Volumetric Properties and Surface Tension of Few-Layer Graphene Nanofluids Based on a Commercial Heat Transfer Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanofluid Preparation
2.2. Characterization Techniques
3. Results and Discussion
3.1. FLG Nanosheets Characterization
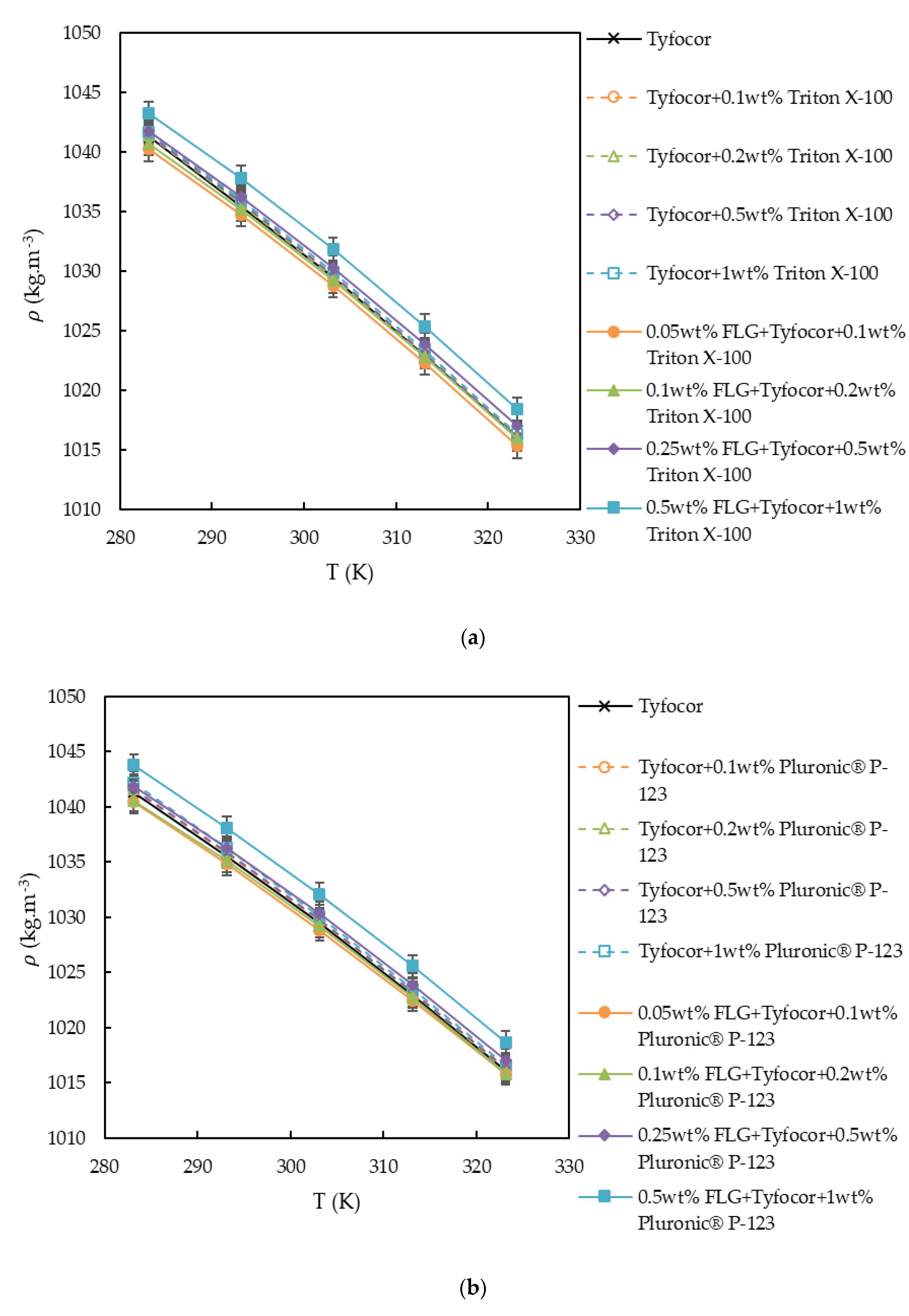
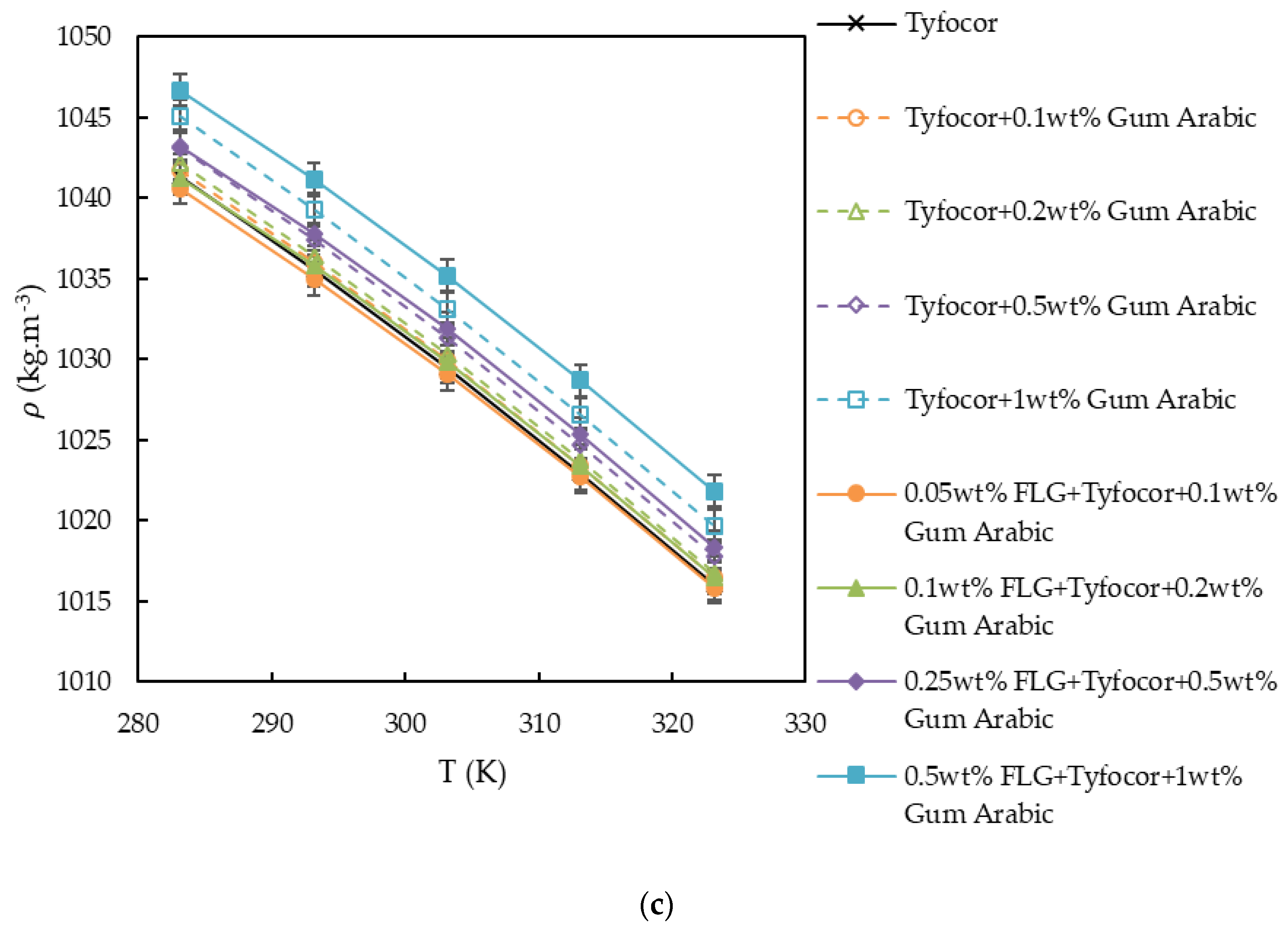
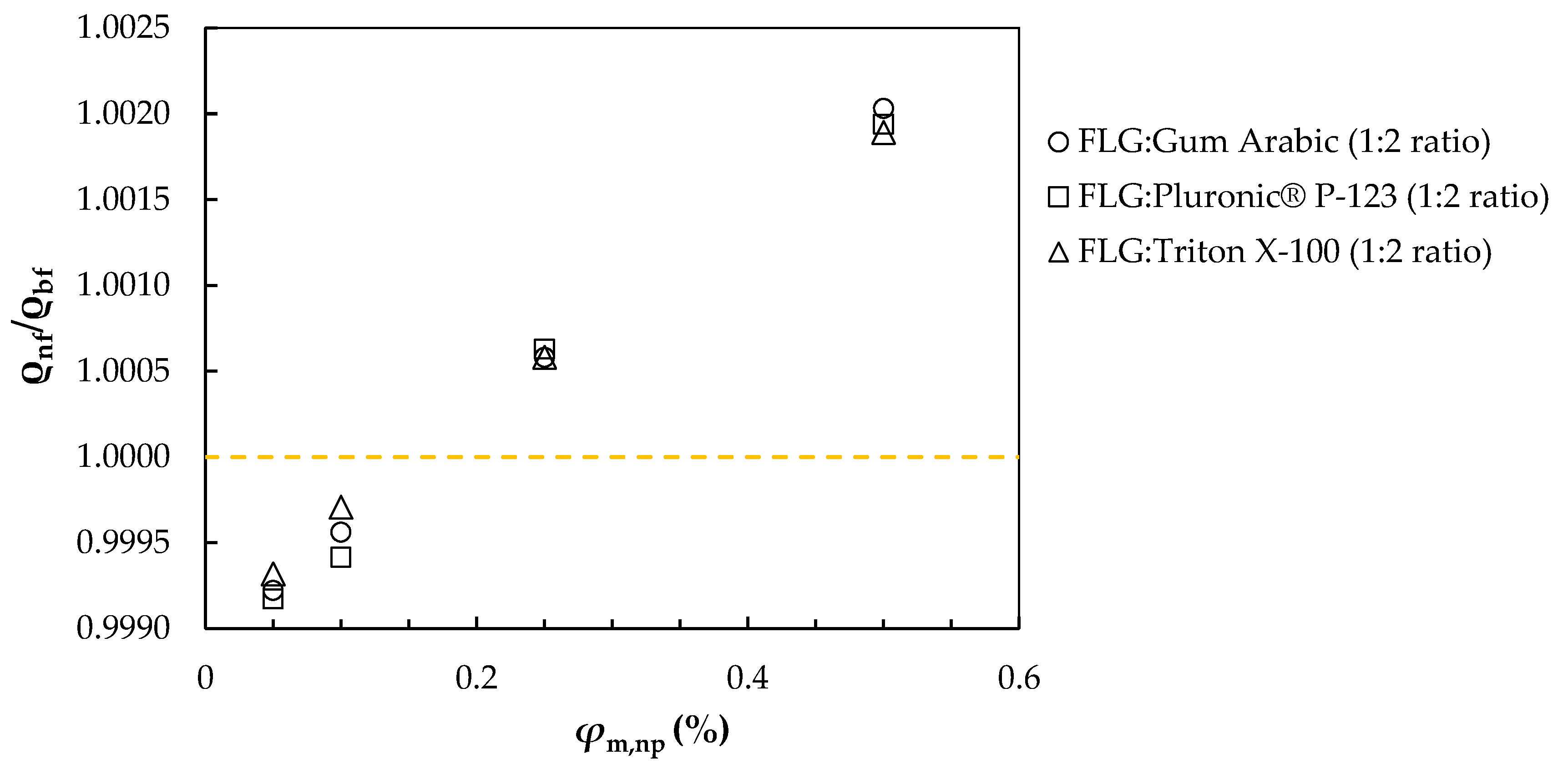
3.2. Density
3.3. Isobaric Thermal Expansivity
3.4. Surface Tension
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| CNTs | carbon nanotubes |
| ST | surface tension (mN.m−1) |
| GO | graphene oxide |
| rGO | reduced graphene oxide |
| SDBS | sodium dodecylbenzene sulfonate |
| FLG | few-layer graphene |
| GA | gum arabic |
| PEO | poly(ethylene oxide) |
| PPO | poly(propylene oxide) |
| SEM | scanning electron microscopy |
| HRTEM | high-resolution transmission electron microscopy |
| TEM | transmission electron microscopy |
| density (kg.m−3) | |
| AAD | absolute average deviation (%) |
| T | temperature (K) |
| ai | fitting parameters |
| St. Dev. | standard deviation |
| CMC | critical micelle concentration |
| PEG | polyethylene glycol |
| mass fraction | |
| isobaric thermal expansivity (K−1) | |
| fGnP | functionalized graphene nanoplatelets |
| PG | propylene glycol |
| W | water |
| Subscripts | |
| nf | nanofluid |
| np | nanoparticles |
| sft | surfactant |
| bf | Base fluid |
| Symbols | |
| ↑ | increase |
| → | stable or constant |
| ↓ | decrease |
References
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles, International Mechanical Engineering Congress and Exhibition; Argonne National Lab: Argonne, IL, USA, 1995; pp. 99–105. [Google Scholar]
- Żyła, G.; Fal, J.; Estellé, P. Thermophysical and dielectric profiles of ethylene glycol based titanium nitride (TiN–EG) nanofluids with various size of particles. Int. J. Heat Mass Transf. 2017, 113, 1189–1199. [Google Scholar] [CrossRef]
- Godson, L.; Raja, B.; Mohan Lal, D.; Wongwises, S. Enhancement of heat transfer using nanofluids—An overview. Renew. Sustain. Energy Rev. 2010, 14, 629–641. [Google Scholar] [CrossRef]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particuology 2009, 7, 141–150. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Estellé, P.; Navas, H.; Desforges, A.; Vigolo, B. Dynamic Viscosity and Surface Tension of Stable Graphene Oxide and Reduced Graphene Oxide Aqueous Nanofluids. J. Nanofluids 2018, 7, 1081–1088. [Google Scholar] [CrossRef]
- Kakaç, S.; Pramuanjaroenkij, A. Review of convective heat transfer enhancement with nanofluids. Int. J. Heat Mass Transf. 2009, 52, 3187–3196. [Google Scholar] [CrossRef]
- Ramesh, G.; Prabhu, N.K. Review of thermo-physical properties, wetting and heat transfer characteristics of nanofluids and their applicability in industrial quench heat treatment. Nanoscale Res. Lett. 2011, 6, 334. [Google Scholar] [CrossRef] [Green Version]
- Kleinstreuer, C.; Feng, Y. Experimental and theoretical studies of nanofluid thermal conductivity enhancement: A review. Nanoscale Res. Lett. 2011, 6, 229. [Google Scholar] [CrossRef] [Green Version]
- Eggers, J.R.; Kabelac, S. Nanofluids revisited. Appl. Therm. Eng. 2016, 106, 1114–1126. [Google Scholar] [CrossRef]
