Property Data Estimation for Hemiformals, Methylene Glycols and Polyoxymethylene Dimethyl Ethers and Process Optimization in Formaldehyde Synthesis
Abstract
1. Introduction
2. Prior Work on Reaction Model and Property Data
3. Modeling Approaches and Discussion of the Results
3.1. Development of the Chemical Model
3.1.1. Numerical Approach for Formaldehyde-Containing Component Systems
3.1.2. Implementation of the Chemical Model in Aspen Plus®
3.1.3. Other Components Systems: Trioxane, Dimethyl Ether (DME), Polyoxymethylene Dimethyl Ethers (OMEn)
3.2. Estimation of Property Data of OME2–10, Polyoxymethylene Glycols (MGn) and Hemiformals (HFn)
3.2.1. Estimation of the Normal Boiling Points of OMEn
3.2.2. Estimation of the Normal Boiling Points of MGn and HFn
3.3. Simulation and Optimization of Formaldehyde Synthesis
Process Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FA | Formaldehyde |
| W | Water |
| MeOH | Methanol |
| MG | Methylene glycol |
| OME1 | Methylal |
| OME | Polyoxymethylen dimethylether |
| HF | Hemiformal |
| TRI | Trioxane |
| DME | Dimethyl ether |
| TB | Normal boiling point |
| TC | Critical temperature |
| pC | Critical pressure |
| ARD | Average of the relative deviations |
| s | Standard deviation |
| STP | Standard temperature and pressure |
| ΔGR | Gibbs free energy |
| ΔHR | Enthalpy of reaction |
Appendix A

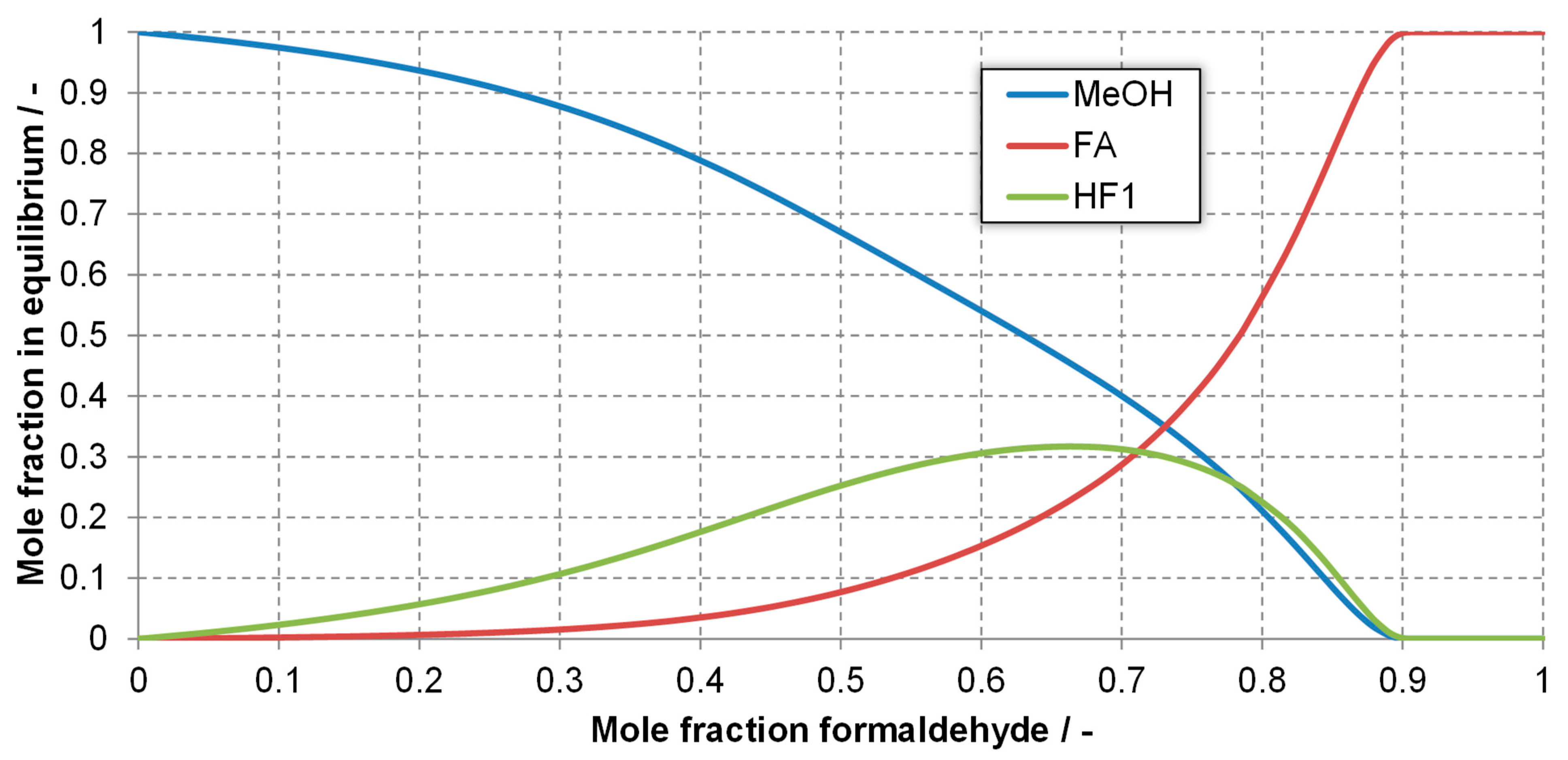
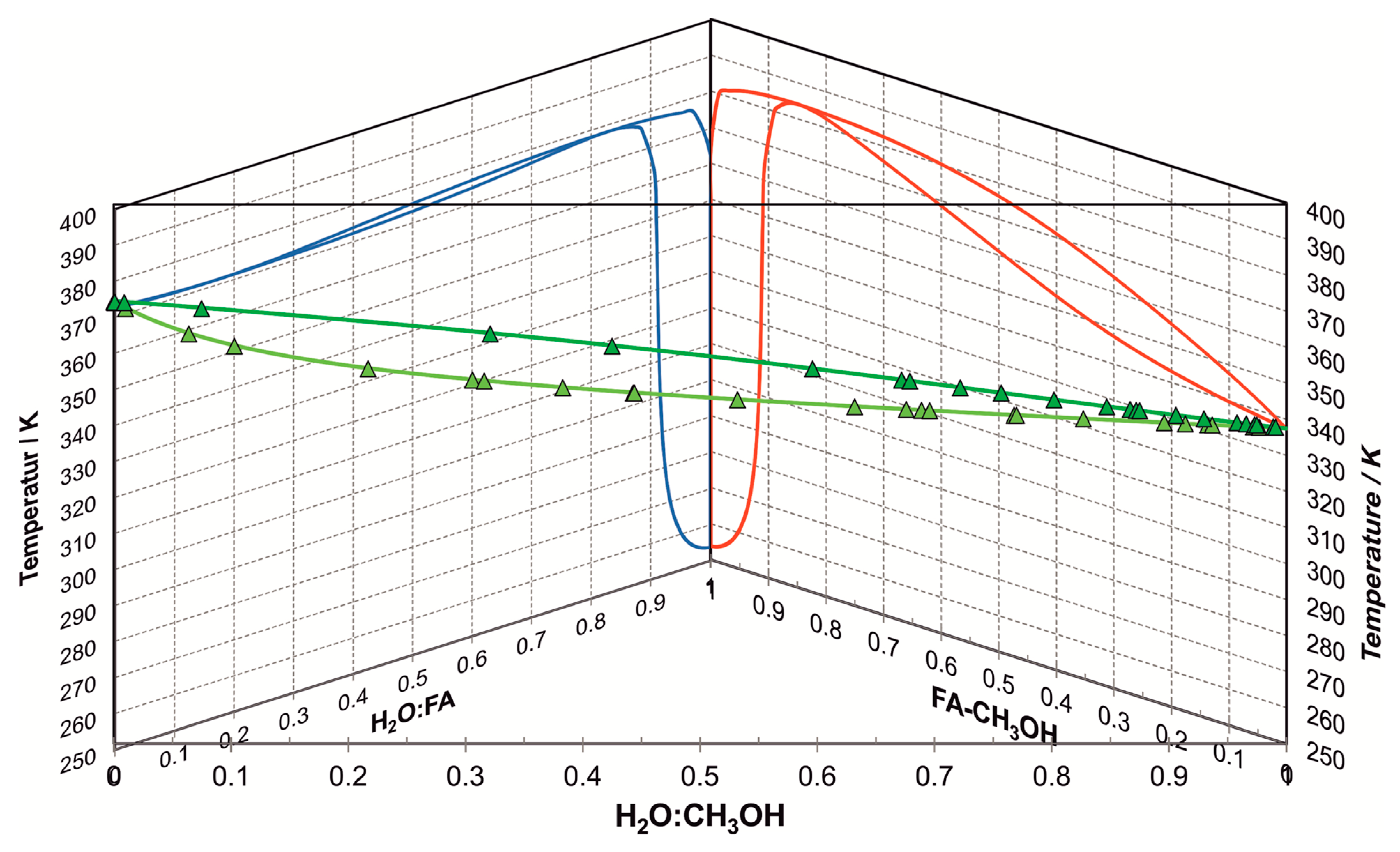
Appendix A.1. Model for Activity-Based Equilibrium Constants
Appendix A.2. UNIFAC Parameters Required for Modeling Component Systems on OME3–5 Synthesis Routes
| NRTL Parameter [21] | UNIFAC Parameter (Regression) | ||
|---|---|---|---|
| 0.3 | 142.2 | ||
| 0.3 | −22.0 | ||
| 251.45 | - | - | |
| 33.22 | - | - | |
| Substance | Molecule Groups | Reference | |
|---|---|---|---|
| Water | H2O | [32] | |
| Formaldehyde | CH2O | [32] | |
| Methanol | CH3OH | [32] | |
| MG1 | CH2(OH)2 | [32] | |
| MG2–10 | (n − 1) | -CH2O- | [32] |
| 2 | -OH | ||
| 1 | -CH2- | ||
| HF1–10 | (n − 1) | -CH2O- | [32] |
| 1 | CH3O- | ||
| 1 | -CH2OH | ||
| Trioxane | (CH2O)3 | Grützner [72] (based on Albert [49]) | |
| Methylal | C3H8O2 | Drunsel [29] (based on Kuhnert [73]) | |
| Dimethylether | 1 | C2H6O | assumption |
| OME2–10 | n | (CH2O)OME | [54] |
| 1 | C3H8O2 | ||
| Number | Molecule Group | R | Q | Reference |
|---|---|---|---|---|
| 1 | -OH | 1.0 | 1.2 | [32] |
| 2 | -CH2O- | 0.9183 | 0.78 | [32] |
| 3 | (CH2O)OME | 0.9183 | 0.78 | [54] |
| 4 | -CH2- | 0.6744 | 0.54 | [32] |
| 5 | -CH3 | 0.9011 | 0.848 | [43] |
| 6 | H2O | 0.9200 | 1.4 | [32] |
| 7 | CH2(OH)2 | 2.6744 | 2.94 | [32] |
| 8 | CH3OH | 1.4311 | 1.432 | [32] |
| 9 | CH3O- | 1.1450 | 1.088 | [32] |
| 10 | -CH2OH | 1.2044 | 1.124 | [32] |
| 11 | (CH2O)3 | 2.754 | 2.34 | Grützner [72] (based on Albert [49]) |
| 12 | C3H8O2 | 2.9644 | 2.716 | Drunsel [29] (based on Kuhnert [73]) |
| 13 | C2H6O | 1.23 | 0.418 | regression |
| i\j | 1 | 2 | 4/5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.0 | 28.1 | 156.4 | 353.5 | 353.5 | −137.1 | 28.1 | −137.1 | 28.1 | 28.1 | 0.0 | 28.1 |
| 2 | 237.7 | 0.0 | 83.4 | 240.0 | 240.0 | 339.7 | 0.0 | 339.7 | −320.6 * | 0.0 | 0.0 | 0.0 |
| 4/5 | 986.5 | 251.5 | 0.0 | 1318.0 | 1318.0 | 697.2 | 251.5 | 697.2 | 251.5 | 251.5 | 0.0 | 251.5 |
| 6 | −229.1 | −149.0 | 300.0 | 0.00 | 0.0 | 289.6 | −149.0 | 289.6 | 80.6 | 28.9 * | 79.6 | −141.0 * |
| 7 | −229.1 | −149.0 | 300.0 | 0.00 | 0.0 | 289.6 | −149.0 | 289.6 | 80.6 | 28.9 * | 0.0 | −149.0 |
| 8 | 249.1 | −180.6 | 16.5 | −181.0 | −181.0 | 0.0 | −180.6 | 0.0 | −16.7 | −71.2 | −69.4 | −180.6 |
