Sub- and Supercritical Water Liquefaction of Kraft Lignin and Black Liquor Derived Lignin
Abstract
:1. Introduction
2. Black Liquor
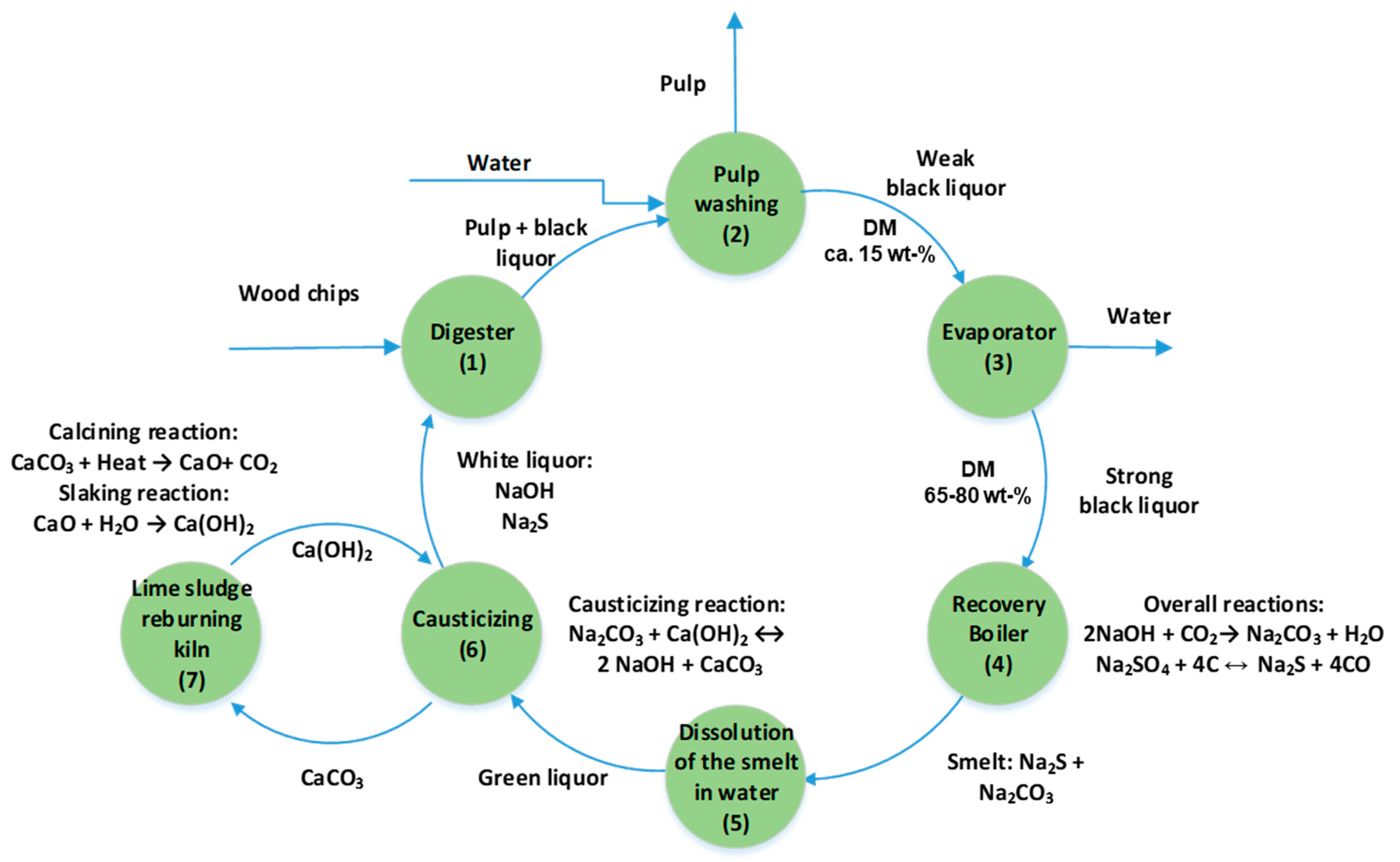
2.1. The Kraft Pulping Process
2.2. Formation of Black Liquor
2.3. The Composition of Black Liquor
3. Hydrothermal Treatment
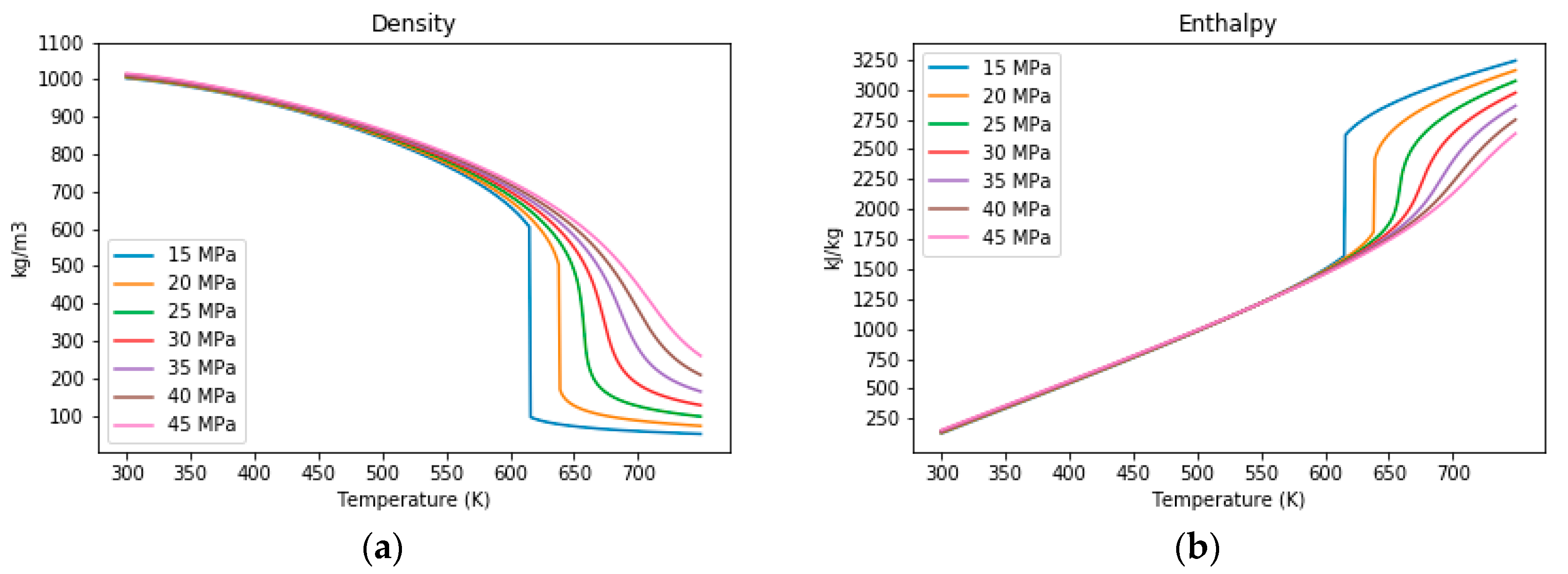
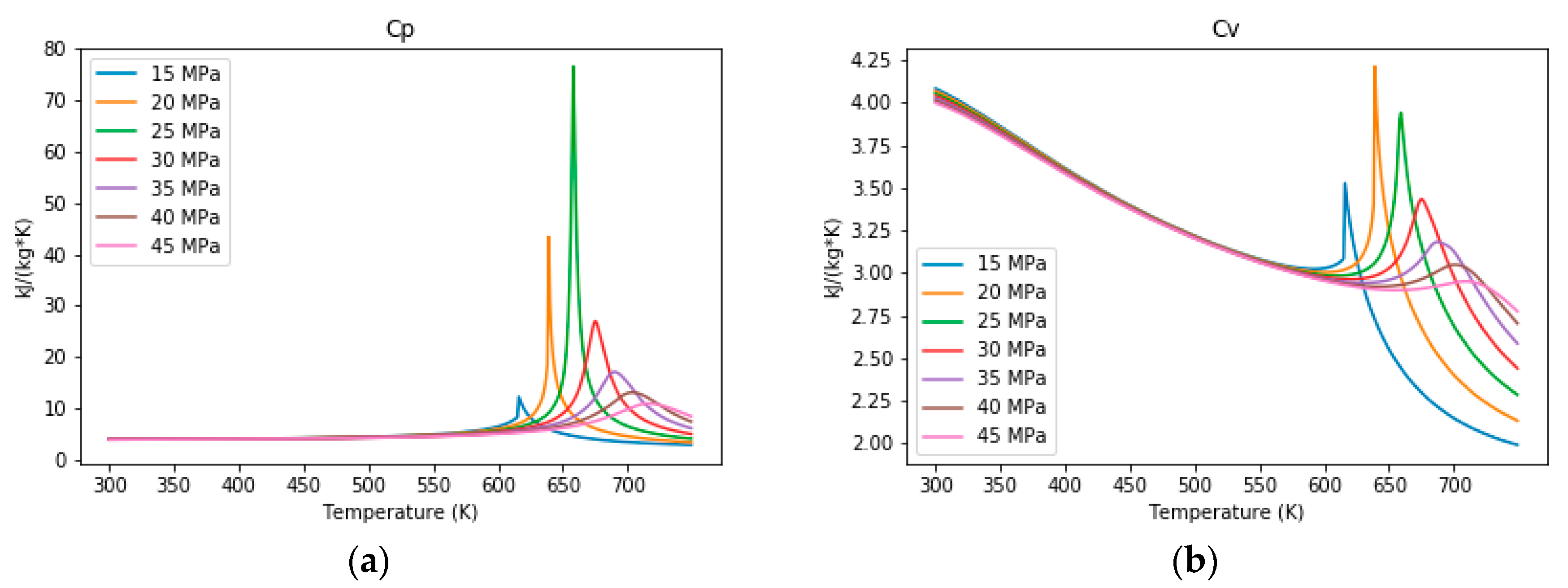
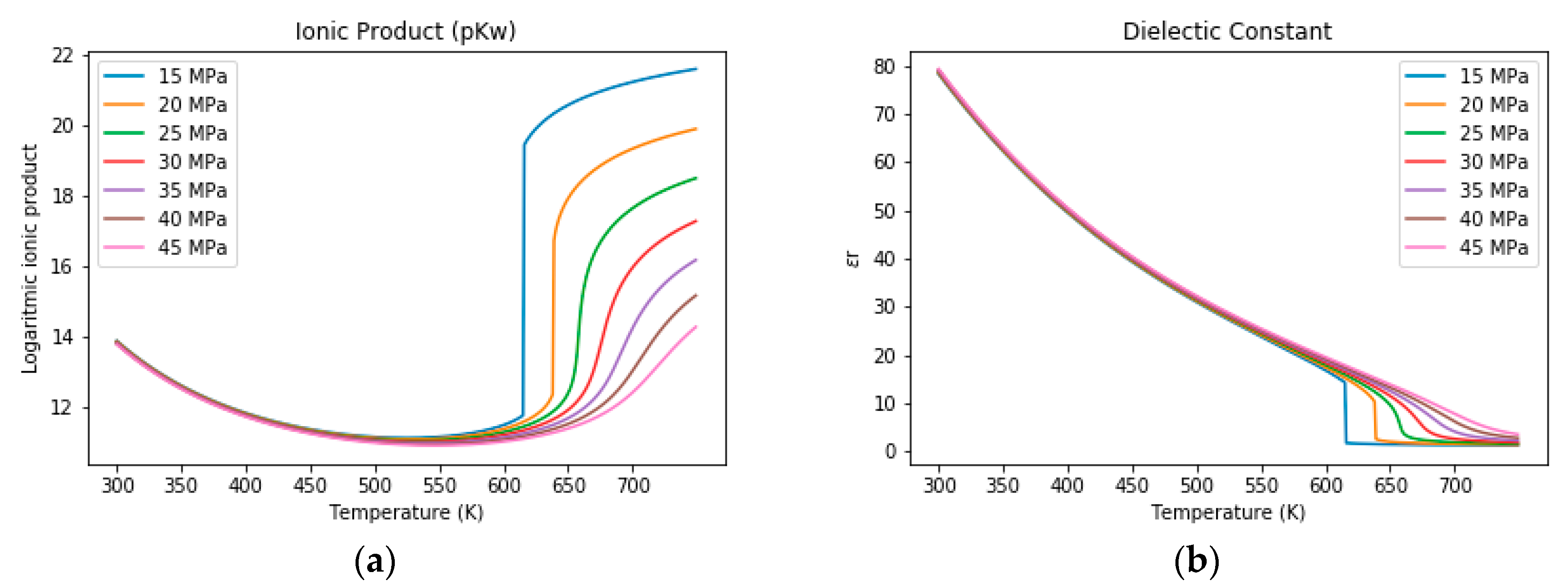
3.1. The Properties of Water under Sub- and Supercritical Conditions
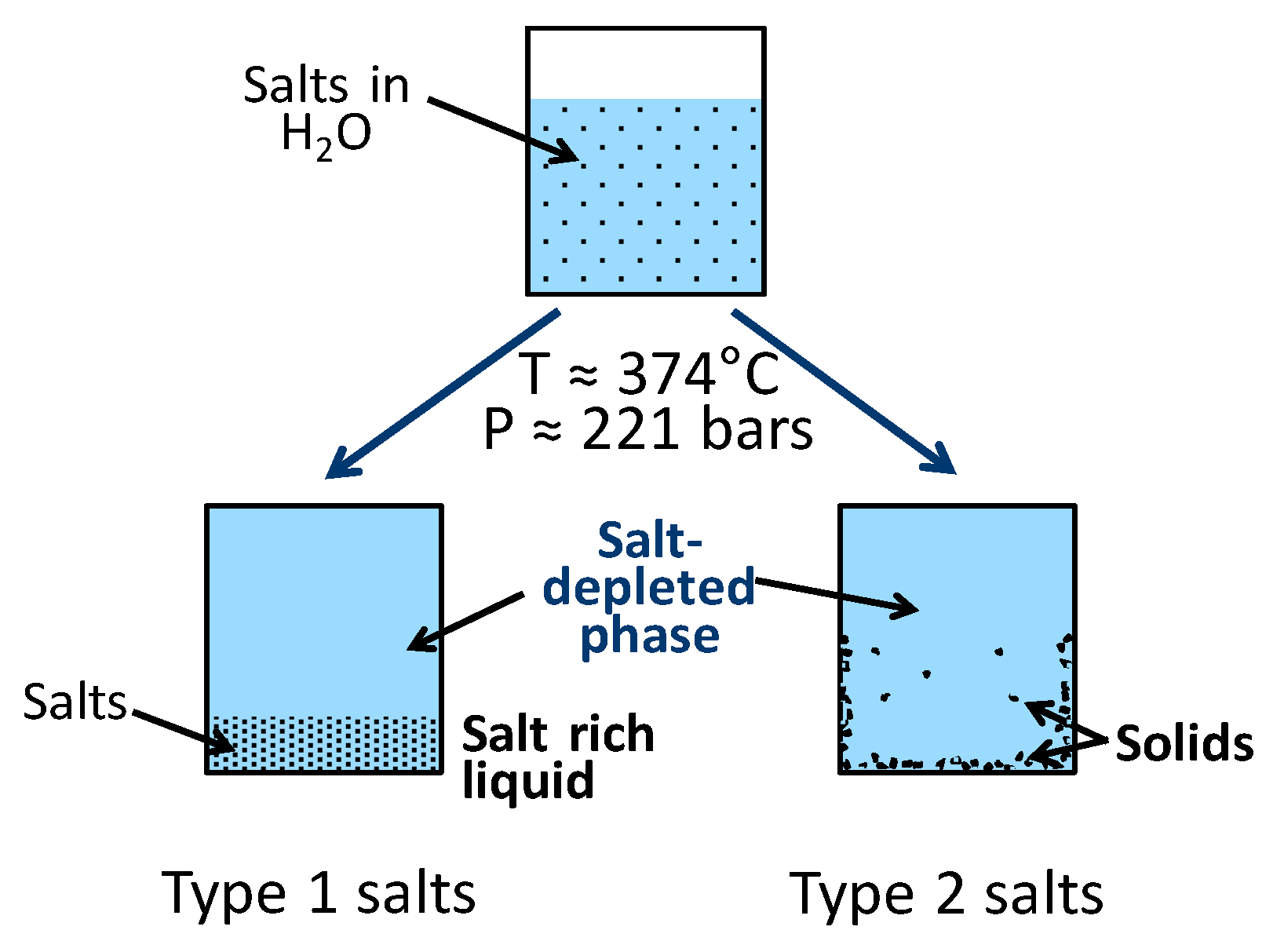
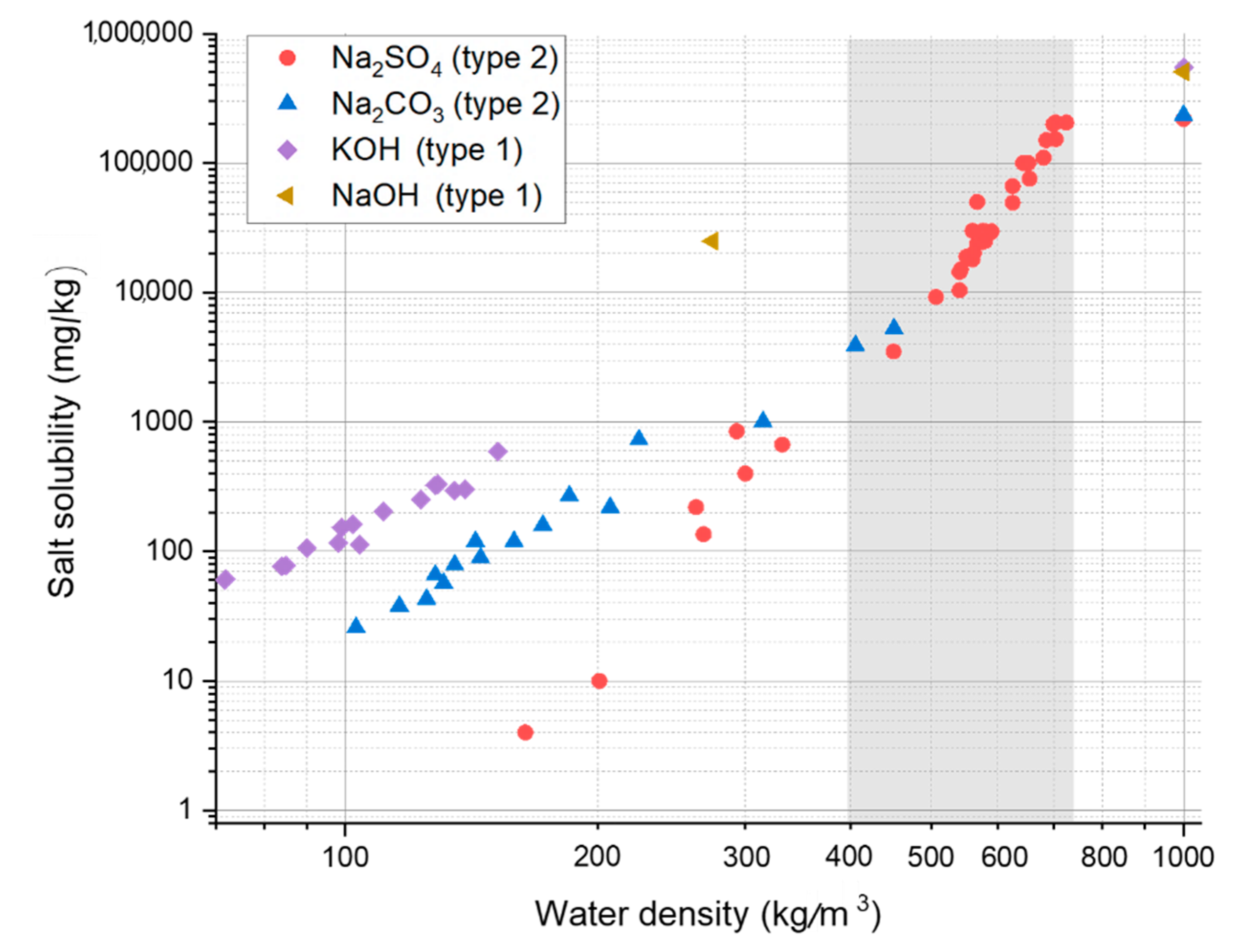
