Gasification of Shenhua Bituminous Coal with CO2: Effect of Coal Particle Size on Kinetic Behavior and Ash Fusibility
Abstract
1. Introduction
2. Materials and Methods
3. Results
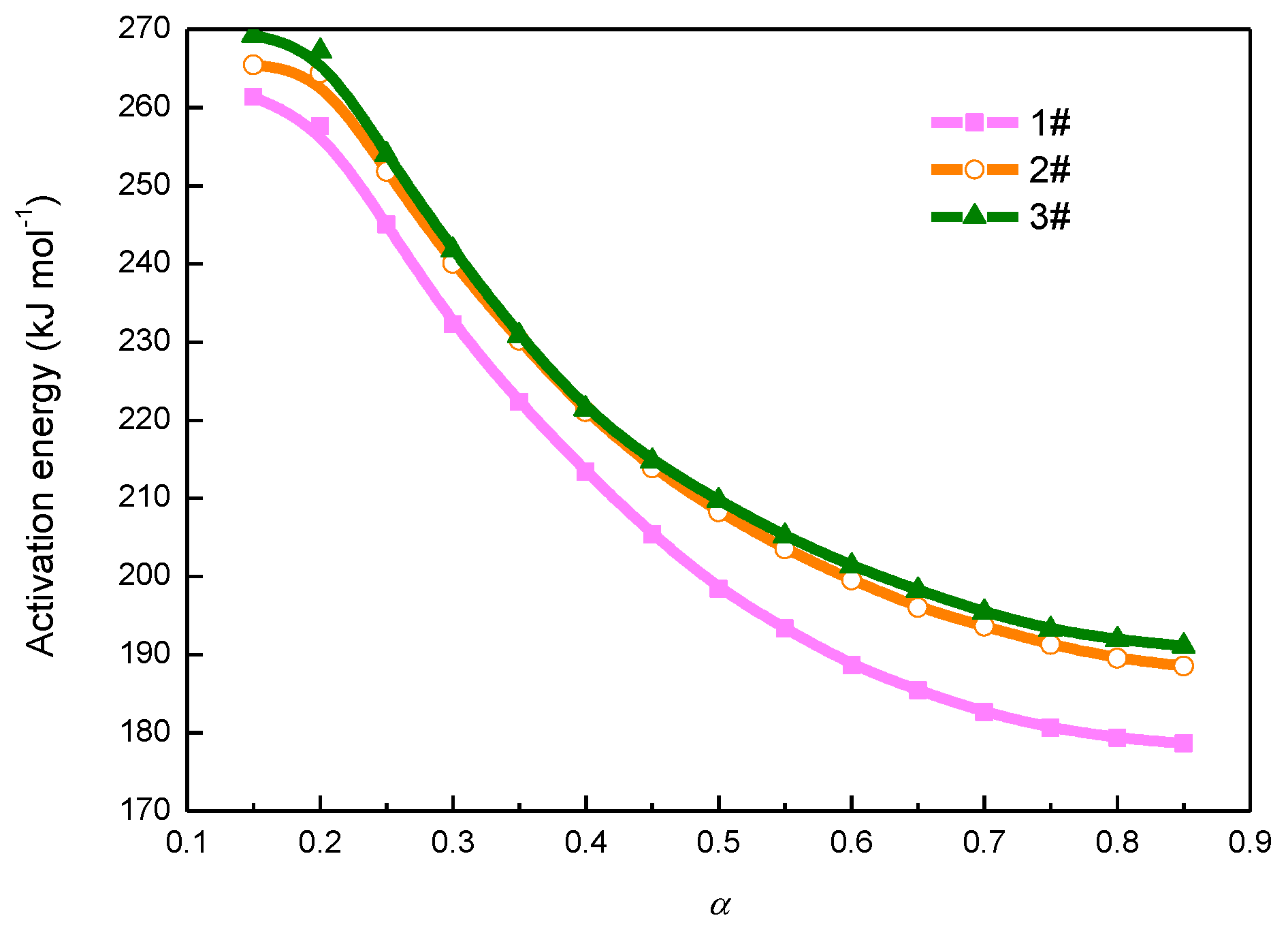
3.1. Kinetic Analysis with Miura Isoconversional Method
3.2. Ash Fusibility of Shenhua Bituminous Coal
3.2.1. Preparation of the Ash
3.2.2. XRF Measurements
3.2.3. Ash Fusion Temperature Test
4. Discussion
4.1. Determination of Coal Particle Size
4.2. Thermal Behavior Based on TG/DTG Curves
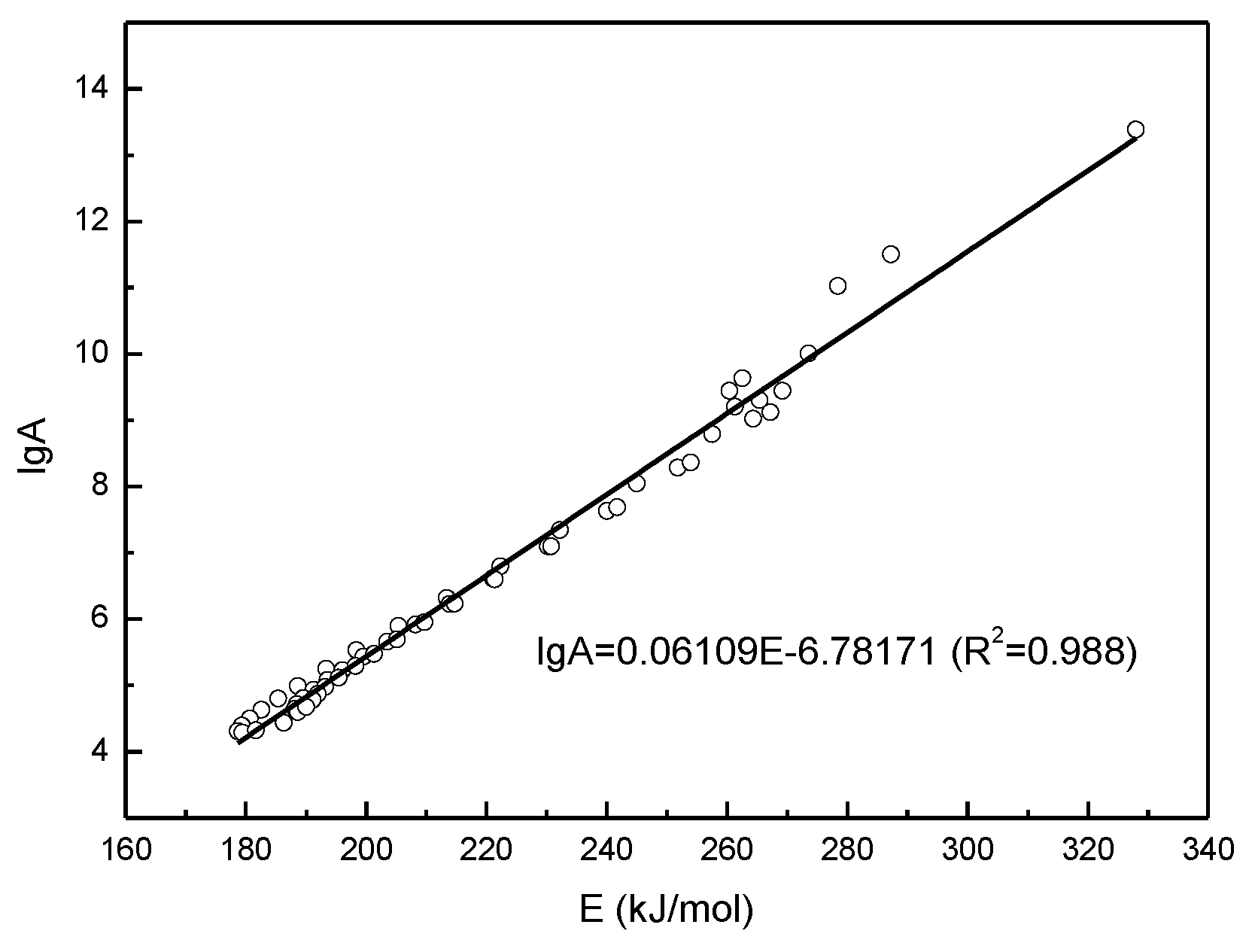
4.3. Kinetic Analysis with Isoconversional Methods
4.4. Ash Fusibility of Coal with Different Particle Size
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tian, B.; Qiao, Y.-Y.; Tian, Y.-Y.; Liu, Q. Investigation on the effect of particle size and heating rate on pyrolysis characteristics of a bituminous coal by TG-FTIR. J. Anal. Appl. Pyrol. 2016, 121, 376–386. [Google Scholar] [CrossRef]
- Domenico, M.D.; Collazzo, G.C.; Pacioni, T.R.; Jose, H.J.; Moreira, R.F.P.M. Gasification of brazilian coal-chars with CO2: Effect of samples’ properties on reactivity and kinetic modeling. Chem. Eng. Commun. 2019, 206, 158–168. [Google Scholar] [CrossRef]