- Azmi, W.H.; Sharma, K.V.; Mamat, R.; Najafi, G.; Mohamad, M.S. The enhancement of effective thermal conductivity and effective dynamic viscosity of nanofluids—A review. Renew. Sustain. Energy Rev. 2016, 53, 1046–1058. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Yu, W.; France, D.M.; Singh, D.; Routbort, J.L. Nanofluids for heat transfer: An engineering approach. Nanoscale Res. Lett. 2011, 6, 182. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177. [Google Scholar] [CrossRef]
- Minea, A.A. Challenges in hybrid nanofluids behavior in turbulent flow: Recent research and numerical comparison. Renew. Sustain. Energy Rev. 2017, 71, 426–434. [Google Scholar] [CrossRef]
- Sundar, L.S.; Sharma, K.V.; Singh, M.K.; Sousa, A.C.M. Hybrid nanofluids preparation, thermal properties, heat transfer and friction factor—A review. Renew. Sustain. Energy Rev. 2017, 68, 185–198. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Cabaleiro, D.; Colla, L.; Barison, S.; Lugo, L.; Fedele, L.; Bobbo, S. Heat Transfer Capability of (Ethylene Glycol + Water)-Based Nanofluids Containing Graphene Nanoplatelets: Design and Thermophysical Profile. Nanoscale Res. Lett. 2017, 12, 53. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, A.K.; Khalid, M.; Rashmi, W.; Gupta, T.C.S.M.; Chan, A. Graphene based nanofluids and nanolubricants—Review of recent developments. Renew. Sustain. Energy Rev. 2016, 63, 346–362. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Mehrali, M.; Saidur, R.; Mehrali, M.; Tahan Latibari, S.; Akhiani, A.R.; Metselaar, H.S.C. A comprehensive review on graphene nanofluids: Recent research, development and applications. Energy Convers. Manag. 2016, 111, 466–487. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Yan, Y.; Reay, D. A review on graphene based nanofluids: Preparation, characterization and applications. J. Mol. Liq. 2019, 279, 444–484. [Google Scholar] [CrossRef]
- Wanic, M.; Cabaleiro, D.; Hamze, S.; Fal, J.; Estellé, P.; Żyła, G. Surface tension of ethylene glycol-based nanofluids containing various types of nitrides: An experimental study. J. Therm. Anal. Calorim. 2020, 139, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Moffat, J.R.; Sefiane, K.; Shanahan, M.E.R. Effect of TiO2 Nanoparticles on Contact Line Stick−Slip Behavior of Volatile Drops. J. Phys. Chem. B 2009, 113, 8860–8866. [Google Scholar] [CrossRef]
- Askounis, A.; Sefiane, K.; Koutsos, V.; Shanahan, M.E.R. Effect of particle geometry on triple line motion of nano-fluid drops and deposit nano-structuring. Adv. Colloid Interface Sci. 2015, 222, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Estellé, P.; Cabaleiro, D.; Żyła, G.; Lugo, L.; Murshed, S.M.S. Current trends in surface tension and wetting behavior of nanofluids. Renew. Sustain. Energy Rev. 2018, 94, 931–944. [Google Scholar] [CrossRef]
- Incropera, F.P.; DeWitt, D.P.; Bergman, T.L.; Lavine, A.S. Introduction to Heat Transfer, 6th ed.; John Wiley: New York, NY, USA, 2007. [Google Scholar]
- Kim, E.J.; Desforges, A.; Speyer, L.; Ghanbaja, J.; Gleize, J.; Estellé, P.; Vigolo, B. Graphene for Water-Based Nanofluid Preparation: Effect of Chemical Modifications on Dispersion and Stability. J. Nanofluids 2017, 6, 603–613. [Google Scholar] [CrossRef]
- Cong, H.-P.; Chen, J.-F.; Yu, S.-H. Graphene-based macroscopic assemblies and architectures: An emerging material system. Chem. Soc. Rev. 2014, 43, 7295–7325. [Google Scholar] [CrossRef]
- Ahammed, N.; Asirvatham, L.G.; Wongwises, S. Effect of volume concentration and temperature on viscosity and surface tension of graphene–water nanofluid for heat transfer applications. J. Therm. Anal. Calorim. 2016, 123, 1399–1409. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Ridha, S.; Abdul Kareem, F.A. Dispersion stability and surface tension of SDS-Stabilized saline nanofluids with graphene nanoplatelets. Colloids Surf. Physicochem. Eng. Asp. 2020, 592, 124584. [Google Scholar] [CrossRef]