3.2. Salt Separation
3.3. Hydrothermal Liquefaction (HTL)
3.4. The Effects of the Process Parameters
3.4.1. Temperature and Pressure
- -
- An increase in temperature leads to higher HHV of the bio-oil due to both a decrease in the oxygen weight fraction and the increased weight fractions of carbon and hydrogen.
- -
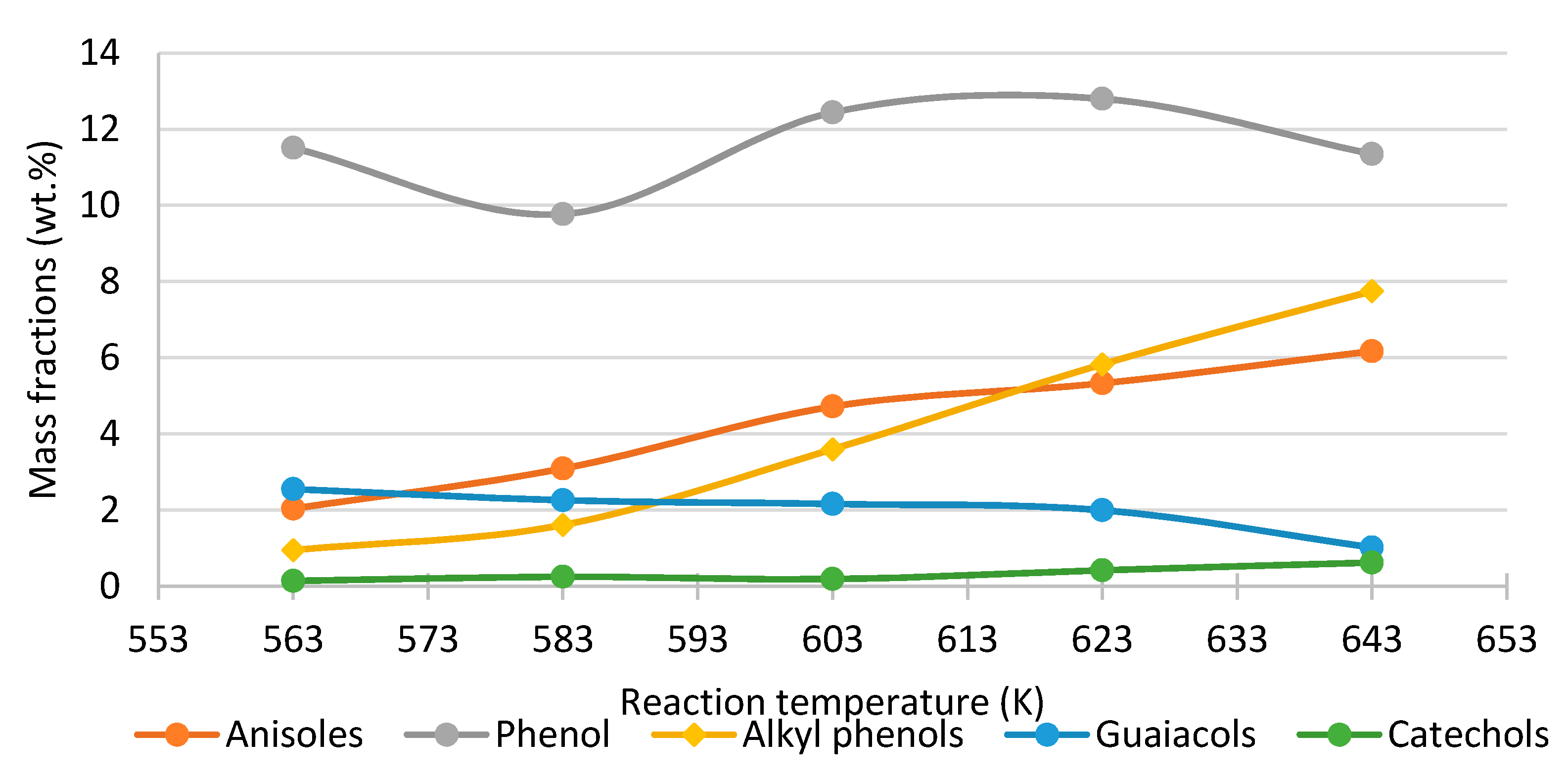
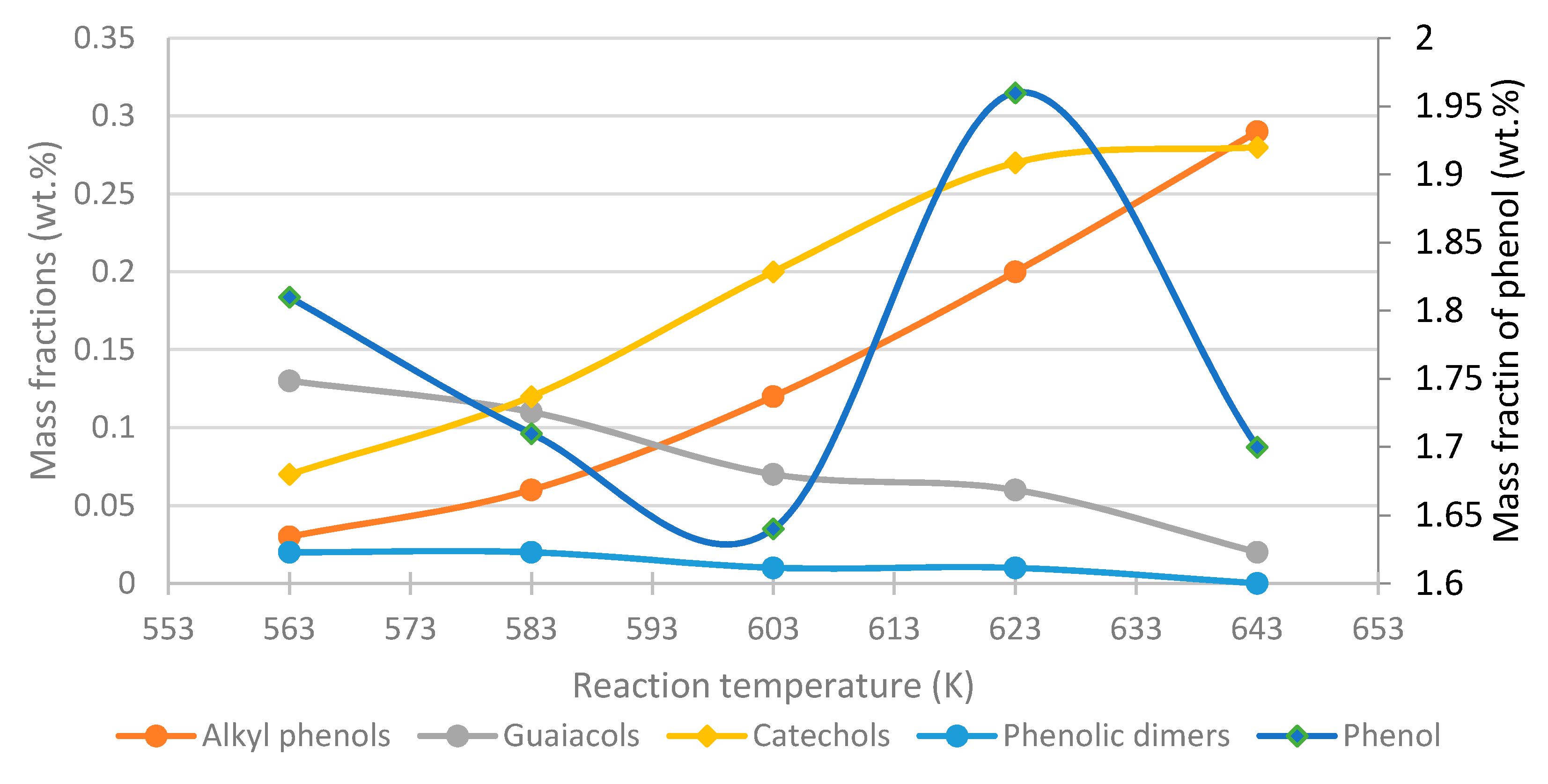
- Phenol, cresol, alkylphenol yields increase with increasing temperature, while the yield of guaiacol decreases, regardless of the process conditions and feed composition.
