Electrogenic Biofilm Development Determines Charge Accumulation and Resistance to pH Perturbation
Abstract
:1. Introduction
2. Materials and Methods
2.1. MFC Construction and Operation
2.2. Analyses
2.2.1. Electrochemical and Chemical Analysis
2.2.2. Molecular Analysis and Microbial Diversity Studies
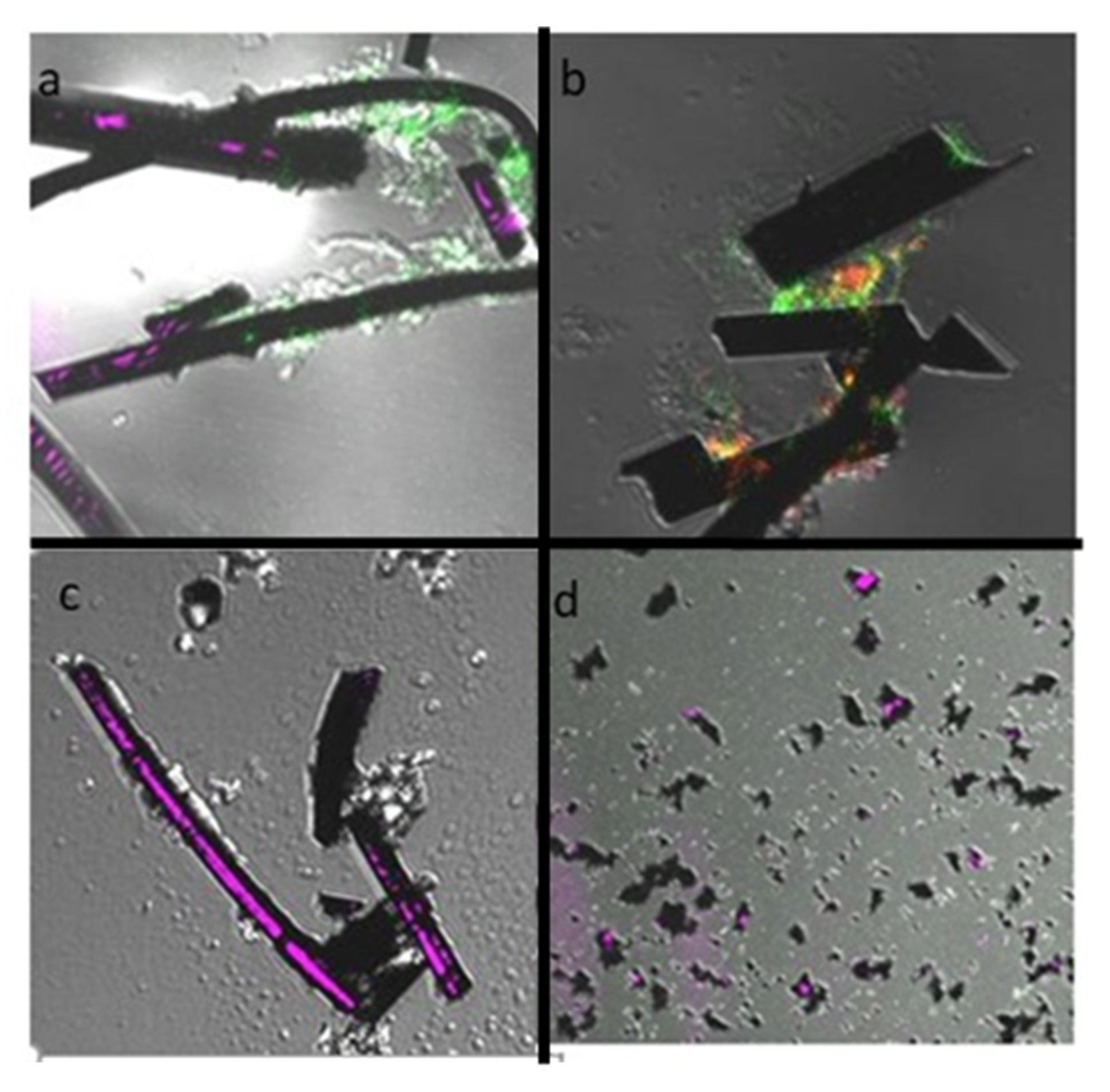
2.2.3. Microscopy
3. Results
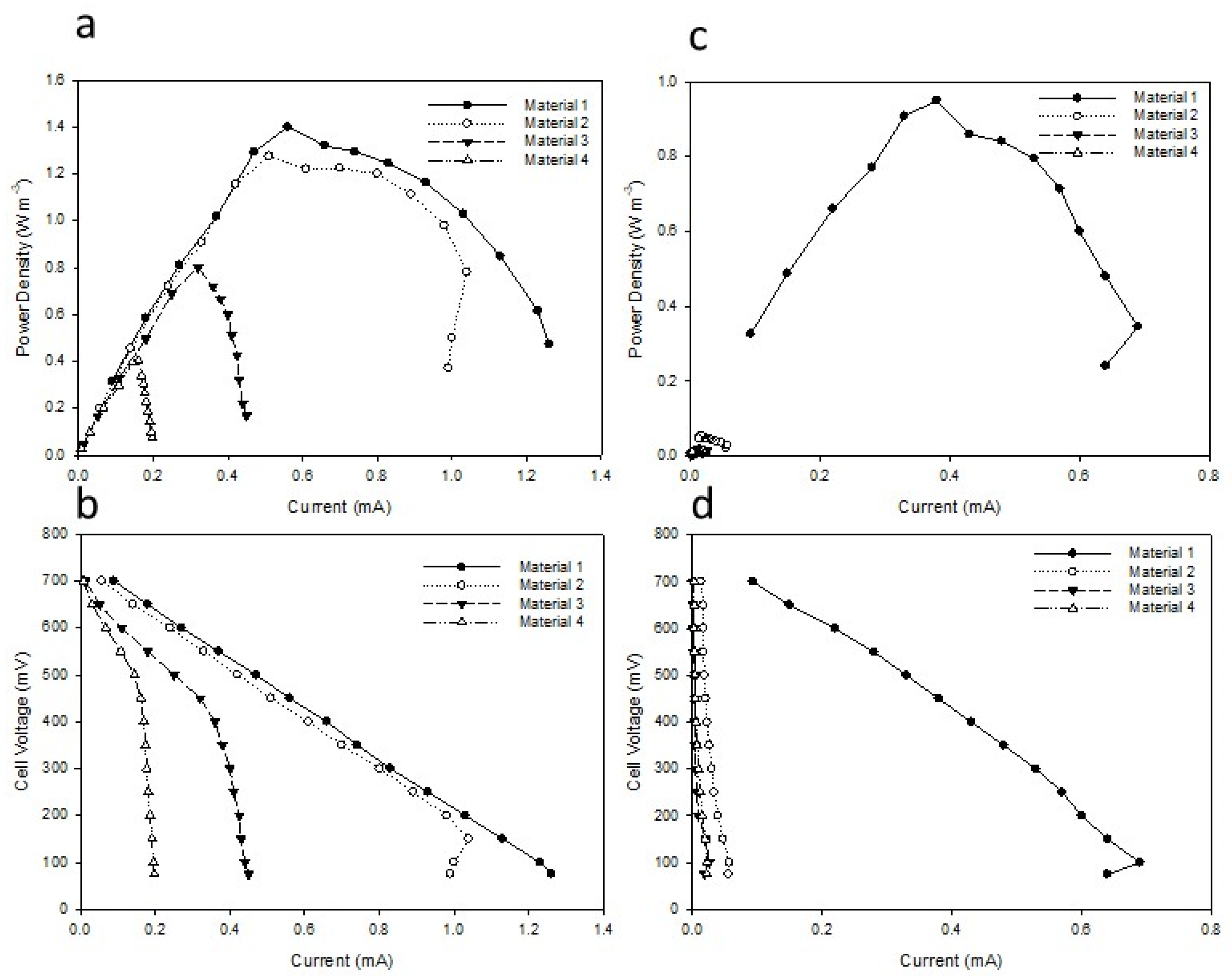
3.1. Electrogenic Biofilm Development with Different Open- and Closed-Circuit-Acclimated Carbon Anode Materials
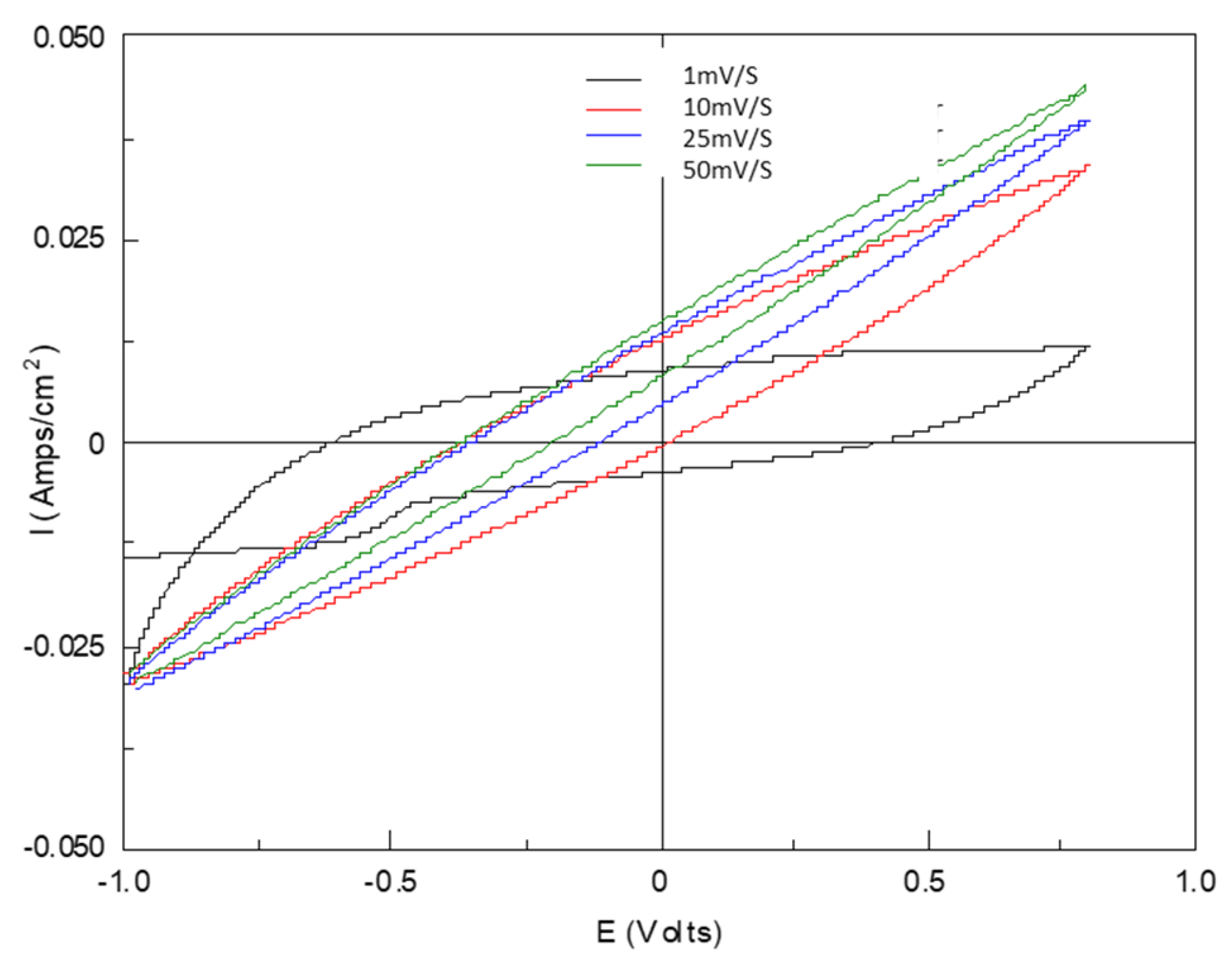
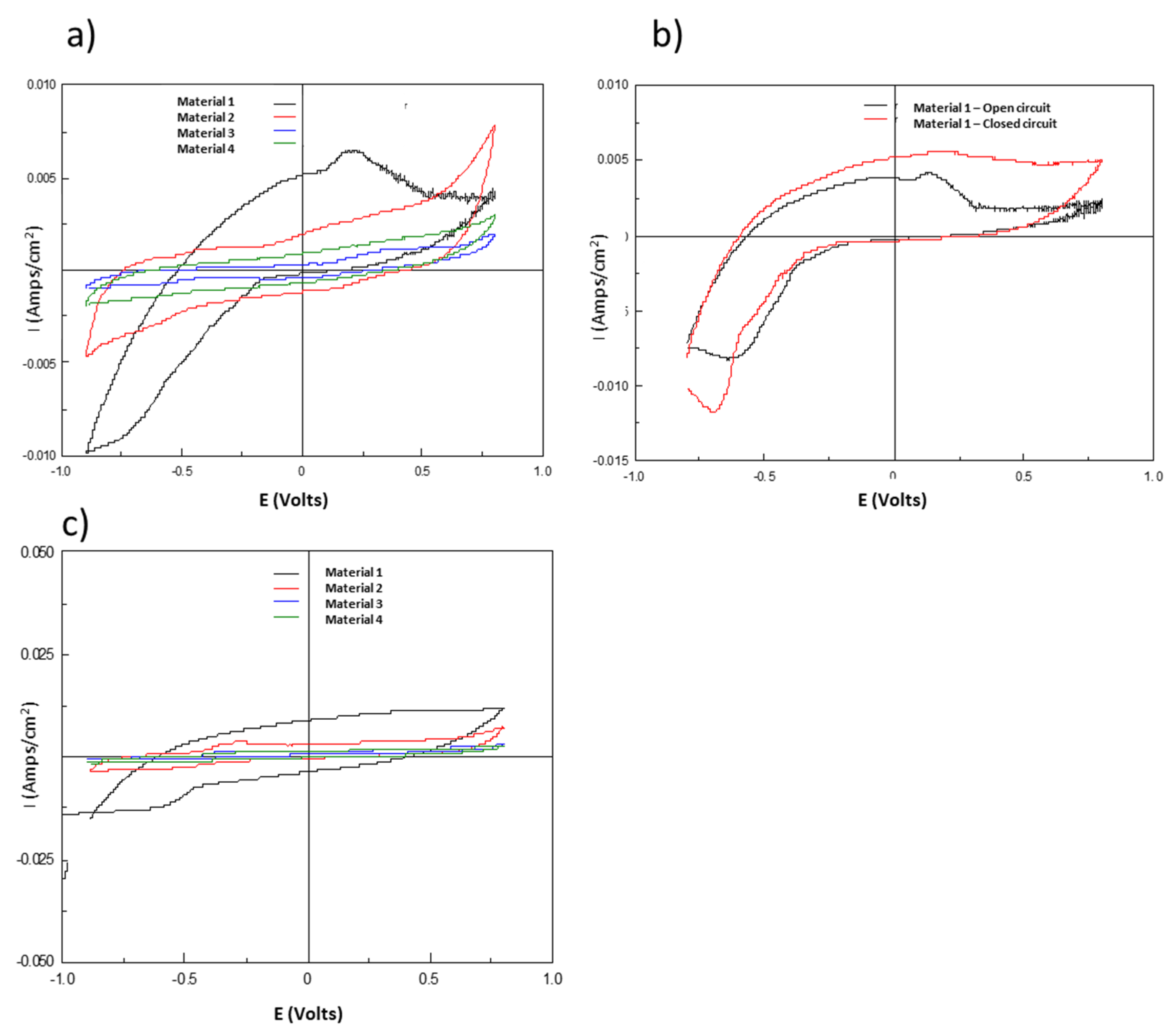
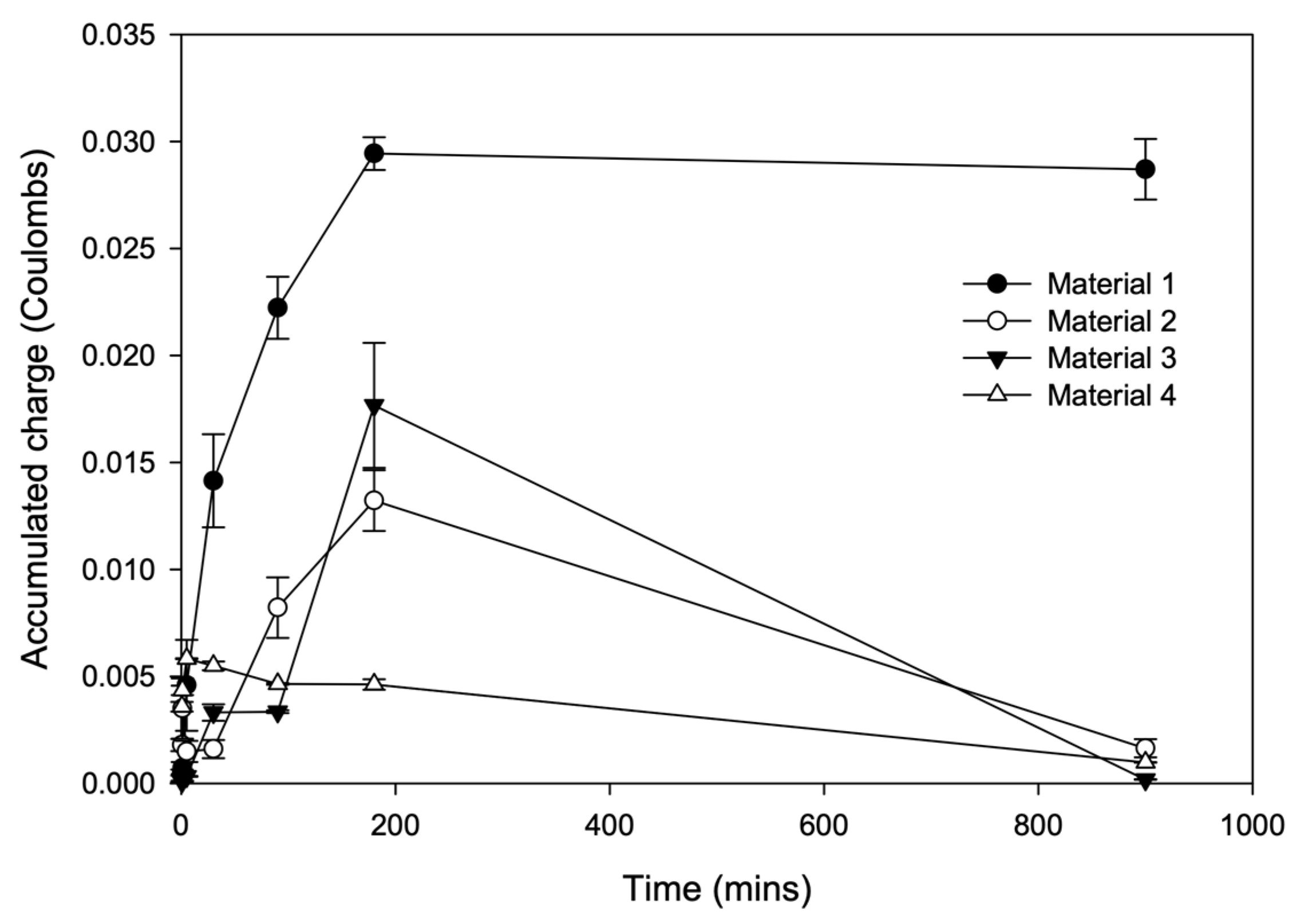
3.2. Biofilm Charge Accumulation and Electrochemical Activity
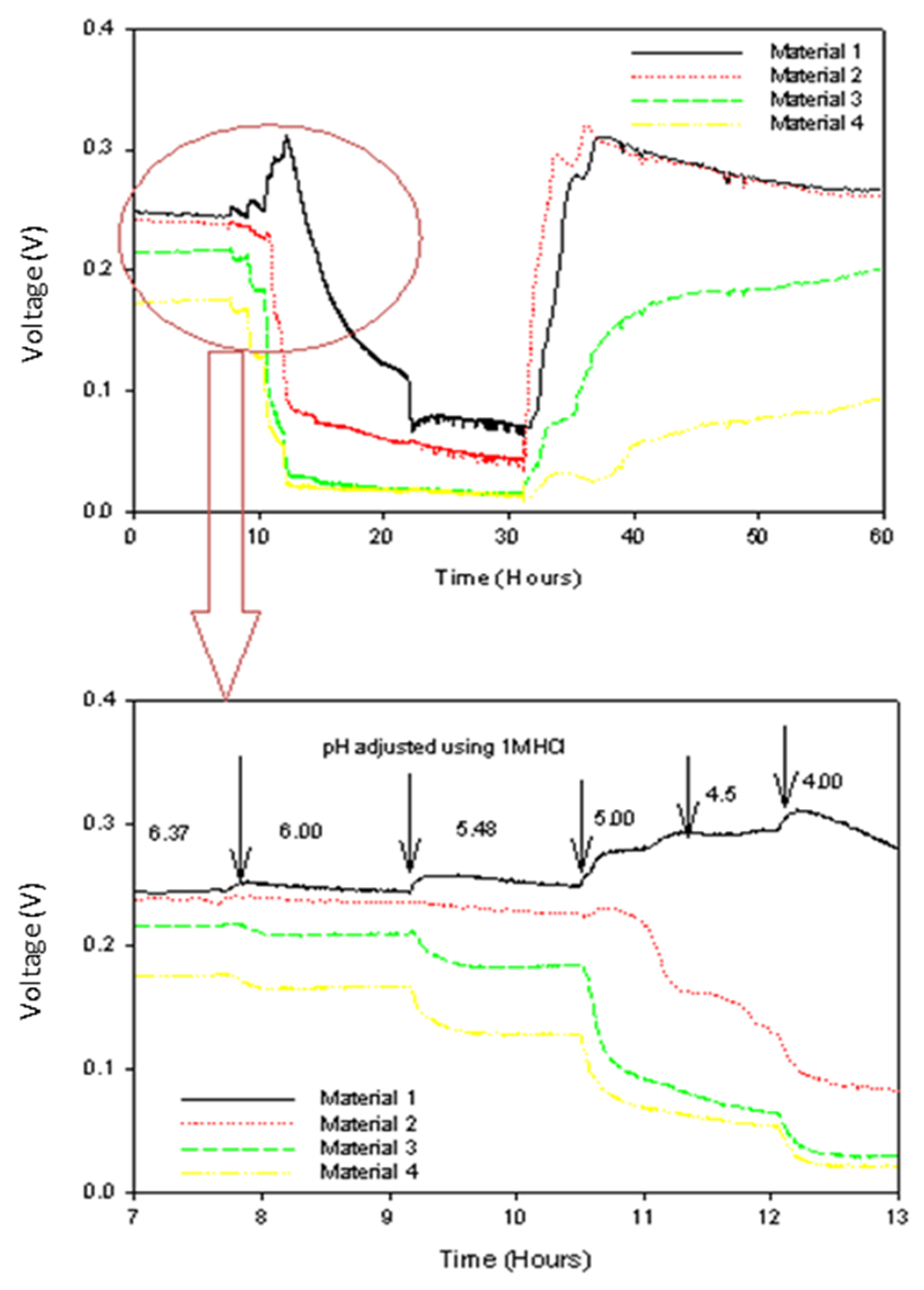
3.3. The Influence of pH Perturbation
4. Discussion
4.1. The Effect of Material Type on Electrogenic Developement
4.2. The Development of Biofilm and Anodic Charge Accumulation
4.3. Biofilm and Resistance to pH Perturbation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stein, N.E.; Hamelers, H.M.V.; van Straten, G.; Keesman, K.J. On-line detection of toxic components using a microbial fuel cell-based biosensor. J. Process Control 2012, 22, 1755–1761. [Google Scholar] [CrossRef]
- Michie, I.S.; Kim, J.R.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. The influence of psychrophilic and mesophilic start-up temperature on microbial fuel cell system performance. Energy Environ. Sci. 2011, 4, 1011–1019. [Google Scholar] [CrossRef]
- Morozan, A.; Stamatin, I.; Stamatin, L.; Dumitru, A.; Scott, K. Carbon electrodes for microbial fuel cells. J. Optoelectron. Adv. Mater. 2007, 9, 221–224. [Google Scholar]
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Li, T.; Dong, Z.; Wang, Y. Modification of carbon felt anodes using double-oxidant HNO3/H2O2 for application in microbial fuel cells. RSC Adv. 2018, 8, 2059–2064. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.Y.; Zhai, D.D.; Si, R.W.; Sun, J.Z.; Liu, X.; Yong, Y.C. Three-Dimensional Electrodes for High-Performance Bioelectrochemical Systems. Int. J. Mol. Sci. 2017, 18, 90. [Google Scholar] [CrossRef] [Green Version]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite Fiber Brush Anodes for Increased Power Production in Air-Cathode Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef]
- Larrosa-Guerrero, A.; Scott, K.; Katuri, K.P.; Godinez, C.; Head, I.M.; Curtis, T. Open circuit versus closed circuit enrichment of anodic biofilms in MFC: Effect on performance and anodic communities. Appl. Microbiol. Biotechnol. 2010, 87, 1699–1713. [Google Scholar] [CrossRef]
- Sleutels, T.H.J.A.; Lodder, R.; Hamelers, H.V.M.; Buisman, C.J.N. Improved performance of porous bio-anodes in microbial electrolysis cells by enhancing mass and charge transport. Int. J. Hydrog. Energy 2009, 34, 9655–9661. [Google Scholar] [CrossRef]
- Xie, X.; Criddle, C.; Cui, Y. Design and fabrication of bioelectrodes for microbial bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 3418–3441. [Google Scholar] [CrossRef]
- Roubaud, E.; Lacroix, R.; Da Silva, S.; Etcheverry, L.; Bergel, A.; Basséguy, R.; Erable, B. Benchmarking of Industrial Synthetic Graphite Grades, Carbon Felt, and Carbon Cloth as Cost-Efficient Bioanode Materials for Domestic Wastewater Fed Microbial Electrolysis Cells. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Schrott, G.D.; Bonanni, P.S.; Robuschi, L.; Esteve-Nuñez, A.; Busalmen, J.P. Electrochemical insight into the mechanism of electron transport in biofilms of Geobacter sulfurreducens. Electrochim. Acta 2011, 56, 10791–10795. [Google Scholar] [CrossRef]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvankar, N.S.; Mester, T.; Tuominen, M.T.; Lovley, D.R. Supercapacitors Based on c-Type Cytochromes Using Conductive Nanostructured Networks of Living Bacteria. ChemPhysChem 2012, 13, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Krige, A.; Sjöblom, M.; Ramser, K.; Christakopoulos, P.; Rova, U. On-Line Raman Spectroscopic Study of Cytochromes’ Redox State of Biofilms in Microbial Fuel Cells. Molecules 2019, 24, 646. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Yu, H.; Yu, H.; Wang, X. Lack of anodic capacitance causes power overshoot in microbial fuel cells. Bioresour. Technol. 2013, 138, 353–358. [Google Scholar] [CrossRef]
