Novel Ni/Ce(Ti)ZrO2 Catalysts for Methane Dry Reforming Prepared in Supercritical Alcohol Media
Abstract
:1. Introduction
2. Materials and Methods
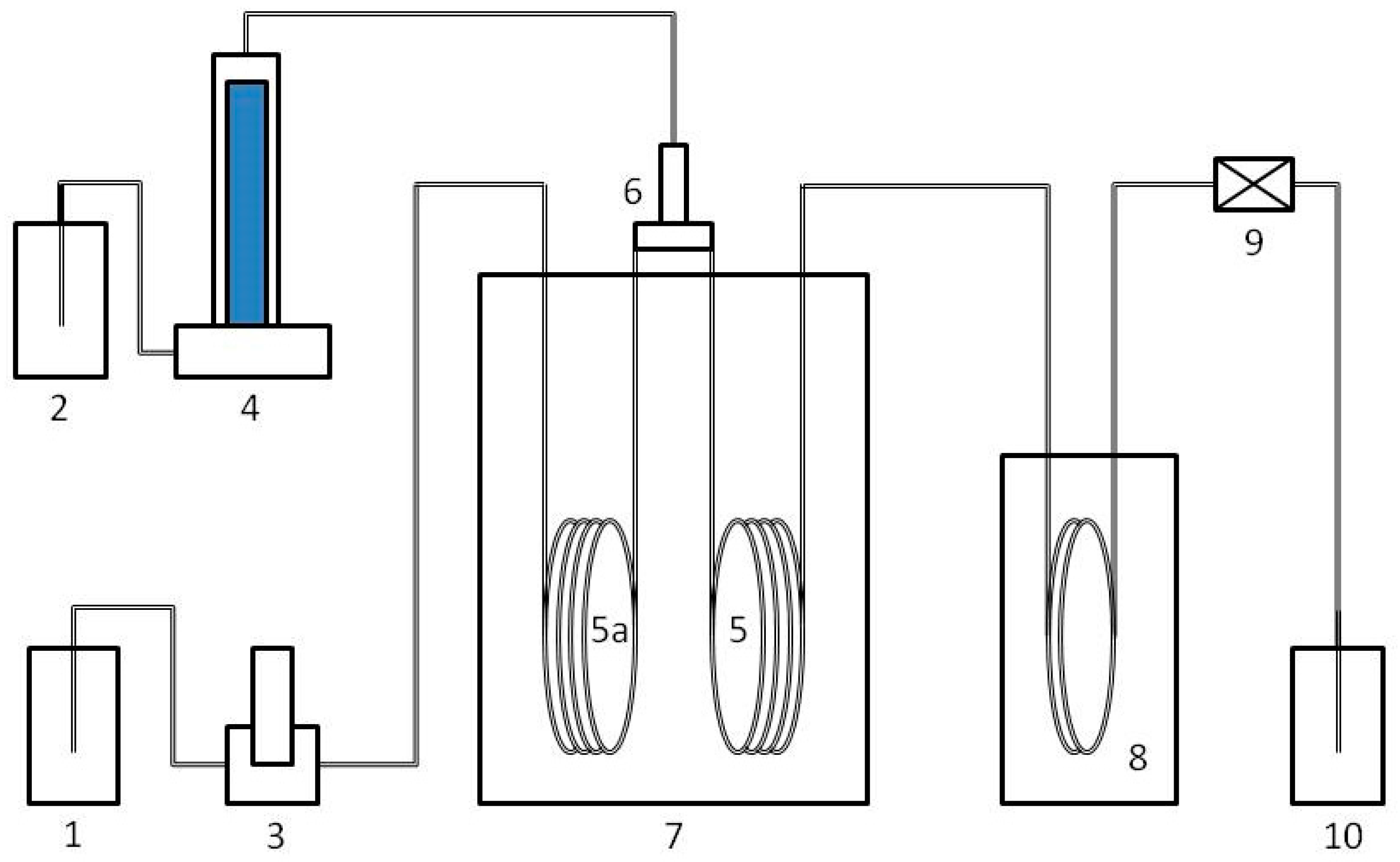
2.1. Support Preparation
2.2. Catalysts Preparation
2.3. Characterization
2.4. Catalytic Reaction
3. Results and Discussion
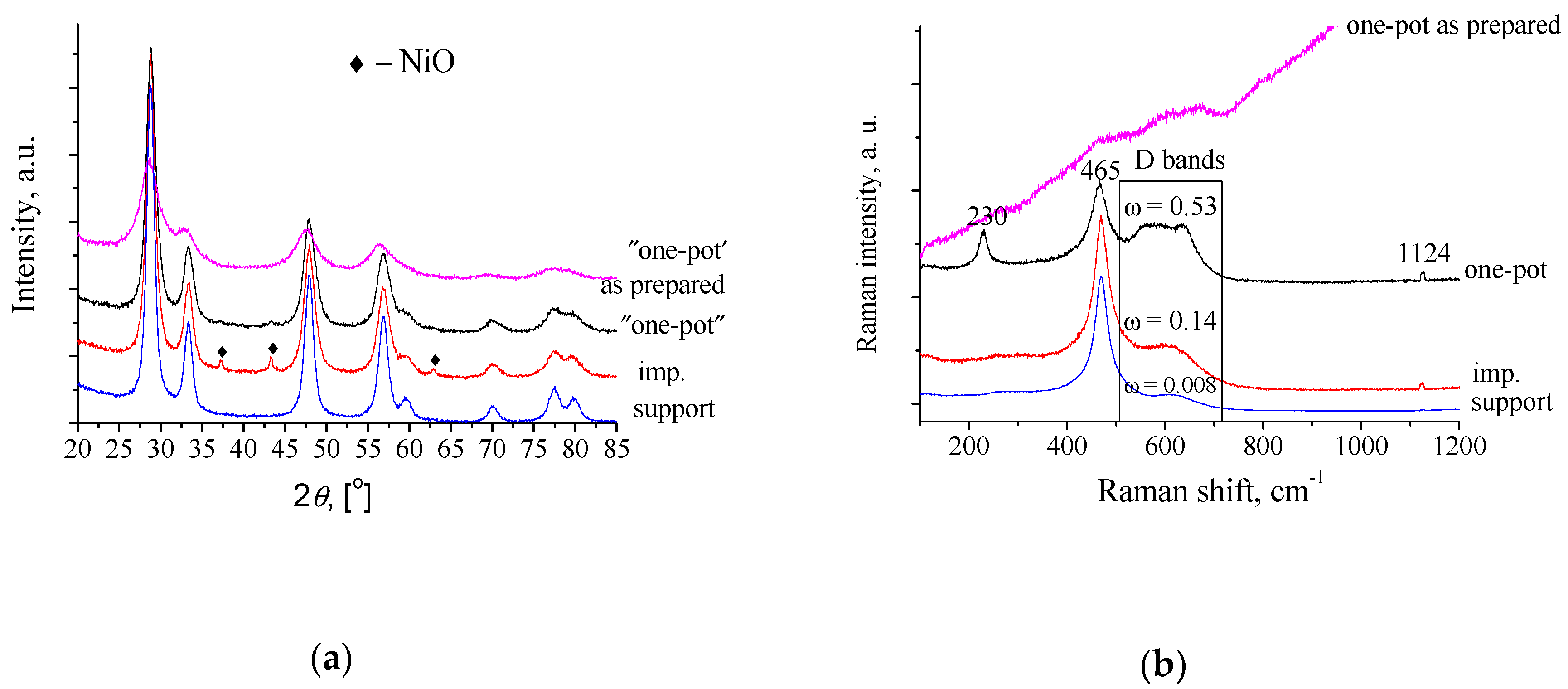
3.1. Structural Properties of Supports and Ni/Ce0.75Zr0.25O2−δ
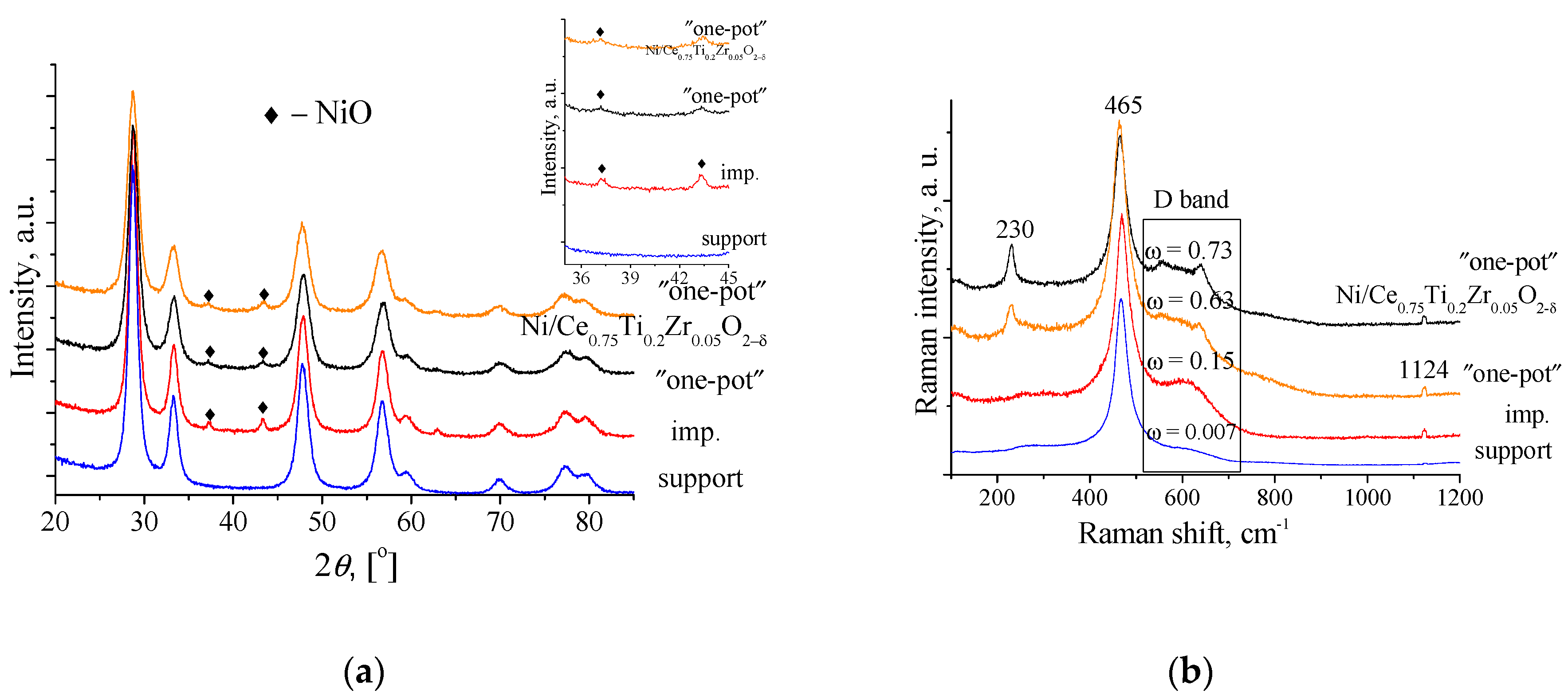
3.2. Structural Properties of Supports Doped by Ti and Catalysts Ni/Ce0.75TixZr0.15-xO2−δ
3.3. Textural Characterization
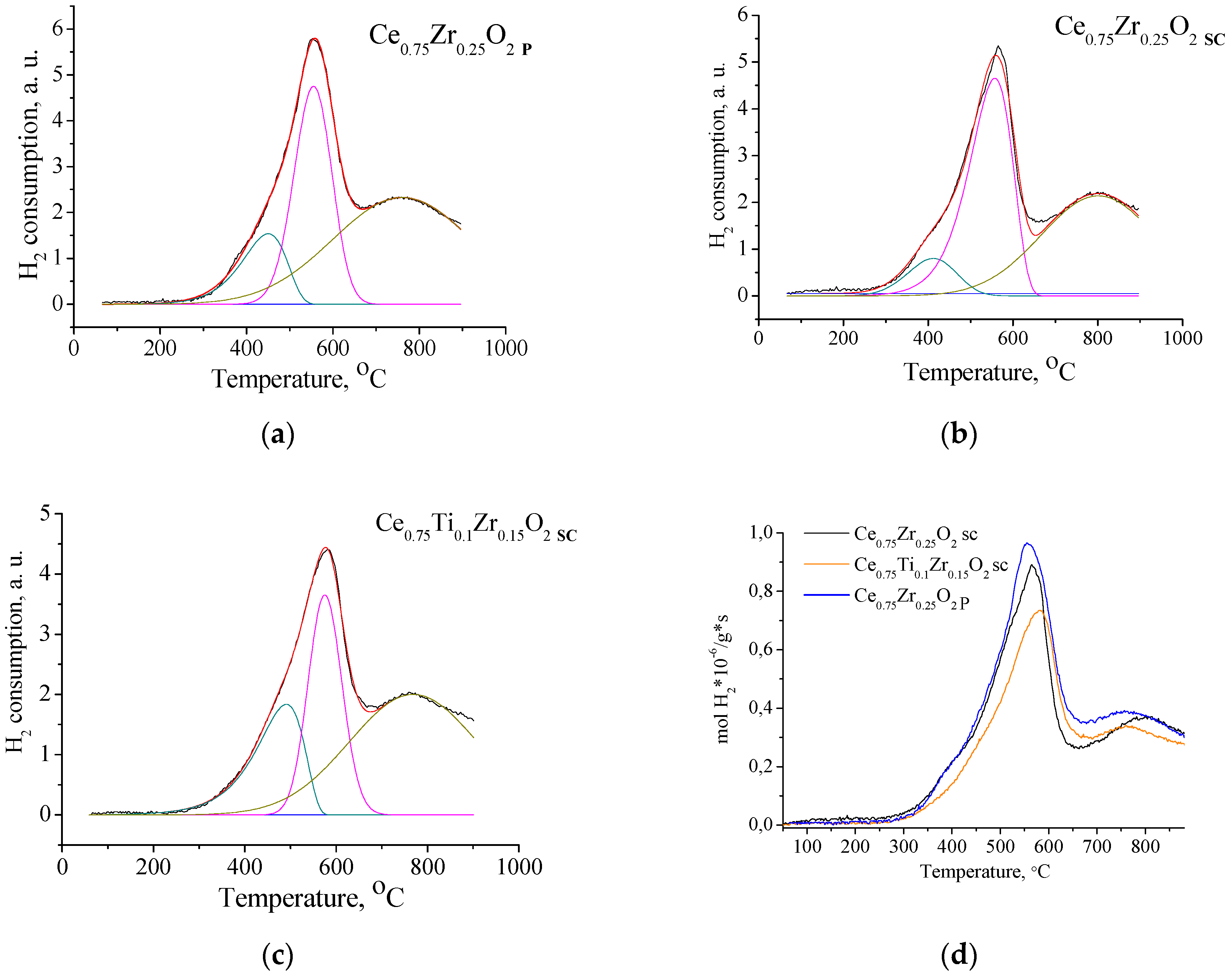
3.4. H2-TPR Characterization
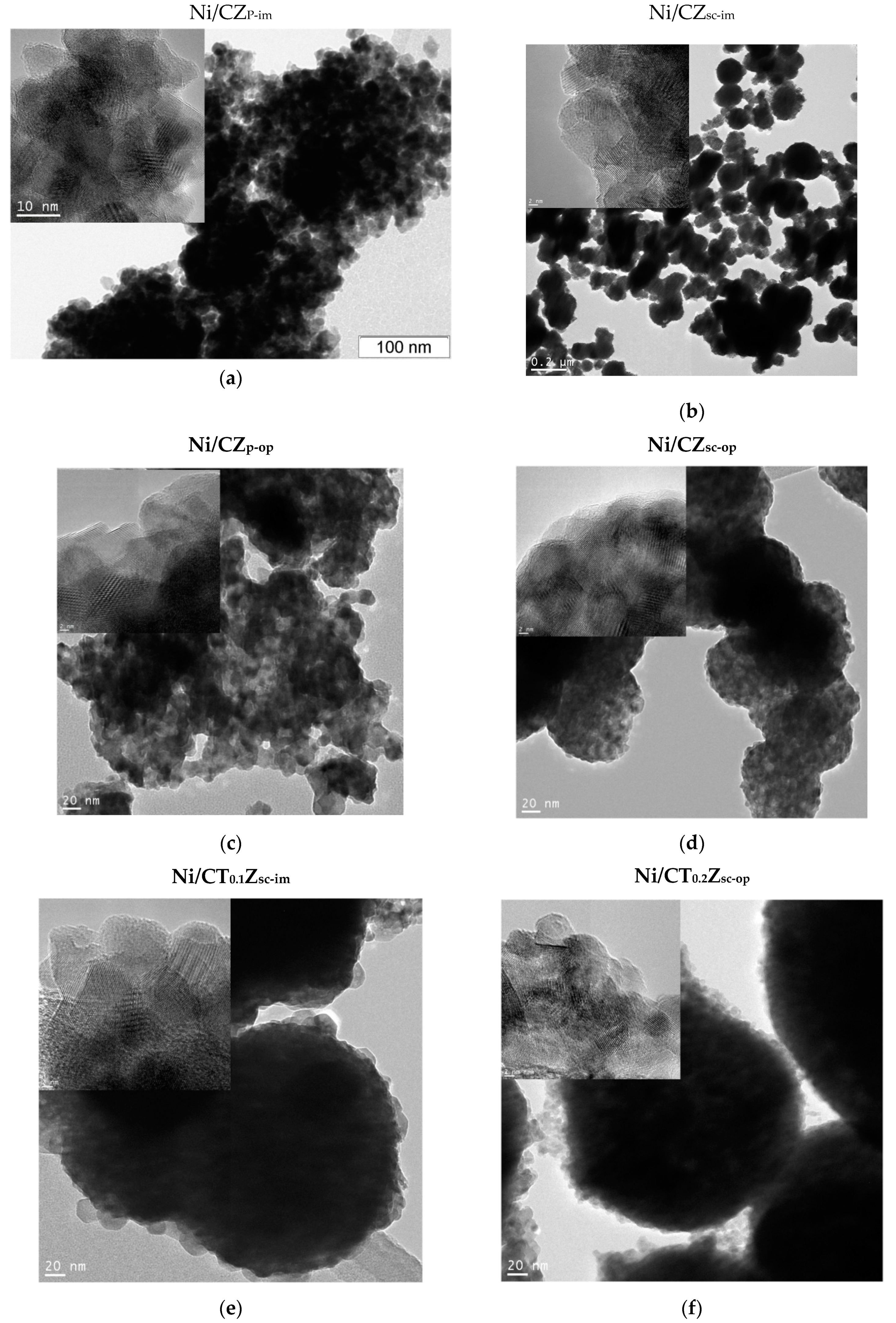
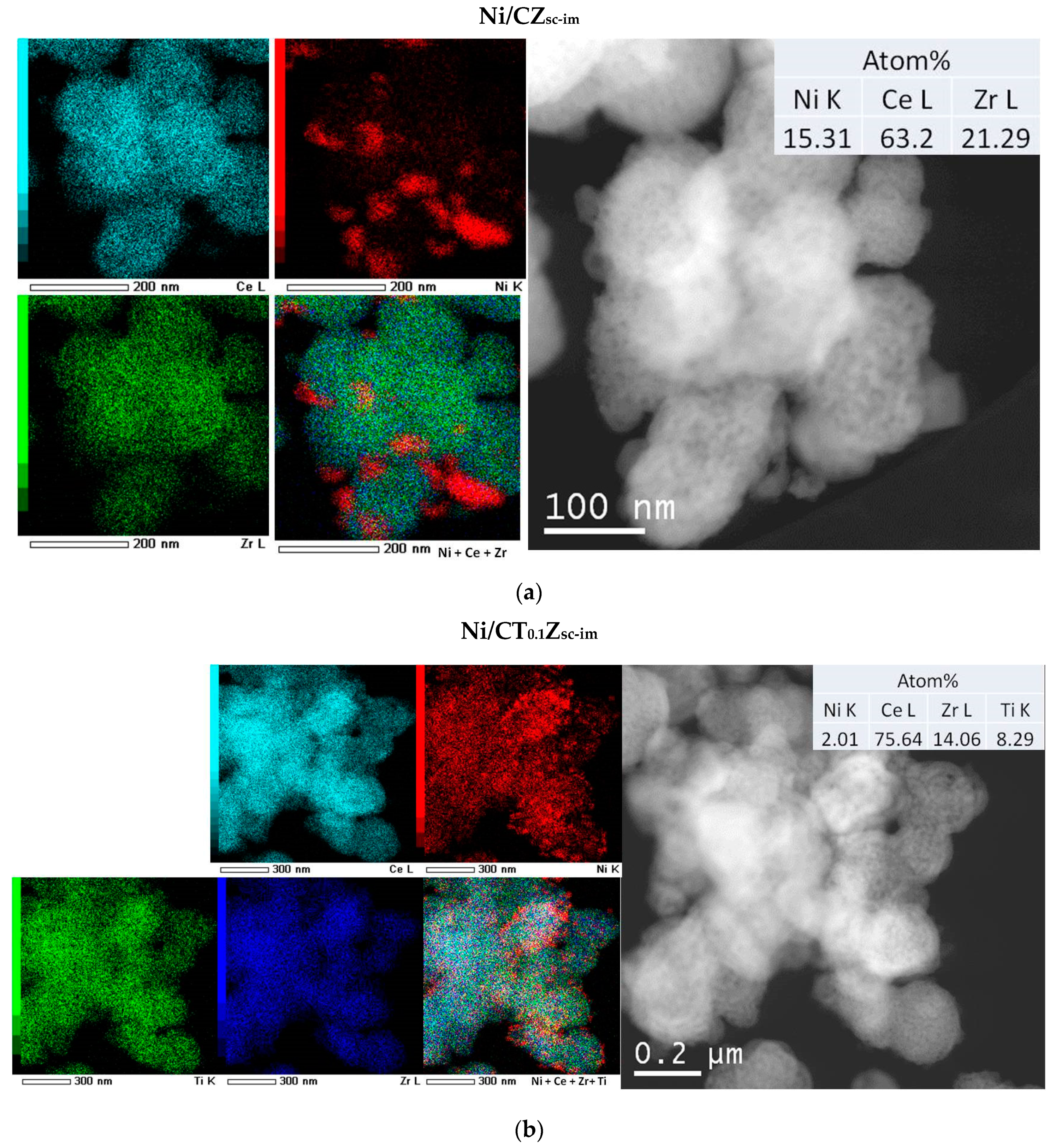
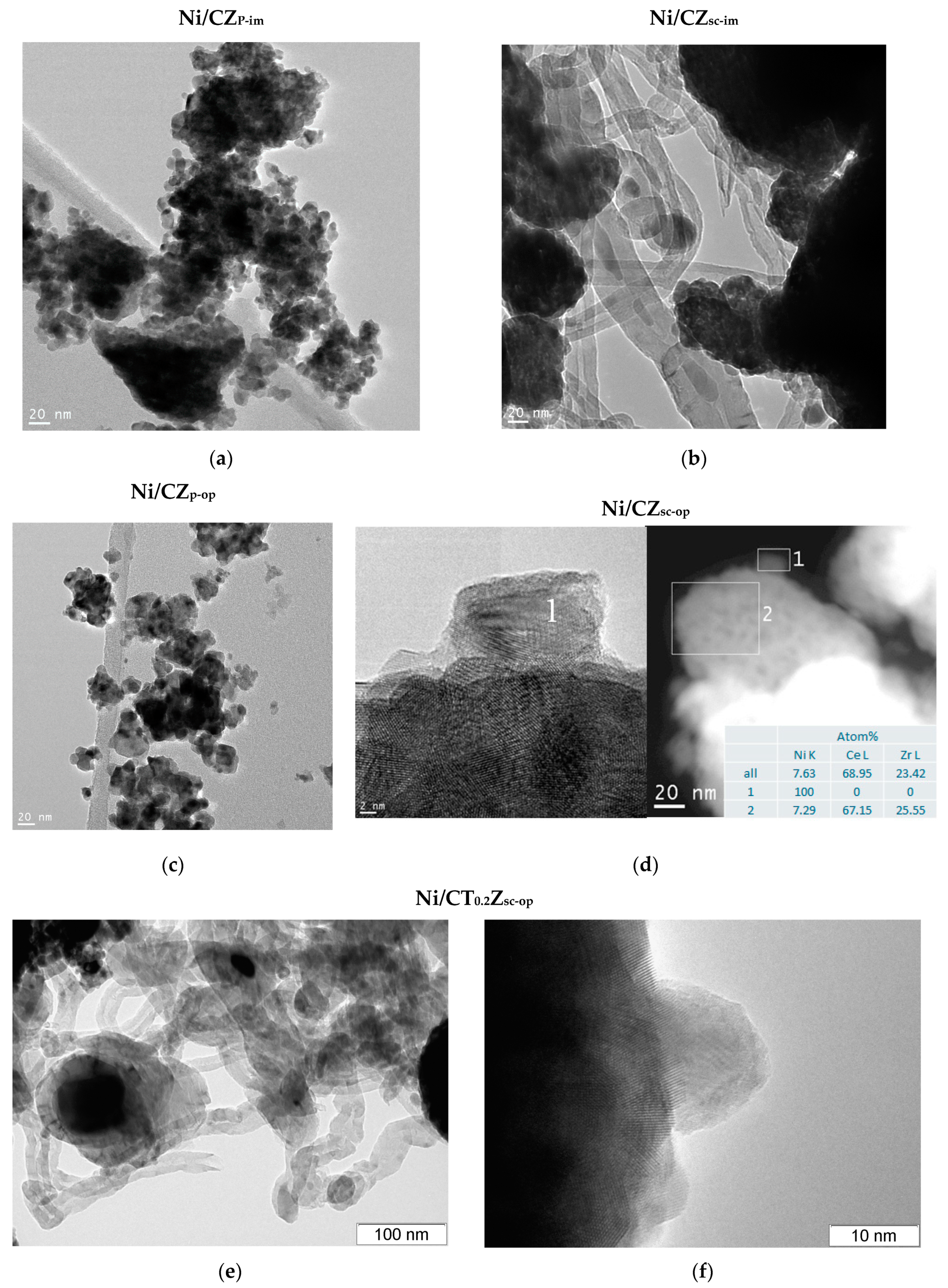
3.5. Morphology of Ceria–Zirconia Mixed Oxides
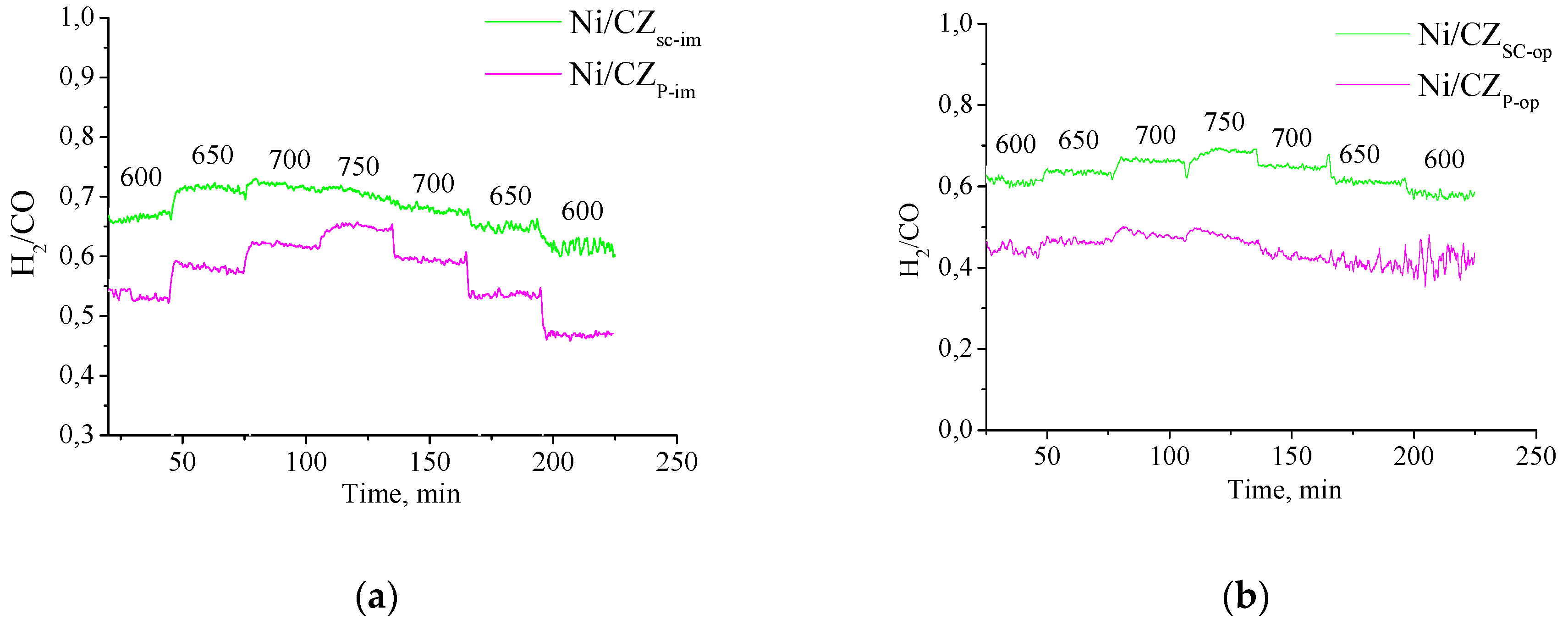
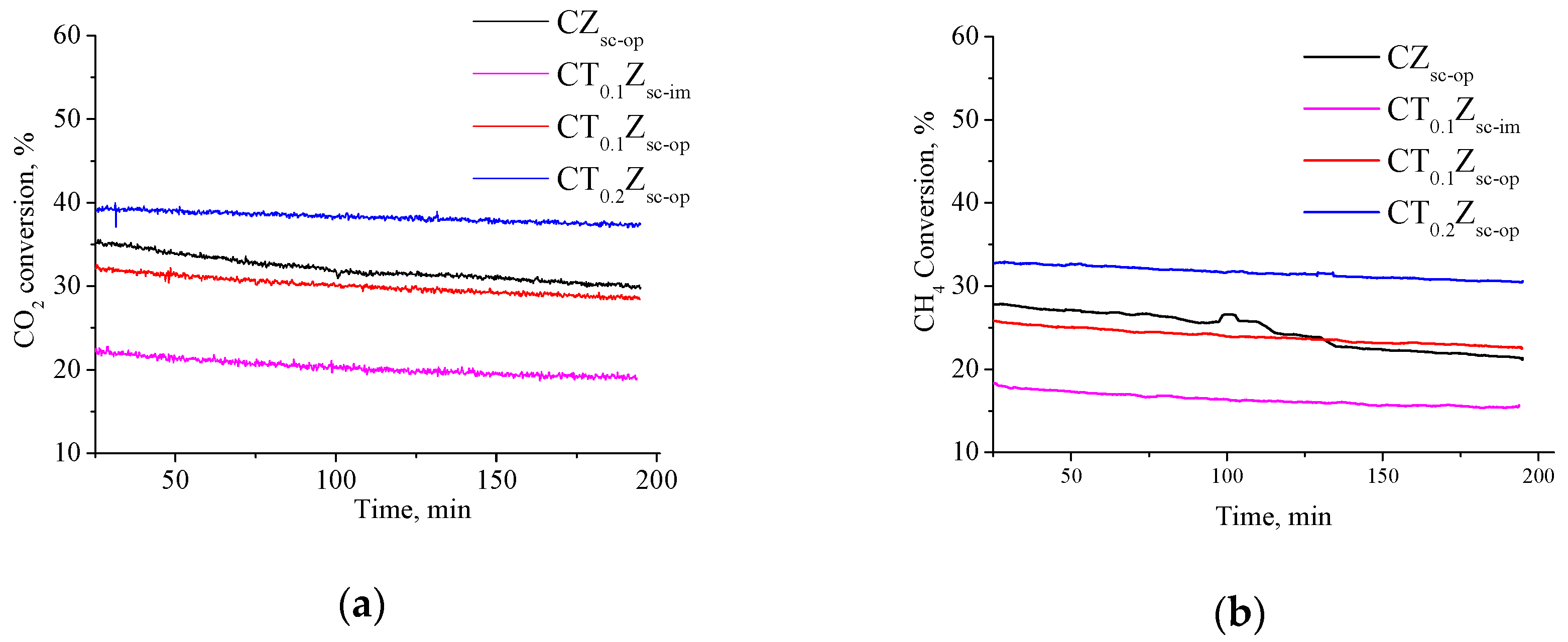
3.6. Methane Dry Reforming Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Usman, M.; Daud, W.W.; Abbas, H.F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A review of CH4CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E.; Kesavan, J.K.; Kumar, S.S.; Selvakumar, K. Dry reforming of methane over Ni supported on Odoped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Takano, A.; Tagawa, T.; Goto, S. Carbon Dioxide Reforming of Methane on Supported Nickel Catalysts. J. Chem. Eng. Jpn. 1994, 27, 727–731. [Google Scholar] [CrossRef] [Green Version]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, S.M.; Won, J.M.; Yang, H.J.; Hong, S.C. Effect of Ce/Ti ratio on the catalytic activity and stability of Ni/CeO2-TiO2 catalyst for dry reforming of methane. Chem. Eng. J. 2015, 280, 433–440. [Google Scholar] [CrossRef]
- Swaan, H.; Kroll, V.; Martin, G.; Mirodatos, C. Deactivation of supported nickel catalysts during the reforming of methane by carbon dioxide. Catal. Today 1994, 21, 571–578. [Google Scholar] [CrossRef]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni-alumina aerogel catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalysis by Ceria and Related Materials, 2nd ed.; Università di Udine: Udine, Italy, 2002; Volume 2, p. 598. [Google Scholar]
