Techno-Economic Analysis of Pressurized Oxy-Fuel Combustion of Petroleum Coke
Abstract
:1. Introduction
- Post combustion: CO2 is removed from the flue gas using techniques such as absorption.
- Pre combustion: Pre-reformed fuel is used, where, after reformation, CO2 is removed from the fuel before combustion.
- Oxy-fuel combustion: Fuel is combusted in oxygen (>95%) instead of air, leading to high CO2 concentrations in the flue gas due to the near absence of nitrogen in the combustion process.
2. Methodology
2.1. Process Simulation Model
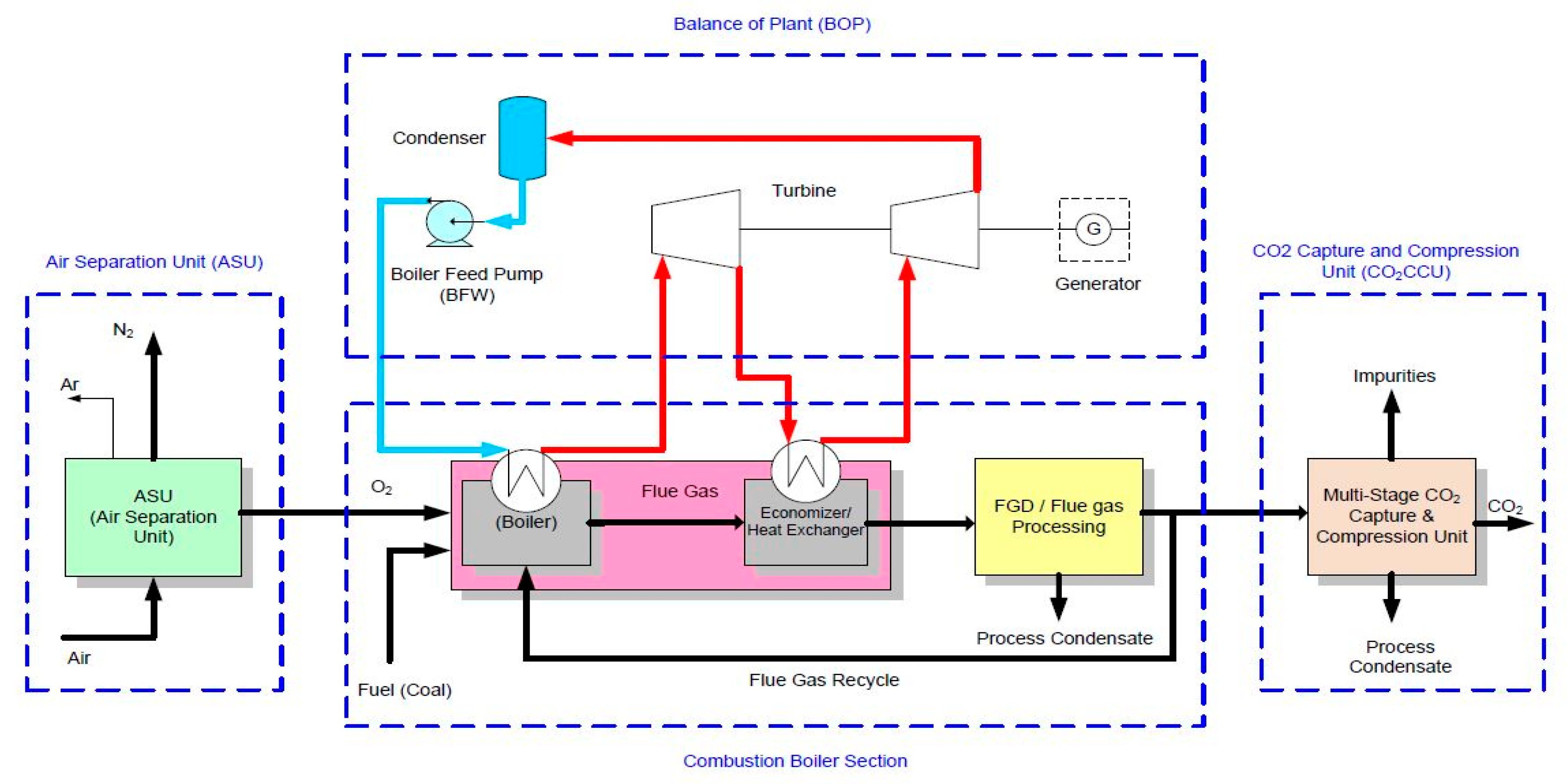
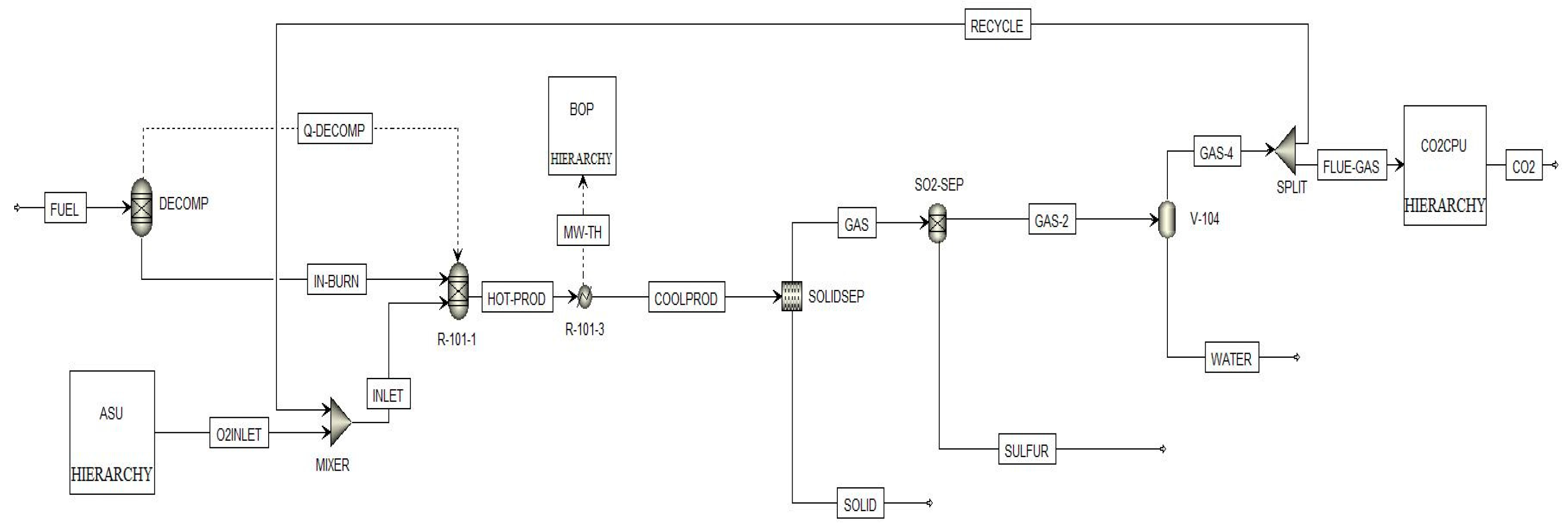
2.1.1. Combustion Boiler Section (CBS)
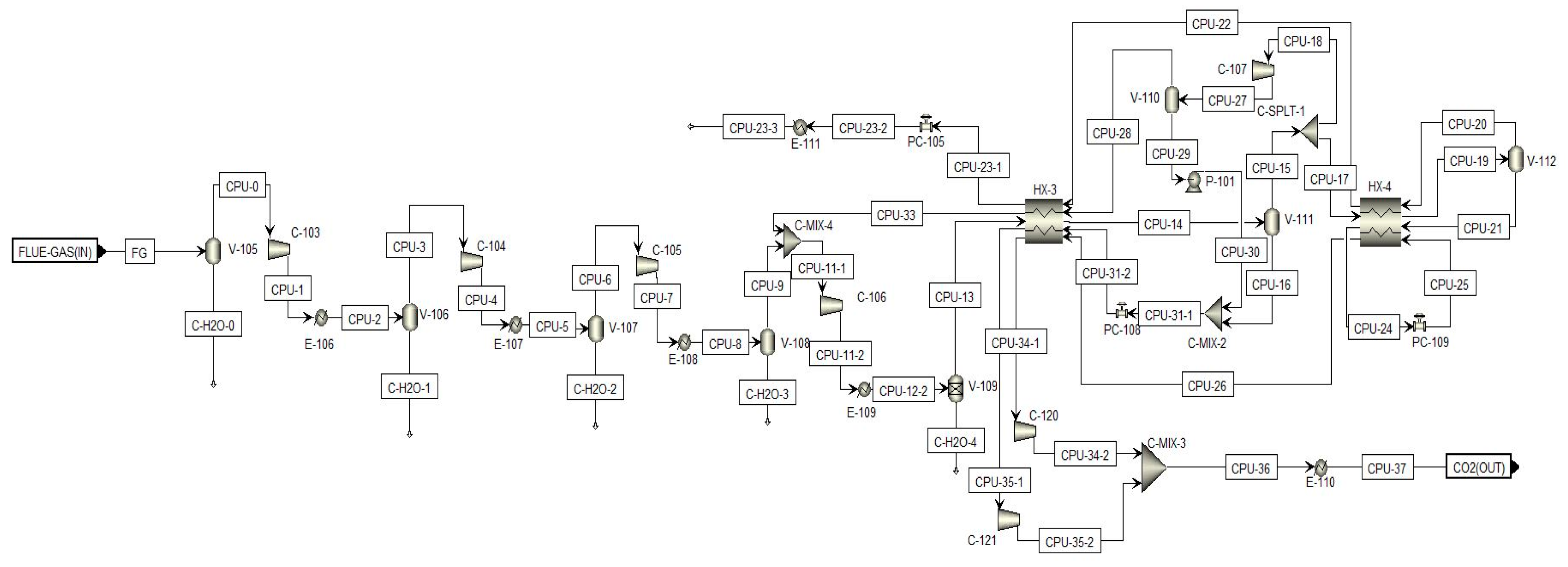
2.1.2. CO2 Capture and Purification Unit (CO2CPU)
2.2. Economic Model
3. Results and Discussion
3.1. Simulation Results
3.1.1. Atmospheric Oxy-Petcoke Combustion
3.1.2. Pressurized Oxy-Petcoke Combustion
3.2. Economic Evaluation
3.2.1. Economic Analysis of Oxy-Petcoke Power Plant in the USA
3.2.2. Economic Analysis of Oxy-Petcoke Plant in the KSA
4. Conclusions