- -
- An increase of pressure at a set temperature will lead to an increase of phenols and cresol as a result of favored hydrolysis (higher water density).
- -
- As for any type of feed, a decrease in water density (increase of temperature or decrease of pressure) will significantly increase the gas yield and decrease the formation of tars/chars.
- -
- The effect of water density in the HTL of lignin has not yet been widely studied, even though it greatly affects the dissociation of lignin. Under supercritical water conditions, liquid-like densities appear to be one way to influence the product distribution and, therefore, the effect of water density on the HTL of black liquor should be studied further.
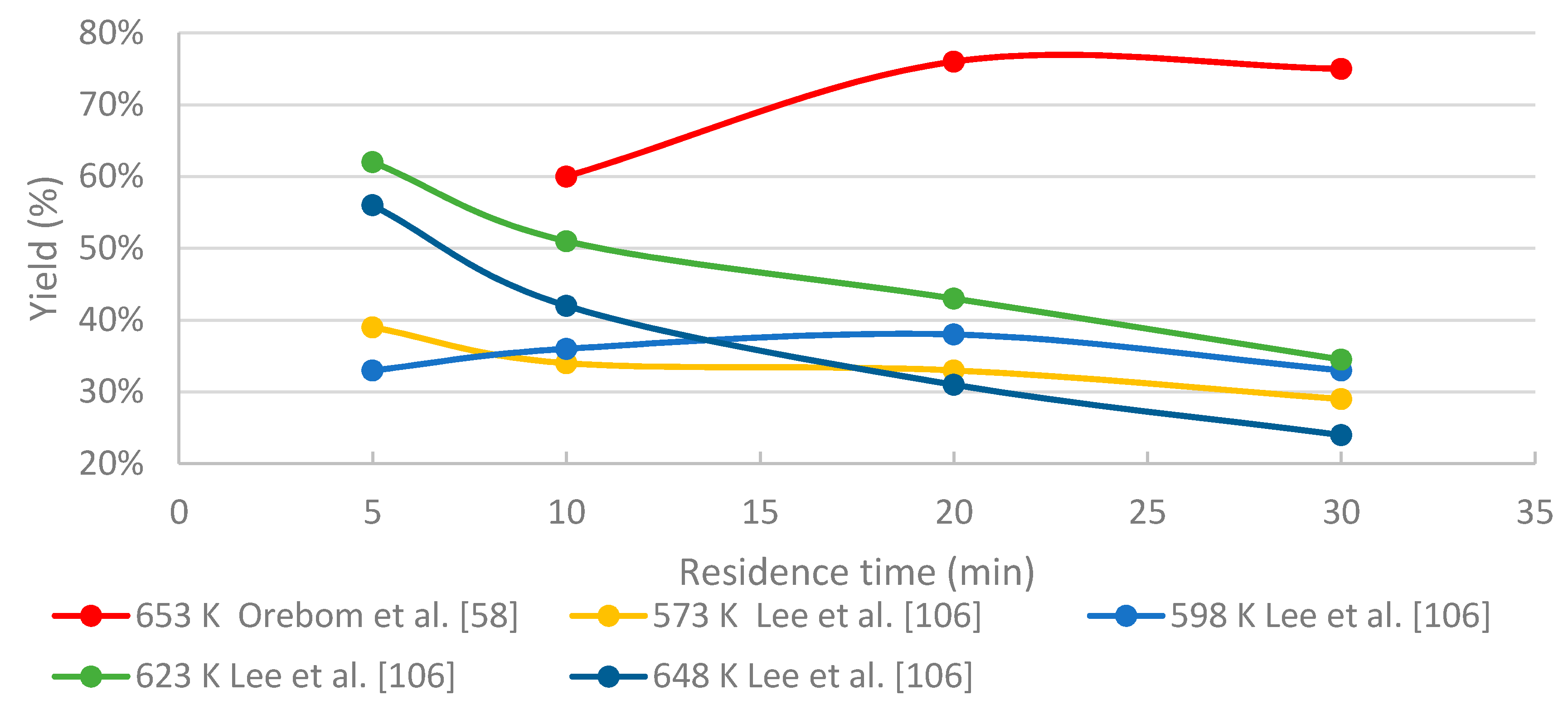
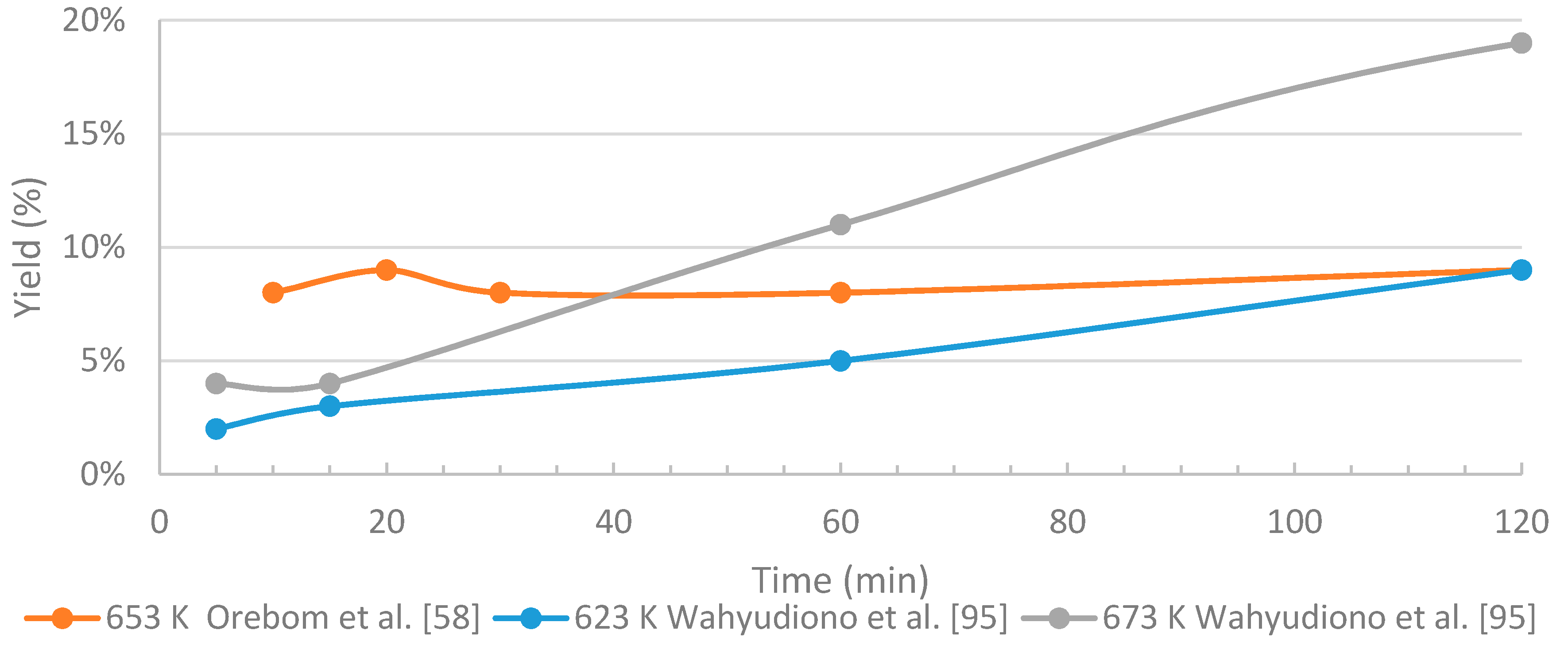
3.4.2. The Effects of Residence Time
- -
- To maximize the yield of certain compounds, such as phenol/catechol, a compromise between temperature and residence time is required.
- -
- Higher temperatures are favorable for producing high bio-oil yields, whereas residence time needs to be reduced significantly to prevent both gasification and char formation.
3.4.3. The Effect of the Dry Matter Content of the Feedstock
3.5. The Performance of the Catalysts
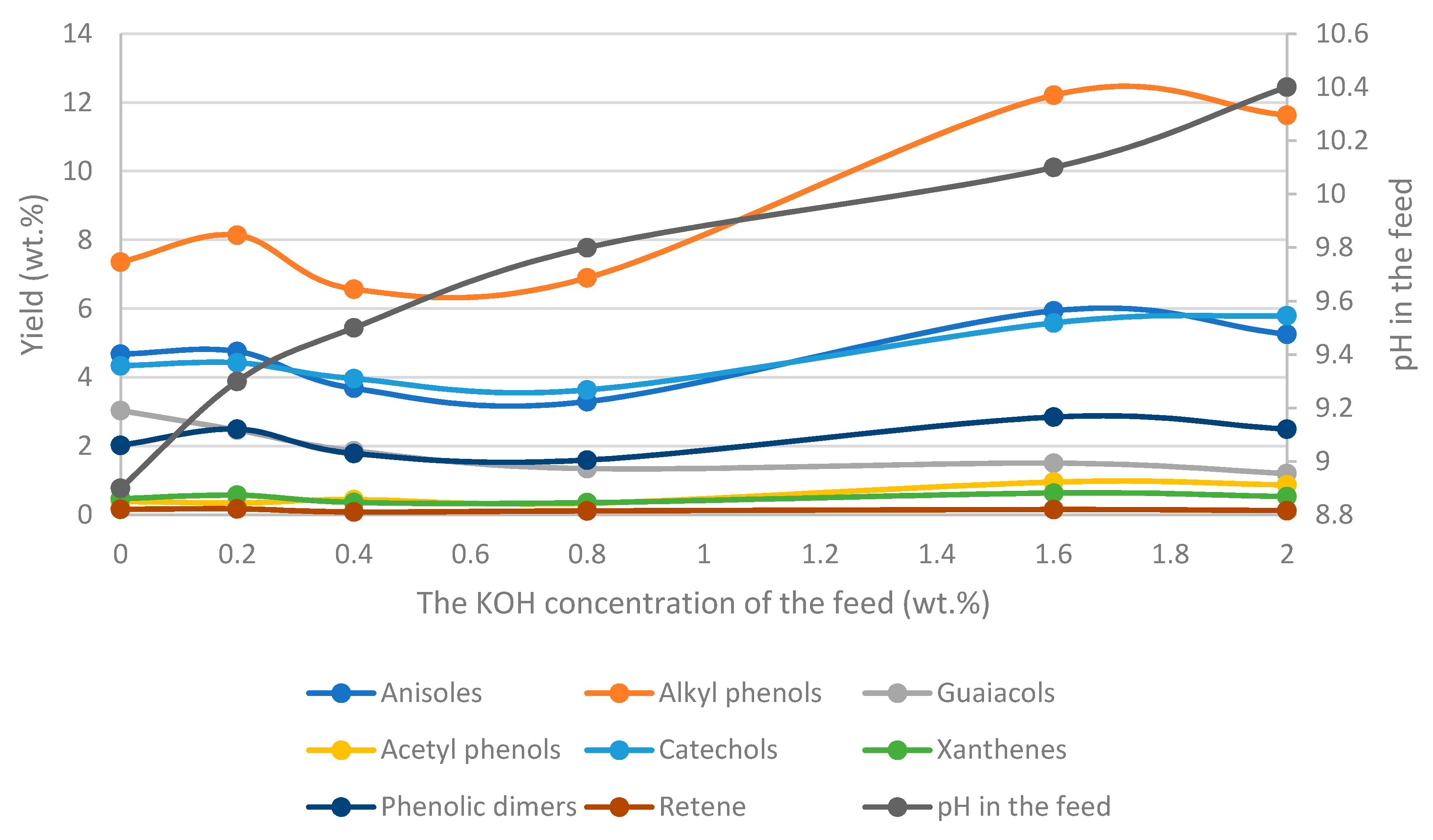
- -
- Potassium carbonate increases the presence of suspended solids in the bio-oil and favors the yields of phenolic compounds.
- -
- Adjusting the Na/K ratio for a CO32− and HO- mixture gives led to similar yields of bio-oils but affects product yields at a high Na/K ratio. On the other hand, a high ratio decreases the amounts of suspended solids (SS) in the oil and increases the proportion of heavy oil.
- -
- Alkali salts catalyzes decarboxylation and demethoxylation reactions. In addition, alkali salts with hydroxy anions enhance deoxygenation reactions.
- -
- Homogeneous catalysts (alkali salts) naturally present in BL affect product yields and composition, but also affect the ash-content in the bio-oil, which increases with increasing salt concentration.
- -
- The pH of the feedstock and product will affect the composition of the bio-oil and the aqueous phase (WSO) as weak acids, such as phenols, may become deprotonated. The final pH will depend on the process parameters.
- -
- Of the few compatible heterogeneous catalysts that are compatible with the high-pH sulfur-rich feed of black liquor, only zirconia (ZrO2) has been studied. Although this appears to impact the quality of the bio-oil, the understanding of the mechanism is very limited. Bearing in mind that the high concentration of salts in black liquor may foul (see Section 3.2) and hence deactivate the catalysts, much research still remains to be done into heterogeneous catalysis with black liquor.
3.6. The Role of Co-Solvents
- -
- Ethanol was found to prevent solid residue formation and limit the conversion of feedstock to WSO.