- Bonura, G.; Cannilla, C.; Frusteri, F. Ceria-gadolinia supported NiCu catalyst: A suitable system for dry reforming of biogas to feed a solid oxide fuel cell (SOFC). Appl. Catal. B Environ. 2012, 121, 135–147. [Google Scholar] [CrossRef]
- Di Monte, R.; Kašpar, J. Nanostructured CeO2-ZrO2 mixed oxides. J. Mater. Chem. 2005, 15, 633–648. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Comparative Study of Ni-based Mixed Oxide Catalyst for Carbon Dioxide Reforming of Methane. Energy Fuels 2008, 22, 3575–3582. [Google Scholar] [CrossRef]
- Efstathiou, A.M.; Christou, S.Y.; Trovarelli, A.; Fornasiero, P. Investigation of the oxygen storage and release kinetics of model and commercial three-way catalytic materials by transient techniques. Catal. Sci. Ser. 2013, 12, 139–221. [Google Scholar]
- Yang, Z.; Wei, Y.; Fu, Z.; Lu, Z.; Hermansson, K. Facilitated vacancy formation at Zr-doped ceria(111) surfaces. Surf. Sci. 2008, 602, 1199–1206. [Google Scholar] [CrossRef]
- Mamontov, E.; Egami, T.; Brezny, R.; Koranne, M.; Tyagi, S. Lattice Defects and Oxygen Storage Capacity of Nanocrystalline Ceria and Ceria-Zirconia. J. Phys. Chem. B 2000, 104, 11110–11116. [Google Scholar] [CrossRef]
- Fornasiero, P.; Dimonte, R.; Rao, G.; Kaspar, J.; Meriani, S.; Trovarelli, A.; Graziani, M. Rh-Loaded CeO2-ZrO2 Solid-Solutions as Highly Efficient Oxygen Exchangers: Dependence of the Reduction Behavior and the Oxygen Storage Capacity on the Structural-Properties. J. Catal. 1995, 151, 168–177. [Google Scholar] [CrossRef]
- Zamar, F.; Trovarelli, A.; De Leitenburg, C.; Dolcetti, G. The direct room-temperature synthesis of CeO2-based solid solutions: A novel route to catalysts with a high oxygen storage/transport capacity. Stud. Surf. Sci. Catal. 1996, 101, 1283–1292. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Methane steam reforming over Ni/Ce-ZrO2 catalyst: Influences of Ce-ZrO2 support on reactivity, resistance toward carbon formation, and intrinsic reaction kinetics. Appl. Catal. A Gen. 2005, 290, 200–211. [Google Scholar] [CrossRef]
- Khan, A.; Sukonket, T.; Saha, B.; Idem, R. Catalytic Activity of Various 5 wt % Ni/Ce0.5Zr0.33M0.17O2−δ Catalysts for the CO2 Reforming of CH4 in the Presence and Absence of Steam. Energy Fuels 2011, 26, 365–379. [Google Scholar] [CrossRef]