| 9 | 237.7 | 0.0 | 83.4 | 240.0 | 240.0 | 339.7 | 0.0 | 339.7 | 0.00 | 0.0 | 0.0 | 0.0 |
| 10 | 249.1 | −180.6 | 16.5 | −181.0 | −181.0 | 0.0 | −180.6 | 0.0 | −187.7 | 0.0 | 0.0 | −180.6 |
| 11 | 237.7 | 3041.2 * | 83.4 | 379.4 | 379.4 | 239.6 | 0.0 | 392.2 | 0.00 | 142.2 | 0.0 | 3041.2 * |
| 12 | 237.7 | 0.0 | 83.4 | 413.4 * | 413.3 * | 410.0 | 0.0 | 0.0 | −22.0 | 0.0 | 0.0 | 26.0 |
| 13 | 0.0 | 0.0 | 0.0 | −447.5 | 0.0 | −364.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 3 | 237.7 | 0.0 | 83.4 | 670.7 | 240.0 | 339.7 | 0.0 | 339.7 | −320.6 * | 141.5 | 0.0 | 0.0 |
Appendix A.3. Linear Regression of Correlations Parameters
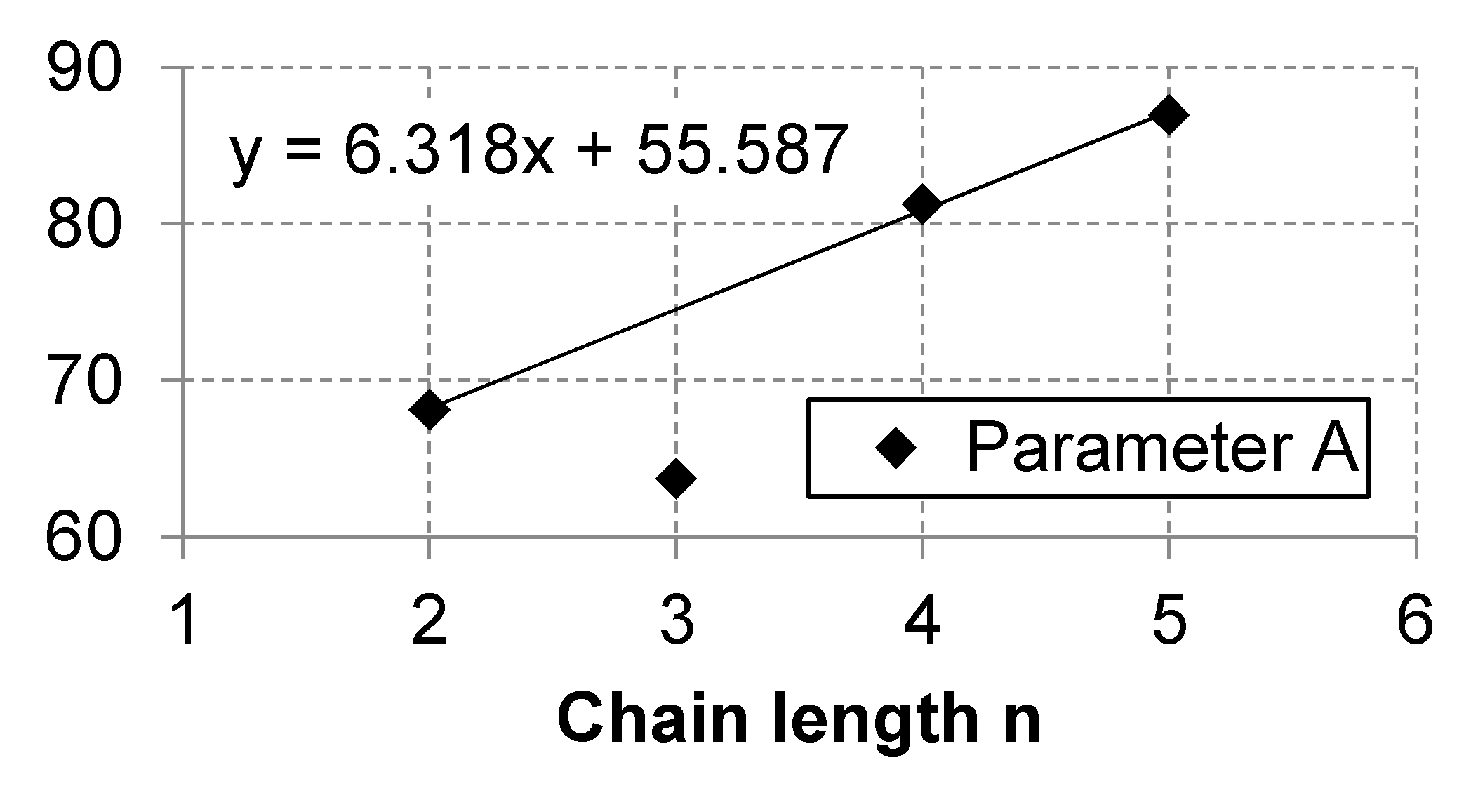
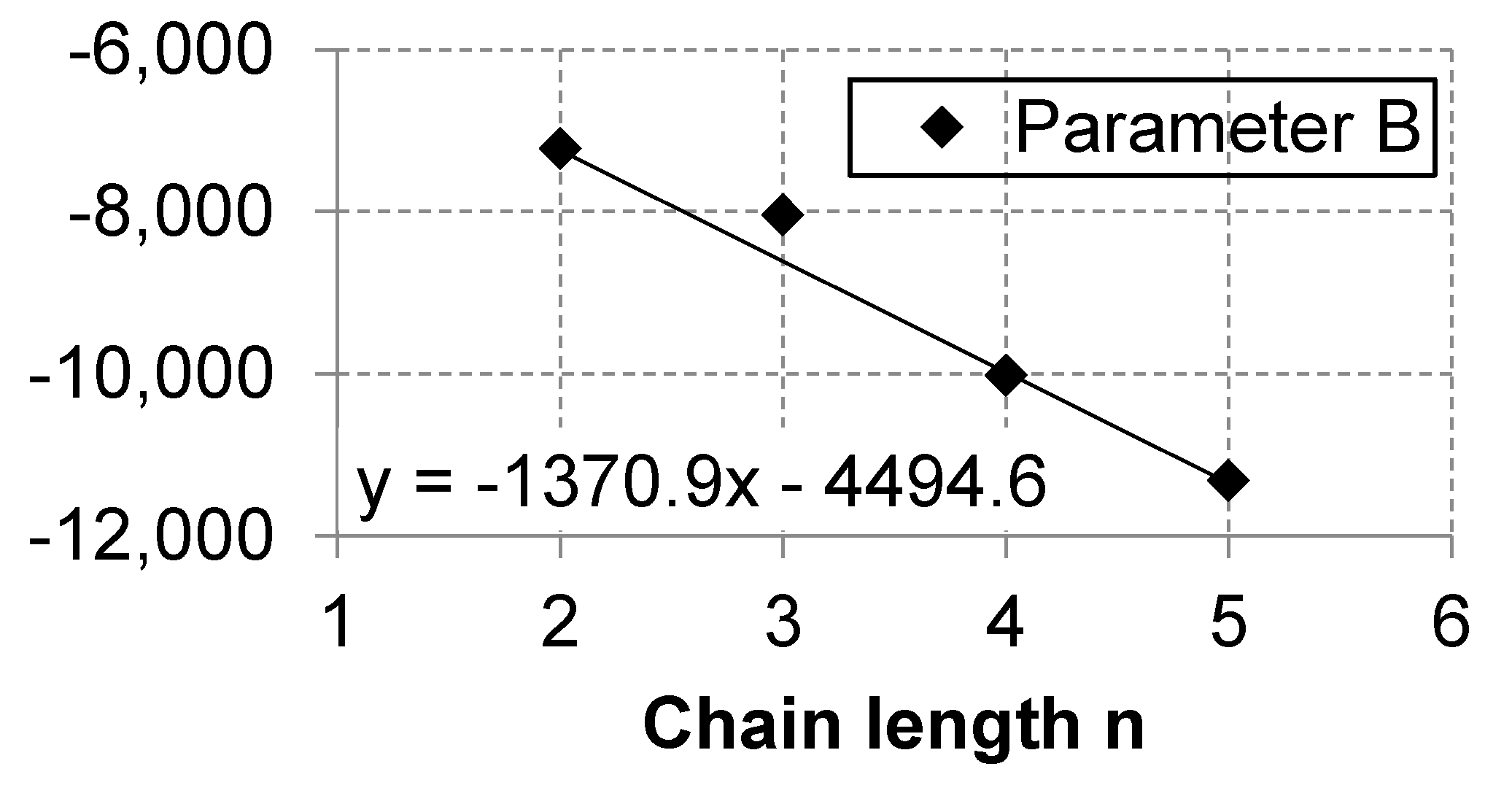
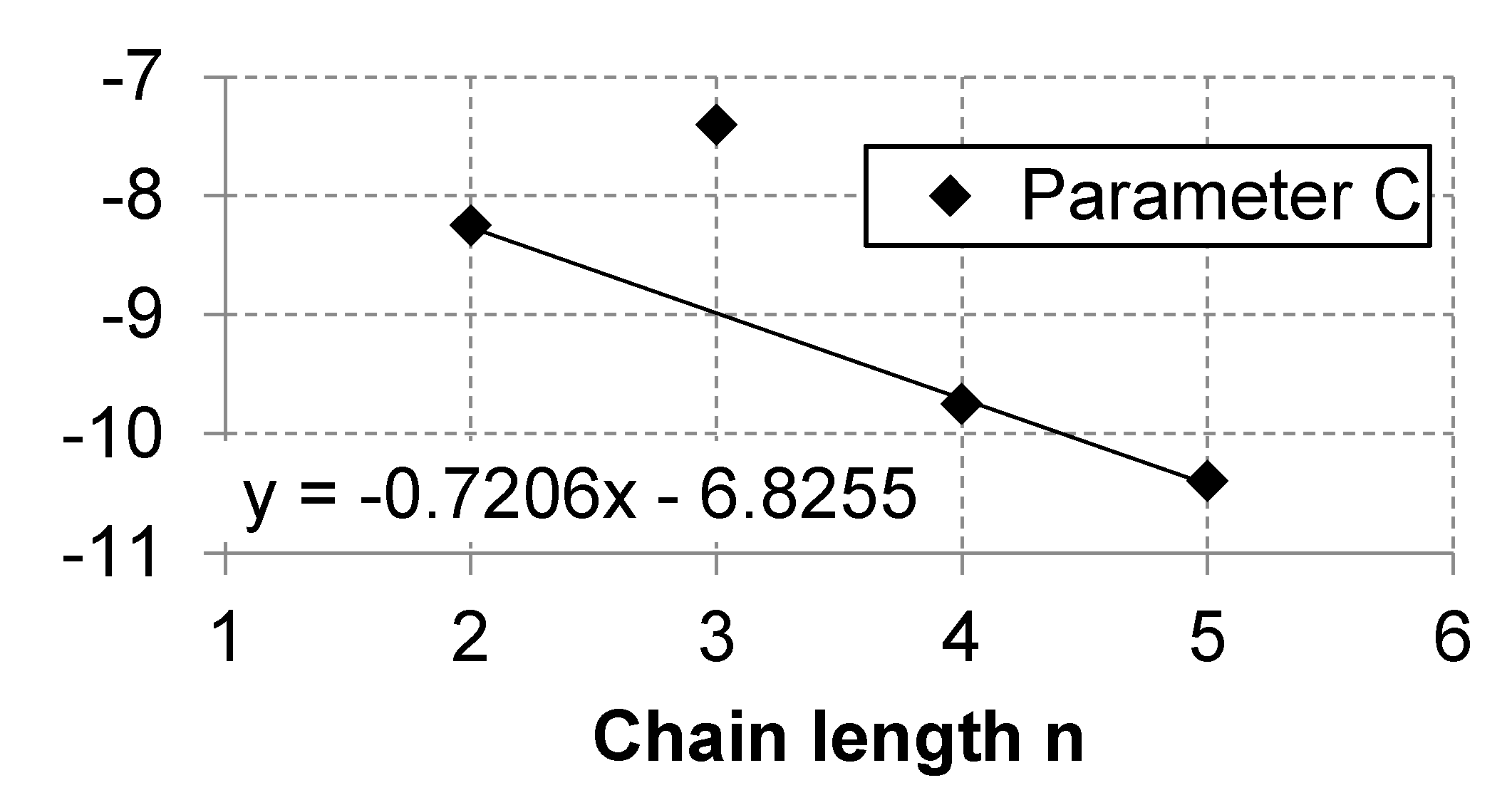
| n = 1 | n = 2 | n = 3 | n = 4 | n = 5 | n = 6 | n = 7 | n = 8 | n = 9 | n = 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| MGn | 133.5 | 178.8 | 224.1 | 269.4 | 314.7 | 360.0 | 405.3 | 450.6 | 495.9 | 541.2 |
| HFn | 86.6 | 131.9 | 177.2 | 222.5 | 267.8 | 313.1 | 358.4 | 403.7 | 449.0 | 494.3 |
References
- Schemme, S.; Samsun, R.C.; Peters, R.; Stolten, D. Power-to-fuel as a key to sustainable transport systems—An analysis of diesel fuels produced from CO2 and renewable electricity. Fuel 2017, 205, 198–221. [Google Scholar] [CrossRef]
- Lumpp, B.; Rothe, D.; Pastötter, C.; Lämmermann, R.; Jacob, E. Oxymethylene Ethers as Diesel Fuel Additives of the Future. MTZ Worldwide eMagazine 2011, 72, 34–38. [Google Scholar] [CrossRef]
- Burger, J.; Siegert, M.; Ströfer, E.; Hasse, H. Poly(oxymethylene) dimethyl ethers as components of tailored diesel fuel: Properties, synthesis and purification concepts. Fuel 2010, 89, 3315–3319. [Google Scholar] [CrossRef]
- Lautenschütz, L.; Oestreich, D.; Seidenspinner, P.; Arnold, U.; Dinjus, E.; Sauer, J. Physico-chemical properties and fuel characteristics of oxymethylene dialkyl ethers. Fuel 2016, 173, 129–137. [Google Scholar] [CrossRef]
- Barro, C.; Parravicini, M.; Boulouchos, K. Neat polyoxymethylene dimethyl ether in a diesel engine; part 1: Detailed combustion analysis. Fuel 2019, 256, 115892. [Google Scholar] [CrossRef]
- Iannuzzi, S.E.; Barro, C.; Boulouchos, K.; Burger, J. POMDME-diesel blends: Evaluation of performance and exhaust emissions in a single cylinder heavy-duty diesel engine. Fuel 2017, 203, 57–67. [Google Scholar] [CrossRef]
- Omari, A.; Heuser, B.; Pischinger, S. Potential of oxymethylenether-diesel blends for ultra-low emission engines. Fuel 2017, 209, 232–237. [Google Scholar] [CrossRef]
- Härtl, M.; Seidenspinner, P.; Jacob, E.; Wachtmeister, G. Oxygenate screening on a heavy-duty diesel engine and emission characteristics of highly oxygenated oxymethylene ether fuel OME1. Fuel 2015, 153, 328–335. [Google Scholar] [CrossRef]
- Zimmermann, A.W.; Gençer, E.; O’Sullivanb, F.; Schmäckera, R. E-fuels as Carbon Capture and Utilization Option for the Industry? A Standardized Techno-Economic Assessment OME3–5. In Proceedings of the 14th International Conference on Greenhouse Gas Control Technologies, GHGT-14, Melbourne, Australia, 22–25 October 2018. [Google Scholar]
- Bongartz, D.; Burre, J.; Mitsos, A. Production of Oxymethylene Dimethyl Ethers from Hydrogen and Carbon Dioxide—Part I: Modeling and Analysis for OME1. Ind. Eng. Chem. Res. 2019, 58, 4881–4889. [Google Scholar] [CrossRef]
- Schemme, S.; Breuer, J.L.; Köller, M.; Meschede, S.; Walman, F.; Samsun, R.C.; Peters, R.; Stolten, D. H2-based synthetic fuels: A techno-economic comparison of alcohol, ether and hydrocarbon production. Int. J. Hydrogen Energy 2019, 45, 5395–5414. [Google Scholar] [CrossRef]
