Infiltrated Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Based Electrodes as Anodes in Solid Oxide Electrolysis Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
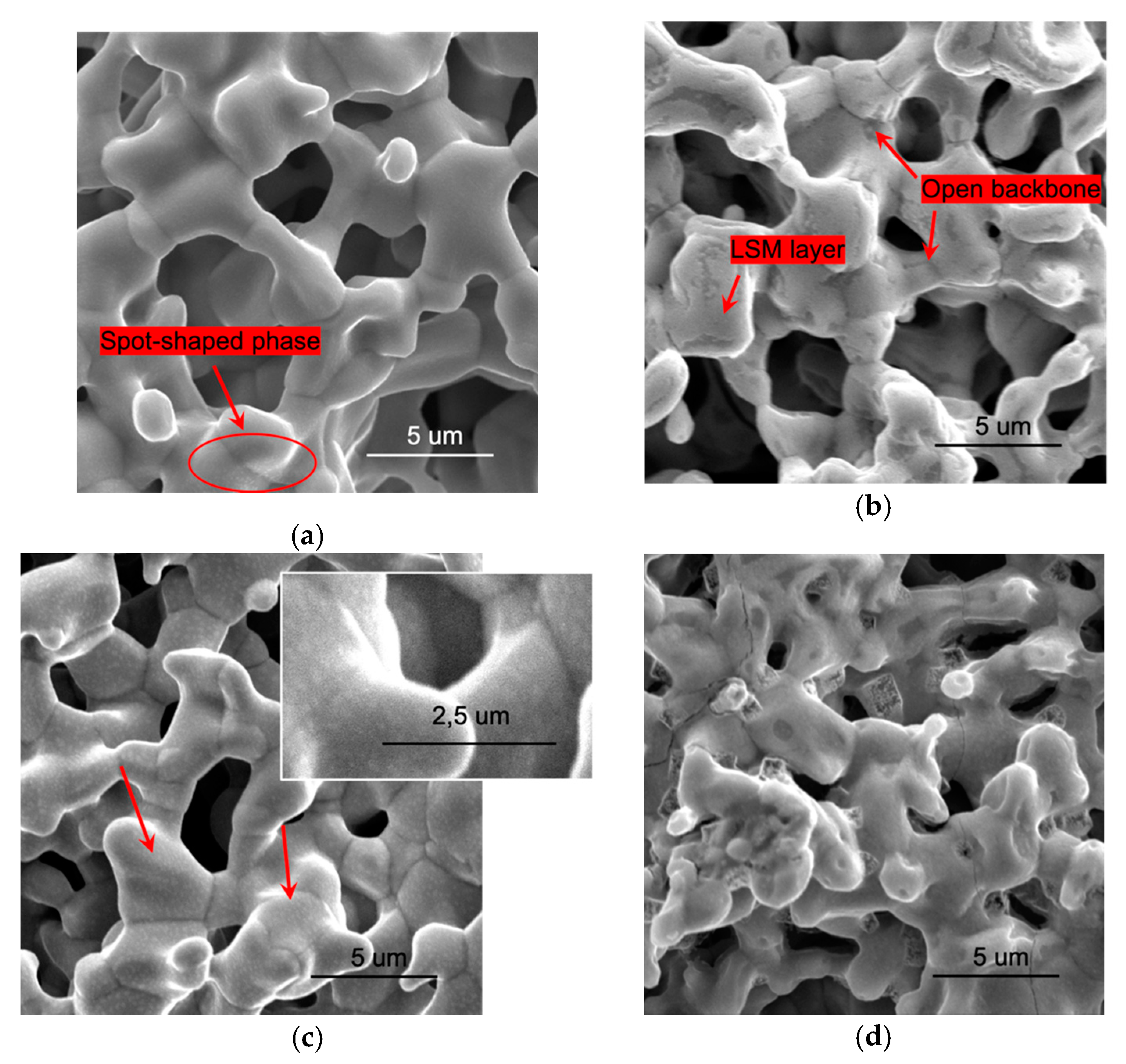
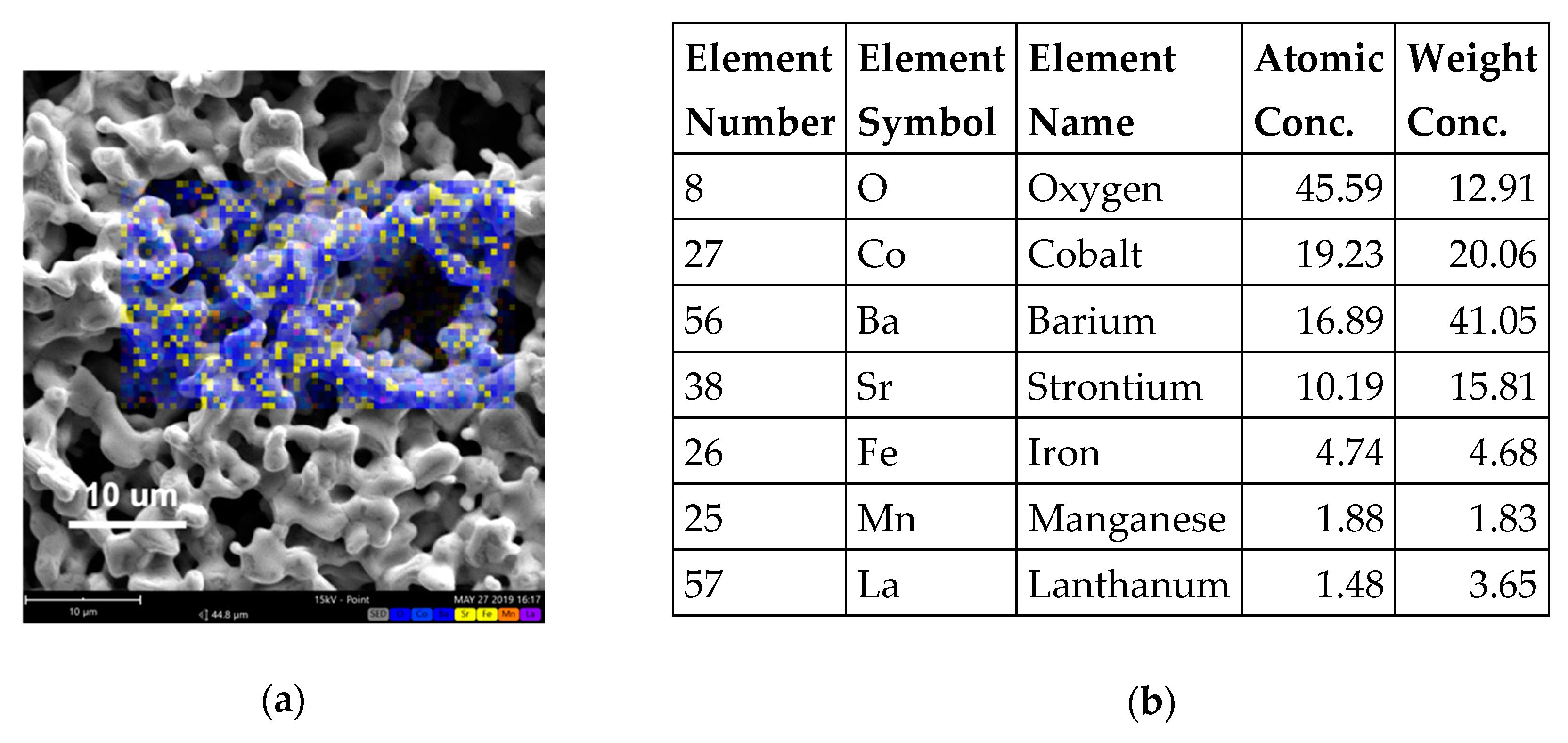
3.1. Microstructural