- Labojko, G.; Kotyczka-Moranska, M.; Plis, A.; Sciazko, M. Kinetic study of polish hard coal and its char gasification using carbon dioxide. Thermochim. Acta 2012, 549, 158–165. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, X.; Cheng, S. Kinetic study of CO2 gasification of coal chars. In Advances in Chemical Engineering II, pts 1-4; Liu, Z., Peng, F., Liu, X., Eds.; Trans Tech Publications: Stafa-Zurich, Switzerland, 2012; Volume 550–553, pp. 2754–2757. [Google Scholar]
- Liu, L.; Cao, Y.; Liu, Q.; Yang, J. Experimental and kinetic studies of coal-CO2 gasification in isothermal and pressurized conditions. RSC Adv. 2017, 7, 2193–2201. [Google Scholar]
- Zeng, X.; Zheng, S.; Zhou, H. The phenomena of secondary weight loss in high-temperature coal pyrolysis. Energy Fuels 2017, 31, 10178–10185. [Google Scholar] [CrossRef]
- Li, F.; Yan, Q.; Huang, J.; Zhao, J.; Fang, Y.; Wang, J. Lignite-char gasification mechanism in mixed atmospheres of steam and CO2 at different pressures. Fuel Process. Technol. 2015, 138, 555–563. [Google Scholar] [CrossRef]
- Fu, W.B.; Wang, Q.H. A general relationship between the kinetic parameters for the gasification of coal chars with CO2 and coal type. Fuel Process. Technol. 2001, 72, 63–77. [Google Scholar] [CrossRef]