- -
- Adding glycerol to black liquor can lead to improvement in the bio-oil heating value, the carbon yield, and the fluidity of the bio crude, but these benefits are limited.
- -
- The addition of phenol to the HTL of lignin with K2CO3 or K2CO3 + KOH improves bio-oil yields and the yields of dimers and monomers, while it tends to reduce the yield of insoluble compounds (dry solids).
- -
- Phenol as a co-solvent gives better results than aliphatic alcohols, probably due to its better reactivity with the reaction intermediates that are subject to repolymerization
4. Reaction Chemistry
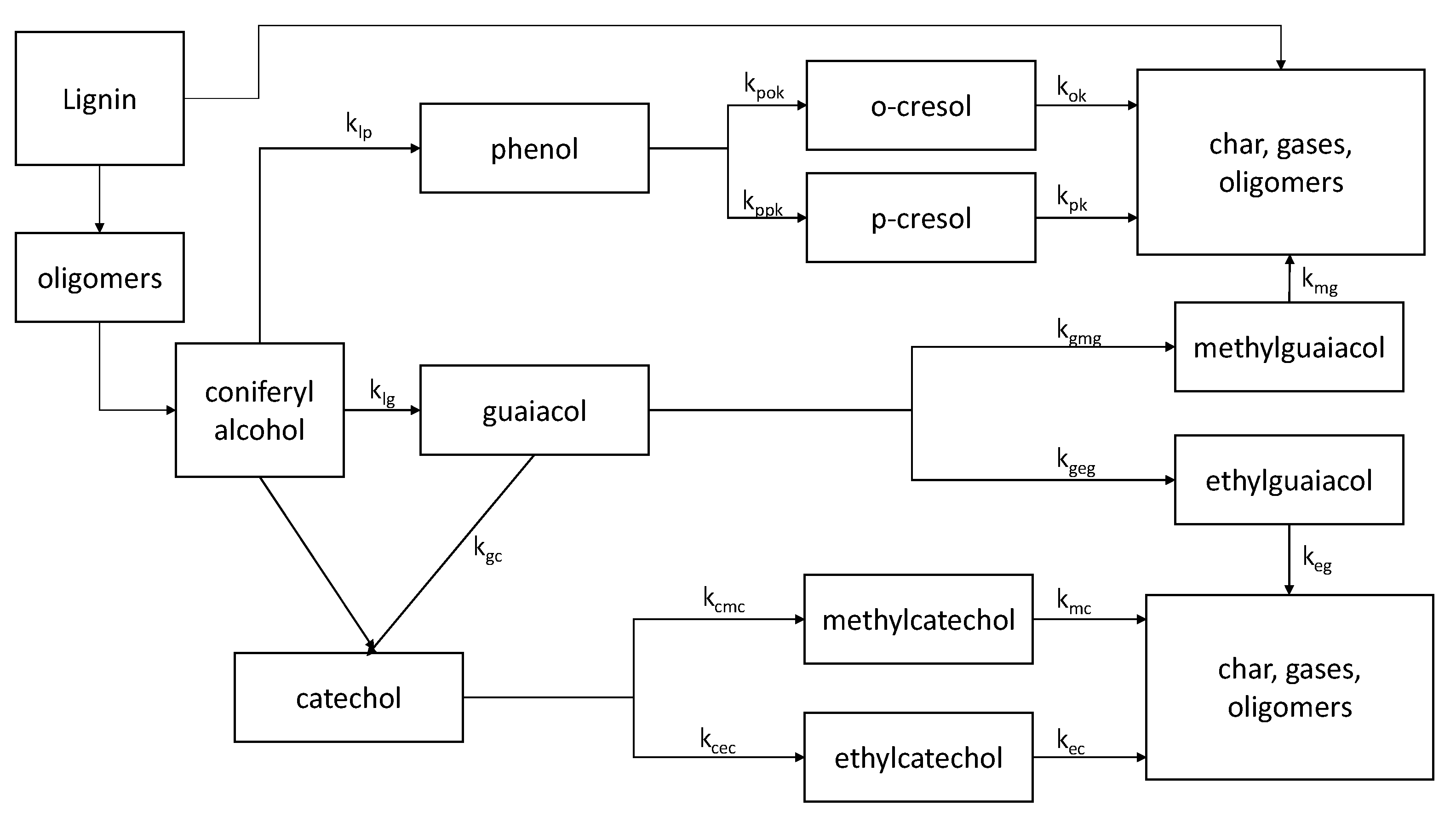
4.1. Reaction Pathways and Kinetics
4.2. Sulfur Chemistry in Supercritical Water
4.2.1. Organosulfur Compounds
4.2.2. Inorganic Species
4.3. Bio-Oil Quality
5. Review of Techno-Economic Analyses of the Hydrothermal Liquefaction of Black Liquor
6. Conclusions
- -
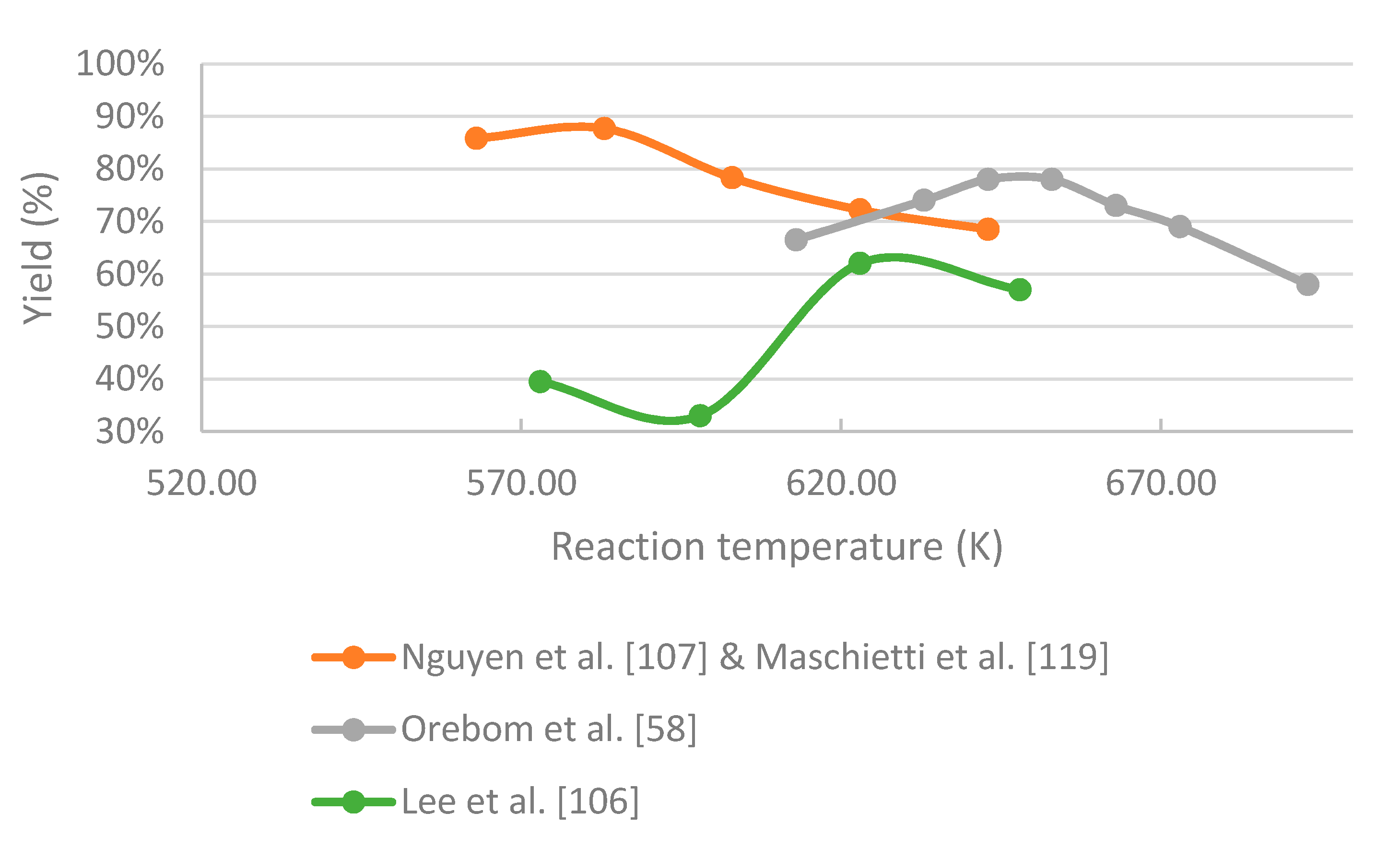
- In the case of black liquor, an increase in temperature favors both repolymerization and gasification reactions, leading to a lower yield of bio-oil. The highest yield of bio-oil of in a continuously operated plant was received under subcritical conditions at around 573 K and residence time of around 12 min. In a batch test conducted slightly above the critical point of water, a bio-oil yield of 70 wt.% was achieved. There is no data from experiments on black liquor under supercritical water conditions in continuous working reactors, so this should be investigated in the future. The density of supercritical water was found to have an effect on the reaction mechanism, and thus on the products. Therefore, the effect of water density should be investigated to maximize the yield of the desired components.
- -
- In HTL processing, the residence time greatly influences the yields of different fractions and the distribution of the chemical compounds within these fractions. When black liquor-derived lignin is treated under near- and supercritical water conditions, relatively short residence times are recommended in order to limit the amount of unwanted products (char, WSO, gaseous compounds). The longer residence time on batch tests seems to improve the quality of the bio-oil, but on the other hand increases the repolymerization, thus use of higher reaction conditions, and shorter residence time might be feasible. Slightly above supercritical conditions, residence time of few minutes may be enough for sufficient conversion degree.
- -
- Co-solvents can stabilize and improve reactions in the HTL of lignin leading to higher bio-oil yields while inhibiting the formation of residual solids. Phenol appeared to be a more promising co-solvent, as even at low concentrations its use led to a better yield of bio-oil. One reason for the better yield was that phenol stabilizes the intermediate products reducing repolymerization to high molecular weight chemical compounds, i.e., char.
- -
- Existing studies have included experiments where either homogeneous or heterogeneous catalysts were used in the HTL of black liquor-derived lignin. The homogeneous catalyst studies focused on the salts that are naturally present in black liquor, i.e., K2CO3, KOH, Na2CO3 and NaOH. A similar bio-oil yields were obtained when only one of the cations (either Na+ or K+) was present. However, when only Na+ cations were present, the amount of suspended solids (SS) in the bio-oil fraction was significantly lower while the amount of heavy oil was higher. The use of ZrO2 as a heterogeneous catalyst decreased the average molecular weight of the bio-oil.
- -
- The initial lignin concentration in the black liquor can also influence the formation of bio-oil and char. The highest yield of bio-oil yield was achieved when the initial lignin concentration was 5 wt.%. Increasing the concentration of the initial lignin led to higher yield of char and lower yield of bio-oil.
- -
- The large concentration of salts present in black liquor are likely to be precipitated from the mainstream when the water density is low, i.e., in the higher ranges of pressure and temperature typically used for HTL, and this may result in reactor fouling. Under these conditions, the decrease in the solubility of the salts that are homogeneous catalysts for organics liquefaction can significantly impact on their catalytic activity.
- -
- The bio-oil produced from black liquor in the HTL process is significantly different from fossil crude oil. For example, the compounds present in the bio-oil are more oxygenated (oxygen content between 10.5 wt.% and 25.9 wt.%), while typical fossil petroleum contains only 1–2 wt.% of oxygen. In addition, bio-oil produced from black liquor may contain some impurities, such as alkali salts, that must be removed before it is suitable for conventional refining processes. To use bio-oil as a feedstock for an oil refinery, the amount of impurities and oxygen in the bio-oil should be reduced to acceptable limits.
- -
- The degradation of lignin in the HTL process is highly complicated and involves a wide range of chemical compounds and reactions. Currently, there is no chemical reaction pathway and kinetic model for the degradation of lignin. However, a model for the degradation of Indulin AT (Kraft Lignin) has been developed, which includes reaction pathways and kinetics for the degradation of lignin under a subcritical water condition. The suitability of the reaction model and kinetics for modeling the degradation of lignin in black liquor must be studied and, presumably, modified. In addition, a reaction pathway and the kinetics of the degradation of black liquor lignin under supercritical water conditions must be developed.
- -
- The profitability of black liquor HTL has been studied in a few TEAs where black liquor is either the main feed or the co-feed to the HTL process. These TEAs have shown that the MSP of biofuel is somewhere between 0.93 $/liter and 1.05 $/liter, while the annual average price of Brent crude oil has fluctuated between 0.36 $/liter and 0.83 $/liter over the last 5 years. Therefore, either the price of fossil crude oil must rise, or the MSP of biofuel must decrease before it will be feasible to apply the process on an industrial scale.
- -
- Increase understanding of continuous operating HTL process applying short residence time (several minutes) under near- and supercritical conditions.
- -
- The effect of inorganic compounds on the reaction mechanisms and kinetics for the degradation of organic compounds of black liquor in near- and supercritical water.
- -
- The solubility and catalytic behavior of alkali salt mixtures (applied in pulping) in near-critical and supercritical water.
- -
- A detailed study of the qualities and costs of corrosion-resistant materials to improve the quality of any future economic evaluations.
Author Contributions
Funding
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C. 2018. Available online: https://www.ipcc.ch/site/assets/uploads/sites/2/2019/06/SR15_Full_Report_High_Res.pdf (accessed on 19 June 2020).
- International Energy Agency Tracking Clean Energy Progress 2017; International Energy Agency: Paris, France, 2017.