- Blanchet, E.; Erable, B.; De Solan, M.-L.; Bergel, A. Two-dimensional carbon cloth and three-dimensional carbon felt perform similarly to form bioanode fed with food waste. Electrochem. Commun. 2016, 66, 38–41. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Huang, Y.; Manohar, A.K.; Mansfeld, F. Effect of electrolyte pH on the rate of the anodic and cathodic reactions in an air-cathode microbial fuel cell. Bioelectrochemistry 2008, 74, 78–82. [Google Scholar] [CrossRef]
- Ren, Z.; Ward, T.E.; Regan, J.M. Electricity Production from Cellulose in a Microbial Fuel Cell Using a Defined Binary Culture. Environ. Sci. Technol. 2007, 41, 4781–4786. [Google Scholar] [CrossRef]
- Torres, C.I.; Kato Marcus, A.; Rittmann, B.E. Proton transport inside the biofilm limits electrical current generation by anode-respiring bacteria. Biotechnol. Bioeng. 2008, 100, 872–881. [Google Scholar] [CrossRef]
- Biffinger, J.C.; Pietron, J.; Bretschger, O.; Nadeau, L.J.; Johnson, G.R.; Williams, C.C.; Nealson, K.H.; Ringeisen, B.R. The influence of acidity on microbial fuel cells containing Shewanella oneidensis. Biosens. Bioelectron. 2008, 24, 900–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penteado, E.D.; Fernandez-Marchante, C.M.; Zaiat, M.; Gonzalez, E.R.; Rodrigo, M.A. Influence of carbon electrode material on energy recovery from winery wastewater using a dual-chamber microbial fuel cell. Environ. Technol. 2017, 38, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Boon, N.; Siciliiano, S.; Verhaege, M.; Verstraete, W. Biofuel cells select for consortia that self-mediate electron transfer. Appl. Environ. Microbiol. 2004, 70, 5373–5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricke, K.; Harnisch, F.; Schroder, U. On the use of cyclic voltammetry for the study of anodic electron transfer in microbial fuel cells. Energy Environ. Sci. 2008, 1, 144–147. [Google Scholar] [CrossRef]