- Dutta, G.; Waghmare, U.V.; Baidya, T.; Hegde, M.S.; Priolkar, K.R.; Sarode, P.R. Origin of Enhanced Reducibility/Oxygen Storage Capacity of Ce1−xTixO2 Compared to CeO2 or TiO2. Chem. Mater. 2006, 18, 3249–3256. [Google Scholar] [CrossRef]
- Luo, M.; Chen, J.; Chen, L.; Lu, J.; Feng, Z.; Li, C. Structure and Redox Properties of CexTi1−xO2 Solid Solution. Chem. Mater. 2001, 13, 197–202. [Google Scholar] [CrossRef]
- Lu, F.; Jiang, B.-B.; Wang, J.; Huang, Z.; Liao, Z.; Yang, Y.; Zheng, J. Promotional effect of Ti doping on the ketonization of acetic acid over a CeO2 catalyst. RSC Adv. 2017, 7, 22017–22026. [Google Scholar] [CrossRef]
- Yashima, M.; Morimoto, K.; Ishizawa, N.; Yoshimura, M. Zirconia-Ceria Solid Solution Synthesis and the Temperature-Time-Transformation Diagram for the 1:1 Composition. J. Am. Ceram. Soc. 1993, 76, 1745–1750. [Google Scholar] [CrossRef]
- Mastelaro, V.R.; Briois, V.; De Souza, D.P.; Silva, C.L. Structural studies of a ZrO2-CeO2 doped system. J. Eur. Ceram. Soc. 2003, 23, 273–282. [Google Scholar] [CrossRef]
- Li, G.; Wang, Q.; Zhao, B.; Shen, M.; Zhou, R. Effect of iron doping into CeO2-ZrO2 on the properties and catalytic behaviour of Pd-only three-way catalyst for automotive emission control. J. Hazard. Mater. 2011, 186, 911–920. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Q.; Li, G.; Zhou, R. Effect of rare earth (La, Nd, Pr, Sm and Y) on the performance of Pd/Ce0.67Zr0.33MO2−δ three-way catalysts. J. Environ. Chem. Eng. 2013, 1, 534–543. [Google Scholar] [CrossRef]
- Le Gal, A.; Abanades, S.; Flamant, G. CO2 and H2O Splitting for Thermochemical Production of Solar Fuels Using Nonstoichiometric Ceria and Ceria/Zirconia Solid Solutions. Energy Fuels 2011, 25, 4836–4845. [Google Scholar] [CrossRef]
- Fang, J.; Bao, H.; He, B.; Wang, F.; Si, D.; Jiang, Z.; Pan, Z.; Wei, S.; Huang, W. Interfacial and Surface Structures of CeO2-TiO2 Mixed Oxides. J. Phys. Chem. C 2007, 111, 19078–19085. [Google Scholar] [CrossRef]