- Kamatchi, R.; Venkatachalapathy, S.; Abhinaya Srinivas, B. Synthesis, stability, transport properties, and surface wettability of reduced graphene oxide/water nanofluids. Int. J. Therm. Sci. 2015, 97, 17–25. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Wang, D.; Jiang, N.; Tan, J.; Fu, J.; Wu, B.; Hu, Y.; Guo, Z. Surface tension of supercooled graphene oxide nanofluids measured with acoustic levitation. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Zheng, Z.Z. Experimental Investigation on Surface Tension of Water-Based Graphene Oxide Nanofluids. Adv. Mater. Res. 2014, 1082, 297–301. [Google Scholar] [CrossRef]
- Alawi, O.A.; Mallah, A.R.; Kazi, S.N.; Sidik, N.A.C.; Najafi, G. Thermophysical properties and stability of carbon nanostructures and metallic oxides nanofluids: Experimental approach. J. Therm. Anal. Calorim. 2019, 135, 1545–1562. [Google Scholar] [CrossRef]
- Amiri, A.; Shanbedi, M.; Dashti, H. Thermophysical and rheological properties of water-based graphene quantum dots nanofluids. J. Taiwan Inst. Chem. Eng. 2017, 76, 132–140. [Google Scholar] [CrossRef]
- Azizi, M.; Honarvar, B. Investigation of thermophysical properties of nanofluids containing poly(vinyl alcohol)-functionalized graphene. J. Therm. Anal. Calorim. 2018, 133, 1259–1269. [Google Scholar] [CrossRef]
- Ijam, A.; Saidur, R.; Ganesan, P.; Moradi Golsheikh, A. Stability, thermo-physical properties, and electrical conductivity of graphene oxide-deionized water/ethylene glycol based nanofluid. Int. J. Heat Mass Transf. 2015, 87, 92–103. [Google Scholar] [CrossRef]
- Karami, H.; Papari-Zare, S.; Shanbedi, M.; Eshghi, H.; Dashtbozorg, A.; Akbari, A.; Mohammadian, E.; Heidari, M.; Sahin, A.Z.; Teng, C.B. The thermophysical properties and the stability of nanofluids containing carboxyl-functionalized graphene nano-platelets and multi-walled carbon nanotubes. Int. Commun. Heat Mass Transf. 2019, 108, 104302. [Google Scholar] [CrossRef]
- Sani, E.; Vallejo, J.P.; Cabaleiro, D.; Lugo, L. Functionalized graphene nanoplatelet-nanofluids for solar thermal collectors. Sol. Energy Mater. Sol. Cells 2018, 185, 205–209. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Pérez-Tavernier, J.; Cabaleiro, D.; Fernández-Seara, J.; Lugo, L. Potential heat transfer enhancement of functionalized graphene nanoplatelet dispersions in a propylene glycol-water mixture. Thermophysical profile. J. Chem. Thermodyn. 2018, 123, 174–184. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Álvarez-Regueiro, E.; Cabaleiro, D.; Fernández-Seara, J.; Fernández, J.; Lugo, L. Functionalized graphene nanoplatelet nanofluids based on a commercial industrial antifreeze for the thermal performance enhancement of wind turbines. Appl. Therm. Eng. 2019, 152, 113–125. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Amiri, A.; Alehashem, M.S.; Dahari, M.; Kazi, S.N. Experimental investigation of thermo-physical properties, convective heat transfer and pressure drop of functionalized graphene nanoplatelets aqueous nanofluid in a square heated pipe. Energy Convers. Manag. 2016, 114, 38–49. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Amiri, A.; Montazer, E.; Arzani, H.K.; Sadri, R.; Dahari, M.; Kazi, S.N. Nanofluid based on activated hybrid of biomass carbon/graphene oxide: Synthesis, thermo-physical and electrical properties. Int. Commun. Heat Mass Transf. 2016, 72, 10–15. [Google Scholar] [CrossRef]
