A Review on the Influence of CO2/Shale Interaction on Shale Properties: Implications of CCS in Shales
Abstract
:1. Introduction
1.1. Development of Shale Formations
1.2. Adsorption Capacity of CO2 in Shales
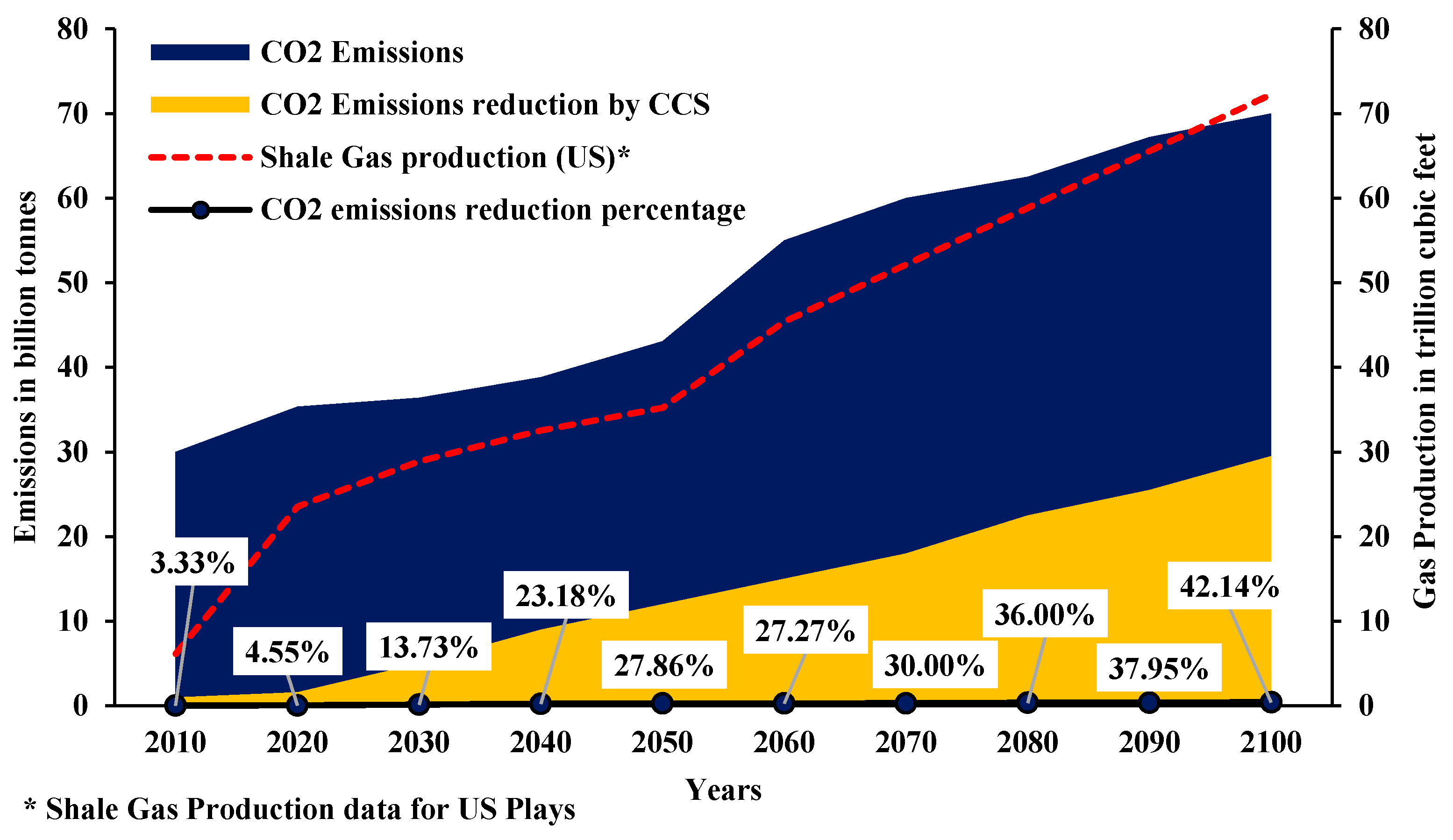
1.3. CCS in Shales
2. CO2-Shale Interaction
2.1. Pore Structure
2.2. Mineral Composition
2.3. Chemical Properties
2.4. Surface Wettability
2.5. Mechanical Properties
3. Environmental Evaluation of CCS
4. Economic Viability of CCS in Shales
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. Carbon Dioxide Capture and Storage; Cambridge Univ. Press: New York, NY, USA, 2005. [Google Scholar]
- de Silva, P.N.K.; Ranjith, P.G.; Choi, S.K. A study of methodologies for CO2 storage capacity estimation of coal. Fuel 2012, 91, 1–15. [Google Scholar] [CrossRef]
- Blunt, M.; Fayers, F.J.; Orr, F.M. Carbon dioxide in enhanced oil recovery. Energy Convers. Manag. 1993, 34, 1197–1204. [Google Scholar] [CrossRef]
- Lackner, K.S. A guide to CO2 sequestration. Science 2003, 300, 1677–1678. [Google Scholar] [CrossRef]
- Holloway, S. An overview of the underground disposal of carbon dioxide. Energy Convers. Manag. 1997, 38, 193–198. [Google Scholar] [CrossRef]
- Tao, Z.; Clarens, A. Estimating the carbon sequestration capacity of shale formations using methane production rates. Environ. Sci. Technol. 2013, 47, 11318–11325. [Google Scholar] [CrossRef]
- Kang, S.M.; Fathi, E.; Ambrose, R.J.; Akkutlu, I.Y.; Sigal, R.F. Carbon dioxide storage capacity of organic-rich shales. SPE J. 2011, 16, 842–855. [Google Scholar] [CrossRef] [Green Version]
- Perera, M.S.A. Influences of CO2 Injection into Deep Coal Seams: A Review. Energy Fuels 2017, 31, 10324–10334. [Google Scholar] [CrossRef]
- Merey, S.; Sinayuc, C. Analysis of carbon dioxide sequestration in shale gas reservoirs by using experimental adsorption data and adsorption models. J. Nat. Gas Sci. Eng. 2016, 36, 1087–1105. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Agarwal, R.K. Numerical simulation of long-term storage of CO2 in Yanchang shale reservoir of the Ordos basin in China. Chem. Geol. 2016, 440, 288–305. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, N.; Xian, X.; Zhou, L.; Tang, J.; Kang, Y.; Wang, H. Supercritical CO2 fracking for enhanced shale gas recovery and CO2 sequestration: Results, status and future challenges. Adv. Geo-Energy Res. 2019, 3, 207–224. [Google Scholar] [CrossRef] [Green Version]
- Guiltinan, E.J.; Cardenas, M.B.; Bennett, P.C.; Zhang, T.; Espinoza, D.N. The effect of organic matter and thermal maturity on the wettability of supercritical CO2 on organic shales. Int. J. Greenh. Gas Control 2017, 65, 15–22. [Google Scholar] [CrossRef]