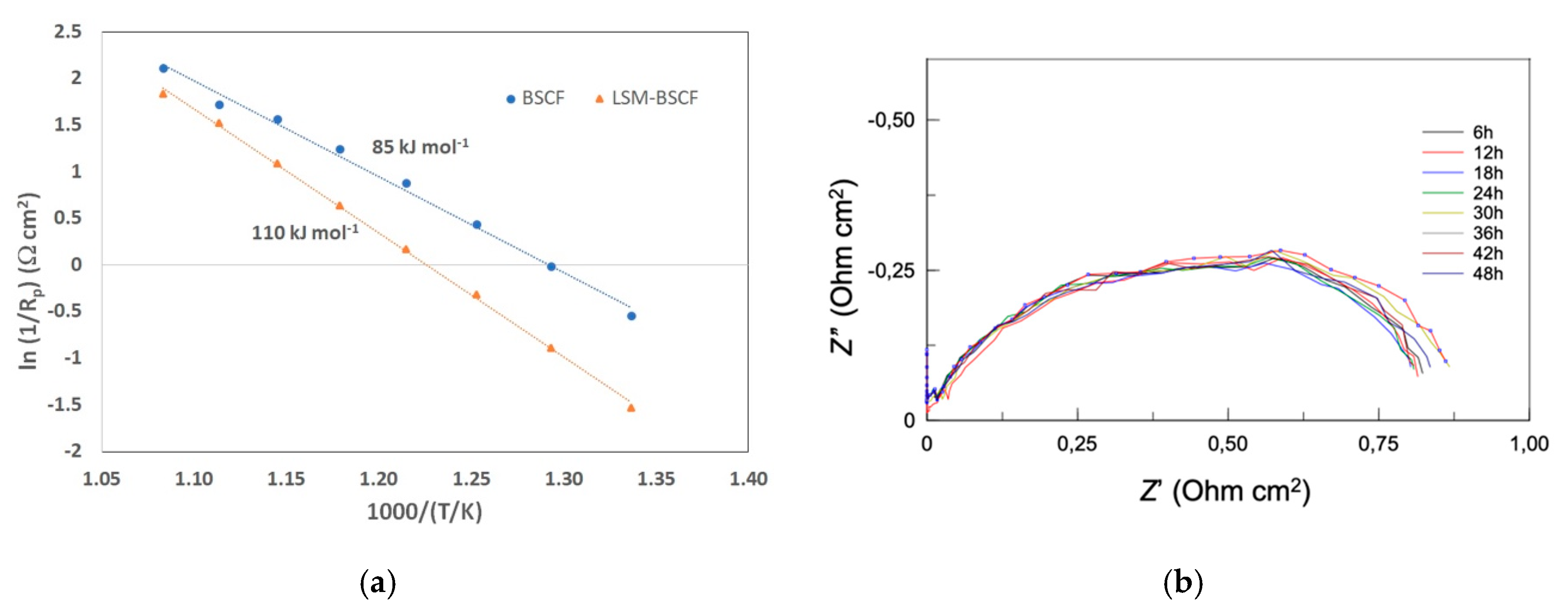
3.2. Electrochemical
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 10 June 2020).