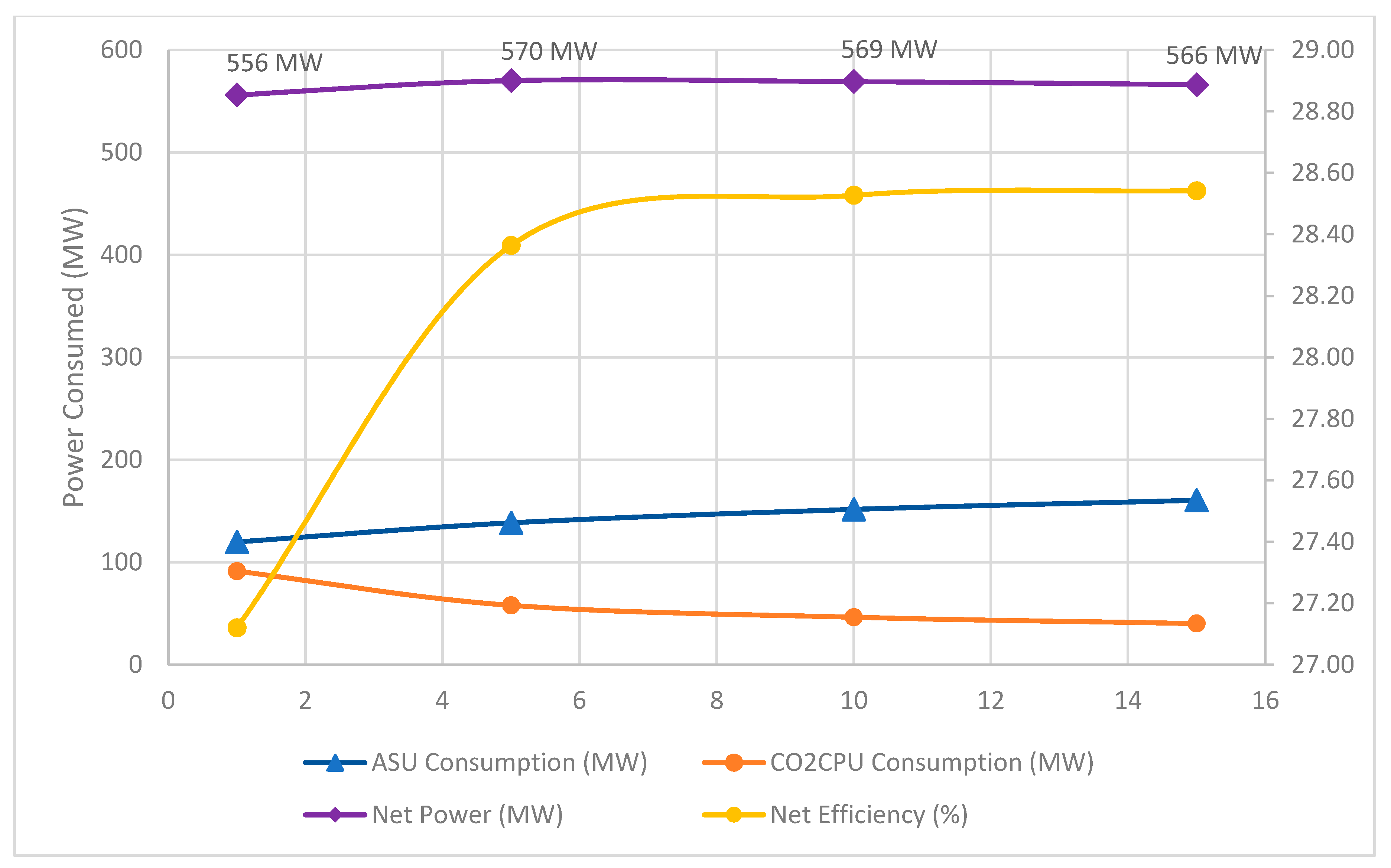
- There appears to be an optimum pressure (at ~10 bars) which maximizes the plant efficiency, plant net power output, and minimizes the LCOE, cost of CO2 avoided and cost of CO2 captured for both the USA and KSA scenarios. This is due to reduced overall power consumption at high pressure. When the pressure is increased, there is a power balance between ASU and CO2CPU. The reduction in CO2CPU power requirement is higher than the increase in ASU power requirement for operating pressure above 1 bar.