- Marsili, E.; Rollefson, J.B.; Baron, D.B.; Hozalski, R.M.; Bond, D.R. Microbial Biofilm Voltammetry: Direct Electrochemical Characterization of Catalytic Electrode-Attached Biofilms. Appl. Environ. Microbiol. 2008, 74, 7329–7337. [Google Scholar] [CrossRef] [Green Version]
- Heering, H.A.; Hirst, J.; Armstrong, F.A. Interpreting the catalytic voltammetry of electroactive enzymes adsorbed on electrodes. J. Phys. Chem. B 1998, 102, 6889–6902. [Google Scholar] [CrossRef]
- Armstrong, F.A. Recent developments in dynamic electrochemical studies of adsorbed enzymes and their active sites. Curr. Opin. Chem. Biol. 2005, 9, 110–117. [Google Scholar] [CrossRef]
- Magnuson, T.S.; Isoyama, N.; Hodges-Myerson, A.L.; Davidson, G.; Maroney, M.J.; Geesey, G.G.; Lovley, D.R. Isolation, characterization and gene sequence analysis of a membrane-associated 89 kDa Fe(III) reducing cytochrome c from Geobacter sulfurreducens. Biochem. J. 2001, 359, 147–152. [Google Scholar] [CrossRef]
- Inoue, K.; Qian, X.; Morgado, L.; Kim, B.-C.; Mester, T.n.; Izallalen, M.; Salgueiro, C.A.; Lovley, D.R. Purification and Characterization of OmcZ, an Outer-Surface, Octaheme c-Type Cytochrome Essential for Optimal Current Production by Geobacter sulfurreducens. Appl. Environ. Microbiol. 2010, 76, 3999–4007. [Google Scholar] [CrossRef] [Green Version]
- Paitier, A.; Godain, A.; Lyon, D.; Haddour, N.; Vogel, T.M.; Monier, J.-M. Microbial fuel cell anodic microbial population dynamics during MFC start-up. Biosens. Bioelectron. 2017, 92, 357–363. [Google Scholar] [CrossRef]
- Chae, K.-J.; Choi, M.-J.; Lee, J.-W.; Kim, K.-Y.; Kim, I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I.; Krajmalnik-Brown, R.; Parameswaran, P.; Marcus, A.K.; Wanger, G.; Gorby, Y.A.; Rittmann, B.E. Selecting Anode-Respiring Bacteria Based on Anode Potential: Phylogenetic, Electrochemical, and Microscopic Characterization. Environ. Sci. Technol. 2009, 43, 9519–9524. [Google Scholar] [CrossRef] [PubMed]