- Kang, S.-H.; Ryu, J.-H.; Park, S.-N.; Byun, Y.-S.; Seo, S.-J.; Yun, Y. Kinetic studies of pyrolysis and char-CO2 gasification on low rank coals. Korean J. Chem. Eng. 2011, 49, 114–119. [Google Scholar] [CrossRef]
- Hanson, S.; Patrick, J.W.; Walker, A. The effect of coal particle size on pyrolysis and steam gasification. Fuel 2002, 81, 531–537. [Google Scholar] [CrossRef]
- Zhu, W.; Song, W.; Lin, W. Effect of the coal particle size on pyrolysis and char reactivity for two types of coal and demineralized coal. Energy Fuels 2008, 22, 2482–2487. [Google Scholar] [CrossRef]
- Kok, M.V.; Ozbas, E.; Karacan, O.; Hicyilmaz, C. Effect of particle size on coal pyrolysis. J. Anal. Appl. Pyrol. 1998, 45, 103–110. [Google Scholar]
- Morris, R.M. Effect of particle-size and temperature on volatiles produced from coal by slow pyrolysis. Fuel 1990, 69, 776–779. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Shen, J.; Zhang, H. Pyrolysis of superfine pulverized coal. Part 1. Mechanisms of methane formation. Energy Convers. Manag. 2014, 87, 1027–1038. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Shen, J.; Zhang, H. Pyrolysis of superfine pulverized coal. Part 2. Mechanisms of carbon monoxide formation. Energy Convers. Manag. 2014, 87, 1039–1049. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Shen, J.; Zhang, H. Pyrolysis of superfine pulverized coal. Part 3. Mechanisms of nitrogen-containing species formation. Energy Convers. Manag. 2015, 94, 130–138. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Y.; Luo, L.; Ma, J.; Zhang, H.; Jiang, X. Pyrolysis of superfine pulverized coal. Part 4. Evolution of functionalities in chars. Energy Convers. Manag. 2017, 134, 32–46. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Pisupati, S.V. Fate of sulfur during entrained-flow gasification of pittsburgh no. 8 coal: Influence of particle size, sulfur forms, and temperature. Energy Fuels 2016, 30, 3241–3250. [Google Scholar] [CrossRef]
- Jayaraman, K.; Kok, M.V.; Gokalp, I. Pyrolysis, combustion and gasification studies of different sized coal particles using TGA-MS. Appl. Therm. Eng. 2017, 125, 1446–1455. [Google Scholar] [CrossRef]
- Miura, K.; Maki, T. A simple method for estimating f(E) and k0(E) in the distributed activation energy model. Energy Fuels 1998, 12, 864–869. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; Wu, J. Kinetic and energy production analysis of pyrolysis of lignocellulosic biomass using a three-parallel gaussian reaction model. Bioresour. Technol. 2016, 211, 502–508. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Wu, J.; Wu, J. Multi-gaussian-daem-reaction model for thermal decompositions of cellulose, hemicellulose and lignin: Comparison of N2 and CO2 atmosphere. Bioresour. Technol. 2014, 166, 87–95. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Wu, J.; Wu, J. A novel gaussian-daem-reaction model for the pyrolysis of cellulose, hemicellulose and lignin. RSC Adv. 2014, 4, 17513–17520. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Wu, J.; Wu, J. TG-MS analysis and kinetic study for thermal decomposition of six representative components of municipal solid waste under steam atmosphere. Waste Manag. 2015, 43, 152–161. [Google Scholar] [CrossRef]
- Song, H.; Liu, G.; Zhang, J.; Wu, J. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method. Fuel Process. Technol. 2017, 156, 454–460. [Google Scholar] [CrossRef]
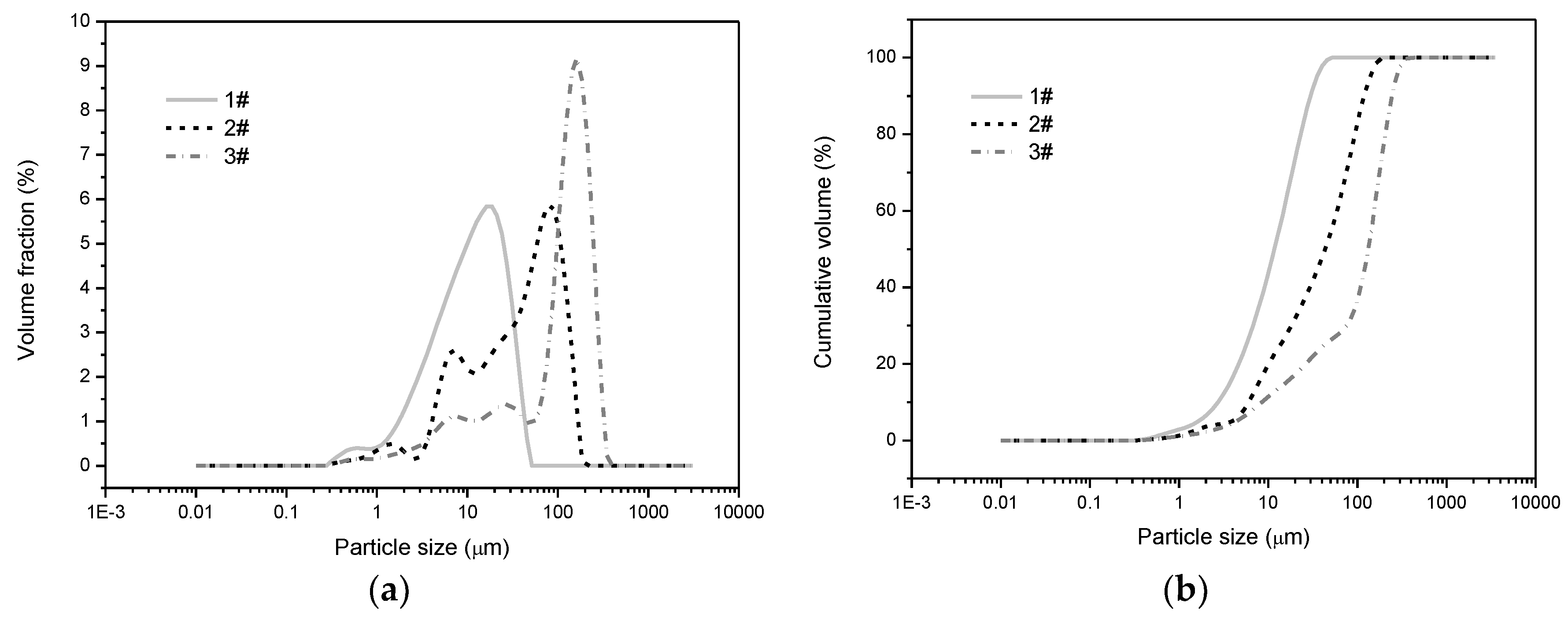

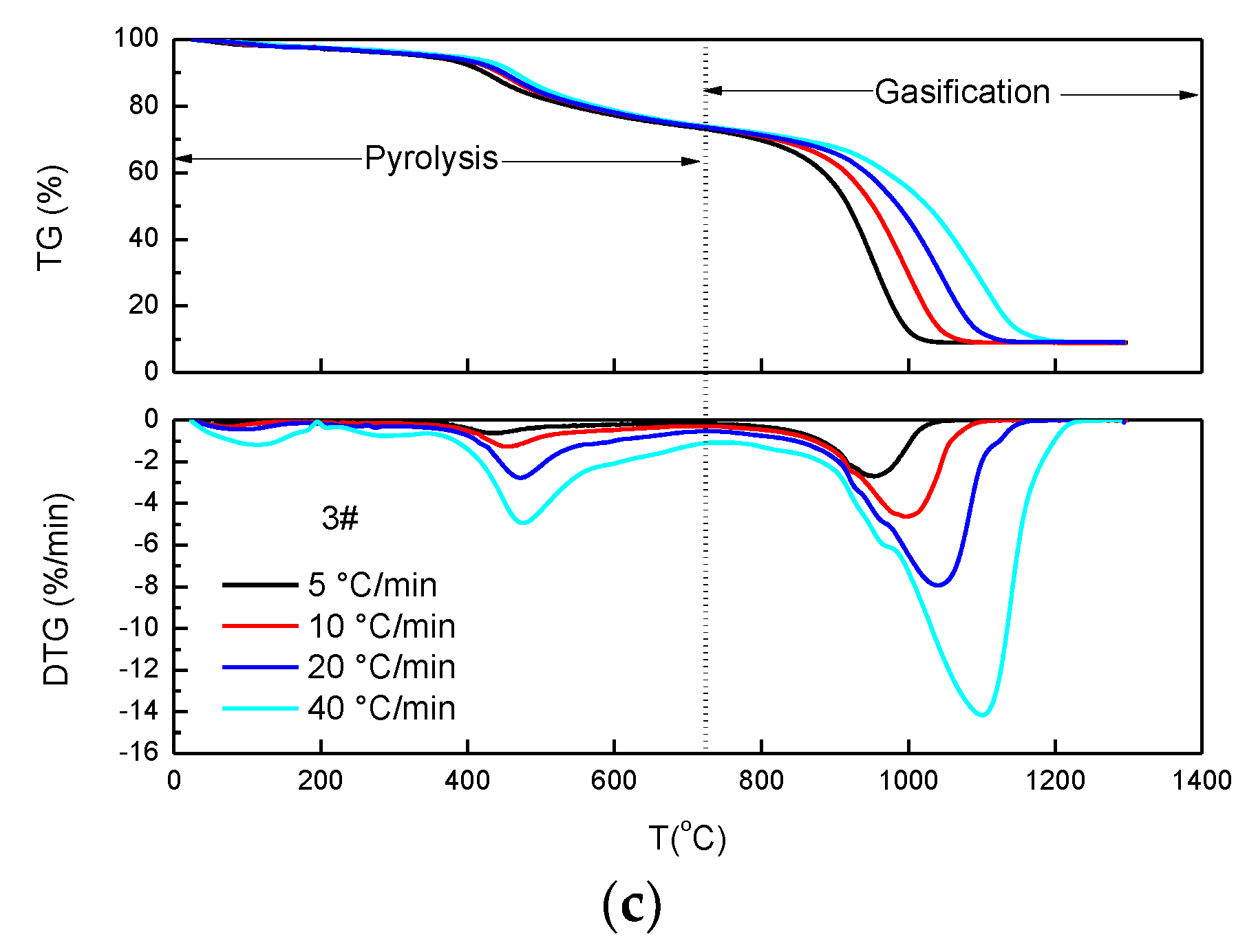
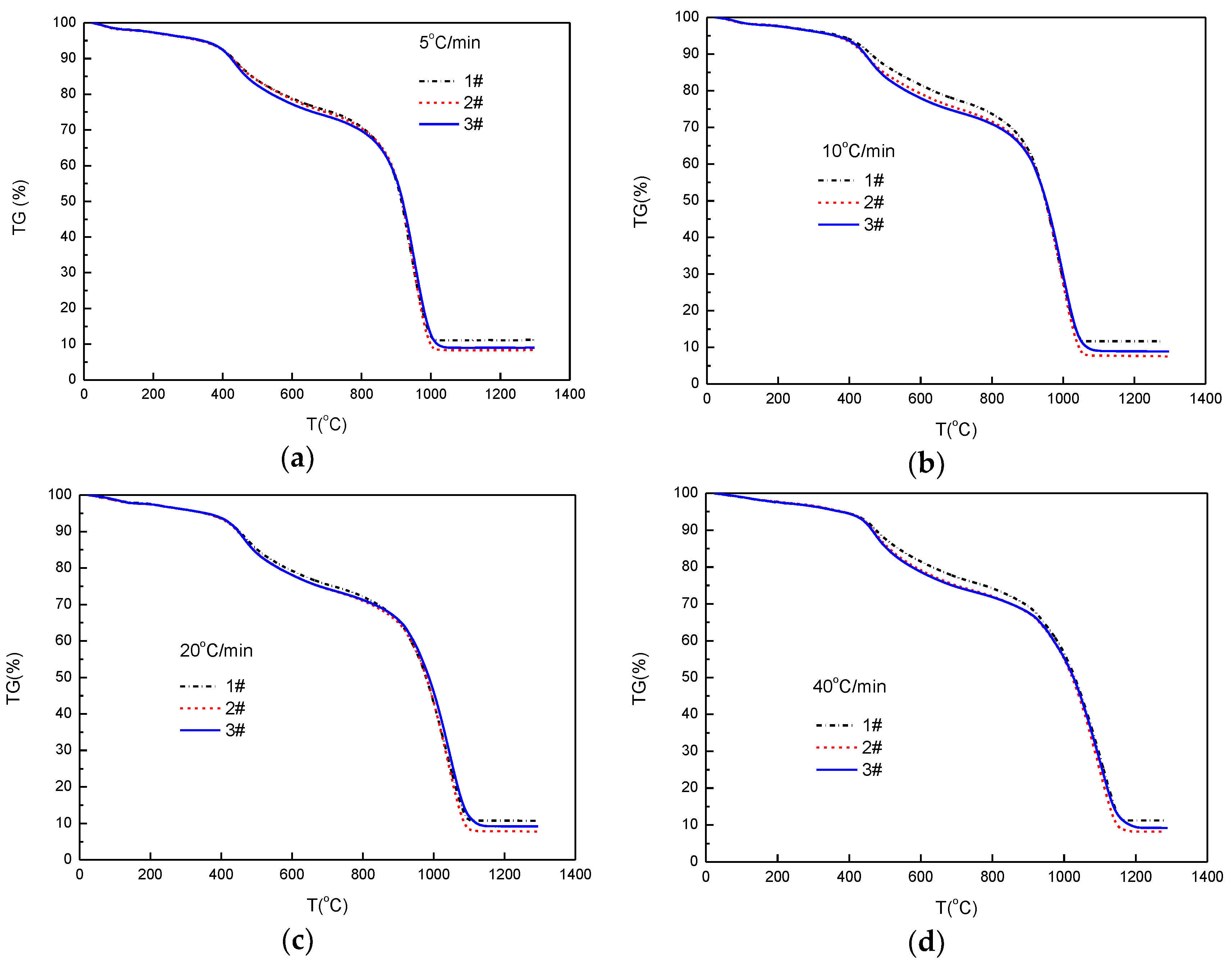

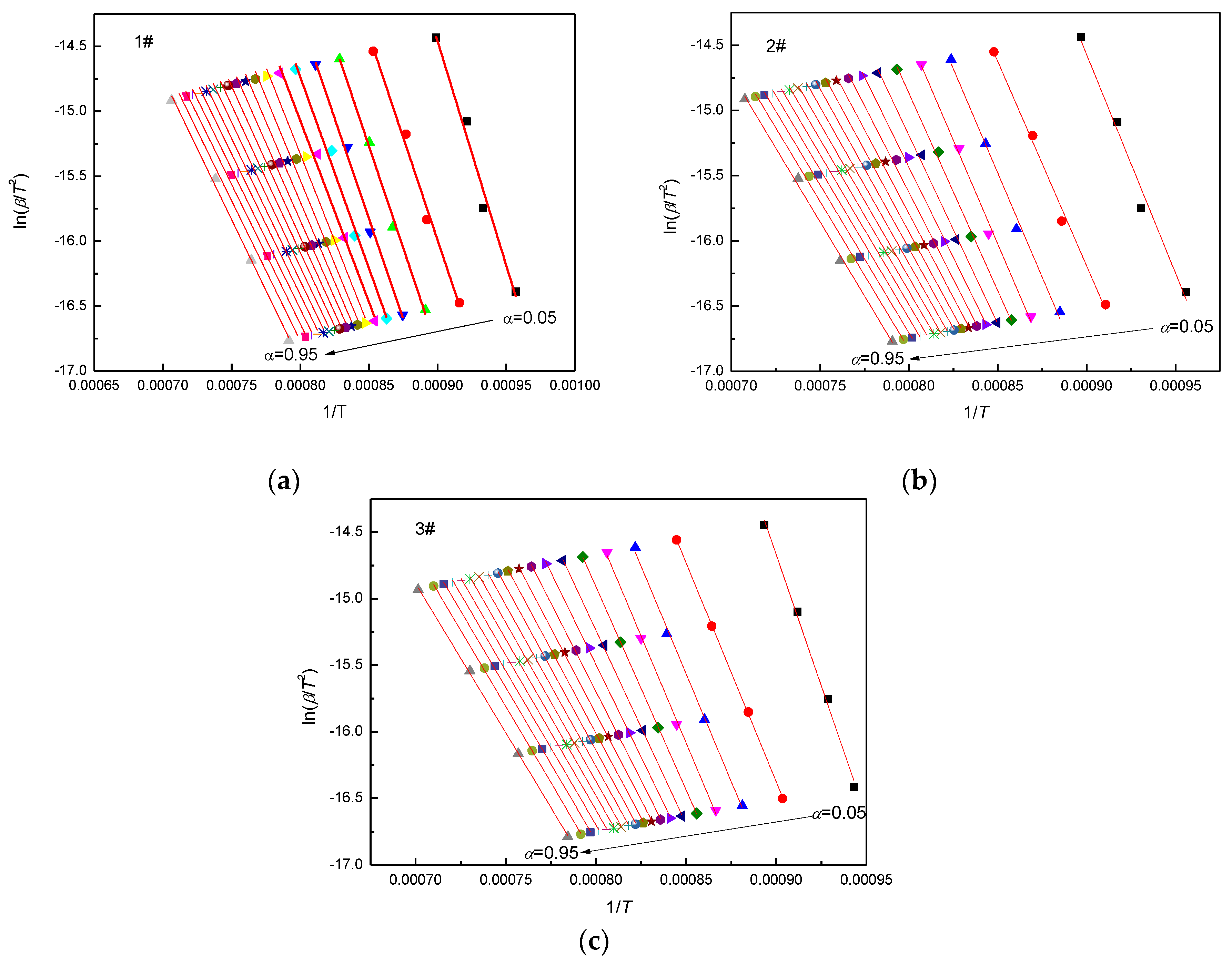
| Sample | Proximate Analysis (ad, %) | Ultimate Analysis (daf, %) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC * | C | H | N | S | O * | |
| 1# | 6.11 | 15.24 | 28.04 | 50.61 | 89.22 | 4.67 | 1.04 | 1.24 | 3.83 |