- Held, M.; Tönges, Y.; Pélerin, D.; Härtl, M.; Wachtmeister, G.; Burger, J. On the energetic efficiency of producing polyoxymethylene dimethyl ethers from CO2 using electrical energy. Energy Environ. Sci. 2019, 12, 1019–1034. [Google Scholar] [CrossRef]
- Burre, J.; Bongartz, D.; Mitsos, A. Production of Oxymethylene Dimethyl Ethers from Hydrogen and Carbon Dioxide—Part II: Modeling and Analysis for OME3–5. Ind. Eng. Chem. Res. 2019, 58, 5567–5578. [Google Scholar] [CrossRef]
- Breitkreuz, C.F.; Schmitz, N.; Ströfer, E.; Burger, J.; Hasse, H. Design of a Production Process for Poly(oxymethylene) Dimethyl Ethers from Dimethyl Ether and Trioxane. Chemie Ingenieur Technik 2018, 90, 1–9. [Google Scholar] [CrossRef]
- Schmitz, N.; Burger, J.; Ströfer, E.; Hasse, H. From methanol to the oxygenated diesel fuel poly(oxymethylene) dimethyl ether: An assessment of the production costs. Fuel 2016, 185, 67–72. [Google Scholar] [CrossRef]
- Ouda, M.; Mantei, F.K.; Elmehlawy, M.; White, R.J.; Klein, H.; Fateen, S.E.K. Describing oxymethylene ether synthesis based on the application of non-stoichiomsetric Gibbs minimisation. React. Chem. Eng. 2018, 3, 277–292. [Google Scholar] [CrossRef]
- Peters, R. Identification and thermodynamic analysis of reaction pathways of methylal and OME-n formation. Energy 2017, 138, 1221–1246. [Google Scholar] [CrossRef]
- Peters, R.; Decker, M.; Eggemann, L.; Schemme, S.; Schorn, F.; Breuer, J.L.; Weiske, S.; Pasel, J.; Samsun, R.C.; Stolten, D. Thermodynamic and ecological preselection of synthetic fuel intermediates from biogas at farm sites. Energy Sustain. Soc. 2020, 10, 4. [Google Scholar] [CrossRef]
- Baranowski, C.J.; Bahmanpour, A.M.; Kröcher, O. Catalytic synthesis of polyoxymethylene dimethyl ethers (OME): A review. Appl. Catal. B Environ. 2017, 217, 407–420. [Google Scholar] [CrossRef]
- Hackbarth, K.; Haltenort, P.; Arnold, U.; Sauer, J. Recent Progress in the Production, Application and Evaluation of Oxymethylene Ethers. Chemie Ingenieur Technik 2018, 90, 1–10. [Google Scholar] [CrossRef]
- Burger, J. A Novel Process for the Production of Diesel Fuel Additives by Hierarchical Design. Ph.D. Thesis, Kaiserslautern Technical University, Kaiserslautern, Germany, 2012. [Google Scholar]
- Oestreich, D. Prozessentwicklung zur Gewinnung von Oxymethylenethern (OME) aus Methanol und Formaldehyd. Ph.D. Thesis, Karlsruher Institut für Technology, Karlsruhe, Germany, 2017. [Google Scholar]
- Bhatelia, T.; Lee, W.J.; Samanta, C.; Patel, J.; Bordoloi, A. Processes for the production of oxymethylene ethers: Promising synthetic diesel additives. Asia-Pac. J. Chem. Eng. 2017, 12, 827–837. [Google Scholar] [CrossRef]
- Weidert, J.-O.; Burger, J.; Renner, M.; Blagov, S.; Hasse, H. Development of an Integrated Reaction–Distillation Process for the Production of Methylal. Ind. Eng. Chem. Res. 2017, 56, 575–582. [Google Scholar] [CrossRef]
- Schmitz, N.; Strofer, E.; Burger, J.; Hasse, H. Conceptual Design of a Novel Process for the Production of Poly(oxymethylene) Dimethyl Ethers from Formaldehyde and Methanol. Ind. Eng. Chem. Res. 2017, 56, 11519–11530. [Google Scholar] [CrossRef]
- Schmitz, N.; Breitkreuz, C.F.; Ströfer, E.; Burger, J.; Hasse, H. Separation of water from mixtures containing formaldehyde, water, methanol, methylal, and poly(oxymethylene) dimethyl ethers by pervaporation. J. Membr. Sci. 2018, 564, 806–812. [Google Scholar] [CrossRef]
- Bertau, M.; Räuchle, K.; Offermanns, H. Methanol—Die Basischemikalie. Chemie In Unserer Zeit 2015, 49, 312–329. [Google Scholar] [CrossRef]