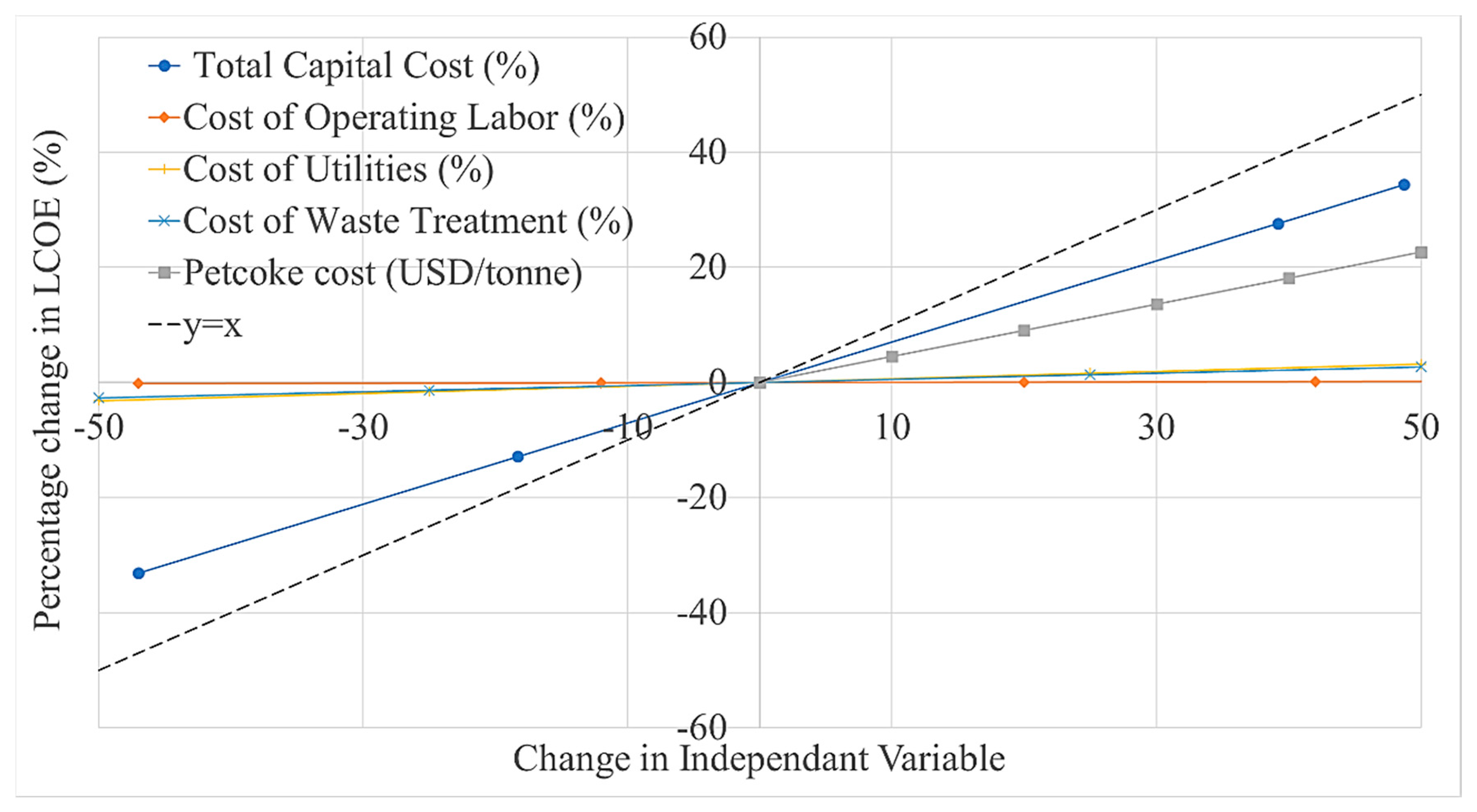
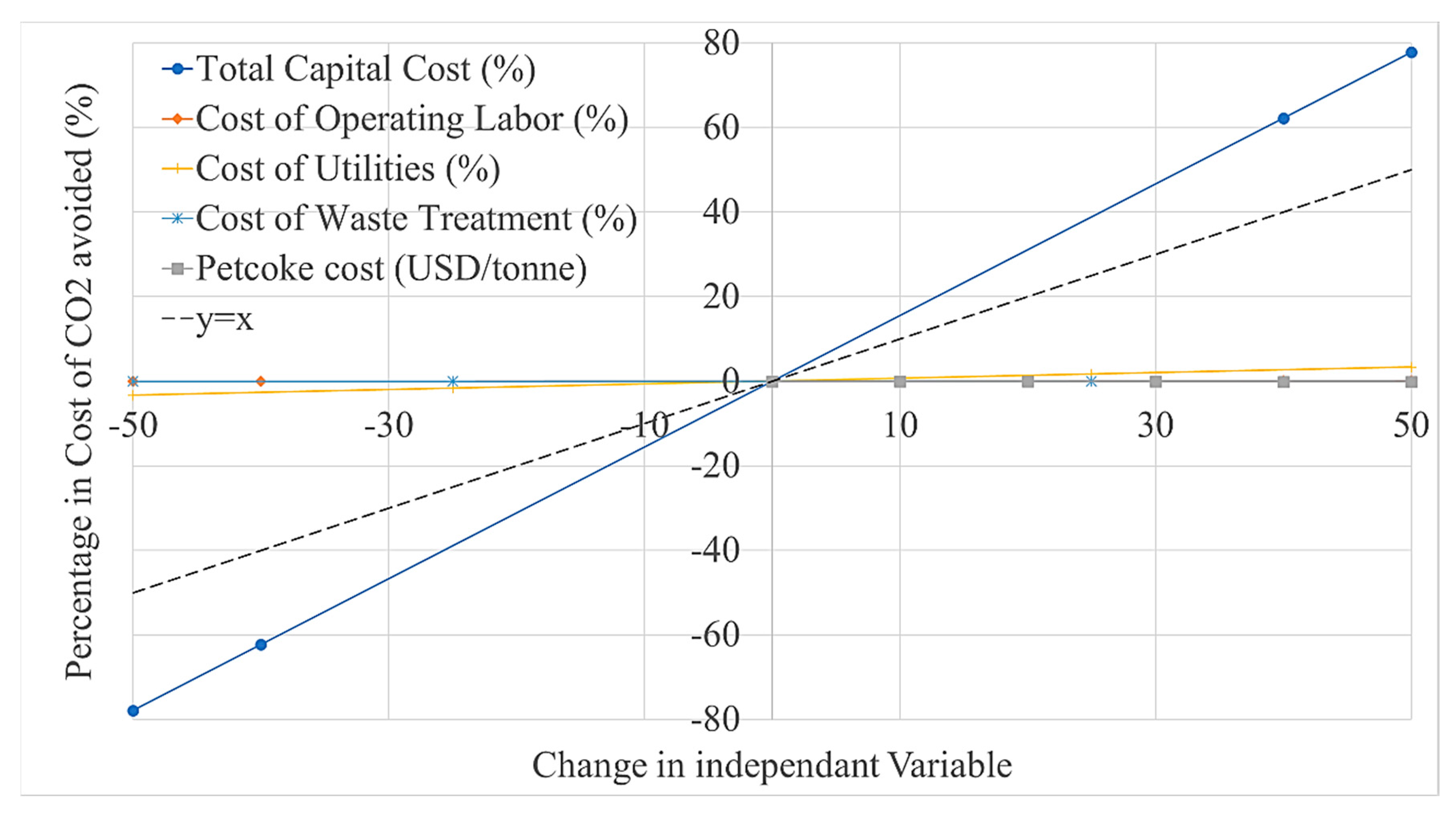
- A sensitivity analysis shows that the LCOE moderately increases when increasing the capital cost of the plant (~0.7% per %) and the cost of petcoke (~0.5% per USD/tonne), but is insensitive to the costs of labour, utilities and waste treatment.