- Masui, T.; Fujiwara, K.; Peng, Y.; Sakata, T.; Machida, K.-I.; Mori, H.; Adachi, G.-Y. Characterization and catalytic properties of CeO2-ZrO2 ultrafine particles prepared by the microemulsion method. J. Alloy. Compd. 1998, 269, 116–122. [Google Scholar] [CrossRef]
- Hadi, A.; Ismail, K.N.; Abu, M.N. Effect of Metals Oxides Loading on the Modification of Microstructure and Phase Transformation of Nanocrystalline CeZrO2 Synthesized Using Water-in-oil-Microemulsion. Procedia Soc. Behav. Sci. 2015, 195, 2051–2060. [Google Scholar] [CrossRef] [Green Version]
- Enzo, S.; Frattini, R.; Delogu, F.; Primavera, A.; Trovarelli, A. Neutron diffraction studies of ceria-zirconia catalysts prepared by high-energy mechanical milling. Nanostruct. Mater. 1999, 12, 673–676. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, M.; Yang, D.; Wu, K. Mechanical alloying of ceramics in zirconia-ceria system. Mater. Sci. Eng. A 1994, 183, L9–L12. [Google Scholar] [CrossRef]
- Inagaki, M.; Kato, E. Hydrothermal Synthesis and Sintering of Fine Powders in CeO2-ZrO2 System. J. Ceram. Soc. Jpn. 1996, 104, 958–962. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Dosaka, K.; Yoshioka, T.; Okuwaki, A.; Torii, K.; Onodera, Y. Sintering of Ceria-Doped Tetragonal Zirconia Crystallized in Organic Solvents, Water, and Air. J. Am. Ceram. Soc. 1992, 75, 552–556. [Google Scholar] [CrossRef]
- Tyrsted, C.; Becker, J.; Hald, P.; Bremholm, M.; Pedersen, J.S.; Chevallier, J.; Cerenius, Y.; Iversen, S.B.; Iversen, B.B. In-Situ Synchrotron Radiation Study of Formation and Growth of Crystalline CexZr1−xO2 Nanoparticles Synthesized in Supercritical Water. Chem. Mater. 2010, 22, 1814–1820. [Google Scholar] [CrossRef]
- Kim, J.-R.; Lee, K.-Y.; Suh, M.-J.; Ihm, S.-K. Ceria–zirconia mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as catalyst support. Catal. Today 2012, 185, 25–34. [Google Scholar] [CrossRef]
- Slostowski, C.; Marre, S.; Babot, O.; Toupance, T.; Aymonier, C. Near- and Supercritical Alcohols as Solvents and Surface Modifiers for the Continuous Synthesis of Cerium Oxide Nanoparticles. Langmuir 2012, 28, 16656–16663. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, E.K.; Habu, T.; Tooriyama, H.; Ohtani, M.; Kobiro, K. Ultra-simple synthetic approach to the fabrication of CeO2-ZrO2 mixed nanoparticles into homogeneous, domain, and core-Shell structures in mesoporous spherical morphologies using supercritical alcohols. J. Supercrit. Fluids 2015, 97, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, M.Y.; Pavlova, S.N.; Krieger, T.A.; Bespalko, Y.N.; Anikeev, V.I.; Chesalov, Y.; Kaichev, V.; Mezentseva, N.V.; Sadykov, V. The Synthesis of Ce1−xZrxO2 Oxides in Supercritical Alcohols and Catalysts for Carbon Dioxide Reforming of Methane on Their Basis. Russ. J. Phys. Chem. B 2017, 11, 1312–1321. [Google Scholar] [CrossRef]
- Adamski, A.; Legutko, P.; Dziadek, K.; Parkhomenko, K.; Aymonier, C.; Sadykov, V.A.; Roger, A.-C. Role of CeO2-ZrO2 Support for Structural, Textural and Functional Properties of Ni-based Catalysts Active in Dry Reforming of Methane. E3S Web Conf. 2019, 108, 02018. [Google Scholar] [CrossRef]
- Sukonket, T.; Khan, A.; Saha, B.; Ibrahim, H.; Tantayanon, S.; Kumar, P.; Idem, R. Influence of the catalyst preparation method, surfactant amount and steam on CO2 reforming of CH4 over 5Ni/Ce0.6Zr0.4O2 Catalysts. Energy Fuels 2011, 25, 864–877. [Google Scholar] [CrossRef]
- Roh, H.-S.; Potdar, H.; Jun, K.-W.; Kim, J.-W.; Oh, Y.-S. Carbon dioxide reforming of methane over Ni incorporated into Ce-ZrO2 catalysts. Appl. Catal. A Gen. 2004, 276, 231–239. [Google Scholar] [CrossRef]
- Marinho, A.L.; Rabelo-Neto, R.C.; Epron, F.; Bion, N.; Toniolo, F.S.; Noronha, F.B. Embedded Ni nanoparticles in CeZrO2 as stable catalyst for dry reforming of methane. Appl. Catal. B Environ. 2020, 268, 118387. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Faga, M.G.; Miletto, I.; Coluccia, S.; Caputo, G.; Sau, S.; Giaconia, A.; Berlier, G. Unravelling the structure and reactivity of supported Ni particles in Ni-CeZrO2 catalysts. Appl. Catal. B Environ. 2013, 138, 353–361. [Google Scholar] [CrossRef]
- Zagaynov, I.; Loktev, A.S.; Arashanova, A.; Ivanov, V.; Dedov, A.; Moiseev, I. Ni(Co)-Gd0.1Ti0.1Zr0.1Ce0.7O2 mesoporous materials in partial oxidation and dry reforming of methane into synthesis gas. Chem. Eng. J. 2016, 290, 193–200. [Google Scholar] [CrossRef]