- Gupta, R.; Soini, M.C.; Patel, M.P.; Parra, D. Levelized cost of solar photovoltaics and wind supported by storage technologies to supply firm electricity. J. Energy Storage 2020, 27, 101027. [Google Scholar] [CrossRef]
- Zsiborács, H.; Hegedüsné Baranyai, N.; Vincze, A.; Zentkó, L.; Birkner, Z.; Kinga, M.; Pintér, G. Intermittent Renewable Energy Sources: The Role of Energy Storage in the European Power System of 2040. Electronics 2019, 8, 729. [Google Scholar] [CrossRef]
- Mogensen, M.B.; Chen, M.; Frandsen, H.L.; Graves, C.; Hansen, J.B.; Hansen, K.V.; Hauch, A.; Jacobsen, T.; Jensen, S.H.; Skafte, T.L.; et al. Reversible solid-oxide cells for clean and sustainable energy. Clean Energy 2019, 3, 175–201. [Google Scholar] [CrossRef]
- Venkataraman, V.; Pérez-Fortes, M.; Wang, L.; Hajimolana, Y.S.; Boigues-Muñoz, C.; Agostini, A.; McPhail, S.J.; Maréchal, F.; Van Herle, J.; Aravind, P.V. Reversible solid oxide systems for energy and chemical applications—Review & perspectives. J. Energy Storage 2019, 24, 100782. [Google Scholar]
- Perna, A.; Minutillo, M.; Jannelli, E. Designing and analyzing an electric energy storage system based on reversible solid oxide cells. Energy Convers. Manag. 2018, 159, 381–395. [Google Scholar] [CrossRef]
- Giarola, S.; Forte, O.; Lanzini, A.; Gandiglio, M.; Santarelli, M.; Hawkes, A. Techno-economic assessment of biogas-fed solid oxide fuel cell combined T heat and power system at industrial scale. Appl. Energy 2018, 211, 689–704. [Google Scholar] [CrossRef]
- Carpanese, M.P.; Panizza, M.; Viviani, M.; Mercadelli, E.; Sanson, A.; Barbucci, A. Study of reversible SOFC/SOEC based on a mixed anionic- protonic conductor. J. Appl. Electrochem. 2015, 45, 657–665. [Google Scholar] [CrossRef]
- Thorel, A.S.; Chesnaud, A.; Viviani, M.; Barbucci, A.; Presto, S.; Piccardo, P.; Ilhan, Z.; Vladikova, D.; Stynov, Z. IDEAL-cell, a high temperature innovative dual mEmbrAne fuel cell. ECS. Trans. 2009, 25, 753–762. [Google Scholar] [CrossRef]
- Gondolini, A.; Mercadelli, E.; Sangiorgi, A.; Sanson, A. Integration of Ni-GDC layer on a NiCrAl metal foam for SOFC application. J. Eur. Ceram. Soc. 2017, 37, 1023–1030. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef]
- Brauns, Z.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Presto, S.; Artini, C.; Pani, M.; Carnasciali, M.M.; Massardo, S.; Viviani, M. Ionic conductivity and local structural features in Ce1−xSmxO2−x/2. Phys. Chem. Chem. Phys. 2018, 20, 28338–28345. [Google Scholar] [CrossRef]
- Chen, G.; Sun, W.; Luo, Y.; He, Y.; Zhang, X.; Zhu, B.; Li, W.; Liu, X.; Ding, Y.; Li, Y.; et al. Advanced Fuel Cell Based on New Nanocrystalline Structure Gd0.1Ce0.9O2 Electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 10642–10650. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tanwar, K.; Suman, R.; Kumar, D.; Upadhyay, S.; Parkash, O.; Uppadhya, S.; Parkash, O. A brief review on ceria based solid electrolytes for solid oxide fuel cells. J. Alloy. Compd. 2019, 781, 984–1005. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.; Viviani, M.; Presto, S. Dy doped SrTiO3: A promising anodic material in solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 19242–19249. [Google Scholar] [CrossRef]