- Costs are lower for the KSA scenario because of the lower interest rate and cost of labour.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stockman, L. Petroleum Coke: The coal hiding in the Tar Sands. Oil Chang. Int. 2013. Available online: http://priceofoil.org/content/uploads/2013/01/OCI.Petcoke.FINALSCREEN.pdf (accessed on 1 July 2020).
- The Engineering ToolBox(2003) Fuels—Higher and Lower Calorific Values. Available online: https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html (accessed on 11 January 2020).
- IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage; Metz, B., Davidson, O., de Coninck, H.C., Loos, M., Meyer, L.A., Eds.; Prepared by Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005; p. 442. [Google Scholar]
- Chen, L.; Yong, S.Z.; Ghoniem, A.F. Oxy-fuel combustion of pulverized coal: Characterization, fundamentals, stabilization and CFD modeling. Prog. Energy Combust. Sci. 2012, 38, 156–214. [Google Scholar] [CrossRef]
- Uchida, T.; Goto, T.; Yamada, T.; Kiga, T.; Spero, C. Oxyfuel combustion as CO2 capture technology advancing for practical use - Callide oxyfuel project. Energy Procedia 2013, 37, 1471–1479. [Google Scholar] [CrossRef]
- Guedea, I.; Lupiañez, C.; Romeo, L.M. Exergetic comparison of different oxyfuel technologies. Int. J. Energy Environ. Eng. 2011, 2, 35–47. [Google Scholar]
- Fu, C.; Gundersen, T. Exergy analysis and heat integration of a coal-based oxy-combustion power plant. Energy Fuels 2013, 27, 7138–7149. [Google Scholar] [CrossRef]
- Hong, J.; Field, R.; Gazzino, M.; Ghoniem, A.F. Operating pressure dependence of the pressurized oxy-fuel combustion power cycle. Energy 2010, 35, 5391–5399. [Google Scholar] [CrossRef] [Green Version]
- Zebian, H.; Gazzino, M.; Mitsos, A. Multi-variable optimization of pressurized oxy-coal combustion. Energy 2012, 38, 37–57. [Google Scholar] [CrossRef] [Green Version]
- Soundararajan, R.; Gundersen, T. Coal based power plants using oxy-combustion for CO2 capture: Pressurized coal combustion to reduce capture penalty. Appl. Therm. Eng. 2013, 61, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Gopan, A.; Kumfer, B.M.; Phillips, J.; Thimsen, D.; Smith, R.; Axelbaum, R.L. Process design and performance analysis of a Staged, Pressurized Oxy-Combustion (SPOC) power plant for carbon capture. Appl. Energy 2014, 125, 179–188. [Google Scholar] [CrossRef]
- Chen, S.; Yu, R.; Soomro, A.; Xiang, W. Thermodynamic assessment and optimization of a pressurized fluidized bed oxy-fuel combustion power plant with CO2 capture. Energy 2019, 175, 445–455. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, W.; Shao, Y.; Liu, X. Energy efficiency analysis of pressurized oxy-coal combustion system utilizing circulating fluidized bed. Appl. Therm. Eng. 2019, 150, 1104–1115. [Google Scholar] [CrossRef]
- Hagi, H.; Nemer, M.; Le Moullec, Y.; Bouallou, C. Pathway for advanced architectures of oxy-pulverized coal power plants: Minimization of the global system exergy losses. Energy Procedia 2013, 37, 1331–1340. [Google Scholar] [CrossRef]
- Shafeen, A. An Exergy-Based Framework for Efficiency Improvement for Integrated Oxy-Fuel Power Generation Systems with CO2 Capture; University of Waterloo: Waterloo, ON, Canada, 2014. [Google Scholar]
- Chen, H.T.; Wei, W. Efficiency enhancement of pressurized oxy-coal power plant with heat integration. Int. J. Energy Res. 2015, 39, 256–264. [Google Scholar] [CrossRef]
- DOE/NETL-2010/1405. Advancing Oxycombustion Technolgoy For Bituminous Coal Power Plants: An R&D Guide, Final Report, April 2012. Available online: https://www.netl.doe.gov/projects/files/AdvancingOxycombustionTechnologyforBituminousCoalPowerPlantsAnRDGuide_040112.pdf (accessed on 1 July 2020).
