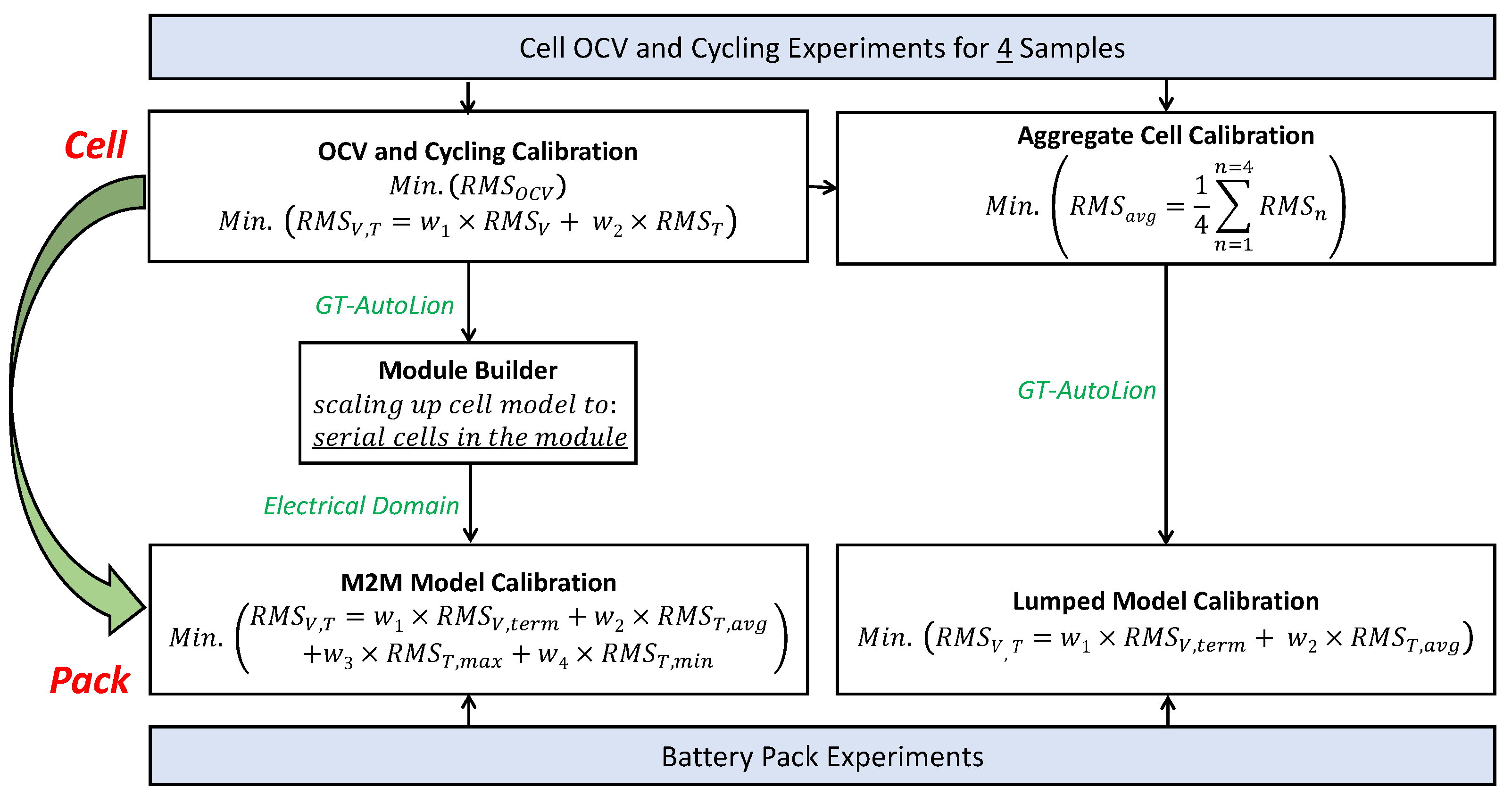
5.1.2. Cycling Calibration
In the cycling calibration optimization stage, the multipliers are used to tune the charge (
) and mass transport (
and
) properties within the cell. The heat transfer coefficient (
h) and the contact resistance (
) are the other two decision variables where intrinsic values are adjusted in the design optimizer. Li et al. [
22] presented the effective intervals of the parameters influencing the cell cycling behavior, showing that the mass and charge transfer coefficients can vary one order of magnitude in the calibration optimization problem. Similarly, Ecker et al. [
42] considered one order of magnitude change in the exchange current density to find the best fit to the experimental voltage curve. Accordingly,
Table 5 presents the lower and upper bounds for the parameters, which are considered in the present cycling calibration optimization. The choice of the limits on the contact resistance (
) and the natural convection heat transfer coefficient (
h) have been made based on Refs. [
19,
41], respectively. Moreover, similarly to the study by Santhanagopalan et al. [
21], the diffusion coefficient inside the cathode particles highly affects the cell discharged capacity. Therefore, the effective range of
has been narrowed to enhance the model predictability.