- Reuss, G.; Disteldorf, W.; Gamer, A.O.; Hilt, A. Formaldehyde. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 15. [Google Scholar] [CrossRef]
- Drunsel, J.-O.; Renner, M.; Hasse, H. Experimental study and model of reaction kinetics of heterogeneously catalyzed methylal synthesis. Chem. Eng. Res. Des. 2012, 90, 696–703. [Google Scholar] [CrossRef]
- Hahnenstein, I.; Albert, M.; Hasse, H.; Kreiter, C.G.; Maurer, G. NMR Spectroscopic and Densimetric Study of Reaction Kinetics of Formaldehyde Polymer Formation in Water, Deuterium Oxide, and Methanol. Ind. Eng. Chem. Res. 1995, 34, 440–450. [Google Scholar] [CrossRef]
- Maiwald, M.; Fischer, H.H.; Ott, M.; Peschla, R.; Kuhnert, C.; Kreiter, C.G.; Maurer, G.; Hasse, H. Quantitative NMR Spectroscopy of Complex Liquid Mixtures: Methods and Results for Chemical Equilibria in Formaldehyde−Water−Methanol at Temperatures up to 383 K. Ind. Eng. Chem. Res. 2003, 42, 259–266. [Google Scholar] [CrossRef]
- Maurer, G. Vapor-Liquid Equilibrium of Formaldehyde– and Water-Containing Multicomponent Mixtures. AIChE J. 1986, 32, 932–948. [Google Scholar] [CrossRef]
- Kuhnert, C.; Albert, M.; Breyer, S.; Hahnenstein, I.; Hasse, H.; Maurer, G. Phase Equilibrium in Formaldehyde Containing Multicomponent Mixtures: Experimental Results for Fluid Phase Equilibria of (Formaldehyde + (Water or Methanol) + Methylal)) and (Formaldehyde + Water + Methanol + Methylal) and Comparison with Predictions. Ind. Eng. Chem. Res. 2006, 45, 5155–5164. [Google Scholar] [CrossRef]
- Albert, M.; García, B.C.; Kreiter, C.; Maurer, G. Vapor-liquid and chemical equilibria of formaldehyde-water mixtures. AIChE J. 1999, 45, 2024–2033. [Google Scholar] [CrossRef]
- Albert, M.; Hasse, H.; Kuhnert, C.; Maurer, G. New Experimental Results for the Vapor−Liquid Equilibrium of the Binary System (Trioxane + Water) and the Ternary System (Formaldehyde + Trioxane + Water). J. Chem. Eng. Data 2005, 50, 1218–1223. [Google Scholar] [CrossRef]
- Albert, M.; Hahnenstein, I.; Hasse, H.; Maurer, G. Vapor–liquid equilibrium of formaldehyde mixtures: New data and model revision. AIChE J. 1996, 42, 1741–1752. [Google Scholar] [CrossRef]
- Ott, M.; Schoenmakers, H.; Hasse, H. Distillation of formaldehyde containing mixtures: Laboratory experiments, equilibrium stage modeling and simulation. Chem. Eng. Process. Process Intensif. 2005, 44, 687–694. [Google Scholar] [CrossRef]
- Grützner, T.; Hasse, H. Solubility of Formaldehyde and Trioxane in Aqueous Solutions. J. Chem. Eng. Data 2004, 49, 642–646. [Google Scholar] [CrossRef]
- O’Connell, J.P.; Gani, R.; Mathias, P.M.; Maurer, G.; Olson, J.D.; Crafts, P.A. Thermodynamic Property Modeling for Chemical Process and Product Engineering: Some Perspectives. Ind. Eng. Chem. Res. 2009, 48, 4619–4637. [Google Scholar] [CrossRef]
- Grützner, T.; Hasse, H.; Lang, N.; Siegert, M.; Ströfer, E. Development of a new industrial process for trioxane production. Chem. Eng. Sci. 2007, 62, 5613–5620. [Google Scholar] [CrossRef]
- Ouda, M.; Yarce, G.; White, R.J.; Hadrich, M.; Himmel, D.; Schaadt, A.; Klein, H.; Jacob, E.; Krossing, I. Poly(oxymethylene) dimethyl ether synthesis—A combined chemical equilibrium investigation towards an increasingly efficient and potentially sustainable synthetic route. React. Chem. Eng. 2017, 2, 50–59. [Google Scholar] [CrossRef]
- Schmitz, N.; Breitkreuz, C.F.; Ströfer, E.; Burger, J.; Hasse, H. Vapor–liquid equilibrium and distillation of mixtures containing formaldehdye and poly(oxymethylene) dimethyl ethers. Chem. Eng. Process. Process Intensif. 2018, 131, 116–124. [Google Scholar] [CrossRef]