- Mehta, T.; Coppi, M.V.; Childers, S.E.; Lovley, D.R. Outer Membrane c-Type Cytochromes Required for Fe(III) and Mn(IV) Oxide Reduction in Geobacter sulfurreducens. Appl. Environ. Microbiol. 2005, 71, 8634–8641. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Phung, N.T.; SeopChang, I.; Kim, B.H.; Sung, H.C. Use of acetate for enrichment of electrochemically active microorganisms and their 16S rDNA analyses. FEMS Microbiol. Lett. 2003, 223, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Kiely, P.; Call, D.; Yates, M.; Regan, J.; Logan, B. Anodic biofilms in microbial fuel cells harbor low numbers of higher-power-producing bacteria than abundant genera. Appl. Microbiol. Biotechnol. 2010, 88, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Clauwaert, P.; Aelterman, P.; Verstraete, W. Tubular Microbial Fuel Cells for Efficient Electricity Generation. Environ. Sci. Technol. 2005, 39, 8077–8082. [Google Scholar] [CrossRef]
- Xie, X.; Hu, L.; Pasta, M.; Wells, G.F.; Kong, D.; Criddle, C.S.; Cui, Y. Three-Dimensional Carbon Nanotube–Textile Anode for High-Performance Microbial Fuel Cells. Nano Lett. 2011, 11, 291–296. [Google Scholar] [CrossRef]
- Liu, Y.; Harnisch, F.; Fricke, K.; Schröder, U.; Climent, V.; Feliu, J.M. The study of electrochemically active microbial biofilms on different carbon-based anode materials in microbial fuel cells. Biosens. Bioelectron. 2010, 25, 2167–2171. [Google Scholar] [CrossRef]
- Fradler, K.R.; Kim, J.R.; Boghani, H.C.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. The effect of internal capacitance on power quality and energy efficiency in a tubular microbial fuel cell. Process Biochem. 2014, 49, 973–980. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Early-stage performance evaluation of flowing microbial fuel cells using chemically treated carbon felt and yeast biocatalyst. Appl. Energy 2018, 222, 369–382. [Google Scholar] [CrossRef]