- Escribano, V.S.; Lopez, E.F.; Panizza, M.; Resini, C.; Amores, J.M.G.; Busca, G. Characterization of cubic ceria–zirconia powders by X-ray diffraction and vibrational and electronic spectroscopy. Solid State Sci. 2003, 5, 1369–1376. [Google Scholar] [CrossRef]
- Romero-Núñez, A.; Díaz, G. High oxygen storage capacity and enhanced catalytic performance of NiO/NixCe1−xO2−δ nanorods: Synergy between Ni-doping and 1D morphology. RSC Adv. 2015, 5, 54571–54579. [Google Scholar] [CrossRef]
- Li, L.; Chen, F.; Lu, J.-Q.; Luo, M.-F. Study of Defect Sites in Ce1−xMxO2−δ (x = 0.2) Solid Solutions Using Raman Spectroscopy. J. Phys. Chem. A 2011, 115, 7972–7977. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chu, W.; Wang, B.; Yang, W.; Zhao, X.S. Mesoporous Ni/Ce1−xNixO2−y heterostructure as an efficient catalyst for converting greenhouse gas to H2 and syngas. Catal. Sci. Technol. 2016, 6, 851–862. [Google Scholar] [CrossRef]
- Pu, Z.-Y.; Lu, J.-Q.; Luo, M.-F.; Xie, Y.-L. Study of Oxygen Vacancies in Ce0.9Pr0.1O2−δ Solid Solution by in Situ X-ray Diffraction and in Situ Raman Spectroscopy. J. Phys. Chem. C 2007, 111, 18695–18702. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Ce3+ and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles. Mater. Chem. Phys. 2012, 131, 666–671. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, N.; Wei, S.; Zhang, Y. Determination of active site densities and mechanisms for soot combustion with O2 on Fe-doped CeO2 mixed oxides. J. Catal. 2010, 276, 16–23. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Kim, J.-R.; Myeong, W.-J.; Ihm, S.-K. Characteristics in oxygen storage capacity of ceria-zirconia mixed oxides prepared by continuous hydrothermal synthesis in supercritical water. Appl. Catal. B Environ. 2007, 71, 57–63. [Google Scholar] [CrossRef]
- Roh, H.-S.; Koo, K.Y.; Yoon, W.L. Combined reforming of methane over co-precipitated Ni-CeO2, Ni-ZrO2 and Ni-Ce0.8Zr0.2O2 catalysts to produce synthesis gas for gas to liquid (GTL) process. Catal. Today 2009, 146, 71–75. [Google Scholar] [CrossRef]
- Slostowski, C.; Marre, S.; Babot, O.; Toupance, T.; Aymonier, C. Effect of Thermal Treatment on the Textural Properties of CeO2 Powders Synthesized in Near- and Supercritical Alcohols. ChemPhysChem 2015, 16, 3493–3499. [Google Scholar] [CrossRef]