- Clematis, D.; Barbucci, A.; Presto, S.; Viviani, M.; Carpanese, M.P. Electrocatalytic activity of perovskite-based cathodes for solid oxide fuel cells. Int. J. Hydrogen Energy 2019, 44, 6212–6222. [Google Scholar] [CrossRef]
- Luo, H.; Wang, H.; Chen, X.; Huang, D.; Zhou, M.; Ding, D. Cation deficiency tuning of LaCoO3 perovskite as bifunctional oxygen electrocatalysts. ChemCatChem 2020, 12, 2768–2775. [Google Scholar] [CrossRef]
- Carpanese, M.P.; Clematis, D.; Bertei, A.; Giuliano, A.; Sanson, A.; Mercadelli, E.; Nicolella, C.; Barbucci, A. Understanding the electrochemical behaviour of LSM-based SOFC cathodes. Part I—Experimental and electrochemical. Solid State Ion. 2017, 301, 106–115. [Google Scholar] [CrossRef]
- Niu, B.; Lu, C.; Yi, W.; Luo, S.; Li, X.; Zhong, X.; Zhao, X.; Xu, B. In-situ growth of nanoparticles-decorated double perovskite electrode materials for symmetrical solid oxide cells. Appl. Catal. B Environ. 2020, 270, 118842. [Google Scholar] [CrossRef]
- Presto, S.; Kumar, P.; Varma, S.; Viviani, M.; Singh, P. Electrical conductivity of NiMo–based double perovskites under SOFC anodic conditions. Int. J. Hydrogen Energy 2018, 43, 4528–4533. [Google Scholar] [CrossRef]
- Rath, M.K.; Lee, K. Superior electrochemical performance of non-precious Co-Ni-Mo alloy catalyst-impregnated Sr2FeMoO6-δ as an electrode material for symmetric solid oxide fuel cells. Electrochim. Acta 2016, 212, 678–685. [Google Scholar] [CrossRef]
- Sharma, R.K.; Burriel, M.; Dessemond, L.; Bassat, J.-M.; Djurado, E. Lan+1NinO3n+1 (n = 2 and 3) phases and composites for solid oxide fuel cell cathodes: Facile synthesis and electrochemical properties. J. Power Sources 2016, 325, 337–345. [Google Scholar] [CrossRef]
- Arias-Serrano, B.I.; Kravchenko, E.; Zakharchuk, K.; Grins, J.; Svensson, G.; Pankov, V.; Yaremchenko, A. Oxygen-Deficient Nd0.8Sr1.2Ni0.8M0.2O4-δ (M = Ni, Co, Fe) Nickelates as Oxygen Electrode Materials for SOFC/SOEC. ECS Trans. 2019, 91, 2387–2397. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Yang, J.; Zong, Z.; Fu, L.; Lian, Z.; Zhang, X.; Wang, X.; Chen, C.; Ma, W.; et al. Nanoscale architecture of (La0.6Sr1.4)0.95Mn0.9B0.1O4 (B = Co, Ni, Cu) Ruddlesden—Popper oxides as efficient and durable catalysts for symmetrical solid oxide fuel cells. Renew. Energy 2020, 157, 840–850. [Google Scholar] [CrossRef]
- Khoshkalam, M.; Tripković, Ð.; Tong, X.; Faghihi-Sani, M.A.; Chen, M.; Vang Hendriksen, P. Improving oxygen incorporation rate on (La0.6Sr0.4)0.98FeO3-δ via Pr2Ni1-xCuxO4+δ surface decoration. J. Power Sources 2020, 457, 228035. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.; Lai, S.Y.; Gerdes, K.; Liu, M. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552–575. [Google Scholar] [CrossRef]
- Jiang, S.P. A review of wet impregnation—An alternative method for the fabrication of high performance and nano-structured electrodes of solid oxide fuel cells. Mat. Sci. Eng. A 2006, 418, 199–210. [Google Scholar] [CrossRef]
- Aguadero, A.; Pérez-Coll, D.; Alonso, J.A.; Skinner, S.J.; Kilner, J. A New family of Mo-Dioped SrCoO3-δ Perovskites for Application in Reversible Solid State Electrochemical Cells. Chem. Mater. 2012, 24, 2655–2663. [Google Scholar] [CrossRef]
- Giuliano, A.; Carpanese, M.P.; Clematis, D.; Boaro, M.; Pappacena, A.; Deganello, F.; Liotta, L.F.; Barbucci, A. Infiltration, Overpotential and Ageing Effects on Cathodes for Solid Oxide Fuel Cells: La0.6Sr0.4Co0.2Fe0.8O3-δ versus Ba0.5Sr0.5Co0.8Fe0.2O3-δ. J. Electrochem. Soc. 2017, 164, F3114–F3122. [Google Scholar] [CrossRef]