- Chong, P.; Erable, B.; Bergel, A. Microbial anodes: What actually occurs inside pores? Int. J. Hydrog. Energy 2019, 44, 4484–4495. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Kang, J.; Lee, J.-H.; Yoon, J. Influence of attached bacteria and biofilm on double-layer capacitance during biofilm monitoring by electrochemical impedance spectroscopy. Water Res. 2011, 45, 4615–4622. [Google Scholar] [CrossRef] [PubMed]
- Manohar, A.K.; Bretschger, O.; Nealson, K.H.; Mansfeld, F. The use of electrochemical impedance spectroscopy (EIS) in the evaluation of the electrochemical properties of a microbial fuel cell. Bioelectrochemistry 2008, 72, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Michie, I.S.; Kim, J.R.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Factors affecting microbial fuel cell acclimation and operation in temperate climates. Water Sci. Technol. 2013, 67, 2568–2575. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wei, J.; Liang, P.; Huang, X. Electricity generation and microbial community changes in microbial fuel cells packed with different anodic materials. Bioresour. Technol. 2011, 102, 10886–10891. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, F. Electrochemical roles of extracellular polymeric substances in biofilms. Curr. Opin. Electrochem. 2017, 4, 206–211. [Google Scholar] [CrossRef]
- Lv, Z.; Xie, D.; Li, F.; Hu, Y.; Wei, C.; Feng, C. Microbial fuel cell as a biocapacitor by using pseudo-capacitive anode materials. J. Power Sources 2014, 246, 642–649. [Google Scholar] [CrossRef]
- Yates, M.D.; Ma, L.; Sack, J.; Golden, J.P.; Strycharz-Glaven, S.M.; Yates, S.R.; Tender, L.M. Microbial Electrochemical Energy Storage and Recovery in a Combined Electrotrophic and Electrogenic Biofilm. Environ. Sci. Technol. Lett. 2017, 4, 374–379. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhou, S.; Xu, N.; Zhuang, L. Electrochemical characterization of anodic biofilms enriched with glucose and acetate in single-chamber microbial fuel cells. Colloids Surf. B Biointerfaces 2011, 82, 641–646. [Google Scholar] [CrossRef]