- Fredenslund, A.; Jones, R.L.; Prausnitz, J.M. Group-contribution estimation of activity coefficients in nonideal liquid mixtures. AIChE J. 1975, 21, 1086–1099. [Google Scholar] [CrossRef]
- Joback, K.G.; Reid, R.C. Estimation of pure-component properties from group-contributions. Chem. Eng. Commun. 1987, 57, 233–243. [Google Scholar] [CrossRef]
- Constantinou, L.; Gani, R. New group contribution method for estimating properties of pure compounds. AIChE J. 1994, 40, 1697–1710. [Google Scholar] [CrossRef]
- Aspen Technology, I. Aspen Physical Property System. In Physical Property Methods and Models; Aspen Technology: Cambridge, MA, USA, 2001. [Google Scholar]
- Gmehling, J.; Rasmussen, P.; Fredenslund, A. Vapor-liquid equilibriums by UNIFAC group contribution. Revision and extension. 2. Ind. Eng. Chem. Process Des. Dev. 1982, 21, 118–127. [Google Scholar] [CrossRef]
- Hahnenstein, I.; Hasse, H.; Kreiter, C.G.; Maurer, G. 1H- and 13C-NMR-Spectroscopic Study of Chemical Equilibria in Solutions of Formaldehyde in Water, Deuterium Oxide, and Methanol. Ind. Eng. Chem. Res. 1994, 33, 1022–1029. [Google Scholar] [CrossRef]
- Albert, M. Thermodynamische Eigenschaften formaldehydhaltiger Mischungen. Ph.D. Thesis, Universität Kaiserslautern, Aachen, Germany, 1999. [Google Scholar]
- Hasse, H.; Hahnenstein, I.; Maurer, G. Revised vapor-liquid equilibrium model for multicomponent formaldehyde mixtures. AIChE J. 1990, 36, 1807–1814. [Google Scholar] [CrossRef]
- Hasse, H.; Maurer, G. Vapor—Liquid equilibrium of formaldehyde-containing mixtures at temperatures below 320 K. Fluid Phase Equilibria 1991, 64, 185–199. [Google Scholar] [CrossRef]
- Albert, M.; Coto García, B.; Kuhnert, C.; Peschla, R.; Maurer, G. Vapor–liquid equilibrium of aqueous solutions of formaldehyde and methanol. AIChE J. 2000, 46, 1676–1687. [Google Scholar] [CrossRef]
- Albert, M.; Hahnenstein, I.; Hasse, H.; Maurer, G. Vapor−Liquid and Liquid−Liquid Equilibria in Binary and Ternary Mixtures of Water, Methanol, and Methylal. J. Chem. Eng. Data 2001, 46, 897–903. [Google Scholar] [CrossRef]
- Schmitz, N.; Friebel, A.; von Harbou, E.; Burger, J.; Hasse, H. Liquid-liquid equilibrium in binary and ternary mixtures containing formaldehyde, water, methanol, methylal, and poly(oxymethylene) dimethyl ethers. Fluid Phase Equilibria 2016, 425, 127–135. [Google Scholar] [CrossRef]
- Kuhnert, C.; Albert, M.; Breyer, S.; Hahnenstein, I.; Hasse, H.; Maurer, G. Phase Equilibrium in Formaldehyde Containing Multicomponent Mixtures: Experimental Results for Fluid Phase Equilibria of (Formaldehyde + (Water or Methanol) + Methylal)) and (Formaldehyde + Water + Methanol + Methylal) and Comparison with Predictions. Ind. Eng. Chem. Res. 2006, 45, 6093–6094. [Google Scholar] [CrossRef][Green Version]
- Hasse, H.; Maurer, G. Kinetics of the poly(oxymethylene) glycol formation in aqueous formaldehyde solutions. Ind. Eng. Chem. Res. 1991, 30, 2195–2200. [Google Scholar] [CrossRef]
- Ott, M.; Fischer, H.H.; Maiwald, M.; Albert, K.; Hasse, H. Kinetics of oligomerization reactions in formaldehyde solutions: NMR experiments up to 373K and thermodynamically consistent model. Chem. Eng. Process. Process Intensif. 2005, 44, 653–660. [Google Scholar] [CrossRef]
- Ott, M. Reaktionskinetik und Destillation formaldehydhaltiger Mischungen. Ph.D. Thesis, TU Kaiserslautern, Kaiserslautern, Germany, 2004. [Google Scholar]
- Ouda, M.; Mantei, F.; Hesterwerth, K.; Bargiacchi, E.; Klein, H.; White, R.J. A hybrid description and evaluation of oxymethylene dimethyl ethers synthesis based on the endothermic dehydrogenation of methanol. React. Chem. Eng. 2018, 3, 676–695. [Google Scholar] [CrossRef]