| 2# | 6.80 | 9.91 | 27.86 | 55.43 | 84.26 | 7.53 | 1.07 | 1.03 | 6.10 |
| 3# | 7.30 | 11.10 | 28.15 | 53.45 | 82.40 | 8.09 | 1.12 | 1.44 | 6.95 |
| 1# | 2# | 3# | |
|---|---|---|---|
| Dv (50)/μm | 11.7 | 43.4 | 132 |
| α | E (kJ mol−1) | k0 (s−1) | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1# | 2# | 3# | 1# | 2# | 3# | 1# | 2# | 3# | |
| 0.05 | 287.24 | 278.47 | 328.01 | 3.15 × 1011 | 1.06 × 1011 | 2.41 × 1013 | 0.975 | 0.978 | 0.994 |
| 0.10 | 262.59 | 260.45 | 273.57 | 4.31 × 109 | 2.78 × 109 | 1.01 × 1010 | 0.988 | 0.990 | 0.999 |
| 0.15 | 261.31 | 265.44 | 269.22 | 1.60 × 109 | 2.01 × 109 | 2.76 × 109 | 0.994 | 0.991 | 0.997 |
| 0.20 | 257.58 | 264.38 | 267.19 | 6.17 × 108 | 1.04 × 109 | 1.32 × 109 | 0.993 | 0.992 | 0.998 |
| 0.25 | 245.02 | 251.78 | 253.96 | 1.11 × 108 | 1.91 × 108 | 2.28 × 108 | 0.989 | 0.996 | 0.999 |
| 0.30 | 232.27 | 240.02 | 241.76 | 2.20 × 107 | 4.25 × 107 | 4.84 × 107 | 0.990 | 0.996 | 0.999 |
| 0.35 | 222.30 | 230.20 | 230.79 | 6.25 × 106 | 1.24 × 107 | 1.24 × 107 | 0.990 | 0.996 | 0.999 |
| 0.40 | 213.39 | 221.10 | 221.41 | 2.08 × 106 | 4.10 × 106 | 3.95 × 106 | 0.990 | 0.996 | 0.999 |
| 0.45 | 205.38 | 213.88 | 214.76 | 7.84 × 105 | 1.68 × 106 | 1.71 × 106 | 0.991 | 0.996 | 0.999 |