- Raghavulu, S.V.; Mohan, S.V.; Goud, R.K.; Sarma, P.N. Effect of anodic pH microenvironment on microbial fuel cell (MFC) performance in concurrence with aerated and ferricyanide catholytes. Electrochem. Commun. 2009, 11, 371–375. [Google Scholar] [CrossRef]
- Stoodley, P.; deBeer, D.; Lappin-Scott, H.M. Influence of electric fields and pH on biofilm structure as related to the bioelectric effect. Antimicrob. Agents Chemother. 1997, 41, 1876–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franks, A.E.; Nevin, K.P.; Jia, H.; Izallalen, M.; Woodard, T.L.; Lovley, D.R. Novel strategy for three-dimensional real-time imaging of microbial fuel cell communities: Monitoring the inhibitory effects of proton accumulation within the anode biofilm. Energy Environ. Sci. 2009, 2, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-H.; Hanna, M.N.; Svensäter, G.; Ellen, R.P.; Cvitkovitch, D.G. Cell Density Modulates Acid Adaptation in Streptococcus mutans: Implications for Survival in Biofilms. J. Bacteriol. 2001, 183, 6875–6884. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, G.S.; Ghangrekar, M.M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Ding, L.; Xu, K.; Ren, H. Influences of initial pH on performance and anodic microbes of fed-batch microbial fuel cells. J. Chem. Technol. Biotechnol. 2011, 86, 1226–1232. [Google Scholar] [CrossRef]
- Jannelli, N.; Anna Nastro, R.; Cigolotti, V.; Minutillo, M.; Falcucci, G. Low pH, high salinity: Too much for microbial fuel cells? Appl. Energy 2017, 192, 543–550. [Google Scholar] [CrossRef] [Green Version]


| Carbon Material Type | Experimental Reactor—Mass (g) | Material Resistance (Ω/4 cm2) | Specific Resistivity (Ω/m2) | Suface BET (Brunauer–Emmett–Teller) SBET (m2/g) |
|---|---|---|---|---|
| Material 1—Felt | 0.15 | 53.9 | 0.2 | 0.97 |
| Material 2—E-Tek carbon paper | 0.11 | 0.7 | 0.08 | 0.87 |