- Winkler, J.; Hendriksen, P.V.; Bonanos, N.; Mogensen, M. Geometric requirements of solid electrolyte cells with a reference electrode. J. Electrochem. Soc. 1998, 145, 1184–1192. [Google Scholar] [CrossRef]
- Carpanese, M.P.; Giuliano, A.; Panizza, M.; Mercadelli, E.; Sanson, A.; Gondolini, A.; Bertei, A.; Sanson, A.; Gondolini, A.; Bertei, A. Experimental Approach for the study of SOFC cathodes. Bulg. Chem. Commun. 2016, 48, 23–29. [Google Scholar]
- Meng, L.; Wang, F.; Wang, A.; Pu, J.; Chi, B.; Li, J. High performance La0.8Sr0.2MnO3-coated Ba0.5Sr0.5Co0.8Fe0.2O3 cathode prepared by a novel solid-solution method for intermediate temperature solid oxide fuel cells. Chin. J. Catal. 2014, 35, 38–42. [Google Scholar] [CrossRef]
- Deganello, F.; Liotta, L.F.; Marcì, G.; Fabbri, E.; Traversa, E. Strontium and iron-doped barium cobaltite prepared by solution combustion synthesis: Exploring a mixed-fuel approach for tailored intermediate temperature solid oxide fuel cell cathode materials. Mater. Renew. Sustain. Energy 2013, 2, 8. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Li, R.; Yang, Y.; Wen, G.; Tian, D.; Lu, X.; Ding, Y.; Chen, Y.; Lin, B. Improving stability and electrochemical performance of Ba0.5Sr0.5Co0.2Fe0.8O3-δ electrode for symmetrical solid oxide fuel cells by Mo doping. J. Alloy. Compd. 2020, 831, 154711. [Google Scholar] [CrossRef]
- Chen, X.J.; Khor, K.A.; Chan, S.H. Electrochemical behavior of La(Sr)MnO3 electrode under cathodic and anodic polarization. Solid State Ion. 2004, 167, 379–387. [Google Scholar] [CrossRef]


| La(NO3)2·2H2O | Sr(NO3)2 | Mn(NO3)3· xH2O | Glycine | PVP |
|---|---|---|---|---|
| 10.477 g | 0.622 g | 7.289 g | 2.468 g 1 | 1.158 g 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majnoni d’Intignano, X.; Cademartori, D.; Clematis, D.; Presto, S.; Viviani, M.; Botter, R.; Barbucci, A.; Cerisola, G.; Caboche, G.; Carpanese, M.P. Infiltrated Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Based Electrodes as Anodes in Solid Oxide Electrolysis Cells. Energies 2020, 13, 3659. https://doi.org/10.3390/en13143659
Majnoni d’Intignano X, Cademartori D, Clematis D, Presto S, Viviani M, Botter R, Barbucci A, Cerisola G, Caboche G, Carpanese MP. Infiltrated Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Based Electrodes as Anodes in Solid Oxide Electrolysis Cells. Energies. 2020; 13(14):3659. https://doi.org/10.3390/en13143659
Chicago/Turabian StyleMajnoni d’Intignano, Xavier, Davide Cademartori, Davide Clematis, Sabrina Presto, Massimo Viviani, Rodolfo Botter, Antonio Barbucci, Giacomo Cerisola, Gilles Caboche, and M. Paola Carpanese. 2020. "Infiltrated Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Based Electrodes as Anodes in Solid Oxide Electrolysis Cells" Energies 13, no. 14: 3659. https://doi.org/10.3390/en13143659
APA StyleMajnoni d’Intignano, X., Cademartori, D., Clematis, D., Presto, S., Viviani, M., Botter, R., Barbucci, A., Cerisola, G., Caboche, G., & Carpanese, M. P. (2020). Infiltrated Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Based Electrodes as Anodes in Solid Oxide Electrolysis Cells. Energies, 13(14), 3659. https://doi.org/10.3390/en13143659