- International Energy Agency Key. World Energy Statistics 2018, 21st ed.; International Energy Agency: Paris, France, 2018. [Google Scholar]
- Sims, R.E.H. Bioenergy Options for a Cleaner Environment in Developed and Developing Countries; Elsevier Science & Technology: Oxford, UK, 2003; ISBN 9780080527949. [Google Scholar]
- International Energy Agency. Global Energy & CO2 Status Report. The Latest trends in Energy and Emissions in 2017; International Energy Agency: Paris, France, 2018. [Google Scholar]
- Slade, R.; Saunders, R.; Gross, R.; Bauen, A. Energy from Biomass: The Size of The Global Resource; UK Energy Research Center: London, UK, 2011. [Google Scholar]
- Hornung, U.; Kruse, A.; Akgül, G. Hydrothermal Liquefaction—Upgrading. In Transformation of Biomass: Theory to Practise; Kruse, A., Ed.; John Wiley & Sons, Ltd.: Hoboken, JN, USA, 2014; ISBN 9781119973270. [Google Scholar]
- Balaman, S.Y. Biomass-Based Production Systems. In Decision-Making for Biomass-Based Production Chains: The Basic Concepts and Methodologies; Academic Press: Cambridge, MA, USA, 2018; pp. 25–54. [Google Scholar]
- Doassans-Carrère, N.; Ferrasse, J.-H.; Boutin, O.; Mauviel, G.; Lédé, J. Comparative Study of Biomass Fast Pyrolysis and Direct Liquefaction for Bio-Oils Production: Products Yield and Characterizations. Energy Fuels 2014, 28, 5103–5111. [Google Scholar] [CrossRef]
- Metsa Group. Sustainability Report. 2018. Available online: https://www.metsagroup.com/en/Documents/Publications/Metsa-Group-sustainability-report-2018.pdf (accessed on 22 June 2020).
- Vakkilainen, E.K.; Välimäki, E. Effect of Lignin Separation to Black Liquor and Recovery Boiler Operation. In Proceedings of the TAPPI’s 2009 Engineering, Pulping, and Environmental Conference, Memphis, TN, USA, 11–14 October 2009. [Google Scholar]
- Licella. A Bridge to a Lower Carbon Future. Available online: https://www.licella.com.au/ (accessed on 27 March 2019).
- RenFuel. Lignin Bio Oils. Available online: https://renfuel.se/?lang=en (accessed on 27 March 2019).
- SunCarbon. Linking the Forest and Petrochemical Industry. Available online: https://www.suncarbon.se/ (accessed on 27 March 2019).
- Licella. Cat-HTRTM—Licella. Available online: https://www.licella.com.au/cat-htr/ (accessed on 27 March 2019).
- Rowlands, W.N.; Humphreys, L.J.; Thew, R.W.C.; Spankie, J.A.; Uloth, V.C.; Watson, P.A.; Pudlas, M.W. Integrated Kraft Pulp Mill and Thermochemical Conversion System. 2015. Available online: https://patents.google.com/patent/WO2016058098A1/en (accessed on 29 August 2018).
- Licella. Pulp & Paper—Licella. Available online: https://www.licella.com.au/pulp-paper/ (accessed on 27 March 2019).
- RenFuel. Technology—RenFuel. Available online: https://renfuel.se/technology/?lang=en (accessed on 27 March 2019).
- SunCarbon. Our Technology—Renewable Fuels from Lignin—SunCarbon. Available online: https://www.suncarbon.se/technology/ (accessed on 27 March 2019).
- SunCarbon. Membrane Pilot at Kraft Pulp Mill—SunCarbon. Available online: https://www.suncarbon.se/2017/02/01/membrane-pilot-at-kraft-pulp-mill/ (accessed on 27 March 2019).
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.; Rosendahl, L. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Jindal, M.K.; Jha, M.K. Hydrothermal liquefaction of wood: a critical review. Rev. Chem. Eng. 2016, 32. [Google Scholar] [CrossRef]
- Tian, C.; Li, B.; Liu, Z.; Zhang, Y.; Lu, H. Hydrothermal liquefaction for algal biorefinery: A critical review. Renew. Sustain. Energy Rev. 2014, 38, 933–950. [Google Scholar] [CrossRef]
- López Barreiro, D.; Ronsse, F.; Brilman, W. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass Bioenergy 2013, 53, 113–127. [Google Scholar]
- Vlaskin, M.S.; Chernova, N.I.; Kiseleva, S.V.; Popel’, O.S.; Zhuk, A.Z. Hydrothermal liquefaction of microalgae to produce biofuels: state of the art and future prospects. Therm. Eng. 2017, 64, 627–636. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, C.; Chen, H.; Tsang, D.C.W.; Luo, G.; Zhang, S.; Chen, J. Hydrothermal liquefaction of agricultural and forestry wastes: state-of-the-art review and future prospects. Bioresour. Technol. 2017, 245, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Park, J.-H. A short review on hydrothermal liquefaction of livestock manure and a chance for Korea to advance swine manure to bio-oil technology. J. Mater. Cycles Waste Manag. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Ramirez, J.; Brown, R.; Rainey, T.; Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A Review of Hydrothermal Liquefaction Bio-Crude Properties and Prospects for Upgrading to Transportation Fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef] [Green Version]
- Biermann, C.J. Pulping Fundamentals. In Handbook of Pulping and Papermaking; Elsevier: Amsterdam, The Netherlands, 1996; ISBN1 10 012647480X. ISBN2 13 9780126474800. [Google Scholar]
- International Energy Agency Black Liquor Gasification. Summary and Conclusions from the IEA Bioenergy ExCo54 Workshop; International Energy Agency: Paris, France, 2007. [Google Scholar]
- Biermann, C.J. Kraft Spent Liquor Recovery. In Handbook Pulping Papermaking; Academic Press: Cambridge, MA, USA, 1996; pp. 101–122. [Google Scholar]
- Sjöström, E. Wood Chemistry: Fundamentals and Applications; Academic Press: Cambridge, MA, USA, 1981; ISBN1 012647480X. ISBN2 9780126474800. [Google Scholar]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Gellerstedt, G. Chemistry of Chemical Pulping. In Pulping Chemistry and Technology; Henriksson, G., Gellerstedt, G., Monica, E.k., Eds.; Walter de Gruyter GmbH & Co.: Berlin, Germany, 2009; ISBN1 9783110213416. ISBN2 9783110483420. ISBN3 9783110213423. [Google Scholar]
- Alén, R.; Niemelä, K. Characterization of Pulping Liquors. In Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Alén, R., Sjöström, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 193–231. ISBN 978-3-662-03898-7. [Google Scholar]
- Theliander, H. Recovery of Cooking Chemicals: The Treatment and Burning of Black Liquor. In Pulping Chemistry and Technology; Ek, M., Henriksson, G., Gellerstedt, G., Eds.; Walter de Gruyter GmbH & Co.: Berlin, Germany, 2009. [Google Scholar]
- Kinnarinen, T.; Golmaei, M.; Jernström, E.; Häkkinen, A. Separation, treatment and utilization of inorganic residues of chemical pulp mills. J. Clean. Prod. 2016, 133, 953–964. [Google Scholar] [CrossRef]
- Bajpai, P. Structure of Lignocellulosic Biomass. In Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer: Singapore, 2016; pp. 7–12. [Google Scholar]
- Stokke, D.D.; Wu, Q.; Han, G.; Stevens, C.V. Introduction to Wood and Natural Fiber Composites, 1st ed.; Stokke, D.D., Wu, Q., Han, G., Stevens, C.V., Eds.; John Wiley & Sons, Incorporate: Hoboken, NJ, USA; ISBN 9780470710913.
- McKendry, P. Energy production from biomass (part 1): overview of biomass. Bioresour. Technol. 2002, 83, 10. [Google Scholar] [CrossRef]
- Van de Wouwer, D.; Boerjan, W.; Vanholme, B. Plant cell wall sugars: sweeteners for a bio-based economy. Physiol. Plant. 2018, 164, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Lancefield, C.S.; Wienk, H.L.J.; Boelens, R.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Identification of a diagnostic structural motif reveals a new reaction intermediate and condensation pathway in kraft lignin formation. Chem. Sci. 2018, 9, 6348–6360. [Google Scholar] [CrossRef] [Green Version]
- Arkell, A.; Olsson, J.; Wallberg, O. Process performance in lignin separation from softwood black liquor by membrane filtration. Chem. Eng. Res. Des. 2014, 92, 1792–1800. [Google Scholar] [CrossRef]
- Alekhina, M.; Ershova, O.; Ebert, A.; Heikkinen, S.; Sixta, H. Softwood kraft lignin for value-added applications: Fractionation and structural characterization. Ind. Crops Prod. 2015, 66, 220–228. [Google Scholar] [CrossRef]
- Sjöström, E. Wood Chemistry: Fundamentals and Applications, 2nd ed.; Academic Press, Inc.: Cambridge, MA, USA, 1993. [Google Scholar]
- Sjöström, E. Preface to the first edition. In Wood Chemistry: Fundamentals and Applications; Academic Press, Inc.: Cambridge, MA, USA, 1965; pp. xiii–xiv. [Google Scholar]
- Demirbas, A. Thermochemical Conversion Processes. In Biofuels; Springer: London, UK, 2009; pp. 261–304. ISBN 978-1-84882-010-4. [Google Scholar]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1. [Google Scholar] [CrossRef]
- Matsumura, Y. Hydrothermal Gasification of Biomass. In Recent Advances in Thermochemical Conversion of Biomass; Pandey, A., Bhaskar, T., Stöcker, M., Sukumaran, R.K., Eds.; Elsevier: Amsterdam, the Netherlands, 2015; p. 676. ISBN 978-0-444-63289-0. [Google Scholar]
- Masek, O. Biochar in thermal and thermochemical biorefineriesdproduction of biochar as a coproduct. In Handbook of Biofuels Production; Luque, R., Lin, C., Wilson, K., Clark, J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 655–671. [Google Scholar]
- Kumar, M.; Olajire Oyedun, A.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Susanti, R.F.; Kim, J.; Yoo, K. pung Supercritical Water Gasification for Hydrogen Production: Current Status and Prospective of High-Temperature Operation. In Supercritical Fluid Technology for Energy and Environmental Applications; Anikeev, V., Fan, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 111–137. ISBN 9780444626967. [Google Scholar]
- Elliott, D.C. Catalytic hydrothermal gasification of biomass. Biofuels, Bioprod. Biorefining 2008, 2, 254–265. [Google Scholar] [CrossRef]
- Zo, H.; Mayr, F. Stability and Performance of Ruthenium Catalysts Based on Refractory Oxide Supports in Supercritical Water Conditions. Energy Fuels 2013, 27, 4739–4747. [Google Scholar]
- Orebom, A.; Verendel, J.J.; Samec, J.S.M. High Yields of Bio Oils from Hydrothermal Processing of Thin Black Liquor without the Use of Catalysts or Capping Agents. ACS Omega 2018, 3, 6757–6763. [Google Scholar] [CrossRef] [PubMed]
- Sengers, J.M.H.L.; Straub, J.; Watanabe, K.; Hill, P.G. Assessment of Critical Parameter Values for H2O and D2O. J. Phys. Chem. Ref. Data 1985, 14, 193–207. [Google Scholar] [CrossRef]
- Wagner, W.; Pruß, A. The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A.; Dahmen, N. Water—A magic solvent for biomass conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Bühler, W.; Dinjus, E.; Ederer, H.J.; Kruse, A.; Mas, C. Ionic reactions and pyrolysis of glycerol as competing reaction pathways in near- and supercritical water. J. Supercrit. Fluids 2002, 22, 37–53. [Google Scholar] [CrossRef]
- IAPWS Release on the Static Dielectric Constant of Ordinary Water Substance for Temperature from 238 K to 873 K and Pressures up to 1000 MPa; Erlangen, Germany. 1997. Available online: http://www.iapws.org/relguide/Dielec.html (accessed on 22 June 2020).
- IAPWS Release on the Ionization Constant of H2O; Lucerne, Switzerland. 2007. Available online: http://www.iapws.org/relguide/Ionization.html (accessed on 22 June 2020).
- Sandquist, J.; Tschentscher, R.; del Alamo Serrano, G. Hydrothermal liquefaction of organic resources in biotechnology: how does it work and what can be achieved? Appl. Microbiol. Biotechnol. 2019, 103, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Jouyban, A.; Soltanpour, S.; Chan, H.-K. A simple relationship between dielectric constant of mixed solvents with solvent composition and temperature. Int. J. Pharm. 2004, 269, 353–360. [Google Scholar] [CrossRef]
- Hodes, M.; Marrone, P.A.; Hong, G.T.; Smith, K.A.; Tester, J.W. Salt precipitation and scale control in supercritical water oxidation—Part A: fundamentals and research. J. Supercrit. Fluids 2004, 29, 265–288. [Google Scholar] [CrossRef]
- Valyashko, V. Phase equilibria of water-salt systems at high temperatures and pressures. In Aqueous Systems at Elevated Temperatures and Pressures; Fernandez-Prini, R., Harvey, A.H., Palmer, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 597–641. ISBN 9780080471990. [Google Scholar]
- Valyashko, V.M. Phase Equilibria in Binary and Ternary Hydrothermal Systems. In Hydrothermal Experimental Data; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 1–133. [Google Scholar]
- Rumyantsev, V.N. Selection of mineralizers in the hydrothermal synthesis and growth of crystals. Sov. Phys. Crystallogr. 1977, 22, 610–613. [Google Scholar]
- Wang, R.; Deplazes, R.; Guo, L.; Vogel, F.; Baudouin, D. Continuous extraction of black liquor salts under hydrothermal conditions. in preparation.