- Zanganeh, K.; Shafeen, A. Auto-Refrigerated Gas Separation System for Carbon Dioxide Capture and Compression. U.S. Patent Application 13/389, 2011. [Google Scholar]
- Towler, G.; Sinnot, R. Chemical Engineering Design Principles, Practice, and Economics of Plant and Process Design, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2008. [Google Scholar]
- Turton, R.; Bailie, R.; Whiting, W.; Shaeiwitz, J. Analysis, Synthesis, and Design of Chemical Processes, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Raibhole, V.N.; Sapali, S.N. Simulation and Parametric Analysis of Cryogenic Oxygen Plant for Biomass Gasification. Mech. Eng. Res. 2012, 2, 97. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Zhu, L.; Chen, X. Complete Equation-Oriented Approach for Process Analysis and Optimization of a Cryogenic Air Separation Unit. Ind. Eng. Chem. Res. 2015, 54, 12096–12107. [Google Scholar] [CrossRef] [Green Version]
- Alfred ong’iro, V.; Ismet Ugursal, A.M.; Al Taweel, G.L. Simulation of Combined Cycle Power Plants. Response 1995, 15, 105–113. [Google Scholar]
- Nazir, S.M.; Bolland, O.; Amini, S. Analysis of combined cycle power plants with chemical looping reforming of natural gas and pre-combustion CO2 capture. Energies 2018, 11, 147. [Google Scholar] [CrossRef] [Green Version]
- Nayak, R.; Mewada, R. Simulation of Coal Gasification Process using ASPEN PLUS. In Proceedings of the International Conference On Current Trends in Technology, Ahmedabad, India, 8–10 December 2011. [Google Scholar]
- AspenPlus Aspen Plus 2004.1: Getting Started Modeling Processes with Solids. 2004. Available online: https://www.coursehero.com/file/39509450/AspenPlus2004-Gettingstartedwithsolidspdf/ (accessed on 1 July 2020).
- Buhre, B.J.P.; Elliott, L.K.; Sheng, C.D.; Gupta, R.P.; Wall, T.F. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.M.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Rubin, E.S.; Davison, J.E.; Herzog, H.J. The cost of CO2 capture and storage. Int. J. Greenh. Gas Control 2015, 40, 378–400. [Google Scholar] [CrossRef]
- IEA Greenhouse Gas R&D Programme (IEA GHG), “CO2 Capture in Low Rank Coal Power Plants, January 2006. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiVqpn7yLLqAhWQFogKHWNlCO4QFjAAegQIBRAB&url=http%3A%2F%2Fieaghg.org%2Fdocs%2FGeneral_Docs%2FReports%2F2006-1%2520Low%2520rank%2520coal%2520report.pdf&usg=AOvVaw2m9E-fZVNI_owdmWx_I82H (accessed on 1 July 2020).
- Doing Business in the Kingdom of Saudi Arabia: A Tax and Legal Guide. Available online: https://www.pwc.com/m1/en/tax/documents/doing-business-guides/doing-business-guide-ksa-2020.pdf (accessed on 1 July 2020).
- U.S Bureau of Labor Statistcs Occupational Employment Statistics. Available online: https://www.bls.gov/oes/current/oes518013.htm (accessed on 1 July 2020).
- EIA. Electric Power Mothly; EIA: Washington, DC, USA, 2018. [Google Scholar]
| Proximate Analysis (Dry Basis) | Coal | Petcoke |
|---|---|---|
| Fixed Carbon | 49.7% | 85.9% |
| Volatile Matter | 39.4% | 11.9% |
| Ash | 10.9% | 2.2% |
| Ultimate Analysis (water ash free) | ||
| Carbon | 71.7% | 80.8% |
| Hydrogen | 5.1% | 3.5% |
| Sulphur | 2.8% | 3.1% |
| Nitrogen | 1.4% | 1.6% |
| Chlorine | 0.3% | 0% |
| Ash | 10.9% | 2.2% |
| Oxygen (by difference) | 7.8% | 8.8% |
| Heating value (HHV) (MJ/kg) | 27.2 | 34.6 |
| Particular | USA | KSA |
|---|---|---|