- Ai, Z.-J.; Chung, C.-Y.; Chien, I.L. DESIGN AND CONTROL OF POLY(OXYMETHYLENE) DIMETHYL ETHERS PRODUCTION PROCESS DIRECTLY FROM FORMALDEHYDE AND METHANOL IN AQUEOUS SOLUTIONS. IFAC-PapersOnLine 2018, 51, 578–583. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Hasse, H.; Maurer, G. Enthalpy change on vaporization of aqueous and methanolic formaldehyde solutions. AIChE J. 1992, 38, 1693–1702. [Google Scholar] [CrossRef]
- Burger, J.; Ströfer, E.; Hasse, H. Production process for diesel fuel components poly(oxymethylene) dimethyl ethers from methane-based products by hierarchical optimization with varying model depth. Chem. Eng. Res. Des. 2013, 91, 2648–2662. [Google Scholar] [CrossRef]
- Hasse, H. Dampf-Flüssigkeits-Gleichgewichte, Enthalpien und Reaktionskinetik in formaldehydhaltigen Mischungen. Ph.D. Thesis, University of Kaiserslautern, Kaiserslautern, Germany, 1990. [Google Scholar]
- Soboleva, O.D.; Kazakova, S.V.; Blazhin, Y.M.; Ogorodnikov, S.K. Liquid-Vapor Equilibrium in the Formaldehyde-Water System. Zhurnal Prikladnoi Khimii 1984, 57, 860–865. [Google Scholar]
- Kogan, L.V.; Blazhin, Y.M.; Ogorodnikov, S.K.; Kafarov, V.V. Liquid-Vapor Equilibrium in the System Formaldehyde-Water (Thermodynamic Verification with Chemical Interaction in the Liquid Phase Taken into Account). Zhurnal Prikladnoi Khimii 1977, 50, 2682–2687. [Google Scholar]
- Walker, J.F. Formaldehyde; Reinhold: New York, NY, USA, 1964. [Google Scholar]
- Savchenko, V.I. Equilibrium in the reaction of trioxane formation in aqueous formaldehyde solutions. Bull. Acad. Sci. USSR Div. Chem. Sci. 1969, 18, 2449–2451. [Google Scholar] [CrossRef]
- Balashov, A.L.; Krasnov, V.L.; Danov, S.M.; Chernov, A.Y.; Sulimov, A.V. Formation of Cyclic Oligomers in Concentrated Aqueous Solutions of Formaldehyde. J. Struct. Chem. 2001, 42, 398–403. [Google Scholar] [CrossRef]
- Drunsel, J.-O. Entwicklung von Verfahren zur Herstellung von Methylal und Ethylal. Ph.D. Thesis, Kaiserslautern Technical University, Kaiserslautern, Germany, 2012. [Google Scholar]
- Gauss, C.F. Abhandlungen zur Methode der Kleinsten Quadrate; P. Stankiewicz: Berlin, Gemany, 1887. [Google Scholar]
- Redlich, O.; Kwong, J.N.S. On the Thermodynamics of Solutions. V. An Equation of State. Fugacities of Gaseous Solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef]
- Grützner, T. Entwicklung eines destillationsbasierten Verfahrens zur Herstellung von Trioxan. Ph.D. Thesis, Institut für Technische Thermodynamik und Thermische Verfahrenstechnik, Universität Stuttgart, Stuttgart, Germany, 2007. [Google Scholar]
- Kuhnert, C. Dampf-Flüssigkeits-Gleichgewichte in mehrkomponentigen formaldehydhaltigen Systemen. Ph.D. Thesis, Universität Kaiserslautern, Kaiserslautern, Germany, 2004. [Google Scholar]
- Haltenort, P.; Hackbarth, K.; Oestreich, D.; Lautenschütz, L.; Arnold, U.; Sauer, J. Heterogeneously catalyzed synthesis of oxymethylene dimethyl ethers (OME) from dimethyl ether and trioxane. Catal. Commun. 2018, 109, 80–84. [Google Scholar] [CrossRef]
- AspenTech. Aspen Physical Property System—Physical Property Models; AspenTech: Cambridge, MA, USA, 2012. [Google Scholar]
- AspenTech. Aspen Plus User Guide V10.2; AspenTech: Cambridge, MA, USA, 2000. [Google Scholar]
- Boyd, R.H. Some Physical Properties of Polyoxymethylene Dimethyl Ethers. J. Polym. Sci. 1961, 50, 133–141. [Google Scholar] [CrossRef]
- Chauvel, A.; Lefebvre, G. Petrochemical Processes; Editions OPHRYS: Paris, France, 2001. [Google Scholar]