- Berrada, N.; Hamze, S.; Desforges, A.; Ghanbaja, J.; Gleize, J.; Maré, T.; Vigolo, B.; Estellé, P. Surface tension of functionalized MWCNT-based nanofluids in water and commercial propylene-glycol mixture. J. Mol. Liq. 2019, 293, 111473. [Google Scholar] [CrossRef] [Green Version]
- Oyinlola, M.A.; Shire, G.S.F.; Moss, R.W. Thermal analysis of a solar collector absorber plate with microchannels. Exp. Therm. Fluid Sci. 2015, 67, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Hamze, S.; Berrada, N.; Cabaleiro, D.; Desforges, A.; Ghanbaja, J.; Gleize, J.; Bégin, D.; Michaux, F.; Maré, T.; Vigolo, B.; et al. Few-layer graphene-based nanofluids with enhanced thermal conductivity. Nanomaterials 2020, 10, 1258. [Google Scholar] [CrossRef] [PubMed]
- Vargaftik, N.B.; Volkov, B.N.; Voljak, L.D. International Tables of the Surface Tension of Water. J. Phys. Chem. Ref. Data 1983, 12, 817–820. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Villarejo, R.; Aguilar, T.; Hamze, S.; Estellé, P.; Navas, J. Experimental analysis of water-based nanofluids using boron nitride nanotubes with improved thermal properties. J. Mol. Liq. 2019, 277, 93–103. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Available online: https://tyfo.de/en/produkt/tyfocor-ls (accessed on 9 June 2020).
- Cabaleiro, D.; Pastoriza-Gallego, M.J.; Piñeiro, M.M.; Legido, J.L.; Lugo, L. Thermophysical properties of (diphenyl ether+biphenyl) mixtures for their use as heat transfer fluids. J. Chem. Thermodyn. 2012, 50, 80–88. [Google Scholar] [CrossRef]
- Available online: https://chemistry.stackexchange.com/questions/45079/does-adding-mass-to-a-liquid-not-water-increase-the-volume (accessed on 9 June 2020).
- Available online: https://www.carolina.com/teacher-resources/interactive/chemistry-lost-volume-demonstration/tr10785.tr (accessed on 9 June 2020).
- Pastoriza-Gallego, M.J.; Casanova, C.; Legido, J.L.; Piñeiro, M.M. CuO in water nanofluid: Influence of particle size and polydispersity on volumetric behaviour and viscosity. Fluid Phase Equilibria 2011, 300, 188–196. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Casanova, C.; Páramo, R.; Barbés, B.; Legido, J.L.; Piñeiro, M.M. A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. J. Appl. Phys. 2009, 106, 064301. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Pastoriza-Gallego, M.J.; Piñeiro, M.M.; Lugo, L. Characterization and measurements of thermal conductivity, density and rheological properties of zinc oxide nanoparticles dispersed in (ethane-1,2-diol+water) mixture. J. Chem. Thermodyn. 2013, 58, 405–415. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Majdan-Cegincara, R. Stability, rheological, magnetorheological and volumetric characterizations of polymer based magnetic nanofluids. Colloid Polym. Sci. 2013, 291, 1977–1987. [Google Scholar] [CrossRef]
- Marcos, M.A.; Cabaleiro, D.; Hamze, S.; Fedele, L.; Bobbo, S.; Estellé, P.; Lugo, L. NePCM Based on Silver Dispersions in Poly(Ethylene Glycol) as a Stable Solution for Thermal Storage. Nanomaterials 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: http://candmz04.brenntag.ca/MSDS/Fr/00070113.pdf (accessed on 9 June 2020).
- Available online: http://www.merckmillipore.com/FR/fr/product/Gum-arabic,MDA_CHEM-104228 (accessed on 9 June 2020).