| Plant Net Output (MWe) [17] | 550 | 550 |
| Capacity Factor (%) [17] | 85 | 85 |
| Economic Life of Plant (years) [17] | 20 | 20 |
| Discount Rate (%) [20,31] | 17.5 | 5 |
| Average Hourly Wage (USD/h) [32] | 31.7 | 18.5 |
| Selling Price of Electricity (cent/kWh) [32] | 12 | 6.7 |
| Selling Price of Captured CO2 (USD/tonne) [32] | 20 | 20 |
| Cost of Petcoke (USD/tonne) | 0 | 0 |
| Parameter | Coal | Petcoke | Petcoke |
|---|---|---|---|
| Adiabatic Flame Temperature, AFT (°C) | 1830 | 1830 | 1866 |
| ASU Flow (kg/h) | 602,713 | 545,523 | 545,304 |
| Fuel Flow (kg/h) | 247,829 | 212,707 | 213,158 |
| Flue Gas Flow to CO2CPU (kg/h) | 752,661 | 727,712 | 728,077 |
| Recycle Ratio (%) | 74.5 | 76.2 | 75.6 |
| CO2 Purity (%) | 96.4 | 96.4 | 96.4 |
| ASU Power Consumption (MW) | 132 | 119.9 | 119.8 |
| CO2CPU Power Consumption (MW) | 93.7 | 90.8 | 91.3 |
| Auxiliaries (MW) | 25.3 | 25.3 | 25.3 |
| Gross Power (MW) | 792.1 | 792.1 | 792.1 |
| Net Power (MW) | 541 | 556.2 | 557.0 |
| Net Efficiency (%) | 28.9 | 27.2 | 27.1 |
| Component | Mole Fraction (%) | ||
|---|---|---|---|
| Coal | Petcoke | Petcoke | |
| (AFT 1830 °C) | (AFT 1830 °C) | (AFT 1866 °C) | |
| O2 | 2.4 | 2.5 | 2.5 |
| CO | 1.3 | 1.4 | 1.8 |
| CO2 | 71.7 | 74.1 | 73.7 |
| H2O | 19.2 | 16.6 | 16.6 |
| SO2 | 0.3 | 0.3 | 0.3 |
| N2 | 2.1 | 2.2 | 2.2 |
| Ar | 2.6 | 2.8 | 2.8 |
| Parameter | 1 bar | 5 bars | 10 bars | 15 bars |
|---|---|---|---|---|
| Total Capital Cost (million USD) | 1545.9 | 1433.7 | 1417.1 | 1428 |
| Bare Module Cost (million USD) | ||||
| CBS | 267.3 | 205.7 | 215 | 209.5 |
| ASU | 451.6 | 463.4 | 470 | 492.5 |
| BOP | 51.3 | 51.3 | 51.3 | 51.3 |
| CO2CPU | 93.9 | 83.6 | 54.6 | 44.1 |
| Cost of Manufacturing (COM) (million USD) | 486.9 | 405.4 | 407.2 | 411.1 |
| Operating Labour (COL) | 1.12 | 1.12 | 1.12 | 1.12 |
| Utilities (COL) | 26.3 | 24.4 | 24.1 | 24.3 |
| Waste Treatment (COL) | 21.6 | 21.1 | 20.4 | 20.2 |
| Raw Materials (CRM) | 0 | 0 | 0 | 0 |
| LCOE (cent/kWh) | 10.45 | 9.45 | 9.37 | 9.43 |
| Cost of CO2 Avoided (USD/tonne) | 78.32 | 66.1 | 65.13 | 65.97 |
| Cost of CO2 Capture (USD/tonne) | 53.86 | 45.46 | 44.79 | 45.37 |
| Parameter | 1 bar | 5 bars | 10 bars | 15 bars |
|---|---|---|---|---|
| LCOE(cent/kWh) | 6.64 | 5.91 | 5.87 | 5.91 |
| Cost of CO2 Avoided (USD/tonne) | 31.9 | 23.04 | 22.57 | 23.08 |
| Cost of CO2 Capture (USD/tonne) | 21.94 | 15.85 | 15.52 | 15.87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamadeh, H.; Toor, S.Y.; Douglas, P.L.; Sarathy, S.M.; Dibble, R.W.; Croiset, E. Techno-Economic Analysis of Pressurized Oxy-Fuel Combustion of Petroleum Coke. Energies 2020, 13, 3463. https://doi.org/10.3390/en13133463
Hamadeh H, Toor SY, Douglas PL, Sarathy SM, Dibble RW, Croiset E. Techno-Economic Analysis of Pressurized Oxy-Fuel Combustion of Petroleum Coke. Energies. 2020; 13(13):3463. https://doi.org/10.3390/en13133463
Chicago/Turabian StyleHamadeh, Hachem, Sannan Y. Toor, Peter L. Douglas, S. Mani Sarathy, Robert W. Dibble, and Eric Croiset. 2020. "Techno-Economic Analysis of Pressurized Oxy-Fuel Combustion of Petroleum Coke" Energies 13, no. 13: 3463. https://doi.org/10.3390/en13133463
APA StyleHamadeh, H., Toor, S. Y., Douglas, P. L., Sarathy, S. M., Dibble, R. W., & Croiset, E. (2020). Techno-Economic Analysis of Pressurized Oxy-Fuel Combustion of Petroleum Coke. Energies, 13(13), 3463. https://doi.org/10.3390/en13133463