The combination of the calibration parameters (multipliers and dimensionless decision variables) needs to be chosen to minimize the total RMS of the voltage and temperature predictions (
), so that the sample-to-sample parameter variability is also minimized.
Table 6 shows the
values for different set of parameters for all four cell samples. It is noted that the contact resistance (
) is considered in all of the combinations [
41] along with the heat transfer coefficient (
h) that couples the cell electrochemical and thermal models.
Comparing the second and third rows in
Table 6 shows that including the diffusion coefficient in the cathode leads to a significant reduction in the
when compared to considering the diffusion coefficient in the anode. Similarly, evaluating the fourth and fifth rows reflects that the cathodic exchange current density is more impactful than the anodic exchange current density. Therefore, the cycling behavior of the battery cells under consideration is governed by cathode’s charge and mass transport properties. Moreover, the sixth row indicates that incorporating the electrolyte diffusivity in addition to the cathodic diffusivity further enhances the model predictability. This is most likely due to the facts that the cathode is made of several different materials (e.g., Ni, Co, and Mn) and the electrolyte includes different solvents (e.g., ethylene carbonate (EC) and ethyl methyl carbonate (EMC)). Including the cathodic exchange current density further reduces the
(the seventh row) while adding the anodic current density (as shown in the eighth row) does not make more improvements.
The next step is to consider the variability of the identified parameters among the cell samples. The variability is defined here as:
where
x stands for each of the factors affecting cell cycling performance and the
,
and
subscripts correspond to the maximum, minimum, and average values among the four cell samples, respectively.
The results for all four samples are summarized in
Table 7. It is seen that all the combinations, except the row in bold, result in 100% or more variability for at least one of the parameters. Therefore, the parameter combination (