| 0.50 | 198.43 | 208.24 | 209.71 | 3.36 × 105 | 8.27 × 105 | 8.87 × 105 | 0.992 | 0.996 | 0.999 |
| 0.55 | 193.36 | 203.54 | 205.16 | 1.77 × 105 | 4.53 × 105 | 4.92 × 105 | 0.992 | 0.997 | 0.999 |
| 0.60 | 188.68 | 199.55 | 201.37 | 9.77 × 104 | 2.70 × 105 | 2.98 × 105 | 0.993 | 0.997 | 0.999 |
| 0.65 | 185.42 | 196.06 | 198.23 | 6.28 × 104 | 1.70 × 105 | 1.94 × 105 | 0.993 | 0.997 | 0.999 |
| 0.70 | 182.62 | 193.63 | 195.46 | 4.24 × 104 | 1.20 × 105 | 1.31 × 105 | 0.994 | 0.997 | 0.999 |
| 0.75 | 180.64 | 191.29 | 193.23 | 3.12 × 104 | 8.51 × 104 | 9.41 × 104 | 0.994 | 0.997 | 0.999 |
| 0.80 | 179.37 | 189.55 | 191.96 | 2.45 × 104 | 6.43 × 104 | 7.40 × 104 | 0.994 | 0.997 | 0.999 |
| 0.85 | 178.68 | 188.52 | 191.11 | 2.04 × 104 | 5.18 × 104 | 6.03 × 104 | 0.995 | 0.997 | 0.999 |
| 0.90 | 179.42 | 188.20 | 190.07 | 1.93 × 104 | 4.43 × 104 | 4.74 × 104 | 0.996 | 0.997 | 0.999 |
| 0.95 | 181.69 | 188.63 | 186.35 | 2.08 × 104 | 3.94 × 104 | 2.72 × 104 | 0.996 | 0.996 | 0.999 |
| Sample Number | DT/°C | ST/°C | HT/°C | FT/°C |
|---|---|---|---|---|
| 1# | 1220 | 1250 | 1270 | 1280 |
| 2# | 1190 | 1200 | 1210 | 1220 |
| 3# | 1180 | 1200 | 1210 | 1220 |
