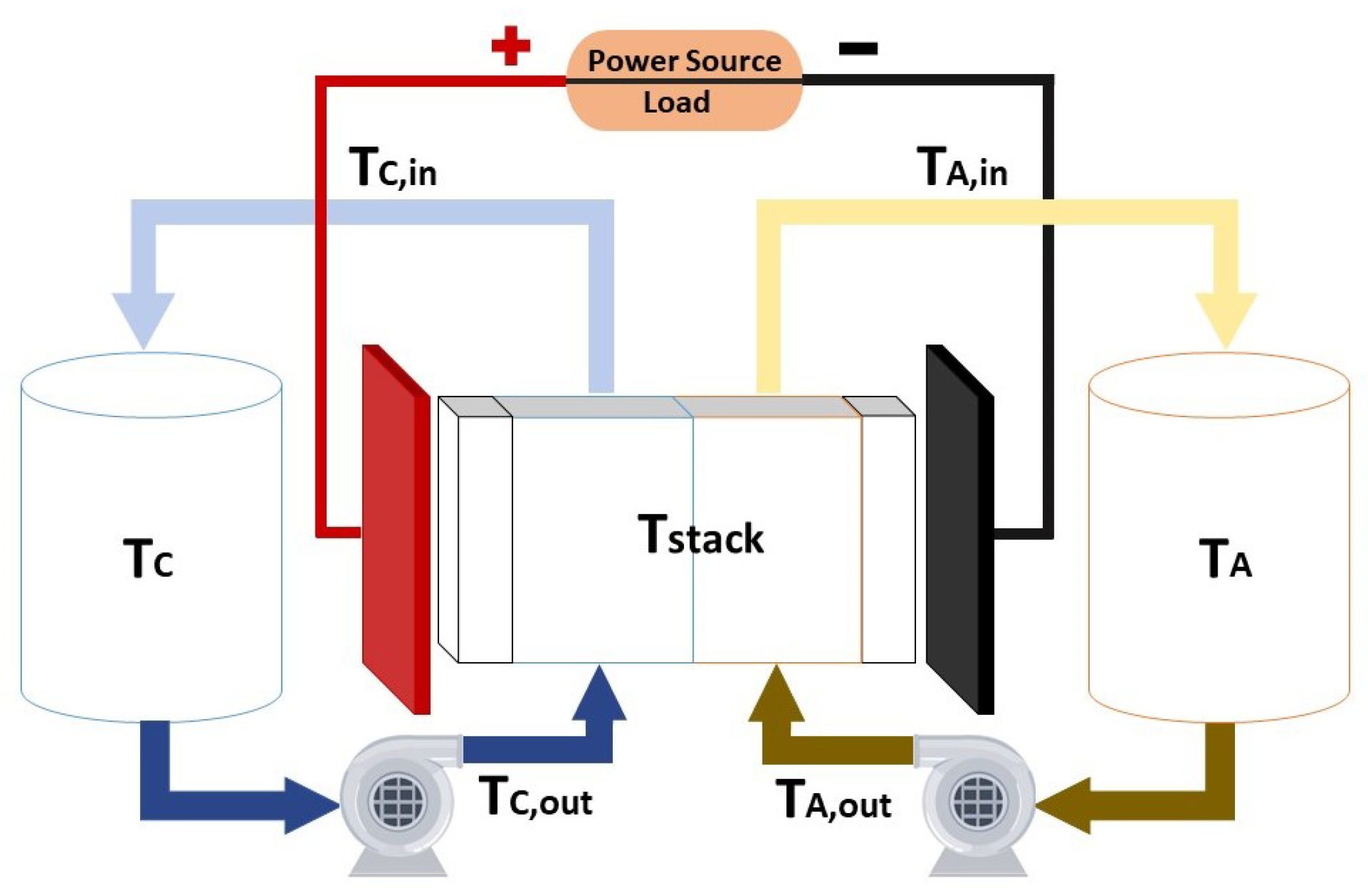
Taking into account the main components of the system, which are the stack, the tanks and the hydraulic transmission line composed by the pipes and pumps, it is possible to model the temperature of each part as it is done on [
112]. It is important to consider the external temperature of the system, which is that of the air (T
).
Figure 3 shows the different points where the temperature of the system is modeled.
The nomenclature of the subscripts of the temperature variables is summarized in
Table 8.
The variation of the different temperatures can be model by using the energy balance equations. Equation (
20) model the behaviour of the temperature in the stack [
110].
where
is the specific heat,
is the density of electrolyte,
Q is the flow rate and
is the heat transfer capability of the stack. The parameters
,
,
and
represent the change of enthalpy, which have an experimental value calculated at 298.15 K for each reaction [
113]. The values of the enthalpies are negative [
110] during the charging process, which yields to the conclusion that the reaction is exothermic. This means that the system releases energy in form of heat to the environment. During the discharging process, the sign of the enthalpy part in (
20) changes, taking place an endothermic reaction. In (
20) also appears the thermal factor due to the ohmic losses produced by the stack resistance,
r, which experimentally changes its value during operation, but for practical purposes it can be assumed constant [
110]. It should be noted that most of the heat generated is due to the ohmic effect, having values between few tens of Joules (currents below 50 A) and hundreds of Joules (for currents in the order of 100 A) [
109].