- Alberton, A.L.; Souza, M.M.V.M.; Schmal, M. Carbon formation and its influence on ethanol steam reforming over Ni/Al2O3 catalysts. Catal. Today 2007, 123, 257–264. [Google Scholar] [CrossRef]







| Code | Method of Synthesis | Composition | Lattice Parameter, Å | Crystallite Size, nm | |
|---|---|---|---|---|---|
| Fluorite Phase | Oxide | NiO | |||
| CZp | Pechini | Ce0.75Zr0.25O2−δ | 5.356 (1) | 10.4 | - |
| Ni/CZP-im | Pechini; impregnated | Ni/Ce0.75Zr0.25O2-δ | 5.357 (1) | 10.7 | 18 |
| Ni/CZP-op | Pechini; «one-pot» | Ni/Ce0.75Zr0.25O2-δ | 5.358 (1) | 11.7 | 70 |
| CZsc | SCS | Ce0.75Zr0.25O2-δ | 5.368 (1) | 8.8 | - |
| Ni/CZsc-im | SCS; impregnated | Ni/Ce0.75Zr0.25O2-δ | 5.368 (1) | 9.4 | 30 |
| Ni/CZsc-op | SCS; «one-pot» | Ni/Ce0.75Zr0.25O2-δ | 5.371 (1) | 8.9 | 19 |
| CT0.1Zsc | SCS; | Ce0.75Ti0.1Zr0.15O2-δ | 5.378 (1) | 10.0 | - |
| CT0.2Zsc | SCS; | Ce0.75Ti0.2Zr0.05O2-δ | 5.393 (1) | 9.0 | - |
| Ni/CT0.1Zsc-im | SCS; impregnated | Ni/Ce0.75Ti0.1Zr0.15O2-δ | 5.379 (1) | 10.4 | 20 |
| Ni/CT0.1Zsc-op | SCS; «one-pot» | Ni/Ce0.75Ti0.1Zr0.15O2-δ | 5.376 (1) | 8.5 | 13 |
| Ni/CT0.2Zsc-op | SCS; «one-pot» | Ni/Ce0.75Ti0.2Zr0.05O2-δ | 5.393 (1) | 8.5 | 15 |
| Code | SSA,m2/g | Vtotal, cm3/g | T max of Peaks, °C/ H2 Consumption, mmol H2 × 10−6 gcomp−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | Total | |||
| CZp | 44 | 0.159 | - | - | 450/ 209 | 555/ 519 | 761/ 752 | - | - | 1484 |
| Ni/CZP-im | 35 | 0.143 | 240/ 143 | 308/ 401 | 449/ 757 | 511/ 199 | 765/ 660 | - | - | 2166 |
| Ni/CZp-op | 14 | 0.160 | 281/ 122 | 341/ 281 | 395/ 80 | 410/ 498 | 579/ 463 | 840/ 421 | - | 1873 |
| CZsc | 21 | 0.150 | - | - | 412/ 116 | 557/ 616 | 801/ 724 | - | - | 1344 |
| Ni/CZsc-im | 20 | 0.144 | 238/ 145 | 313/ 402 | 380/ 143 | 451/ 605 | 557/ 371 | 816/ 703 | - | 2192 |
| Ni/CZsc-op | 13 | 0.126 | 255/ 347 | 294/ 119 | 350/ 249 | 475/ 748 | 764/ 572 | - | - | 2016 |
| CT0.1Zsc | 34 | 0.165 | - | - | 491/ 256 | 575/ 338 | 769/ 581 | - | - | 1185 |
| Ni/CT0.1Zsc-im | 23 | 0.184 | 245/ 131 | 339/ 429 | 416/ 95 | 505/ 662 | 588/ 277 | 644/ 77 | 812/ 619 | 2306 |
| Ni/CT0.1Zsc-op | 16 | 0.143 | 257/ 262 | 319/ 108 | 340/ 314 | 413/ 328 | 532/ 762 | 725/ 613 | - | 2396 |