- Cabaleiro, D.; Segovia, J.J.; Martín, M.C.; Lugo, L. Isobaric heat capacity at high pressure, density, and viscosity of (diphenyl ether + biphenyl) mixtures. J. Chem. Thermodyn. 2016, 93, 86–94. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Pastoriza-Gallego, M.J.; Gracia-Fernández, C.; Piñeiro, M.M.; Lugo, L. Rheological and volumetric properties of TiO2-ethylene glycol nanofluids. Nanoscale Res. Lett. 2013, 8, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korolovych, V.F.; Bulavin, L.A.; Prylutskyy, Y.I.; Khrapatiy, S.V.; Tsierkezos, N.G.; Ritter, U. Influence of Single-Walled Carbon Nanotubes on Thermal Expansion of Water. Int. J. Thermophys. 2014, 35, 19–31. [Google Scholar] [CrossRef]
- Nayak, A.K.; Singh, R.K.; Kulkarni, P.P. Measurement of volumetric thermal expansion coefficient of various nanofluids. Tech. Phys. Lett. 2010, 36, 696–698. [Google Scholar] [CrossRef]
- Nayak, A.K.; Singh, R.K.; Kulkarni, P.P. Thermal expansion characteristics of Al2O3 nanofluids: More to understand than understood. Appl. Phys. Lett. 2009, 94, 094102. [Google Scholar] [CrossRef]
- Radulovic, J.; Sefiane, K.; Shanahan, M.E.R. On the effect of pH on spreading of surfactant solutions on hydrophobic surfaces. J. Colloid Interface Sci. 2009, 332, 497–504. [Google Scholar] [CrossRef]
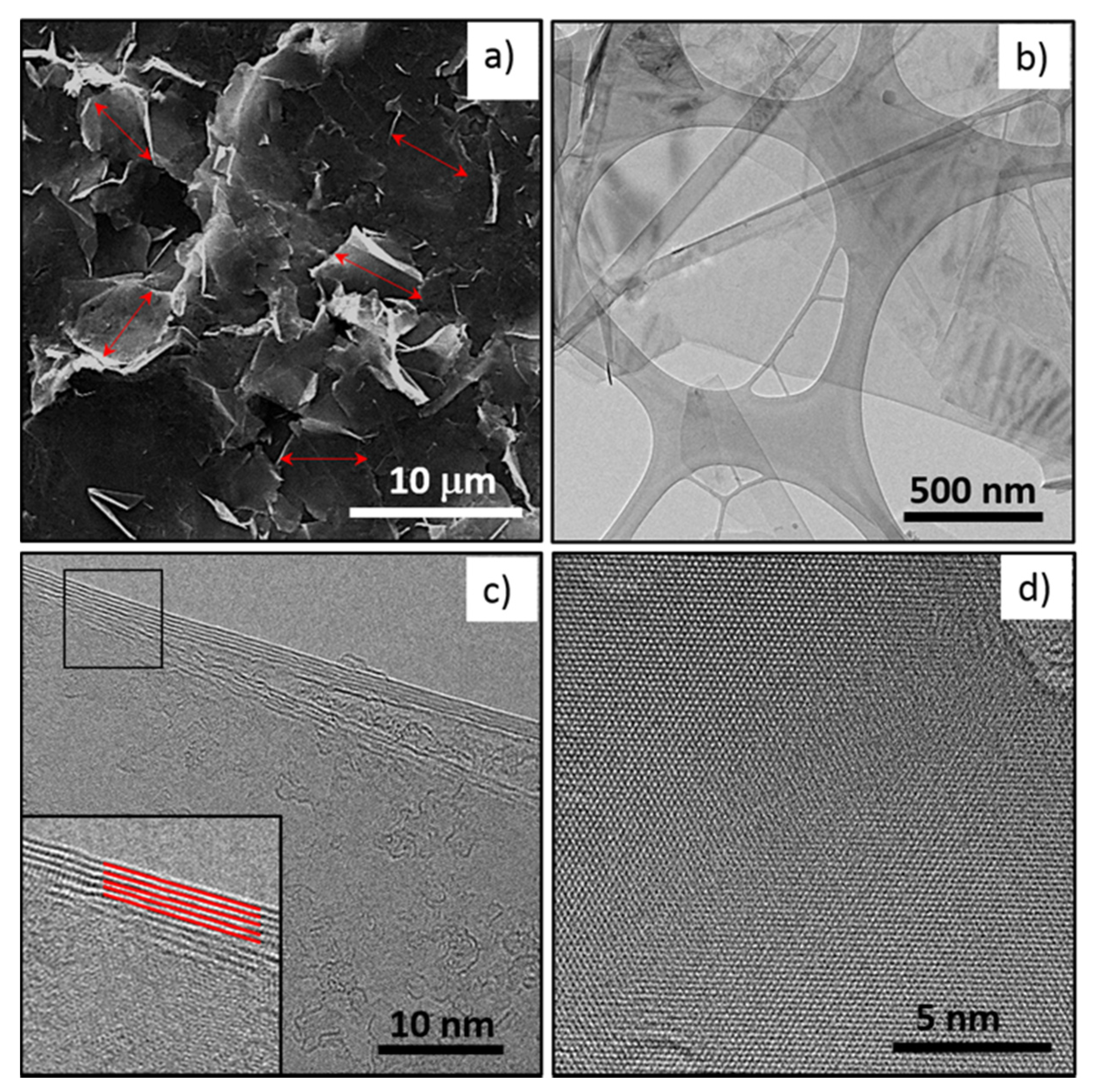
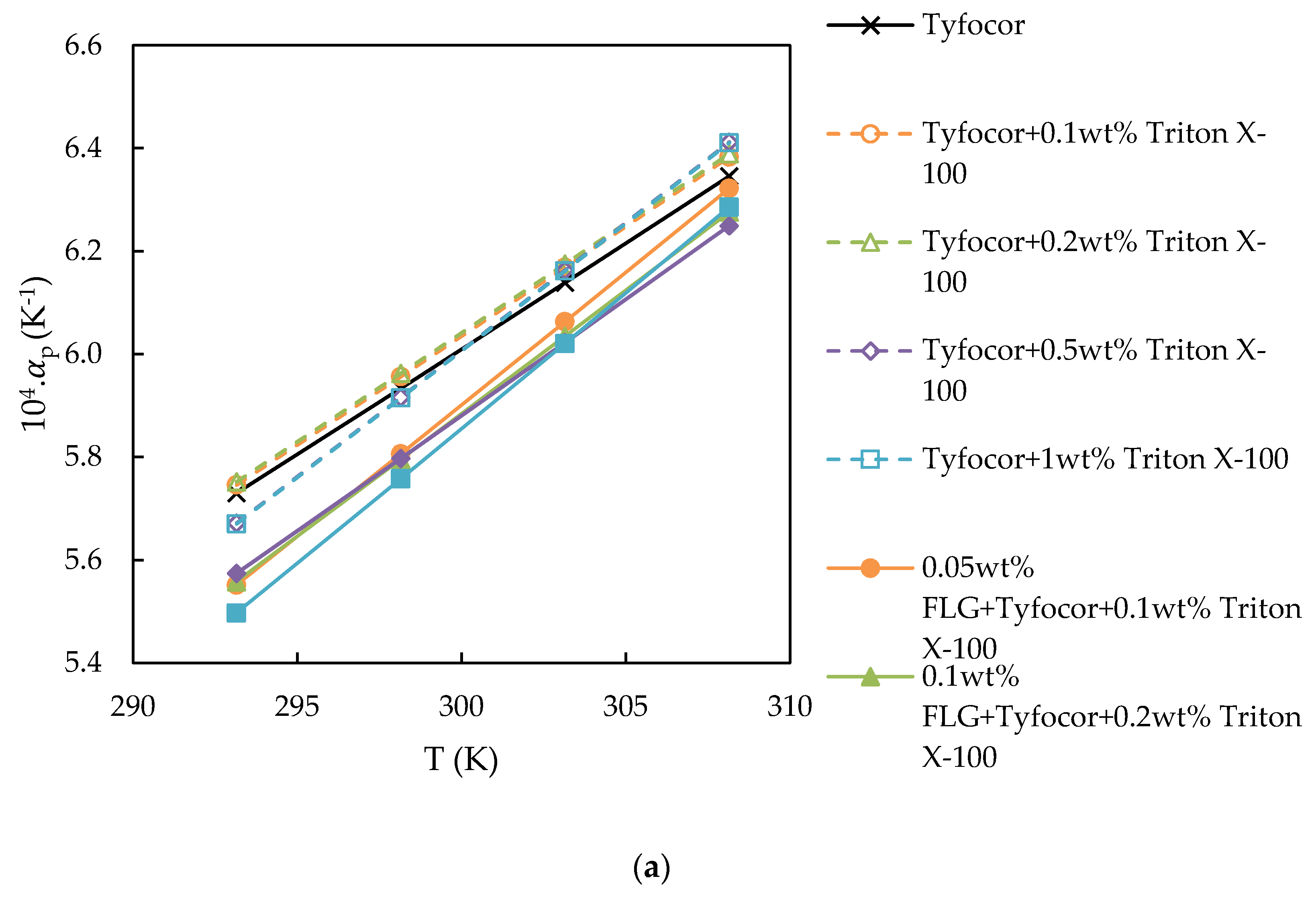
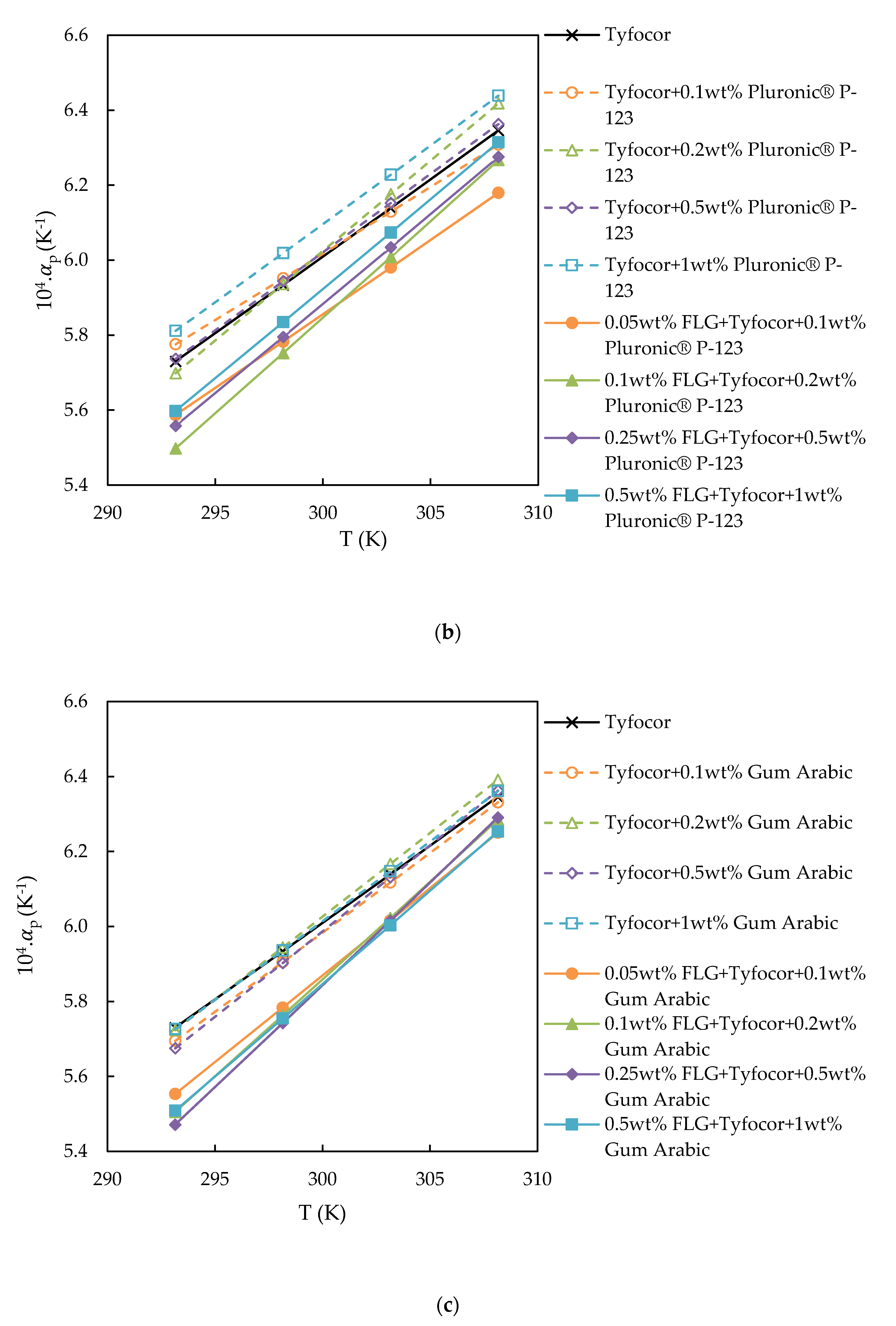
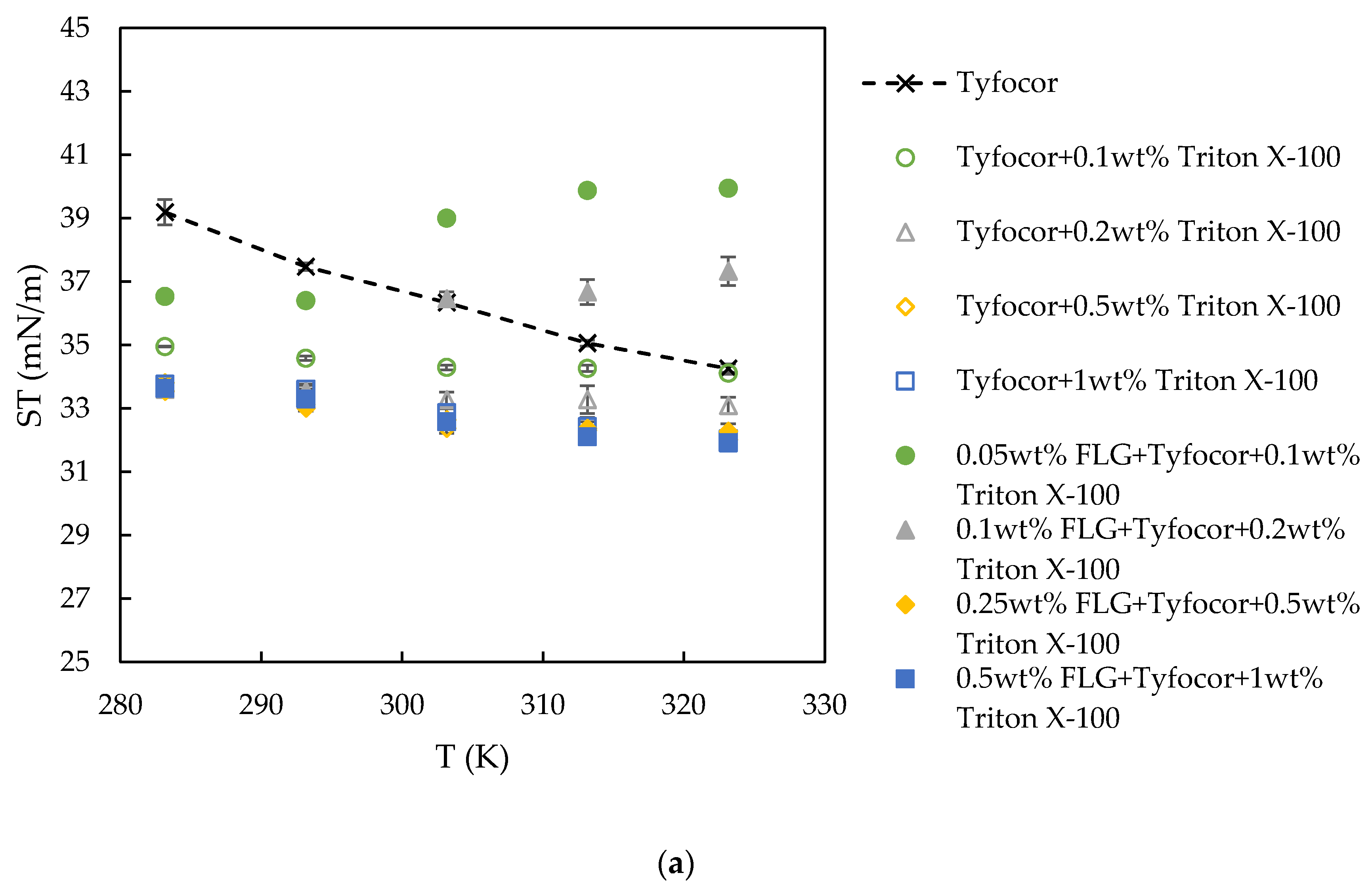
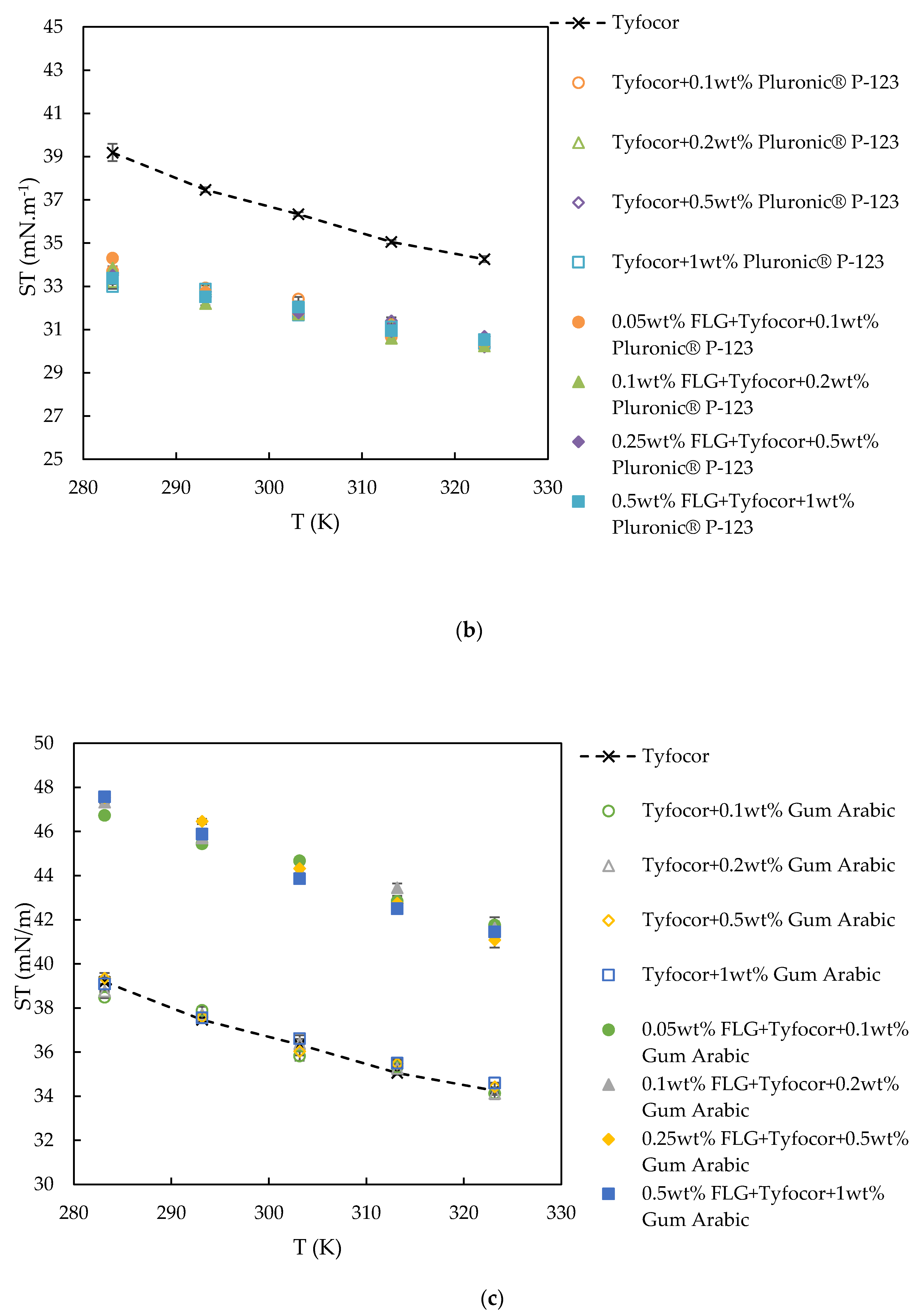
| Reference | Nanoparticle | Base Fluid | Surfactant (np:sft Ratio) | Surface Tension Technique | Main Result with NP Loading | |
|---|---|---|---|---|---|---|
| Type | Concentration | |||||