In the tank and transmission line corresponding to the catholyte part of the system, the temperature equations are:
For the case of the negative side of the system, the same equations model the behaviour of the temperature in a symmetrical way respect to
Figure 3. If it is assumed that the four parts in which the pipes are divided are equals and the flow rate that circulates along the pipes is the same, then same temperatures will exist on both sides of the system.
Table 9 summarizes all parameters of the thermal model.
There exist different studies done in terms of thermal parameters of RFBs. This is the case of [
114], that presents a dynamic electro-thermal model. A thermal model with heat exchangers is presented in [
115], proposing an effective cooling strategy.
5.3. Hydraulic Model
A part of the system that has great importance is the hydraulic part. The pumps are dynamic elements, and their choice in terms of the maximum flow rate they can pump, will depend on the operating conditions of the system. For that reason, it is necessary to determine the power of the pump need to make the electrolytes flow taking into account possible losses due to pressure and friction. The power of the pump affects the temperature model, as can be seen from Equation (
23). Moreover, taking into account that to maximize the efficiency of the VRFB, it is necessary to minimize losses (such as pump power), the obtaining of a correct model will allow to obtain the optimal flow rate that plays the most important role in a flow battery.
The value of the pump power can be obtained through the calculation of the losses in the stack and pipes, following the principle of energy conservation [
116,
117]. The power loss is the product between the pressure drop in the system and the flow rate
Q:
The pressure drop in the pipes can be calculated using the principles of fluid mechanics. In some studies, appears that it can be directly calculated considering two different parts. On the one hand, the friction pressure drop
which comes from fluid viscosity, which is calculated by (
25) [
117]. On the other hand, the losses due to a change in flow direction of the pipes
given by Equation (
26).
The pressure drop in the stack can be calculated considering the pressure drop in the electrodes, that have been found to contribute considerably to the total pressure drop of the system.
Then, the pressure drop of the system is the sum of the previous pressure drops:
Parameters of the hydraulic model are summarized in
Table 10.
7. Conclusions and Future Direction
This paper has introduced the concept of redox flow batteries, contextualizing them within the current energy situation and comparing them with other type of energy storage systems.
The main characteristics and its operation have been explained taking into account the equations that characterize its behaviour, as well as the expressions needed to design the battery sizing for a specific conditions of power and energy. A review in the literature of the existing models has been shown, explaining in detail one of the most used and cited. This dynamic model is decomposed in three main parts that are interconnected: the electrochemical, the thermal and the hydraulic. Attending to the need for optimal control and characterization of the RFB, a discussion about the existing control strategies that are mainly used within the field of redox flow batteries has been presented. Similarly, a discussion about the different methods used to estimate the SOC has been presented, introducing the use of state observers. Finally, some studies have been presented that calibrate the models estimating their parameters and variables. All these techniques constitute an excellent alternative to include complex and expensive instrumentation in the RFB. It has been prove in the literature that these techniques offer very interesting results.
Modelling plays a key role for control purposes inside the RFB. It allows to describe the real system behaviour, providing information and knowledge of the system operation. Moreover, these models are very important to analyse and improve the control systems, observers and parameter estimators. In this sense, until now different studies have been designed with the purpose to obtain a realistic model of a VRFB. Most of them are lumped parameter models that describe with simplicity the behaviour of the RFB, obtaining a behavior which closely resembles the real system. There are few studies that present more complex models that take into account undesired effects such as corrosion, degradation or unexpected reactions. In this scenario, more research will appear in the following years to manage with more realistic models, that will allow to perform more tests to analyze the behaviour of a specific RFB, reducing the R&D (Research and Development) costs. For these purposes, it will be necessary to analyze in which scenarios the use of a lumped or distributed parameter model will be the best option, attending to the practical use that will be given to it. Furthermore, if a distributed parameter model is selected, an analysis of the level of distribution will be required.
Looking to the future, there are different directions in the research of RFB systems. Within the field of control and supervision, research in terms of flow management and parameter estimation will continue to grow, following methodologies such as those described in
Section 6. In particular, the search for online estimators will be one of the key studies in the future, to estimate the different parameters. Therefore, integrating these parameters in the control laws will improve their robustness. Inside this field, a determination of the main parameters to estimate will be required. In addition, parameters that vary over the time and are currently considered constant to simplify the analysis, such as the ohmic resistance or the standard electrode potential, will be online estimated obtaining a more realistic adjustment. It will also be necessary to determine what parameters are affected by the undesired effects such as degradation or corrosion, and its level of affection. As a battery system, it will be important to define the state of health (SOH) which until now has not been studied in detail. Another field of study will be the detection of failures, characterizing the possible failures, what they are due to and the level of damage that they cause within the system. Taking into account all these objectives, a large number of experimental tests will be carried out, being decisive to correctly define the working conditions, the variables to be measured and the instrumentation to be used, seeking a balance between efficiency and experimental cost.
The automation will contribute to the reduction of costs and will open the door to the implementation of fault detection and isolation mechanisms. This will improve their performance and make them even more secure and reliable devices.
Other important investigations will correspond to develop an efficient integration of RFBs in energetic environments such as renewable energy plants, or microgrids.