| Ni/CT0.2Zsc-op | 11 | 0.102 | - | 291/ 392 | 353/ 128 | 461/ 1153 | 587/ 384 | 756/ 641 | - | 2700 |
| Code | T, °C | X(CH4), % | X(CO2), % | S(CO), % | Concentration CO, *10−3 mol/L | S (H2), % | Concentration H2, *10−3 mol/L | H2/CO |
|---|---|---|---|---|---|---|---|---|
| Ni/CZp-im | 700 750 | 33 48 | 40 55 | 73 75 | 3.9 5.4 | 56 44 | 2.3 3.5 | 0.59 0.54 |
| Ni/CZp-op | 700 750 | 9 16 | 13 23 | 65 69 | 1.1 2.1 | 44 62 | 0.5 1.1 | 0.42 0.46 |
| Ni/CZsc-im | 700 750 | 29 39 | 32 42 | 75 76 | 3.1 4.2 | 62 65 | 2.1 3.0 | 0.69 0.71 |
| Ni/CZsc-op | 700 750 | 28 42 | 35 47 | 73 73 | 3.4 4.6 | 65 65 | 2.2 3.2 | 0.65 0.68 |
| Ni/CT0.1Zsc-im | 700 750 | 23 33 | 23 34 | 75 74 | 1.6 2.6 | 32 33 | 1.0 1.7 | 0.65 0.68 |
| Ni/CT0.1Zsc-op | 700 750 | 28 39 | 34 45 | 74 75 | 3.4 4.5 | 35 37 | 1.3 2.0 | 0.40 0.44 |
| Ni/CT0.2Zsc-op | 700 750 | 36 50 | 43 54 | 76 76 | 4.2 5.5 | 48 52 | 2.5 3.6 | 0.58 0.63 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bespalko, Y.; Smal, E.; Simonov, M.; Valeev, K.; Fedorova, V.; Krieger, T.; Cherepanova, S.; Ishchenko, A.; Rogov, V.; Sadykov, V. Novel Ni/Ce(Ti)ZrO2 Catalysts for Methane Dry Reforming Prepared in Supercritical Alcohol Media. Energies 2020, 13, 3365. https://doi.org/10.3390/en13133365
Bespalko Y, Smal E, Simonov M, Valeev K, Fedorova V, Krieger T, Cherepanova S, Ishchenko A, Rogov V, Sadykov V. Novel Ni/Ce(Ti)ZrO2 Catalysts for Methane Dry Reforming Prepared in Supercritical Alcohol Media. Energies. 2020; 13(13):3365. https://doi.org/10.3390/en13133365
Chicago/Turabian StyleBespalko, Yuliya, Ekaterina Smal, Mikhail Simonov, Konstantin Valeev, Valeria Fedorova, Tamara Krieger, Svetlana Cherepanova, Arcady Ishchenko, Vladimir Rogov, and Vladislav Sadykov. 2020. "Novel Ni/Ce(Ti)ZrO2 Catalysts for Methane Dry Reforming Prepared in Supercritical Alcohol Media" Energies 13, no. 13: 3365. https://doi.org/10.3390/en13133365
APA StyleBespalko, Y., Smal, E., Simonov, M., Valeev, K., Fedorova, V., Krieger, T., Cherepanova, S., Ishchenko, A., Rogov, V., & Sadykov, V. (2020). Novel Ni/Ce(Ti)ZrO2 Catalysts for Methane Dry Reforming Prepared in Supercritical Alcohol Media. Energies, 13(13), 3365. https://doi.org/10.3390/en13133365