- Panzer, E.; Emig, G. Verfahrensoptimierung der technischen Formaldehydsynthese am Silberkatalysator; Lehrstuhl für Technische Chemie I, Universität Erlangen-Nürnberg: Nürnberg, Germany, 2000. [Google Scholar]
- Álvarez, V.H.; Mattedi, S.; Iglesias, M.; Gonzalez-Olmos, R.; Resa, J.M. Phase equilibria of binary mixtures containing methyl acetate, water, methanol or ethanol at 101.3 kPa. Phys. Chem. Liq. 2011, 49, 52–71. [Google Scholar] [CrossRef]
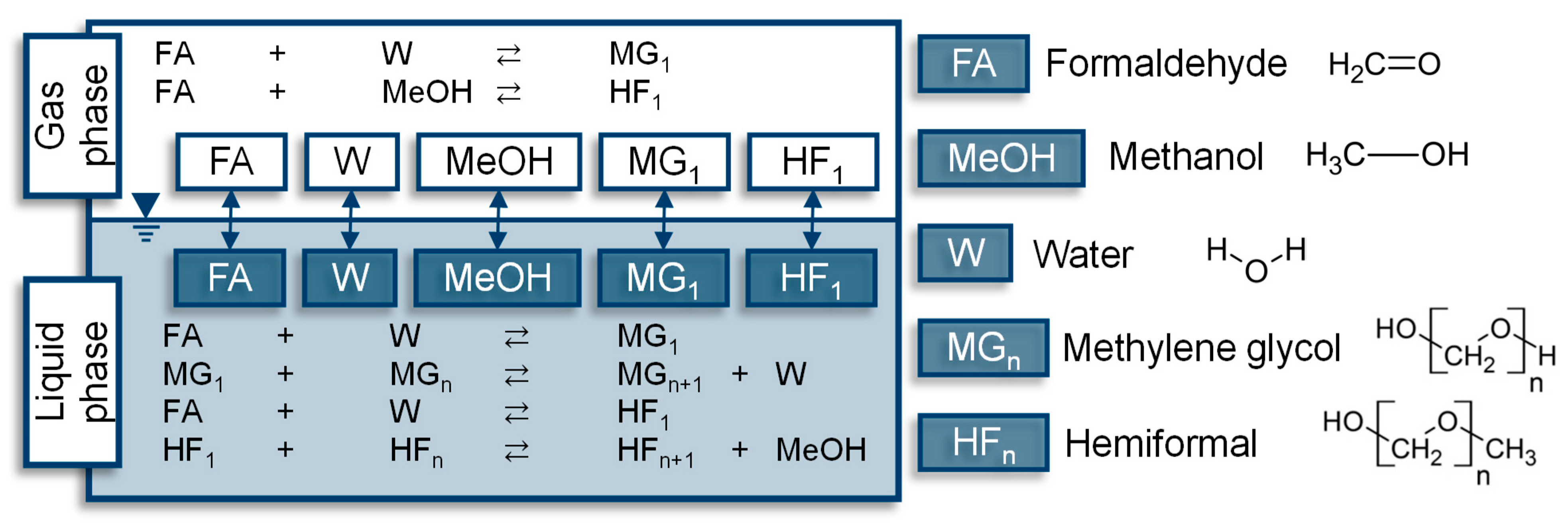

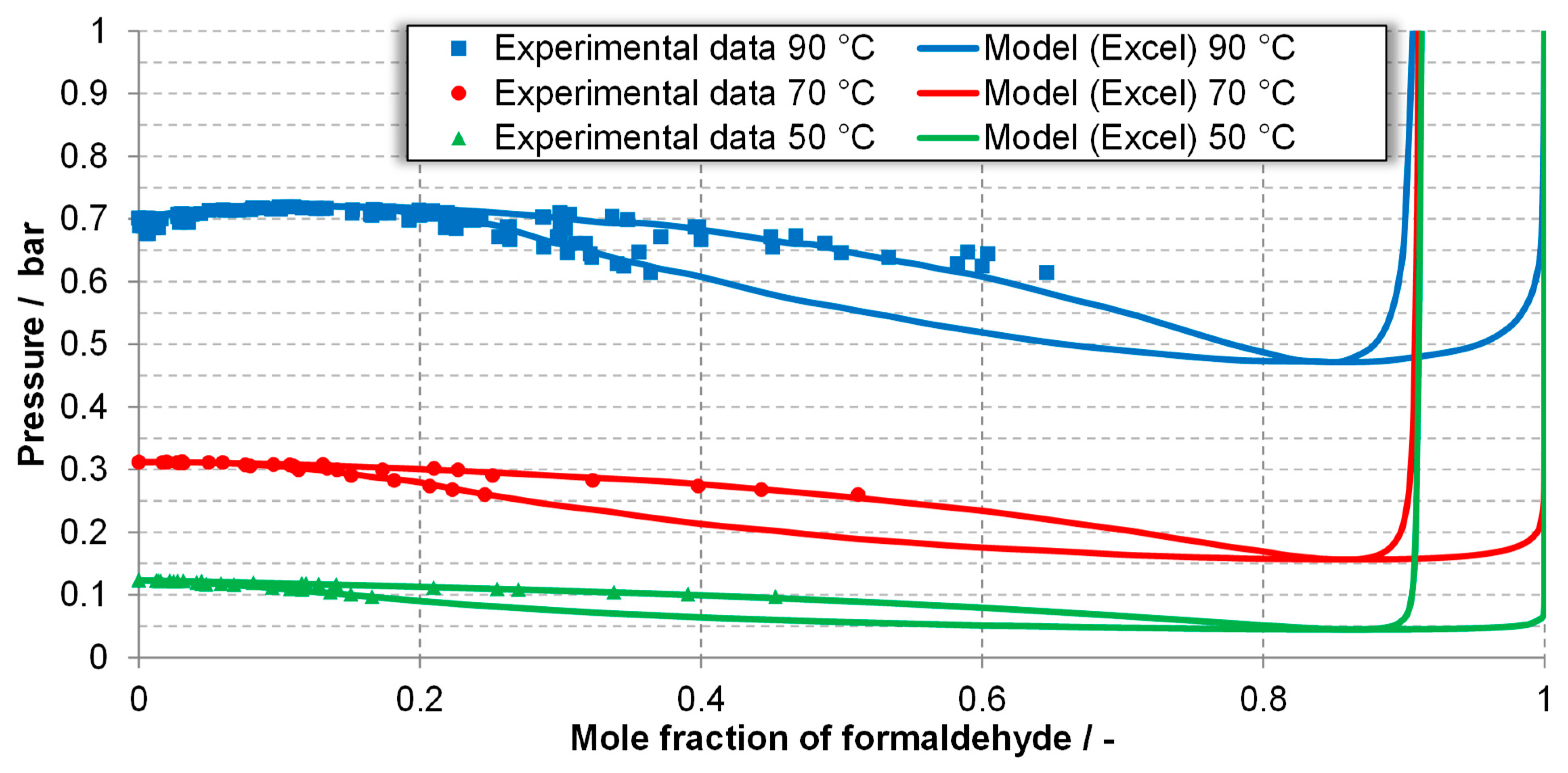
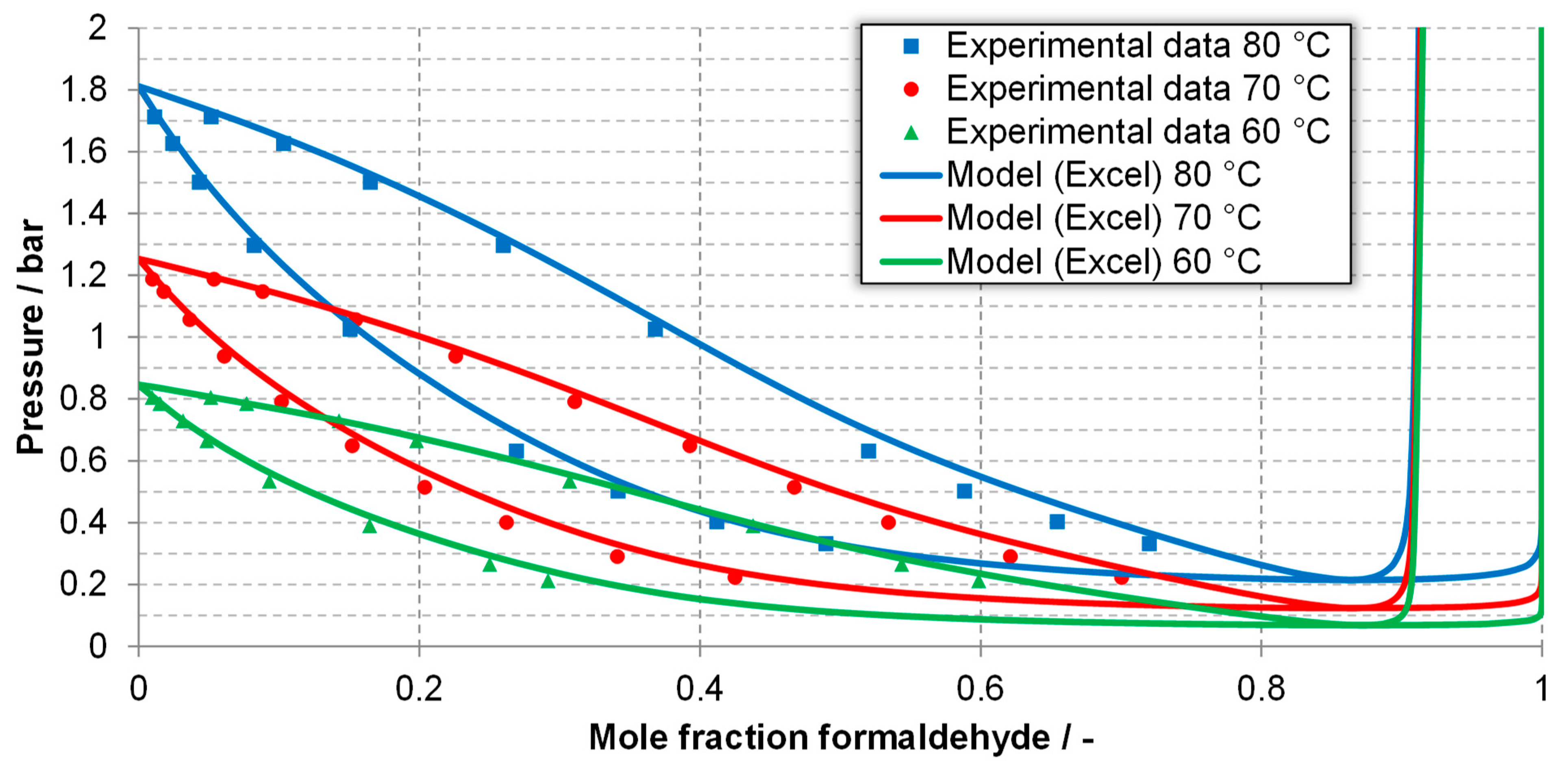
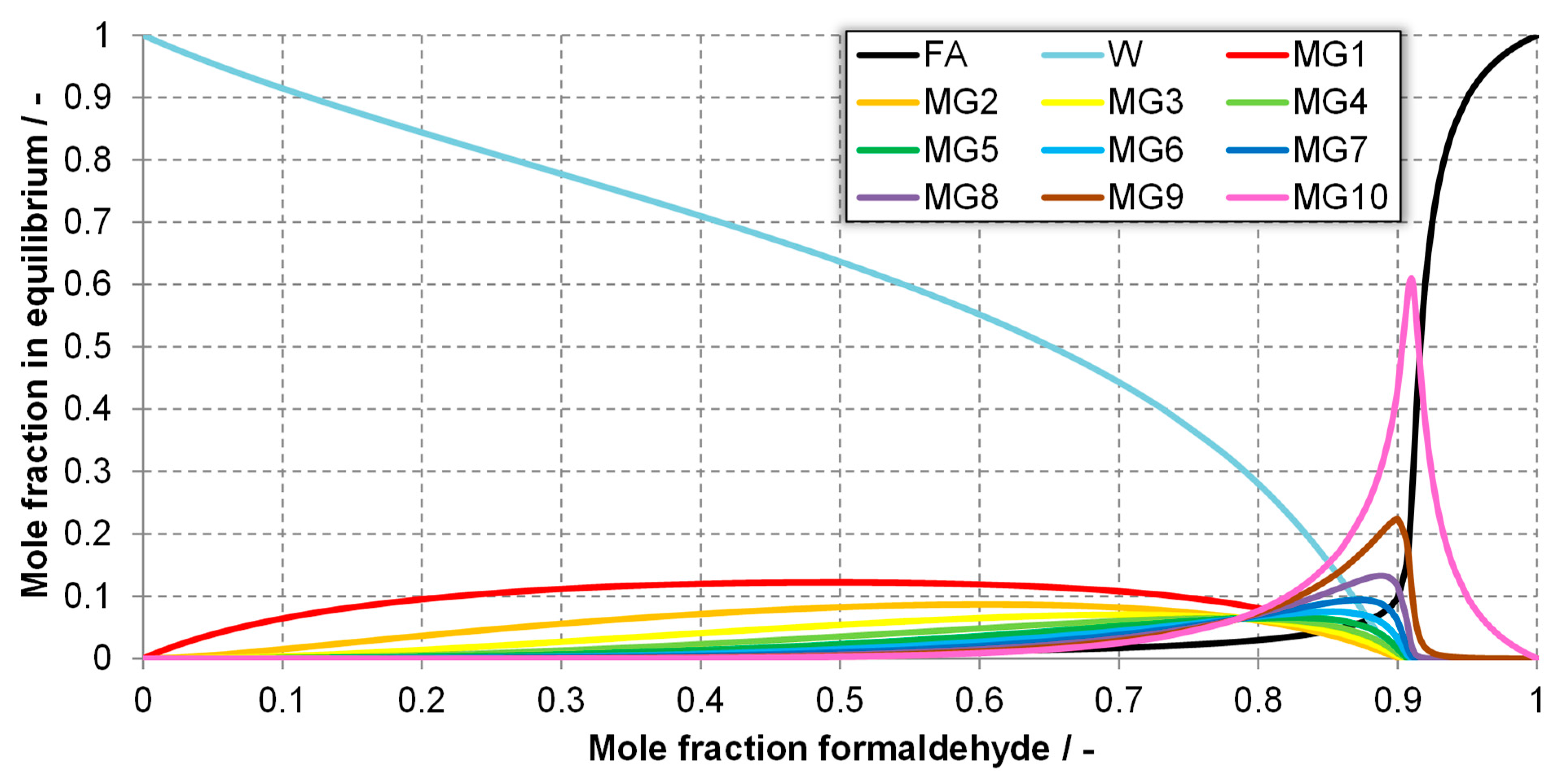

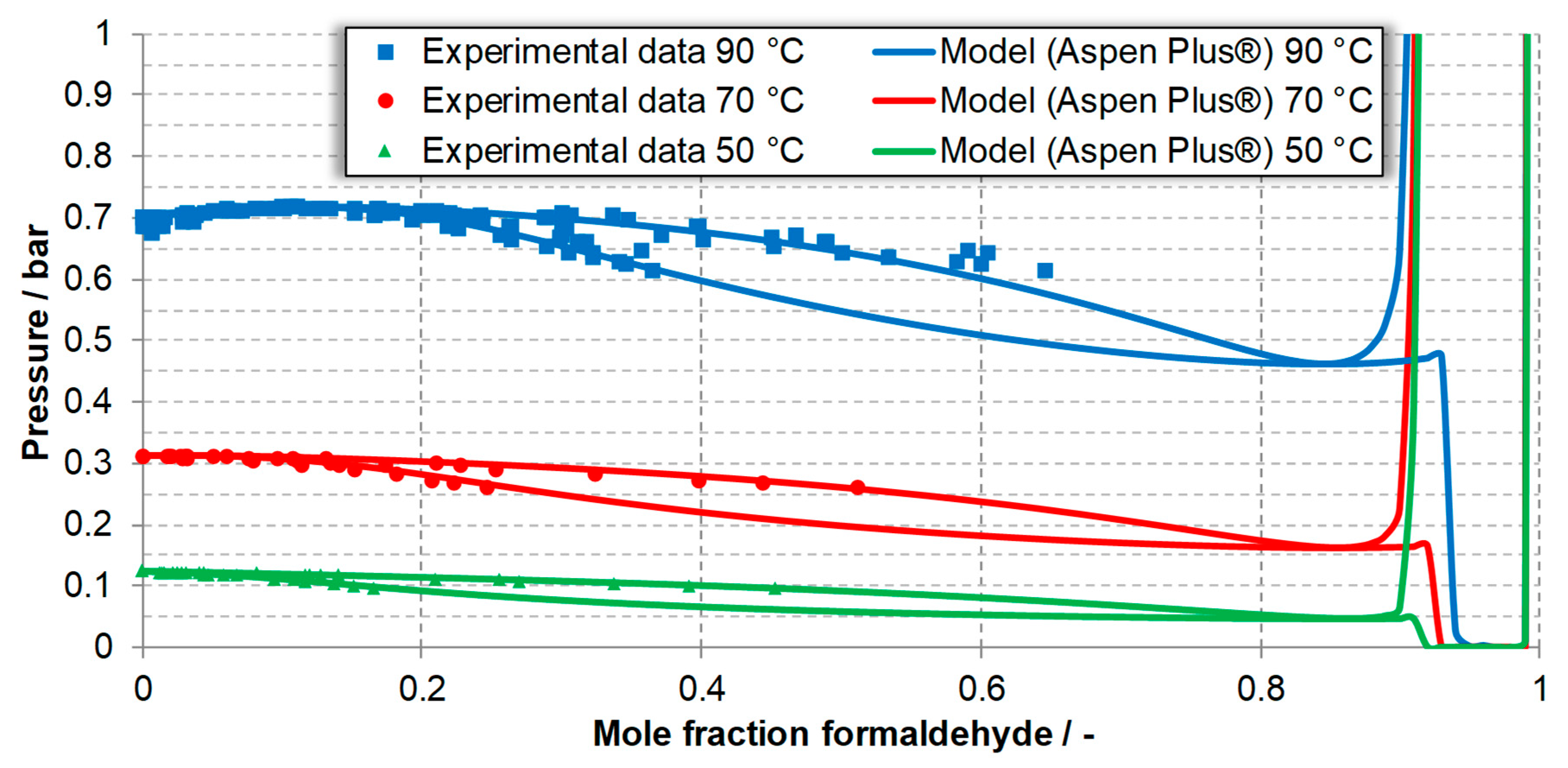

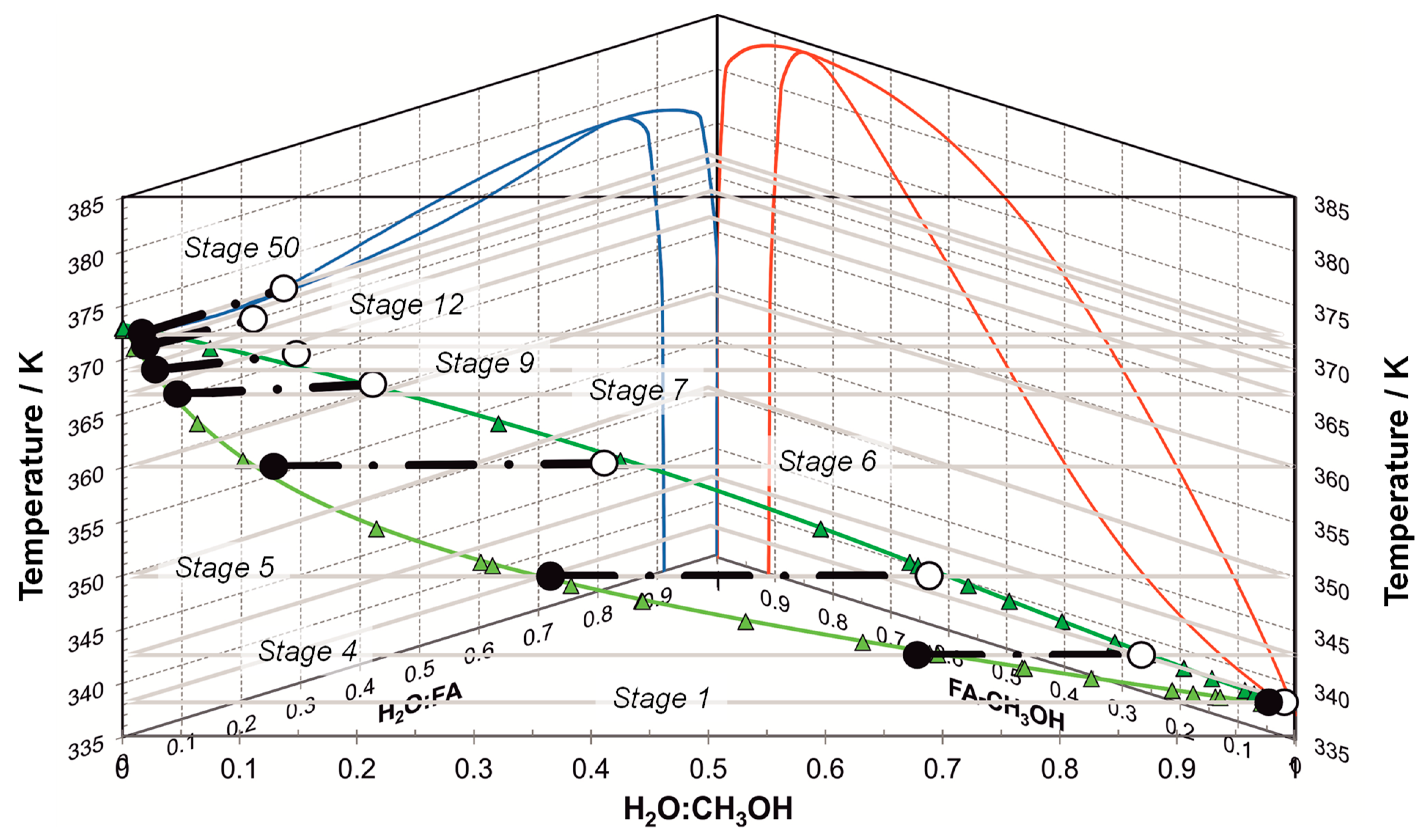
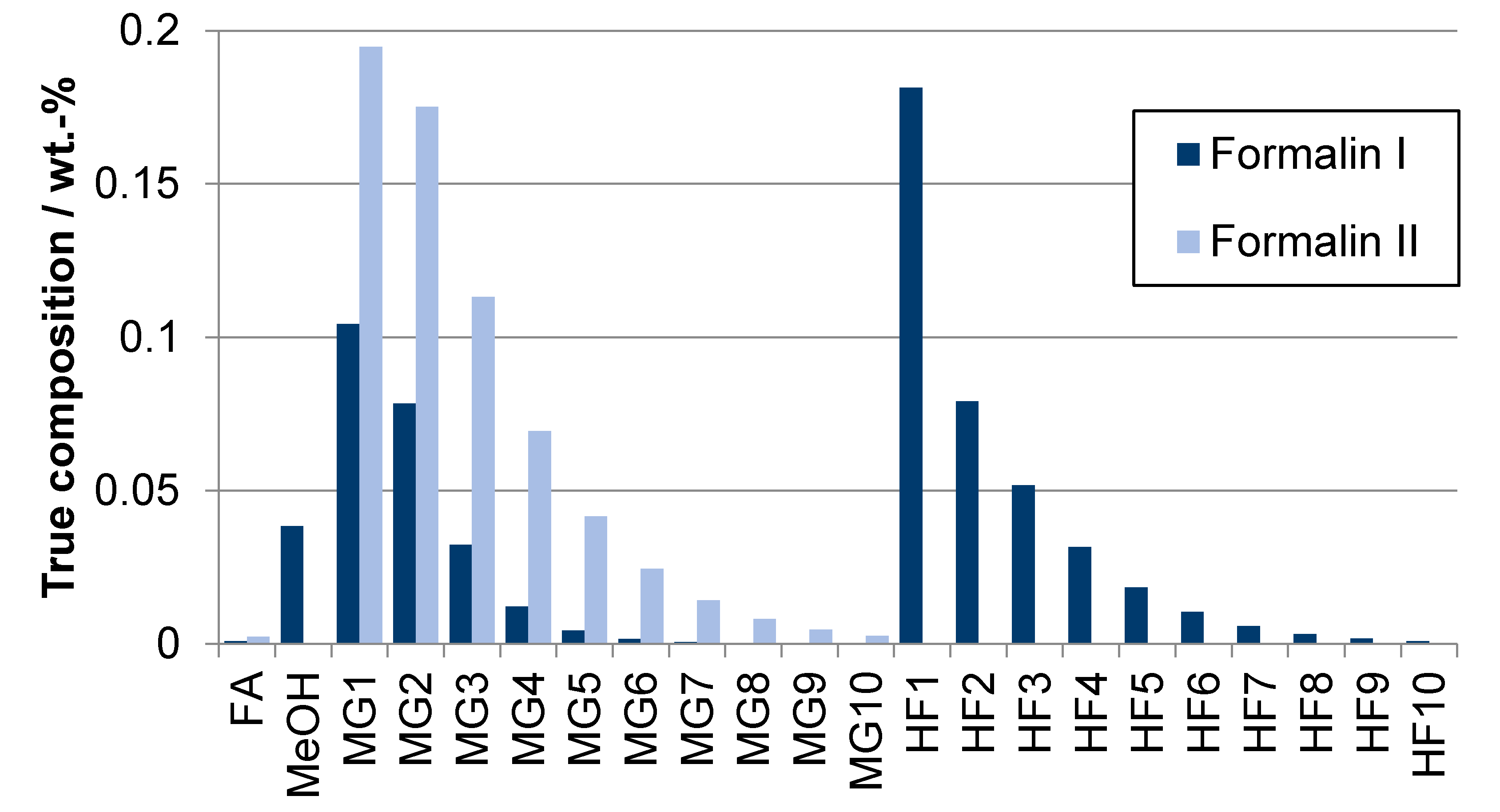
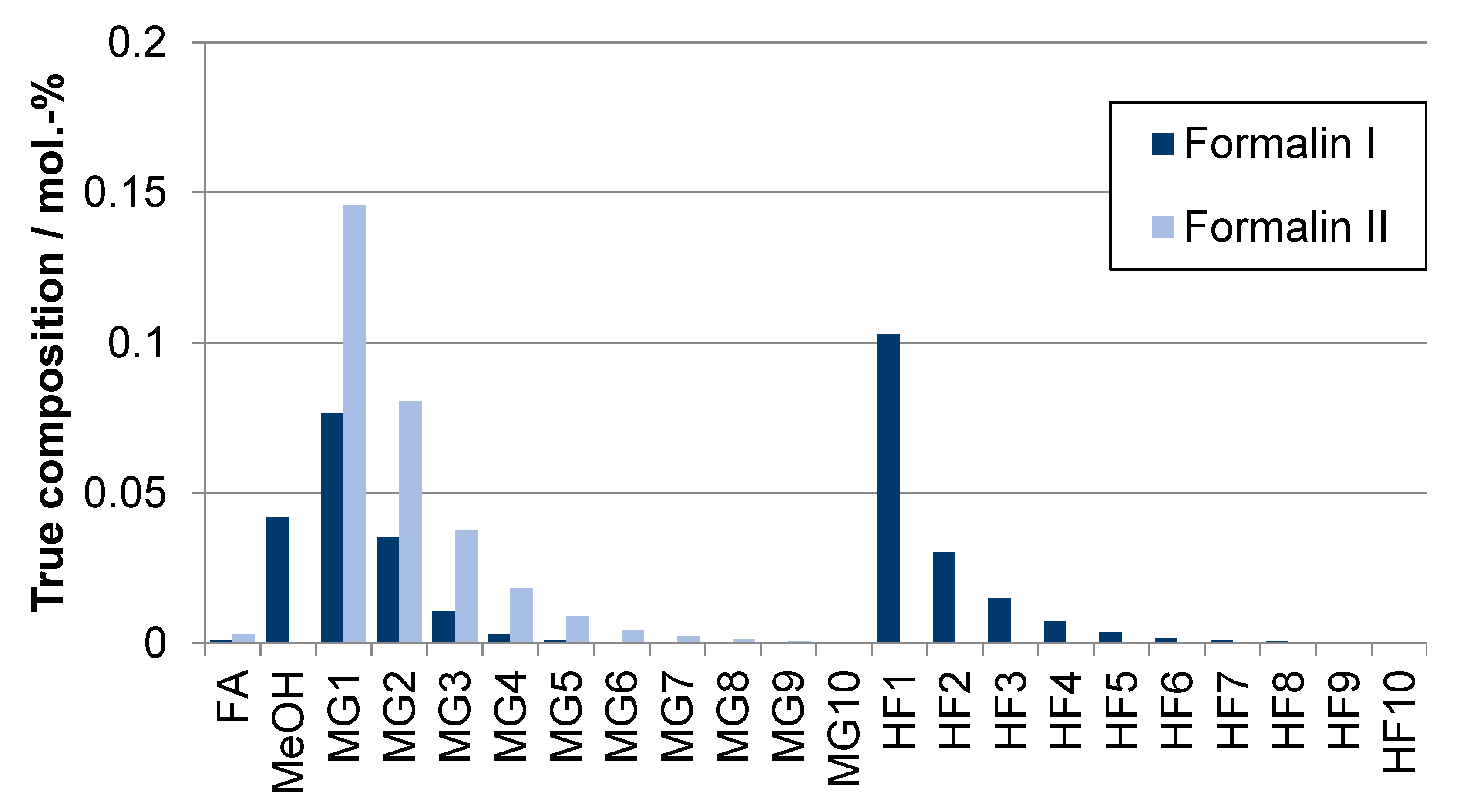
| Component System | Diagram of the Modeled Phase Equilibria and Experimental Data | Boiling Point Line | Dew Point Line | |||
|---|---|---|---|---|---|---|
| ARD % | s % | ARD % | s % | |||
| FA-W system generated using Excel | 90 °C | Figure 3, experimental data [32,34,64,65] | 1.23 | 1.27 | 1.17 | 0.86 |
| 70 °C | 0.65 | 0.63 | 0.57 | 0.41 | ||
| 50 °C | 0.48 | 0.53 | 0.69 | 0.47 | ||
| FA-MeOH system generated using Excel | 80 °C | Figure 4, experimental data [65] | 6.43 | 5.35 | 3.40 | 2.73 |
| 70 °C | 6.30 | 5.63 | 6.34 | 4.47 | ||
| 60 °C | 3.36 | 3.88 | 5.45 | 5.35 | ||
| 1 | –242.1 | 17180 | 33.547 | 3.131 | 73.85 | 600 | –11.7 | 1.945 |
| 2 | 151.9215 | –8666.63 | –21.5084 | 0.230 | –2.097 | –49.15 | 0.0 | 0.0 |
| 3 | 152.41 | –8508.3 | –21.7035 | 0.259 | –1.635 | –53 | 0.0 | 0.0 |
| 4 | 152.372 | –8502.62 | –21.6948 | 0.199 | –1.682 | –53 | 0.0 | 0.0 |
| 5 | 152.367 | –8491.15 | –21.6964 | 0.200 | –1.709 | –53 | 0.0 | 0.0 |
| 6 | 152.363 | –8470.65 | –21.7035 | 0.252 | –1.728 | –53 | 0.0 | 0.0 |
| 7 | 152.358 | –8470 | –21.7015 | 0.232 | –1.741 | –53 | 0.0 | 0.0 |
| 8 | 152.351 | –8477 | –21.696 | 0.187 | –1.751 | –53 | 0.0 | 0.0 |
| 9 | 152.334 | –8468.75 | –21.6961 | 0.205 | –1.759 | –53 | 0.0 | 0.0 |
| 10 | 152.3375 | –8464.4 | –21.698 | 0.216 | –1.765 | –53 | 0.0 | 0.0 |
| Component System | Diagram of Modeled Phase Equilibria and Experimental Data | Boiling Point Line | Dew Point Line | |||
|---|---|---|---|---|---|---|
| ARD % | s % | ARD % | s % | |||
| FA-W system generated using Aspen Plus® | 90 °C | Figure 7, Experimental data [32,34,64,65] | 1.29 | 1.48 | 1.66 | 1.25 |
| 70 °C | 0.74 | 0.74 | 0.57 | 0.38 | ||
| 50 °C | 0.43 | 0.41 | 1.23 | 0.90 | ||
| FA-MeOH system generated using Aspen Plus® | 80 °C | Figure 8, experimental data [65] | 1.88 | 1.83 | 3.77 | 4.88 |
| 70 °C | 2.52 | 2.55 | 3.07 | 2.40 | ||
| 60 °C | 1.50 | 1.39 | 2.88 | 3.04 | ||
| Aspen Plus® Database | Case 1: Estimation Using the Molecular Structure | Case 2: Estimation Using the Molecular Structure and Boiling Point | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OME1 | 315.0 | 497.0 | 39.5 | 312.9 0.7% | 477.4 3.9% | 42.2 6.8% | 315.0 0.0% | 480.7 3.3% | 42.2 (6.8%) |
| DME | 248.3 | 400.0 | 53.7 | 267.6 7.8% | 427.9 7.0% | 49.1 8.6% | 248.3 0.0% | 397.2 0.7% | 49.1 (8.6%) |
| TRI | 387.7 | 604.0 | 58.2 | 373.1 3.8% | 585.0 3.2% | 59.6 2.5% | 397.7 0.0% | 607.8 0.6% | 59.6 (2.5%) |
| OMEn Chain Length n | A | B | C | D | TB/°C | Reference |
|---|---|---|---|---|---|---|
| 2 | 75.01 | −7223.44 | 0.0 | −8.25 | 104.7 | [77] |
| 3 | 70.59 | −8042.31 | 0.0 | −7.41 | 155.6 | [77] |
| 4 | 88.12 | −10017.28 | 0.0 | −9.75 | 201.3 | [77] |
| 5 | 93.85 | −11323.17 | 0.0 | −10.40 | 241.7 | [77] |
| 6 | 100.40 | −12720.00 | 0.0 | −11.15 | 276.6 | * |