,
h,
,
,
) highlighted in bold in
Table 6 and
Table 7 has been considered in the cycling calibration optimization herein.
Table 8 summarizes the cycling calibration optimization results for all four samples. The RRMSEs/
for voltage and temperature predictions are approximately 1%/0.98 and 3%/0.7 for all samples.
The sample-to-sample parameter variability reported in
Table 8 can be explained by qualitative analysis of the experimental observations.
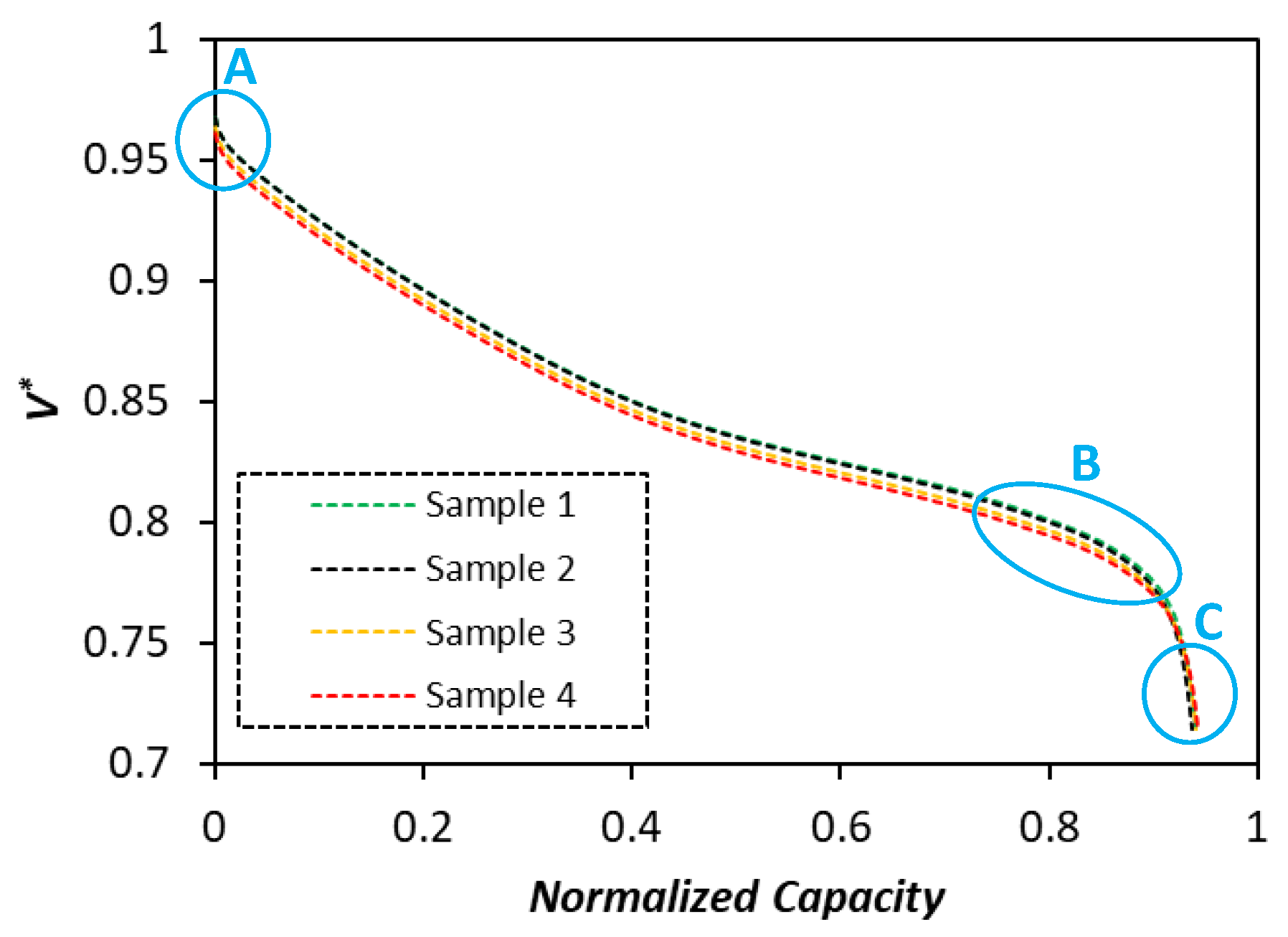
Figure 6 illustrates the experimentally observed discharge curves under 1 C cycling rate for all four samples. Three different regions (A, B, and C) where different parameters govern the cell cycling behavior are marked on the discharge curve.
First, it can be noticed that samples 1 and 2 have almost identical cycling characteristics, while samples 3 and 4 experience similar cycling behaviors. This observation is reflected in the calibrated parameters for the corresponding samples. It is seen that samples 1 and 2 show higher voltage levels in the region A, where the kinetic overpotential is dominant. The calibration optimization results in
Table 8 are in agreement with the experimental data, where samples 1 and 2 show higher values for the cathode exchange current densities (
) in comparison with samples 3 and 4. The parameter identification for the electrolyte diffusivity (
) predicts higher values for samples 1 and 2 than for samples 3 and 4. This prediction is also well aligned with the actual observations in the region B, where samples 1 and 2 show better performance (higher voltage levels) than samples 3 and 4 at the downward turning point of the discharge curve where mass transport limitations of the electrolyte phase play a role. The calibrated values of the diffusion coefficient within the cathode particles (
) for samples 1 and 2 are lower than samples 3 and 4. These predictions are confirmed by the observed experiments in the region C where solid phase mass transport limitations are dominant. Thus, samples 3 and 4 have slightly higher discharged capacities than samples 1 and 2.