| Ahammed et al. [27] | Graphene | 0–0.15 vol.% | Water | SDBS (5 vol.%) | Bubble pressure method | ST↓ |
| Cabaleiro et al. [5] | Graphene oxide and reduced graphene oxide | 0–0.1 vol.% | Water | No surfactant | Pendant drop method | ST↓ |
| Ilyas et al. [28] | Graphene nanoplatelets | 0–0.25 wt.% | Saline aqueous media (NaCl) | SDS (1:1.5) | Pendant drop method | ST↓ |
| Kamatchi et al. [29] | Reduced graphene oxide | 0–0.3 g/l | Water | No surfactant | Bubble pressure method | ST↑ |
| Liu et al. [30] | Graphene oxide | 0–0.12 wt.% | Water | No surfactant | Oscillation droplet method | ST↑ |
| Zheng et al. [31] | Graphene oxide | 0–1 wt.% | Water | Not mentioned | Ring method | ST↑ |
| Reference | Nanoparticle | Base Fluid | Surfactant | Measuring Technique | Main Result with NP Loading | |
|---|---|---|---|---|---|---|
| Type | Concentration | |||||
| Alawi et al. [32] | Pentaethylene glycol-(thermally)-treated graphene nanoplatelets | 0–0.1 wt.% | Water | No surfactant | Vibrating U-tube | ρ↑ |
| Amiri et al. [33] | Amine-treated graphene quantum dots | 0–0.02 wt.% | Water | No surfactant | Vibrating U-tube | ρ→ |
| Azizi et al. [34] | Functionalized few-layer graphene | 0.025, 0.05, 0.1 wt.% | Water, ethylene glycol | PVA | Vibrating U-tube | ρ ↑ |
| Cabaleiro et al. [16] | Functionalized graphene nanoplatelets | 0.1, 0.25, 0.5 wt.% | Ethylene glycol: water (10:90) | No surfactant | Vibrating U-tube | ρ ↑, αp↓ |
| Ijam et al. [35] | Graphene oxide nanosheets | 0–0.1 wt.% | Ethylene glycol: water (40:60) | No surfactant | Vibrating U-tube | ρ ↓ |
| Karami et al. [36] | Carboxyl-functionalized graphene nanoplatelets | 0.1, 0.2 wt.% | Water | No surfactant | Vibrating U-tube | ρ→↑ |