- Ihara, K.; Ueda, H.; Sue, K.; Nonaka, T.; Arai, K. Decomposition of Sodium Thiosulfate and Sodium Thiocyanate in Supercritical Water. J. Japan Inst. Energy 2006, 85, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Regler, J.W.; Vogel, F. Continuous salt precipitation and separation from supercritical water. Part 2. Type 2 salts and mixtures of two salts. J. Supercrit. Fluids 2010, 52, 113–124. [Google Scholar] [CrossRef]
- Schubert, M.; Aubert, J.; Müller, J.B.; Vogel, F. Continuous salt precipitation and separation from supercritical water. Part 3: Interesting effects in processing type 2 salt mixtures. J. Supercrit. Fluids 2012, 61, 44–54. [Google Scholar] [CrossRef]
- Kruse, A.; Forchheim, D.; Gloede, M.; Ottinger, F.; Zimmermann, J. Brines in supercritical biomass gasification: 1. Salt extraction by salts and the influence on glucose conversion. J. Supercrit. Fluids 2010, 53, 64–71. [Google Scholar] [CrossRef]
- Makaev, S.V.; Bitokhov, T.M.; Kravchuk, K.G.; Urusova, M.A.; Valyashko, V.M. Salt deposition from hydrothermal solutions in a flow reactor. Russ. J. Phys. Chem. B 2011, 5, 1045–1055. [Google Scholar]
- Schubert, M.; Müller, J.B.; Vogel, F. Continuous hydrothermal gasification of glycerol mixtures: Effect of glycerol and its degradation products on the continuous salt separation and the enhancing effect of K3PO4 on the glycerol degradation. J. Supercrit. Fluids 2014, 95, 364–372. [Google Scholar] [CrossRef]
- De Blasio, C.; De Gisi, S.; Molino, A.; Simonetti, M.; Santarelli, M.; Björklund-Sänkiaho, M. Concerning operational aspects in supercritical water gasification of kraft black liquor. Renew. Energy 2019, 130, 891–901. [Google Scholar] [CrossRef]
- Voisin, T.; Erriguible, A.; Ballenghien, D.; Mateos, D.; Kunegel, A.; Cansell, F.; Aymonier, C. Solubility of inorganic salts in sub- and supercritical hydrothermal environment: Application to SCWO processes. J. Supercrit. Fluids 2017, 120, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Rogak, S. Solubility of Na2SO4, Na2CO3 and their mixture in supercritical water. J. Supercrit. Fluids 2004, 30, 359–373. [Google Scholar] [CrossRef]
- Príkopský, K.; Wellig, B.; von Rohr, P.R. SCWO of salt containing artificial wastewater using a transpiring-wall reactor: Experimental results. J. Supercrit. Fluids 2007, 40, 246–257. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Schmidt, A.J.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Albrecht, K.O.; Hallen, R.T.; Holladay, J.E. Process development for hydrothermal liquefaction of algae feedstocks in a continuous- fl ow reactor. ALGAL 2013, 2, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Kiyonaga, E.; Yamamura, Y.; Shimizu, Y.; Minowa, T.; Noda, Y.; Matsumura, Y. Gasification of Catalyst-Suspended Chicken Manure in Supercritical Water. J. Chem. Eng. JAPAN 2008, 41, 433–440. [Google Scholar] [CrossRef]
- Elliott, D.C.; Butner, R.S.; Neuenschwander, G.G.; Zacher, A.H.; Hart, T.R. Methods and Apparatus for Catalytic Hydrothermal Gasification of Biomass. 2008. Available online: https://patents.google.com/patent/US8241605/3Den (accessed on 3 May 2019).
- Antal, M.J.; Allen, S.G.; Schulman, D.; Xu, X.; Divilio, R.J. Biomass Gasification in Supercritical Water. Ind. Eng. Chem. Res. 2000, 39, 4040–4053. [Google Scholar] [CrossRef]
- Marrone, P.A.; Hodes, M.; Smith, K.A.; Tester, J.W. Salt precipitation and scale control in supercritical water oxidation — part B: commercial / full-scale applications. J. Supercrit. Fluids 2004, 29, 289–312. [Google Scholar] [CrossRef]
- Peng, G.; Zambrano Varela, R.; Reid Dick, G.; Juillard, F.; Bertschy, L.; De Boni, E.; Vogel, F.; Varela, R.Z.; Dick, G.R.; Juillard, F.; et al. Continuous Phosphorus Recovery and Biogas Generation from Sewage Sludge by Hydrothermal Processing, in preparation. Nat. Energy 2018. [Google Scholar]
- Favrat, D.; MARECHAL, F.; Ensinas, A.V.; Mian, A. Apparatus for Salt Separation Under Supercritical Water Conditions. U.S. Patent WO2016113685A1, 16 January 2015. [Google Scholar]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Regler, J.W.; Vogel, F. Continuous salt precipitation and separation from supercritical water. Part 1: Type 1 salts. J. Supercrit. Fluids 2010, 52, 99–112. [Google Scholar] [CrossRef]
- Reimer, J.; Peng, G.; Viereck, S.; De Boni, E.; Breinl, J.; Vogel, F. A novel salt separator for the supercritical water gasification of biomass. J. Supercrit. Fluids 2016, 117, 113–121. [Google Scholar] [CrossRef]
- Viereck, S.; Jovanovic, Z.R.; Haselbacher, A.; Steinfeld, A. Investigation of Na2SO4 removal from a supercritical aqueous solution in a dip-tube salt separator. J. Supercrit. Fluids 2018, 133, 146–155. [Google Scholar] [CrossRef]
- Lyckeskog, H.N. Hydrothermal Liquefaction of Lignin into Bio-Oil Influence of the Reaction Conditions and Stability of the Bio-Oil Produced; Chalmers University of Technology: Gothenburg, Sweden, 2016. [Google Scholar]
- Basu, P. Hydrothermal Conversion of Biomass. In Biomass Gasification, Pyrolysis and Torrefaction—Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018; pp. 331–372. [Google Scholar]
- Sasaki, M.; Goto, M. Recovery of phenolic compounds through the decomposition of lignin in near and supercritical water. Chem. Eng. Process. Process Intensif. 2008, 47, 1609–1619. [Google Scholar]
- Schuler, J.; Hornung, U.; Dahmen, N.; Sauer, J. Lignin from bark as a resource for aromatics production by hydrothermal liquefaction. GCB Bioenergy 2019, 11, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Yong, T.L.-K.; Matsumura, Y. Kinetic Analysis of Lignin Hydrothermal Conversion in Sub- and Supercritical Water. Ind. Eng. Chem. Res. 2013, 52, 5626–5639. [Google Scholar] [CrossRef]
- Yong, T.L.-K.; Matsumura, Y. Reaction Kinetics of the Lignin Conversion in Supercritical Water. Ind. Eng. Chem. Res. 2012, 51, 11975–11988. [Google Scholar] [CrossRef]
- Forchheim, D. Optimisation of the Reaction Parameters in a Batch Reactor and a CSTR for the Recovery of Phenol from Hydrothermal Biomass Liquefaction. 2014. Available online: https://publikationen.bibliothek.kit.edu/1000042417 (accessed on 27 August 2018).
- Forchheim, D.; Hornung, U.; Kruse, A.; Sutter, T. Kinetic Modelling of Hydrothermal Lignin Depolymerisation. Waste Biomass Valorization 2014, 5, 985–994. [Google Scholar] [CrossRef]
- Tran, K.-Q. Fast hydrothermal liquefaction for production of chemicals and biofuels from wet biomass – The need to develop a plug-flow reactor. Bioresour. Technol. 2016, 213, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hallen, R.T.; Sealock, L.J. Aqueous catalyst systems for the water-gas shift reaction. 2. Mechanism of basic catalysis. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 431–435. [Google Scholar] [CrossRef]
- Elliott, D.C.; Sealock, L.J. Aqueous catalyst systems for the water-gas shift reaction. 1. Comparative catalyst studies. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 426–431. [Google Scholar] [CrossRef]
- Tomani, P. The lignoboost process. Cellul. Chem. Technol. 2010, 44, 53. [Google Scholar]
- Lee, H.; Jae, J.; Ha, J.-M.; Suh, D.J. Hydro- and solvothermolysis of kraft lignin for maximizing production of monomeric aromatic chemicals. Bioresour. Technol. 2016, 203, 142–149. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Åmand, L.-E.; Vamling, L.; Olausson, L.; Andersson, S.-I.; Theliander, H. The effect of temperature on the catalytic conversion of Kraft lignin using near-critical water. Bioresour. Technol. 2014, 170, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugano, M.; Takagi, H.; Hirano, K.; Mashimo, K. Hydrothermal liquefaction of plantation biomass with two kinds of wastewater from paper industry. J. Mater. Sci. 2008, 43, 2476–2486. [Google Scholar] [CrossRef]
- Cheng, S.; Wilks, C.; Yuan, Z.; Leitch, M.; Xu, C. (Charles) Hydrothermal degradation of alkali lignin to bio-phenolic compounds in sub/supercritical ethanol and water–ethanol co-solvent. Polym. Degrad. Stab. 2012, 97, 839–848. [Google Scholar] [CrossRef]
- Belkheiri, T.; Vamling, L.; Dieu, T.; Lyckeskog, H.; Maschietti, M.; Olausson, L.; Andersson, S.-I.; Åmand, L.-E.; Theliander, H. Kraft Lignin Depolymerization in Near–Critical Water: Effect of Changing Co-Solvent. Cellulose Chem. Technol. 2014, 48, 813–818. [Google Scholar]
- Arturi, K.R.; Strandgaard, M.; Nielsen, R.P.; Søgaard, E.G.; Maschietti, M. Hydrothermal liquefaction of lignin in near-critical water in a new batch reactor: Influence of phenol and temperature. J. Supercrit. Fluids 2017, 123, 28–39. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal Liquefaction of Kraft Lignin in Subcritical Water: Influence of Phenol as Capping Agent. Energy Fuels 2018, 32, 5923–5932. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of kraft lignin in sub-critical water: The Influence of the Sodium and Potassium Fraction. Biomass Convers. Biorefinery 2018, 8, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.D.H.; Maschietti, M.; Belkheiri, T.; Åmand, L.E.; Theliander, H.; Vamling, L.; Olausson, L.; Andersson, S.I. Catalytic depolymerisation and conversion of Kraft lignin into liquid products using near-critical water. J. Supercrit. Fluids 2014, 86, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Belkheiri, T.; Mattsson, C.; Andersson, S.-I.; Olausson, L.; Åmand, L.-E.; Theliander, H.; Vamling, L. Effect of pH on Kraft Lignin Depolymerisation in Subcritical Water. Energy Fuels 2016, 30, 4916–4924. [Google Scholar]
- Solantausta, Y.; Anacker, C.; Armbruster, U.; Meier, D.; Appelt, J.; Martin, A.; Strüven, J.O.; Eidam, P.; Mirodatos, C.; Schuurman, Y.; et al. Liquid Fuels from Lignin by Hydrothermal Liquefaction and Deoxygenation (LIGNOHTL). Final Report. 2018. Available online: https://forestvalue.org/wp-content/uploads/2018/07/wwnet_jc4_final_reporting_lignoHTL.pdf (accessed on 3 May 2019).