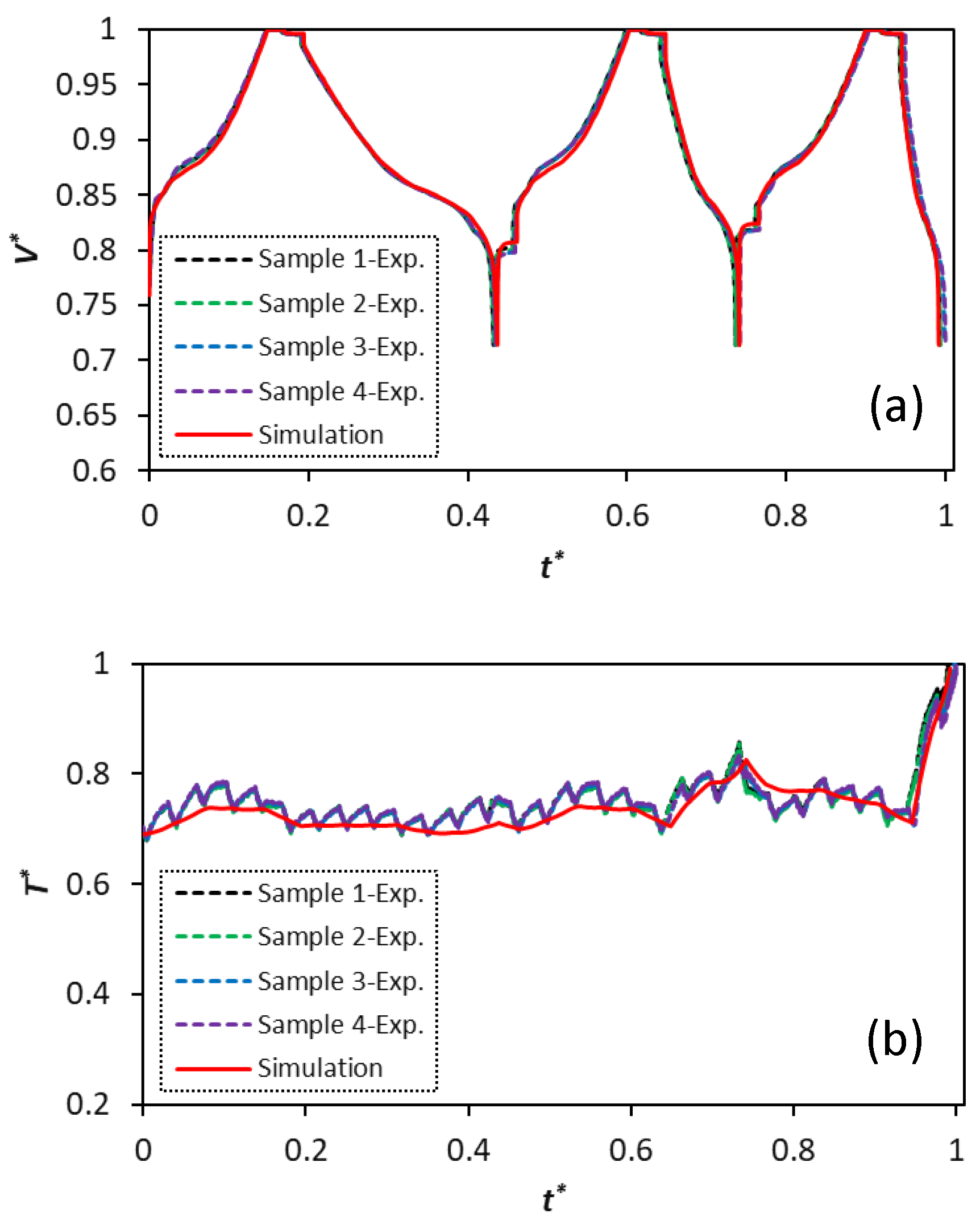
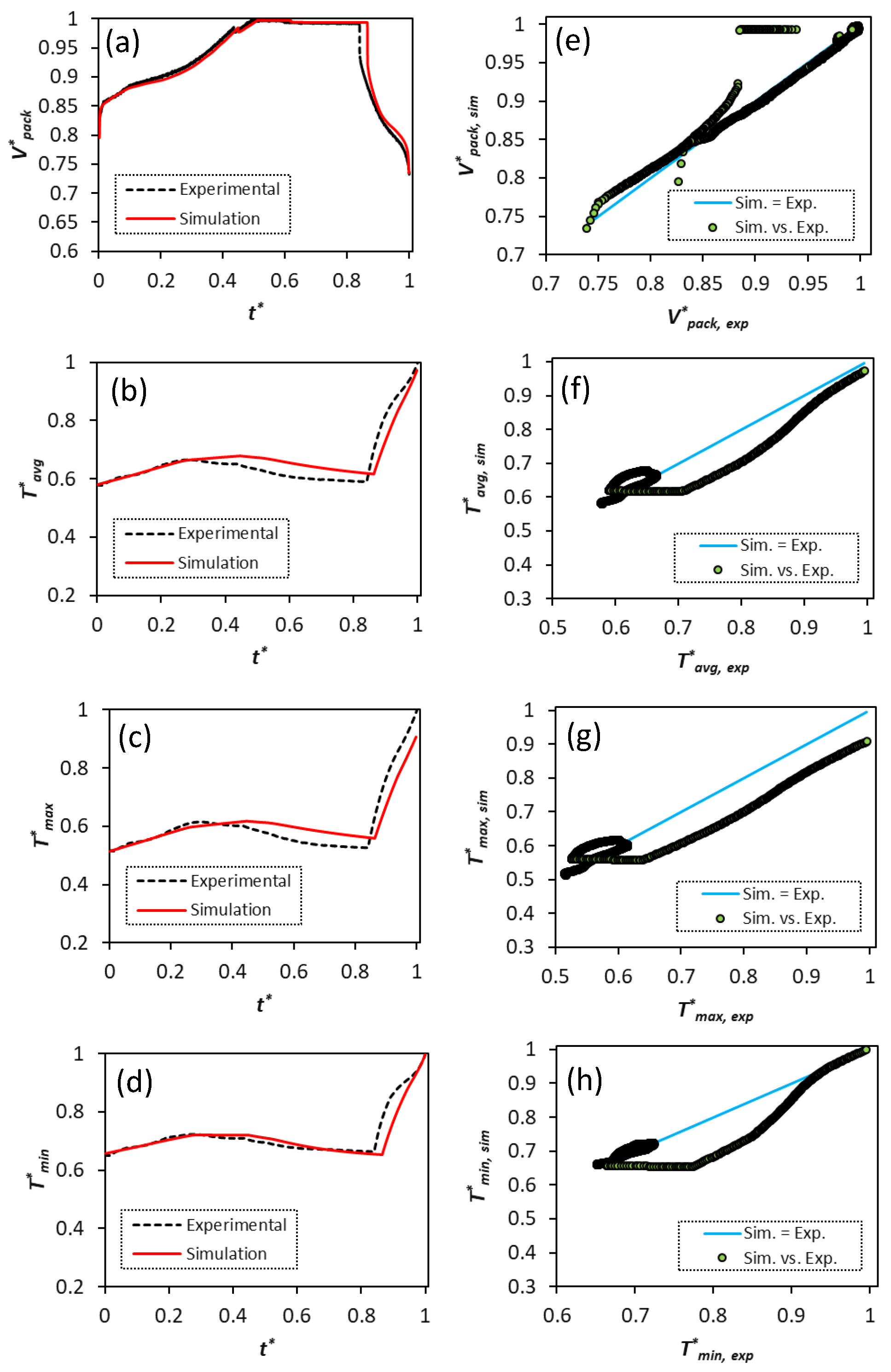
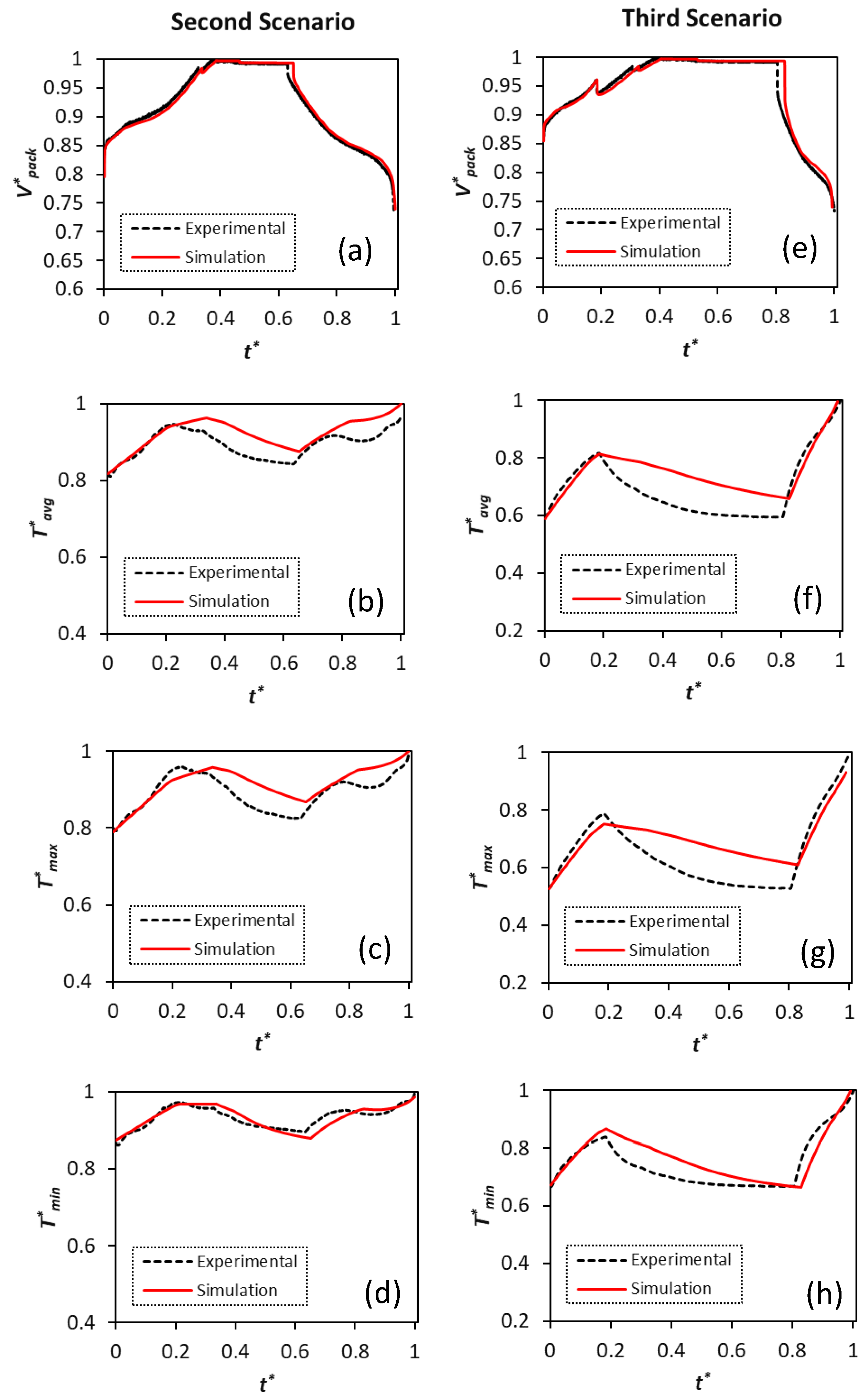
Figure 7 compares the simulated voltage and temperature profiles with the experimental data for all four samples. The cells undergo three consecutive cycles, including C/3 CC-CV charge-rest-C/5 discharge, C/3 CC-CV charge-rest-C/2 discharge, and C/3 CC-CV charge-rest-1C discharge.
The predicted voltage profiles excellently capture the experimentally measured profiles for all four samples. The trend for the temperature profiles is also well captured in the simulations. Nevertheless, it should be mentioned that there is a slight increase in the voltage deviation at the end of discharge for lower C-rates, where the model predicts a higher discharged capacity for the cells than the experimental values. The mass transport limitations play the key role in the cell overpotential increase and they further affect voltage simulation predictions at the end of discharge with higher cycling currents. This phenomenon is common for electrochemistry-based models, which contain a diverse set of parameters to be identified, as it has also been highlighted by Weißhar et al. [
43].
The cell overpotential and cycling current both contribute to heat generation inside the cell causing the temperature to rise.
Figure 6a–d indicate that all samples show the lowest level of temperature during the C/5 discharge process (
) when the operating current is at its lowest value. The maximum temperature is observed in the 1 C discharge cycle when the end of discharge voltage is reached and the cell overpotential is at its highest value during the corresponding cycle (
).