| Sani et al. [37] | Functionalized graphene nanoplatelets | 0.005, 0.05 wt.% | Havoline® XLC Premixed 50/50, | SDBS(0.125 wt.%) | Vibrating U-tube | ρ ↑ |
| Vallejo et al. [38] | Functionalized graphene nanoplatelets | 0.25–1 wt.% | Propylene glycol: water (30:70) | No surfactant | Vibrating U-tube | ρ ↑, αp↓ |
| Vallejo et al. [39] | Functionalized graphene nanoplatelets | 0.25–1 wt.% | Havoline® XLC Premixed 50/50, | SDBS(0.125 wt.%) | Vibrating U-tube | ρ ↑, αp↓ |
| Yarmad et al. [40] | Functionalized graphene nanoplatelets | 0–0.1 wt.% | Water | No surfactant | Vibrating U-tube | ρ ↑ |
| Yarmand et al. [41] | Activate carbon/graphene hybrid | 0.02, 0.04, 0.06 wt.% | Ethylene glycol | No surfactant | Vibrating U-tube | ρ ↑ |
| Tyfocor® LS | Base Fluids | Nanofluids | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (%) | 0 | 0.1 | 0.2 | 0.5 | 1.0 | 0.1 | 0.2 | 0.5 | 1.0 |
| (%) | 0 | 0 | 0 | 0 | 0 | 0.05 | 0.1 | 0.25 | 0.5 |
| FLG nanofluids based on Tyfocor® LS and Triton X-100 | |||||||||
| a0/kg·m−3 | 1043.8 | 1038.3 | 1038.4 | 1005.5 | 1005.7 | 991.41 | 1007.5 | 1021.4 | 987.16 |
| (a1)/kg·m−3.K−1 | 0.53736 | 0.57746 | 0.57689 | 0.79449 | 0.79449 | 0.87035 | 0.76469 | 0.67879 | 0.91615 |
| (103.a2)/kg·m−3.K−2 | −1.93 | −2.00 | −2.00 | −2.36 | −2.36 | −2.46 | −2.29 | −2.14 | −2.54 |
| (102.St. Dev.)/kg·m−3 | 3.6 | 1.8 | 2.0 | 2.3 | 2.3 | 0.8 | 3.4 | 1.8 | 0.8 |
| FLG nanofluids based on Tyfocor® LS and Pluronic® P-123 | |||||||||
| a0/kg·m−3 | 1043.8 | 1070 | 1012.5 | 1041.3 | 1044.1 | 1044.7 | 990.15 | 1008.8 | 1012.1 |
| (a1)/kg·m−3.K−1 | 0.53736 | 0.36499 | 0.74983 | 0.55741 | 0.54941 | 0.5107 | 0.87577 | 0.76411 | 0.75897 |
| (103.a2)/kg·m−3.K−2 | −1.93 | −1.64 | −2.29 | −1.96 | −1.96 | −1.86 | −2.46 | −2.29 | −2.29 |