| 7 | 106.72 | −14091.90 | 0.0 | −11.87 | 307.9 | * |
| 8 | 113.04 | −15462.80 | 0.0 | −12.59 | 335.7 | * |
| 9 | 119.36 | −16833.70 | 0.0 | −13.31 | 360.7 | * |
| 10 | 125.67 | −18204.60 | 0.0 | −14.03 | 383.1 | * |
| A | B | C | D | TB/°C | Reference | |
|---|---|---|---|---|---|---|
| MG1 | 11.0768 | 1997.20 | −142.72 | 0.0 | 178.8 | [32] |
| HF1 | 18.0125 | 5125.00 | 0.0 | 0.0 | 109.5 | [32] |
| n = 1 | n = 2 | n = 3 | n = 4 | n = 5 | n = 6 | n = 7 | n = 8 | n = 9 | n = 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| MGn | 178.8 | 241.6 | 292.5 | 338.3 | 378.6 | 413.6 | 444.8 | 472.7 | 497.6 | 520.1 |
| HFn | 109.5 | 172.3 | 223.2 | 268.9 | 309.3 | 344.3 | 375.5 | 403.4 | 428.3 | 450.8 |
| n = 1 | n = 2 | n = 3 | n = 4 | n = 5 | n = 6 | n = 7 | n = 8 | n = 9 | n = 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| MGn | 356.9 | 400.1 | 443.3 | 486.9 | 531.3 | 577.0 | 624.2 | 673.3 | 724.9 | 779.3 |
| HFn | 282.8 | 327.4 | 371.3 | 415.0 | 458.9 | 503.4 | 549.0 | 596.1 | 645.0 | 696.1 |
| OMEn | 223.9 * | 280.7 | 333.2 | 377.5 | 414.4 | 442.9 | 469.4 | 493.2 | 515.1 | 536.0 |
| Equation | ||||
|---|---|---|---|---|
| Equation (10) | 52.63 | 85.33 | −19.73 | 92.05 |
| Equation (11) | −52.63 | −85.33 | 19.73 | −92.05 |
| Equation (12) | −176.14 | −156.67 | −217.82 | −155.35 |
| Equation (13) | −689.57 | −676.46 | −720.34 | −673.22 |
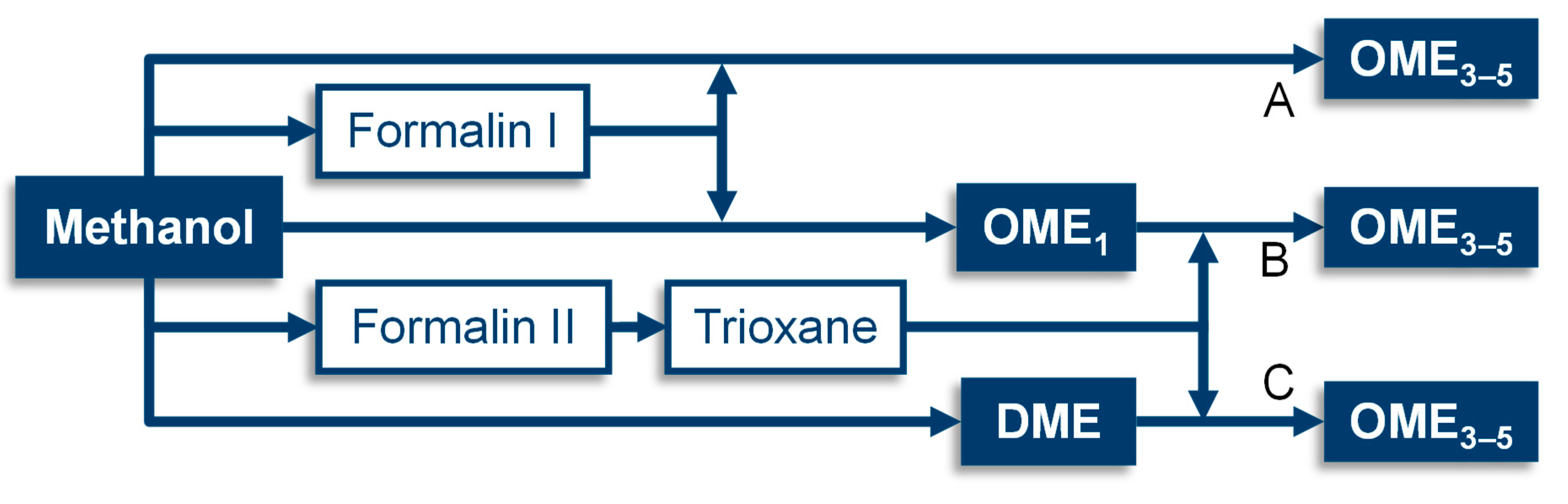
| Product | Stoichiometric Weight Fraction/wt.-% | Route | Absorbent | ||
|---|---|---|---|---|---|
| FA | Water | Methanol | |||
| Formalin Ia | 40.7 | 40.7 | 18.6 | A | Freshwater |
| Formalin Ib | 40.7 | 40.7 | 18.6 | B | OME1 wastewater (5.0 wt.-% FA) |
| Formalin II | 50.0 | 50.0 | 0.0 | B, C | TRI wastewater (1.0 wt.-% FA) |
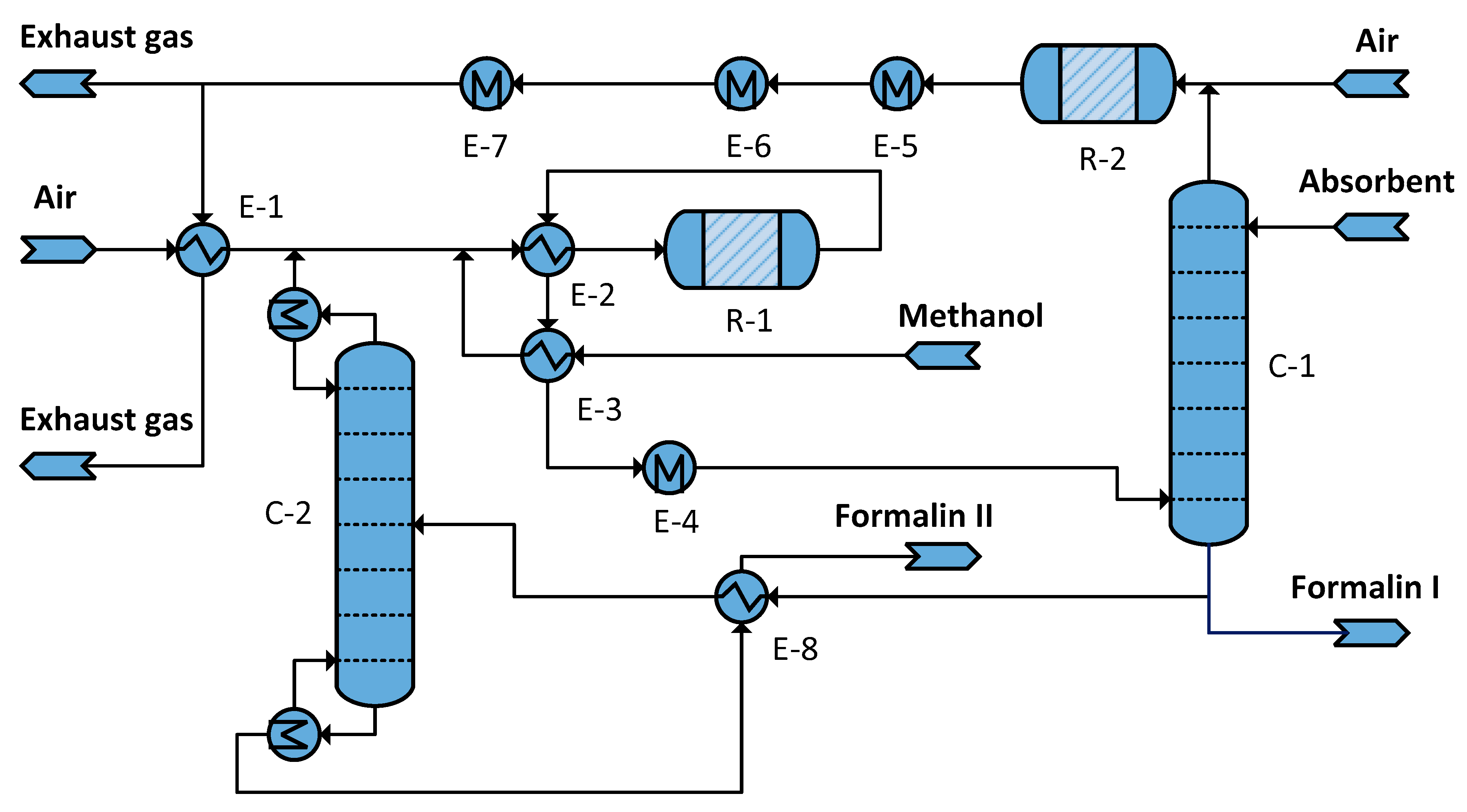
| Unit | Formalin Ia Heat MJ/kg | Formalin Ib Heat MJ/kg | Formalin II Heat MJ/kg | Temperature Level °C | Heating or Cooling Utility |
|---|---|---|---|---|---|
| Reactor R-1 | −2.183 | −2.119 | −2.665 | 630 | HP steam (250 °C) |
| Steam generator E-5 | −1.964 | −1.830 | −2.385 | 260 | HP steam (250 °C) |
| Steam generator E-6 | −0.264 | −0.256 | −0.322 | 185 | MP steam (175 °C) |
| Steam generator E-7 | −0.174 | −0.168 | −0.212 | 135 | LP steam (125 °C) |
| Column Reboiler C-2 | - | - | 4.892 | 99 | LP steam (125 °C) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schemme, S.; Meschede, S.; Köller, M.; Samsun, R.C.; Peters, R.; Stolten, D. Property Data Estimation for Hemiformals, Methylene Glycols and Polyoxymethylene Dimethyl Ethers and Process Optimization in Formaldehyde Synthesis. Energies 2020, 13, 3401. https://doi.org/10.3390/en13133401
Schemme S, Meschede S, Köller M, Samsun RC, Peters R, Stolten D. Property Data Estimation for Hemiformals, Methylene Glycols and Polyoxymethylene Dimethyl Ethers and Process Optimization in Formaldehyde Synthesis. Energies. 2020; 13(13):3401. https://doi.org/10.3390/en13133401
Chicago/Turabian StyleSchemme, Steffen, Sven Meschede, Maximilian Köller, Remzi Can Samsun, Ralf Peters, and Detlef Stolten. 2020. "Property Data Estimation for Hemiformals, Methylene Glycols and Polyoxymethylene Dimethyl Ethers and Process Optimization in Formaldehyde Synthesis" Energies 13, no. 13: 3401. https://doi.org/10.3390/en13133401
APA StyleSchemme, S., Meschede, S., Köller, M., Samsun, R. C., Peters, R., & Stolten, D. (2020). Property Data Estimation for Hemiformals, Methylene Glycols and Polyoxymethylene Dimethyl Ethers and Process Optimization in Formaldehyde Synthesis. Energies, 13(13), 3401. https://doi.org/10.3390/en13133401