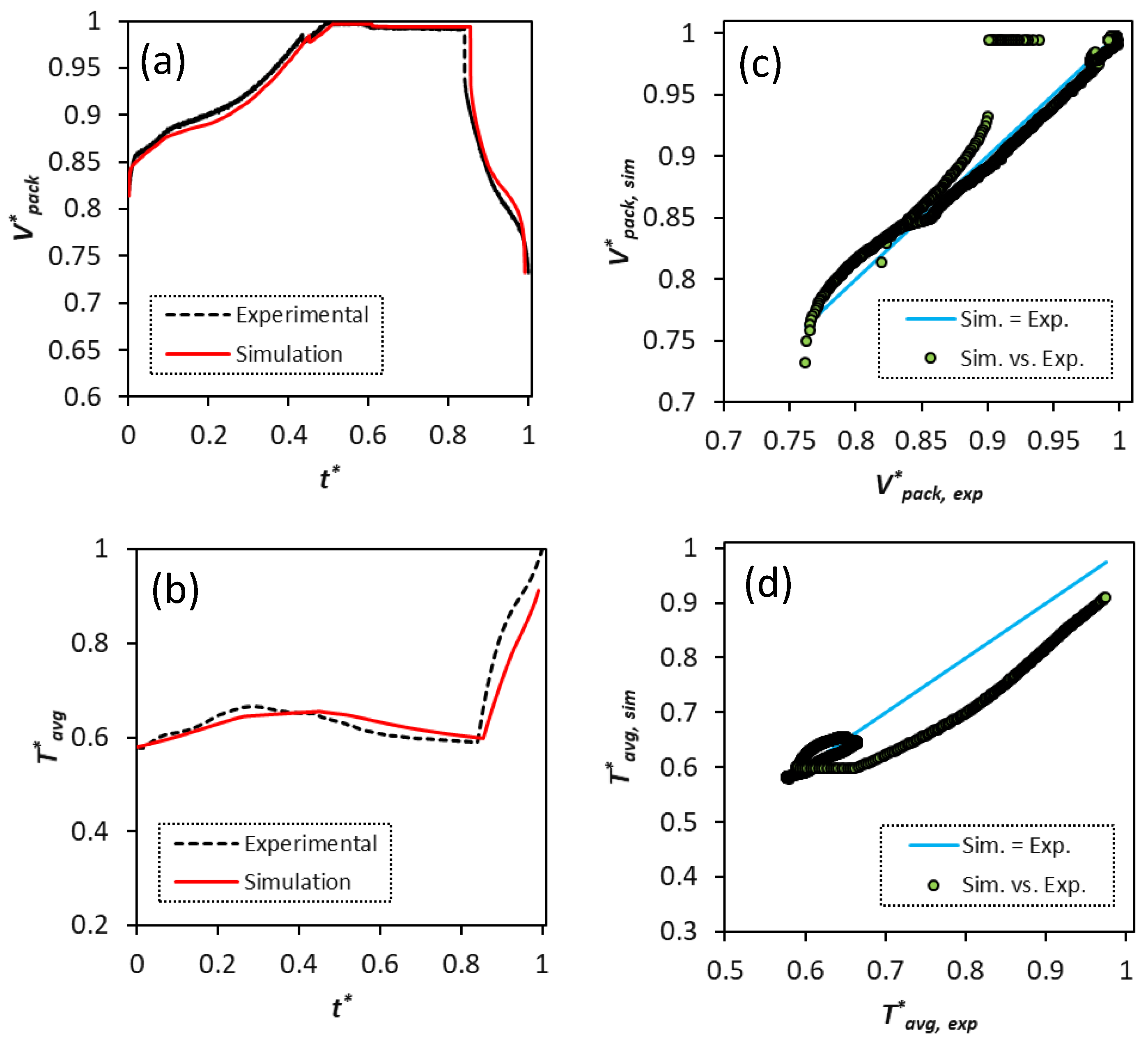
The cycling calibration optimization results for the aggregate cell are provided in
Table 9. The average RRMSEs/
for the voltage and temperature are 1.08%/0.98 and 3.07%/0.68, respectively.
Figure 8 shows the results for the aggregate cell calibration optimization. The predictions for voltage and temperature are compared with the actual profiles that were experienced by samples 1–4. The aggregate cell simulated voltage shows deviations from the experiments at the end of discharge states similar to the calibration results for each cell sample.