| (102.St. Dev.)/kg·m−3 | 3.7 | 1.8 | 1.8 | 2.1 | 1.9 | 1.9 | 0.9 | 2.2 | 2.1 |
| FLG nanofluids based on Tyfocor® LS and Gum Arabic | |||||||||
| a0/kg·m−3 | 1043.8 | 1037.1 | 1029.3 | 1022.8 | 1041.9 | 1013.3 | 988.12 | 977.14 | 1003.7 |
| (a1)/kg·m−3.K−1 | 0.53736 | 0.5826 | 0.64217 | 0.68855 | 0.57746 | 0.72345 | 0.89554 | 0.98172 | 0.8294 |
| (103.a2)/kg·m−3.K−2 | −1.93 | −2.00 | −2.11 | −2.18 | −2.00 | −2.21 | −2.50 | −2.64 | −2.39 |
| (102.St. Dev.)/kg·m−3 | 3.7 | 1.1 | 2.1 | 1.5 | 1.8 | 2.7 | 0.4 | 0.9 | 3.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamze, S.; Cabaleiro, D.; Bégin, D.; Desforges, A.; Maré, T.; Vigolo, B.; Lugo, L.; Estellé, P. Volumetric Properties and Surface Tension of Few-Layer Graphene Nanofluids Based on a Commercial Heat Transfer Fluid. Energies 2020, 13, 3462. https://doi.org/10.3390/en13133462
Hamze S, Cabaleiro D, Bégin D, Desforges A, Maré T, Vigolo B, Lugo L, Estellé P. Volumetric Properties and Surface Tension of Few-Layer Graphene Nanofluids Based on a Commercial Heat Transfer Fluid. Energies. 2020; 13(13):3462. https://doi.org/10.3390/en13133462
Chicago/Turabian StyleHamze, Samah, David Cabaleiro, Dominique Bégin, Alexandre Desforges, Thierry Maré, Brigitte Vigolo, Luis Lugo, and Patrice Estellé. 2020. "Volumetric Properties and Surface Tension of Few-Layer Graphene Nanofluids Based on a Commercial Heat Transfer Fluid" Energies 13, no. 13: 3462. https://doi.org/10.3390/en13133462
APA StyleHamze, S., Cabaleiro, D., Bégin, D., Desforges, A., Maré, T., Vigolo, B., Lugo, L., & Estellé, P. (2020). Volumetric Properties and Surface Tension of Few-Layer Graphene Nanofluids Based on a Commercial Heat Transfer Fluid. Energies, 13(13), 3462. https://doi.org/10.3390/en13133462