- Schuler, J.; Hornung, U.; Kruse, A.; Dahmen, N.; Sauer, J. Hydrothermal Liquefaction of Lignin. J. Biomater. Nanobiotechnol. 2017, 08, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Sato, T.; Inomata, H.; Smith, R.L.; Arai, K.; Kruse, A.; Dinjus, E. Chemical Reactions of C 1 Compounds in Near-Critical and Supercritical Water. Chem. Rev. 2004, 104, 5803–5822. [Google Scholar] [CrossRef] [PubMed]
- Dieu Huyen, T.; Maschietti, M.; Dieu Huyen Nguyen, T.; Belkheiri, T.; Åmand, L.-E.; Theliander, H.; Vamling, L.; Olausson, L.; Andersson, S.-I. Catalytic hydrothermal conversion of LignoBoost Kraft lignin for the production of bio-oil and aromatic chemicals. In Proceedings of the International Chemical Recovery Conference, Tampere, Finland, 8–13 June 2014; Nieminen, M., Lampinen, P., Eds.; Finnish Recovery Boiler Committee: Tampere, Finland, 2014; Volume 2, pp. 252–261. [Google Scholar]
- Välimäki, A. Mustalipeän hydrotermisen nesteytyksen vaikutus bioöljyn laatuun (Improving bio-oil quality in hydrothermal liquefaction of black liquor). Master’s Thesis, Aalto University, Aalto, Finland, 2017. [Google Scholar]
- Sricharoenchaikul, V. Assessment of black liquor gasification in supercritical water. Bioresour. Technol. 2009, 100, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Barham, D.; Reeve, D.W. Chloride and Potassium in the Kraft Recovery Cycle: A Practical Guide. Pulp Pap. Can. Ont. 1990, 91, 185–190. [Google Scholar]
- Minowa, T.; Zhen, F.; Ogi, T. Cellulose decomposition in hot-compressed water with alkali or nickel catalyst. J. Supercrit. Fluids 1998, 13, 253–259. [Google Scholar] [CrossRef]
- Melin, K.; Välimäki, A.; Oasmaa, A.; Lehtonen, J. The Effect of Hydrothermal Liquefaction of Black Liquor in Bio-oil Quality. Eur. Biomass Conf. Exhib. Proc. 2019, 1144–1145. [Google Scholar]
- Peng, C.; Zhang, G.; Han, J.; Li, X. Hydrothermal conversion of lignin and black liquor for phenolics with the aids of alkali and hydrogen donor. Carbon Resour. Convers. 2019, 2, 141–150. [Google Scholar] [CrossRef]
- Osada, M.; Hiyoshi, N.; Sato, O.; Arai, K.; Shirai, M. Effect of Sulfur on Catalytic Gasification of Lignin in Supercritical Water. Energy Fuels 2007, 21, 1400–1405. [Google Scholar]
- Al’myasheva, O.V.; Korytkova, E.N.; Maslov, A.V.; Gusarov, V. Preparation of Nanocrystalline Alumina under Hydrothermal Conditions. Inorg Mater 2005, 41, 460–467. [Google Scholar] [CrossRef]
- Kauppi, E.I.; Honkala, K.; Krause, A.O.I.; Kanervo, J.M.; Lefferts, L. ZrO2 Acting as a Redox Catalyst. Top. Catal. 2016, 59, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Forchheim, D.; Hornung, U.; Kempe, P.; Kruse, A.; Steinbach, D. Influence of RANEY Nickel on the Formation of Intermediates in the Degradation of Lignin. Int. J. Chem. Eng. 2012, 2012, 589749. [Google Scholar] [CrossRef] [Green Version]
- Hupa, M. Recovery boiler chemical principles. TAPPI Kraft Recover. Course 2007, 2007, 2. [Google Scholar]
- Buncel, E.; Stairs, R.A. Solvent Effects in Chemistry; Wiley Publishing: Hoboken, NJ, USA, 2015; ISBN 9781119030980. [Google Scholar]
- Isa, K.M.; Abdullah, T.A.T.; Ali, U.F.M. Hydrogen donor solvents in liquefaction of biomass: A review. Renew. Sustain. Energy Rev. 2018, 81, 1259–1268. [Google Scholar] [CrossRef]
- Okuda, K.; Umetsu, M.; Takami, S.; Adschiri, T. Disassembly of lignin and chemical recovery—Rapid depolymerization of lignin without char formation in water–phenol mixtures. Fuel Process. Technol. 2004, 85, 803–813. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Effects of various solvents on the liquefaction of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2008, 49, 3498–3504. [Google Scholar] [CrossRef]
- Wu, X.Y.; Lü, X.Y. Hydrolysis kinetics of benzyl phenyl ether in high temperature liquid water. Chin. Chem. Lett. 2011, 22, 733–737. [Google Scholar] [CrossRef]
- Sasaki, M.; Goto, M. Conversion of biomass model compound under hydrothermal conditions using batch reactor. Fuel 2009, 88, 1656–1664. [Google Scholar] [CrossRef]
- Wu, X.; Fu, J.; Lu, X. Kinetics and Mechanism of Hydrothermal Decomposition of Lignin Model Compounds. Ind. Eng. Chem. Res. 2013, 52, 5016–5022. [Google Scholar] [CrossRef]
- Sato, T.; Sekiguchi, G.; Saisu, M.; Watanabe, M.; Adschiri, T.; Arai, K. Dealkylation and Rearrangement Kinetics of 2-Isopropylphenol in Supercritical Water. Ind. Eng. Chem. Res. 2002, 41, 3124–3130. [Google Scholar] [CrossRef]
- Penninger, J.M.; Kersten, R.J.; Baur, H.C. Reactions of diphenylether in supercritical water—mechanism and kinetics. J. Supercrit. Fluids 1999, 16, 119–132. [Google Scholar] [CrossRef]
- Schuler, J. Hydrothermal Liquefaction of Lignin. Ph.D. Thesis, Karlsruhe Institute of Technology, Karlsruhe, Germany, 2019. [Google Scholar]
- Timko, M.T.; Ghoniem, A.F.; Green, W.H. Upgrading and desulfurization of heavy oils by supercritical water. J. Supercrit. Fluids 2015, 96, 114–123. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, Y.; Lin, Y. The catalytic effects of minerals on aquathermolysis of heavy oils. Fuel 2004, 83, 2035–2039. [Google Scholar] [CrossRef]
- Scott, D.; Radlein, D.; Piskorz, J.; Majerski, P.; DeBruijn, T.J. Upgrading of bitumen in supercritical fluids. Fuel 2001, 80, 1087–1099. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Nichols, D.A.; Siskin, M.; Murugan, R.; Balasubramanian, M. Reactions in High-Temperature Aqueous Media. Chem. Rev. 2001, 101, 837–892. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Murugan, R.; Balasubramanian, M.; Greenhill, J.V.; Siskin, M.; Brons, G. Aqueous High-Temperature Chemistry of Carbo—And Heterocycles 16 1 Model Sulfur Compounds: A Study of Hydrogen Sulfide Generation. Energy Fuels 1991, 823–834. [Google Scholar] [CrossRef]
- Yang, B.; Tian, S.; Zhao, S. A study of thermal decomposition of alkanethiols in pressure reactor. Fuel Process. Technol. 2006, 87, 673–678. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Timko, M.T.; Class, C.A.; Bonomi, R.E.; Kida, Y.; Hernandez, H.H.; Tester, J.W.; Green, W.H. Supercritical water desulfurization of organic sulfides is consistent with free-radical kinetics. Energy Fuels 2013, 27, 6108–6117. [Google Scholar] [CrossRef]
- Vogelaar, B.M.; Makkee, M.; Moulijn, J.A. Applicability of supercritical water as a reaction medium for desulfurisation and demetallisation of gasoil. Fuel Process. Technol. 1999, 61, 265–277. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Barcock, R.A.; Balasubramanian, M.; Greenhill, J.V.; Siskin, M.; Olmstead, W.N. Aqueous High-Temperature Chemistry of Carbo- and Heterocycles. 21. Reactions of Sulfur-Containing Compounds in Supercritical Water at 460 °C. Energy Fuels 1994, 8, 498–506. [Google Scholar] [CrossRef]
- Kida, Y.; Class, C.A.; Concepcion, A.J.; Timko, M.T.; Green, W.H. Combining experiment and theory to elucidate the role of supercritical water in sulfide decomposition. Phys. Chem. Chem. Phys. 2014, 16, 9220–9228. [Google Scholar] [CrossRef] [PubMed]
- Lewan, M.D. Sulphur-radical control on petroleum formation rates. Nature 1998, 391, 164–166. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Murugan, R.; Siskin, M. Aqueous high-temperature chemistry of carbo- and heterocycles. 15. Aquathermolysis of arenethiols and aryl sulfides in the presence and absence of sodium bisulfite. Energy Fuels 1990, 4, 577–584. [Google Scholar] [CrossRef]
- Goldstein, T.P.; Aizenshtat, Z. Thermochemical sulfate reduction a review. J. Therm. Anal. 1994, 42, 241–290. [Google Scholar] [CrossRef]
- Zhang, T.; Amrani, A.; Ellis, G.S.; Ma, Q.; Tang, Y. Experimental investigation on thermochemical sulfate reduction by H2S initiation. Geochim. Cosmochim. Acta 2008, 72, 3518–3530. [Google Scholar] [CrossRef]
- Yuan, S.; Chou, I.; Burruss, R.C.; Wang, X. Disproportionation and thermochemical sulfate reduction reactions in S–H2O–CH4 and S–D2O–CH4 systems from 200 to 340 °C at elevated pressures. Geochimica Cosmochimica Acta 2013, 118, 263–275. [Google Scholar] [CrossRef]
- Thom, J.; Anderson, G.M. The role of thermochemical sulfate reduction in the origin of Mississippi Valley-type deposits. I. Experimental results. Geofluids 2008, 16–26. [Google Scholar] [CrossRef]
- Meshoulam, A.; Ellis, G.S.; Said Ahmad, W.; Deev, A.; Sessions, A.L.; Tang, Y.; Adkins, J.F.; Liu, J.; Gilhooly, W.P.; Aizenshtat, Z.; et al. Study of thermochemical sulfate reduction mechanism using compound specific sulfur isotope analysis. Geochim. Cosmochim. Acta 2016, 188, 73–92. [Google Scholar] [CrossRef]
- Vaughan, D.J. Sulfide Mineralogy and Geochemistry: Introduction and Overview. Rev. Mineral. Geochemistry 2006, 61, 1–5. [Google Scholar] [CrossRef]
- Xia, X.; Ellis, G.S.; Ma, Q.; Tang, Y. Compositional and stable carbon isotopic fractionation during non-autocatalytic thermochemical sulfate reduction by gaseous hydrocarbons. Geochim. Cosmochim. Acta 2014, 139, 472–486. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.S.; Ma, Q.; Amrani, A.; Tang, Y. Kinetics of uncatalyzed thermochemical sulfate reduction by sulfur-free paraffin. Geochim. Cosmochim. Acta 2012, 96, 1–17. [Google Scholar] [CrossRef]
- Amrani, A.; Zhang, T.; Ma, Q.; Ellis, G.S.; Tang, Y. The role of labile sulfur compounds in thermochemical sulfate reduction. Geochim. Cosmochim. Acta 2008, 72, 2960–2972. [Google Scholar] [CrossRef]
- Ma, Q.; Ellis, G.S.; Amrani, A.; Zhang, T.; Tang, Y. Theoretical study on the reactivity of sulfate species with hydrocarbons. Geochim. Cosmochim. Acta 2008, 72, 4565–4576. [Google Scholar] [CrossRef]
- Ellis, A.; Giggenbach, W. Hydrogen sulphide ionization and sulphur hydrolysis in high temperature solution. Geochim. Cosmochim. Acta 1971, 35, 247–260. [Google Scholar] [CrossRef]
- Oana, S.; Ishikawa, H. Sulfur isotopic fractionation between sulfur and sulfuric acid in the hydrothermal solution of sulfur dioxide. Geochem. J. 1966, 1, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Yakaboylu, O.; Harinck, J.; Gerton Smit, K.G.; de Jong, W. Supercritical water gasification of manure: A thermodynamic equilibrium modeling approach. Biomass and Bioenergy 2013, 59, 253–263. [Google Scholar] [CrossRef]
- Meier, D.; Windt, M. Analysis of Bio-Oils. In Transformation of Biomass: Theory to Practice; Hornung, A., Ed.; John Wiley & Sons, Incorporate: Hoboken, NJ, USA, 2014; pp. 227–256. ISBN 9781118693643. [Google Scholar]
- Schobert, H. Composition, classification, and properties of petroleum. In Chemistry of Fossil Fuels and Biofuels; Cambridge University Press: Cambridge, UK, 2013; pp. 174–191. [Google Scholar]
- Demirbas, A.; Alidrisi, H.; Balubaid, M.A. API gravity, sulfur content, and desulfurization of crude oil. Pet. Sci. Technol. 2015, 33, 93–101. [Google Scholar] [CrossRef]
- Funkenbusch, L.T.; Mullins, M.E.; Vamling, L.; Belkhieri, T.; Srettiwat, N.; Winjobi, O.; Shonnard, D.R.; Rogers, T.N. Technoeconomic assessment of hydrothermal liquefaction oil from lignin with catalytic upgrading for renewable fuel and chemical production. Wiley Interdiscip. Rev. Energy Environ. 2019, 8, e319. [Google Scholar] [CrossRef] [Green Version]
- Ong, B.H.Y.; Walmsley, T.G.; Atkins, M.J.; Walmsley, M.R.W. Hydrothermal liquefaction of Radiata Pine with Kraft black liquor for integrated biofuel production. J. Clean. Prod. 2018, 199, 737–750. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration Spot Prices for Crude Oil and Petroleum Products. Available online: https://www.eia.gov/dnav/pet/pet_pri_spt_s1_m.htm (accessed on 2 April 2019).
- Pedersen, T.H.; Hansen, N.H.; Pérez, O.M.; Cabezas, D.E.V.; Rosendahl, L.A. Renewable hydrocarbon fuels from hydrothermal liquefaction: A techno-economic analysis. Biofuels, Bioprod. Biorefining 2018, 12, 213–223. [Google Scholar] [CrossRef]
- Magdeldin, M.; Kohl, T.; Järvinen, M. Techno-economic assessment of the by-products contribution from non-catalytic hydrothermal liquefaction of lignocellulose residues. Energy 2017, 137, 679–695. [Google Scholar] [CrossRef]
- Tews, I.J.; Zhu, Y.; Drennan, C.; Elliott, D.C.; Snowden-Swan, L.J.; Onarheim, K.; Solantausta, Y.; Beckman, D. Biomass Direct Liquefaction Options. TechnoEconomic and Life Cycle Assessment; Pacific Northwest National Lab: Richland, WA, USA, 2014.