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | SO3 | K2O | Na2O | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1# | 43.61 | 21.99 | 5.33 | 18.92 | 0.68 | 0.92 | 5.78 | 1.44 | 0.98 | 0.14 |
| 2# | 39.36 | 19.66 | 6.00 | 20.60 | 0.65 | 0.93 | 9.86 | 1.18 | 1.22 | 0.12 |
| 3# | 43.55 | 20.97 | 4.28 | 17.03 | 0.76 | 1.03 | 9.23 | 1.21 | 1.65 | 0.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, Z.; Zhao, R.; Wu, J. Gasification of Shenhua Bituminous Coal with CO2: Effect of Coal Particle Size on Kinetic Behavior and Ash Fusibility. Energies 2020, 13, 3313. https://doi.org/10.3390/en13133313
Zhang J, Wang Z, Zhao R, Wu J. Gasification of Shenhua Bituminous Coal with CO2: Effect of Coal Particle Size on Kinetic Behavior and Ash Fusibility. Energies. 2020; 13(13):3313. https://doi.org/10.3390/en13133313
Chicago/Turabian StyleZhang, Jinzhi, Zhiqi Wang, Ruidong Zhao, and Jinhu Wu. 2020. "Gasification of Shenhua Bituminous Coal with CO2: Effect of Coal Particle Size on Kinetic Behavior and Ash Fusibility" Energies 13, no. 13: 3313. https://doi.org/10.3390/en13133313
APA StyleZhang, J., Wang, Z., Zhao, R., & Wu, J. (2020). Gasification of Shenhua Bituminous Coal with CO2: Effect of Coal Particle Size on Kinetic Behavior and Ash Fusibility. Energies, 13(13), 3313. https://doi.org/10.3390/en13133313