| Material 3—Engineered carbon tubes | 1.3 | 0.2 | 12.2 | |
| Material 4—Graphite rod | 2.29 | 1.2 | 1.00 × 10−5 | 14 |
| Material | DNA (ng/μL) | |
|---|---|---|
| Open Circuit | Closed Circuit | |
| 1 | 582 | 1209 |
| 2 | 142 | 1107 |
| 3 | 597 | 767 |
| 4 | 70 | 819 |
| Planktonic | 158 | 120 |
| Open Circuit | Closed Circuit | |||||||
|---|---|---|---|---|---|---|---|---|
| Reductive Sweep (towards a Positive Ean) | Oxidative Sweep (towards a Negative Ean) | Reductive Sweep (towards a Positive Ean) | Oxidative Sweep (towards a Negative Ean) | |||||
| Material | Voltage Ean (V) | ΔI/ΔVmax (ΔI/ΔVAn) | Voltage Ean (V) | ΔI/ΔVmax (ΔI/ΔVAn) | Voltage Ean (V) | ΔI/ΔVmax (ΔI/ΔVAn) | Voltage Ean (V) | ΔI/ΔVmax (ΔI/ΔVAn) |
| 1 | n/a | n/a | −0.6 to −0.15 | −0.01 | n/a | n/a | −0.49 | −0.03 |
| 2 | −0.5 | 0.004 | −0.55 | −0.005 | −0.39 | 0.021 | −0.44 | −0.011 |
| 3 | n/d | n/d | −0.53 | −0.0025 | −0.39 | 0.016 | −0.4 | −0.006 |
| 4 | −0.51 | 0.002 | −0.55 | −0.0025 | −0.38 | 0.006 | −0.39 | −0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michie, I.S.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Electrogenic Biofilm Development Determines Charge Accumulation and Resistance to pH Perturbation. Energies 2020, 13, 3521. https://doi.org/10.3390/en13143521
Michie IS, Dinsdale RM, Guwy AJ, Premier GC. Electrogenic Biofilm Development Determines Charge Accumulation and Resistance to pH Perturbation. Energies. 2020; 13(14):3521. https://doi.org/10.3390/en13143521
Chicago/Turabian StyleMichie, Iain S., Richard M. Dinsdale, Alan J. Guwy, and Giuliano C. Premier. 2020. "Electrogenic Biofilm Development Determines Charge Accumulation and Resistance to pH Perturbation" Energies 13, no. 14: 3521. https://doi.org/10.3390/en13143521
APA StyleMichie, I. S., Dinsdale, R. M., Guwy, A. J., & Premier, G. C. (2020). Electrogenic Biofilm Development Determines Charge Accumulation and Resistance to pH Perturbation. Energies, 13(14), 3521. https://doi.org/10.3390/en13143521