| Origin | Country | Density | Total Dry Solids | Total Hemicelluloses | Total Lignin | Ash | Sodium | Ref. |
|---|---|---|---|---|---|---|---|---|
| (kg/m3) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | |||
| Softwood (ND c) Black liquor | Sweden | 1095 | 183 | 3.56 | 63.8 | 84.3 | 37.8 | [46] |
| Softwood (Pine) Black liquor | Finland | 1128 | 218 | 3.6a | 56.3 | -b | 28.8 | [47] |
| C | H | O | N | S | Na | K | Cl | Others |
|---|---|---|---|---|---|---|---|---|
| wt. % | wt. % | wt. % | wt. % | wt. % | wt. % | wt. % | wt. % | wt. % |
| 38 | 3.6 | 33.1 | 0.1 | 4.8 | 19.1 | 0.9 | 0.2 | 0.2 |
| Na2S | Na2SO4 | Na2S2O3 | Na2SO3 | NaOH | Na2CO3 | Others |
|---|---|---|---|---|---|---|
| wt. % | wt. % | wt. % | wt. % | wt. % | wt. % | wt. % |
| 17 | 12 | 14 | 7 | 6 | 32 | 12 |
| Ions | HO− | Cl− | CO32− | SO42− | S2O32− | SO32− | HS− | S2− |
|---|---|---|---|---|---|---|---|---|
| Mg2+ | 2 | 1 | 2 | 2 | Not stable a | - | - | - |
| Ca2+ | 2 | 1 | 2 | 2 | - | - | - | |
| Na+ | 1 | 1 | 2 | 2 | - | 1 | 1 | |
| K+ | 1 | 1 | 1 | 2 | - | - | - |
| Feed | Reactor Type | Size/Capa-City | Moi-Sture | C | H | O a | S | N | Na | Ash | HHV | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | (MJ/kg) | ||||
| Kraft lignin | Batch | 30 mL | - | - | - | - | - | - | - | - | - | [106] |
| Ligno-Boost lignin | Continuous, catalytic Fixed Bed Reactor | 1 kg/hr | 3.6 | 65.6 | 5.7 | 26 | 1.85 | - | - | 0.8 | 27.7 | [107] |
| Black liquor | Batch | 10 mL | - | 61.8 | 6.1 | 23.0 | 2.29 | 0.31 | 0.2 | - | - | [58] |
| Black liquor | Batch | 200 mL | - | 30.5 | 3.2 | 66.7 b | 0 | - | 6.5 | 2.5 | - | [108] |
| Alkaline lignin | Batch | 14 mL | - | 49 | 4.4 | 46.6 b | 0 | - | - | - | [109] | |
| Ligno-Boost lignin | Contnuous, catalytic Fixed Bed Reactor | 1 kg/hr | 32.6 | 65.6 | 5.7 | 26 | - | - | - | 0.8 | 27.7 | [110] |
| Alkaline lignin | Batch | 5 mL | - | - | - | - | - | - | - | - | - | [95] |
| Indulin AT | CSTR c | 99 mL | 3.6 | 64.4 | 6.7 | 27.1 | 1.8 | - | - | 1.9 | 26.8 | [111] |
| Ligno-Boost lignin | Contnuous, catalytic Fixed Bed Reactor | 2 kg/hr | 32.6 | 65.6 | 5.7 | 26 | 1.8 | - | 0.2 | 0.8 | 27.7 | [112,113] |
| Ligno-Boost lignin | Contnuous, catalytic Fixed Bed Reactor | 1 kg/hr | 32.6 | 65.6 | 5.7 | 26 | 1.8 | - | 0.2 | 0.8 | 27.7 | [114,115] |
| Kraft Black liqour | Batch | 1000 mL | 66 | 33.7 | 3.9 | 38.4 | 4.3 | 0.1 | 18.0 | 50.1 | 14.1 | [116] |
| Feed | Reactor | Max Mass Yield a | Energy Yield d | Process | Resi-Dence Time | Ref. |
|---|---|---|---|---|---|---|
| Type | Conditions | |||||
| Kraft lignin (Sigma-Aldrich) | Batch | 62 wt.% | - | Temperature 623 K | 5 min | [106] |
| LignoBoost lignin | Continuous, catalytic Fixed Bed Reactor | 87.7 wt.% | 98.5% | Temperature 583 K, Pressure 25 MPa, Phenol 4.0 wt.%, K2CO3 1.6 wt.%, Solid catalyst: ZrO2 | 10–13 min | [107,119] |
| Black liquor | Batch | 78 wt.% | - | Temperatures of 643 K and 653 K corresponding pressures of ~ 340 bar and ~410 bar, initial lignin 5 wt.% | 1 h | [58] |
| Alkali lignin (Sigma-Aldrich) | Batch | 90 wt.% b | - | Temperature 593 K, ethanol 50% (vol-%), Initial H2 pressure 5 MPa | 2 h | [109] |
| Kraft Black Liquor (soft wood) | Batch | 70 wt.% c | 52.2% d | Temperature 623 K, Na2CO3 addition 13.2 wt.% of black liquor dry matter | 45 min | [116] |
| Feedstock | Reaction Condition | Density (g/cm3) | Reaction Time | ||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 60 min | 240 min | |||
| Alkaline lignin | Batch 623 K | 0.62 | 0.2 wt.% | 0.4 wt.% | 0.9 wt.% | 1.3 wt.% | 2.5 wt.% |
| 0.67 | 0.3 wt.% | 0.6 wt.% | 1.3 wt.% | 1.6 wt.% | 3.3 wt.% | ||
| Batch 673 K | 0.16 | 4.5 wt.% | 5.0 wt.% | 6.0 wt.% | 7.1 wt.% | 8.5 wt.% | |
| 0.35 | 4.5 wt.% | 5.5 wt.% | 6.3 wt.% | 7.5 wt.% | 10.2 wt.% | ||
| 0.47 | 4.5 wt.% | 5.5 wt.% | 6.3 wt.% | 7.5 wt.% | 10.5 wt.% | ||
| 0.52 | 4.5 wt.% | 6.1 wt.% | 8.0 wt.% | 8.0 wt.% | 11.8 wt.% | ||
| Feed | Catalysts | T (K) | P (MPa) | Solvents | Max bio-Oil Yield | Optimal Catalyst | Reac-tion Time | Ref |
|---|---|---|---|---|---|---|---|---|
| Ligno-boost lignin | K2CO3, KOH, Na2CO3, NaOH | 623 | 25 | Phenol 4 wt.% | 73.8 wt.% | Na/(Na + K) ratios of 0 and 1.0 | 6 min | [113] |
| Ligno-boost lignin | K2CO3, ZrO2 (pellets) | 623 | 25 | Phenol 4.0–4.7 wt.% | 72.2 wt.% | K2CO3 1.6 wt.% | 11 min | [114] |
| Feed-stock | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Alkali lignin (from Sigma-Aldrich) | Reaction condition | Solvent | Ethanol (vol%) | 0 | 25 | 50 | 75 | 100 | [109] |
| Batch, 573 K, 4 h | Yield (wt.%) | Degraded lignin | 49.0 | 51.0 | 87.0 | 48.0 | 15.0 | ||
| Water soluble product | 8.0 | 10.0 | 4.0 | 5.0 | 10.0 | ||||
| Solid residue | 13.0 | 25.0 | 2.0 | 31.0 | 55.0 | ||||
| Total gas | 2.0 | 6.0 | 4.0 | 5.0 | 3.0 | ||||
| Kraft lignin (from Sigma-Aldrich) | Reaction condition | Solvent | Water-to-Ethanol ratio (wt.%/ wt.%) | 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | [106] |
| Batch, 573 K, 30 min | Yield (wt.%) | Bio-oil | 29.0 | 64.0 | 69.0 | 23.0 | 19.0 | ||
| Solid residue | 57.0 | 20.0 | 16.0 | 66.0 | 63.0 | ||||
| Content in bio-oil | MAC | 12.0 | 8.0 | 10.0 | 27.0 | 36.0 | |||
| Batch, 623 K, 30 min | Yield (wt.%) | Bio-oil | 35.0 | 47.0 | 23.0 | 22.0 | 25.0 | ||
| Solid residue | 40.0 | 19.0 | 48.0 | 50.0 | 53.0 | ||||
| Content in bio-oil | MAC | 12.0 | 7.0 | 11.0 | 14.0 | 17.5 |
| Feedstock | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Indulin AT | Reaction condition | Solvent | Phenol (wt.%) | 0.00 | 3.40 | 6.50 | 9.70 | [111] | |
| Batch, 573 K, 200 bar, 15 min, K2CO3 | Yield (wt.%) (phenol-free basis) | Biocrude | 33.9 | 77.3 | 87.6 | 102.3 | |||
| WSO | 0.2 | 4.4 | 4.9 | 3.5 | |||||
| Insolubles | 54.4 | 11.3 | 7.1 | 1.4 | |||||
| C-recovery | 86.2 | 102.3 | 82.8 | 95.5 | |||||
| Total yield (wt.%) | 1-ring aromatics | 0.8 | 8.0 | 10.8 | 11.5 | ||||
| 2-ring aromatics | 0.0 | 1.0 | 1.6 | 9.7 | |||||
| Elemental composition (wt.%) | C | 69.9 | 67.3 | 72.0 | 72.3 | ||||
| H | 5.7 | 6.1 | 6.4 | 6.2 | |||||
| S | 0.6 | 0.7 | 0.7 | 0.6 | |||||
| O | 23.6 | 25.9 | 21.0 | 20.5 | |||||
| Higher heating value (MJ/kg) | HHV | 29.8 | 30.4 | 31.1 | 31.2 | ||||
| Ligno-Boost Lignin | Reaction condition | Solvent | Phenol (wt.%) | 2.00 | 3.00 | 4.00 | 5.00 | 10.00 | [112] |
| Continuous, catalytic Fixed Bed Reactor, 623 K, 250 bar, 15 min, K2CO3 | Yield (wt.%) (phenol-free basis) | Bio-oil | 62.7 | 59.3 | 58.4 | 63.6 | 58.3 | ||
| WSO | 21.3 | 20.9 | 26.6 | 25.5 | 14.7 | ||||
| Char | 13.8 | 13.6 | 14.6 | 12.6 | 12.3 | ||||
| Total gas | 2.0 | 6.0 | 4.0 | 5.0 | 3.0 | ||||
| Total yield (wt.%) | Catechols | 0.2 | 0.3 | 0.0 | 0.1 | 1.1 | |||
| Anisoles | 5.0 | 6.1 | 5.3 | 4.9 | 7.1 | ||||
| Alkyl Phenols | 2.4 | 3.4 | 3.2 | 3.3 | 4.6 | ||||
| Guaiacols | 3.9 | 3.6 | 2.9 | 2.5 | 1.4 | ||||
| Phenolic dimers | 0.8 | 1.6 | 1.9 | 3.2 | 2.9 | ||||
| Elemental composition (wt.%) | C | 73.9 | 72.9 | 74.3 | 72.1 | 77.2 | |||
| H | 6.9 | 6.3 | 6.6 | 6.3 | 6.9 | ||||
| S | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | ||||
| O | 17.9 | 9.7 | 18.2 | 20.6 | 14.8 | ||||
| Na | 0.3 | 0.2 | 0.1 | 0.2 | 0.1 | ||||
| Higher heating value (MJ/kg) | HHV | 32.1 | 30.8 | 31.8 | 30.4 | 33.6 |
| Feed | Process Type | C | H | OA | S | Na | K | HHV | Mw | Mn | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | MJ/kg | g/mol | g/mol | |||
| Kraft lignin | Batch | 11.0–22.0 | 0.53 | 28.3–34.7 | 500–1160 | 200–450 | [106] | ||||
| Ligno-Boost lignin | CSTR | 70.0–76.0 | 6.1–6.8 | 15.0–21.0 | 0.25–0.56 | <0.01–0.011 | 1.7–1.4 | 31.03–32.73 | [107] | ||
| Black liquor | Batch | 80.7 | 8.6 | 10.45 | 0.45 | <0.01 | <0.01 | 550 | [58] | ||
| Alkaline lignin | Batch | 530–1453 | 260–566 | [109] | |||||||
| Ligno-Boost lignin | Continuous, catalytic Fixed Bed Reactor | [110] | |||||||||
| Ligno-Boost lignin | Continuous, catalytic Fixed Bed Reactor | 70.0–76.0 | 6.1–6.8 | 15.0–21.0 | 0.25–0.56 | <0.01–0.011 | 1.7–1.4 | 31.03–32.73 | [107] | ||
| Alkaline lignin | Batch | [95] | |||||||||
| Indulin AT | CSTR | 67.3–72.4 | 5.5–6.5 | 20.5–25.9 | 0.41--0.72 | 29.8–32.1 (dry) | [111] | ||||
| Ligno-Boost lignin | Continuous, catalytic Fixed Bed Reactor | 72.1–74.3 | 6.3–6.9 | 14.8–20.6 | 0.3–0.4 | 0.1–0.3 | 0.5–0.7 | 30.4–33.6 | [112] | ||
| Ligno-Boost lignin | Continuous, catalytic Fixed Bed Reactor | 72.4–74.3 | 6.0–6.6 | 18.3–20.3 | 0.3–0.4 | 0.1–1.0 | 0.03–0.5 | 30.0–31.2 | [113] | ||
| Ligno-Boost lignin | Continuous, catalytic Fixed Bed Reactor | 74.2–74.9 | 6.5–6.9 | 15–17 | 0.29–0.38 | <0.01 | 0.46–1.90 | 31.83–31.93 | [114] | ||
| Ligno-Boost lignin | Continuous, catalytic Fixed Bed Reactor | 74.0–75.8 | 5.7–6.8 | 13.8–17.3 | 0.2–0.4 | <0.01–0.02 | [115] |
| Feedstock | Conver-Sion Technology | Upgra-Ding | Integration | MSP of the Fuel ($/liter) | MSP of the Fuel ($/LGE) | Ref |
|---|---|---|---|---|---|---|
| Black liquor + Kraft lignin | HTL | HDT | Integrated with a pulp mill | 0.93–1.02 | [170] | |
| Black liquor + Radiata pine | HTL | HDT | Integrated with a pulp mill | 1.05 | 0.95 | [171] |
| Wood and glycerol | HTL | HDT | Stand-alone | 0.82–1.14 | [173] | |
| Forest residue | HTL | HDT | Stand-alone | 2.00 | [175] | |
| Forest residue | Pyrolysis | HDT | Stand-alone | 3.09 | [175] | |
| Lignocellulosic forest residue | HTL | HDO | Stand-alone | 0.85–2.38 A | [174] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lappalainen, J.; Baudouin, D.; Hornung, U.; Schuler, J.; Melin, K.; Bjelić, S.; Vogel, F.; Konttinen, J.; Joronen, T. Sub- and Supercritical Water Liquefaction of Kraft Lignin and Black Liquor Derived Lignin. Energies 2020, 13, 3309. https://doi.org/10.3390/en13133309
Lappalainen J, Baudouin D, Hornung U, Schuler J, Melin K, Bjelić S, Vogel F, Konttinen J, Joronen T. Sub- and Supercritical Water Liquefaction of Kraft Lignin and Black Liquor Derived Lignin. Energies. 2020; 13(13):3309. https://doi.org/10.3390/en13133309
Chicago/Turabian StyleLappalainen, Jukka, David Baudouin, Ursel Hornung, Julia Schuler, Kristian Melin, Saša Bjelić, Frédéric Vogel, Jukka Konttinen, and Tero Joronen. 2020. "Sub- and Supercritical Water Liquefaction of Kraft Lignin and Black Liquor Derived Lignin" Energies 13, no. 13: 3309. https://doi.org/10.3390/en13133309
APA StyleLappalainen, J., Baudouin, D., Hornung, U., Schuler, J., Melin, K., Bjelić, S., Vogel, F., Konttinen, J., & Joronen, T. (2020). Sub- and Supercritical Water Liquefaction of Kraft Lignin and Black Liquor Derived Lignin. Energies, 13(13), 3309. https://doi.org/10.3390/en13133309