- Zhan, J.; Soo, E.; Fogwill, A.; Cheng, S.; Cai, H.; Zhang, K.; Chen, Z. A systematic reservoir simulation study on assessing the feasibility of CO2 sequestration in shale gas reservoirs with potential enhanced gas recovery. Carbon Manag. Technol. Conf. C 2017 Glob. CCUS Innov. Nexus 2017, 1, 33–44. [Google Scholar]
- Hoffman, A.; Gustaf, O.; Andreas, L. Shale Gas and Hydraulic Fracturing: Framing the Water Issue; Ineko: Stockholm, Sweden, 2014; Volume 34. [Google Scholar]
- Nuttall, B.; Eble, C.; Drahovzal, J.; Bustin, R. Analysis of Devonian Black Shales in Kentucky for Potential Carbon Dioxide Sequestration and Enhanced Natural; Kentucky Geol. Surv.: Lexington, KY, USA, 2005. [Google Scholar]
- Li, X.; Feng, Z.; Han, G.; Elsworth, D.; Marone, C.; Saffer, D. Hydraulic Fracturing in Shale with H2O, CO2 and N2. In 49th US Rock Mechanics/Geomechanics Symposium; American Rock Mechanics Association: San Francisco, CA, USA, 2015; pp. 1–8. [Google Scholar]
- Zhenyun, S.; Weidong, S.; Yanzeng, Y.; Yong, L.; Zhihang, L.; Xiaoyu, W.; Li, Q.; Zhang, D.; Wang, Y. An experimental study on the CO2/sand dry-frac process. Nat. Gas Ind. B 2015, 1, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Hui, D.; Pan, Y.; Luo, P.; Zhang, Y.; Sun, L.; Lin, C. Effect of supercritical CO2 exposure on the high-pressure CO2 adsorption performance of shales. Fuel 2019, 247, 57–66. [Google Scholar] [CrossRef]
- EIA. Outlook for Shale gas and Tight Oil Development in the U.S.; Energy Information Administration: Washington, DC, USA, 2013. [Google Scholar]
- King, G.E. Thirty Years of Gas Shale Fracturing: What Have We Learned? In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: Florence, Italy, 2010. [Google Scholar]
- Wang, L.; Torres, A.; Xiang, L.; Fei, X.; Naido, A.; Wu, W. A Technical Review on Shale Gas Production and Unconventional Reservoirs Modeling. Nat. Resour. 2015, 06, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Vesovic, V.; Wakeham, W.; Olchowy, G.; Sengers, J.; Watson, J.; Millat, J. The transport properties of carbon dioxide. J. Phys. Chem. 1990, 19, 763–808. [Google Scholar] [CrossRef]
- Vermylen, J.P. Geomechanical Studies of the Barnett Shale. Ph.D Thesis, Stanford University, Stanford, CA, USA, 2011. [Google Scholar]
- Jin, Z.; Firoozabadi, A. Effect of water on methane and carbon dioxide sorption in clay minerals by Monte Carlo simulations. Fluid Phase Equilib. 2014, 382, 10–20. [Google Scholar] [CrossRef]
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Potential for enhanced gas recovery and CO2 storage in the Marcellus Shale in the Eastern United States. Int. J. Coal Geol. 2013, 118, 95–104. [Google Scholar] [CrossRef]
- Lyu, Q.; Long, X.; Ranjith, P.G.; Tan, J.; Kang, Y.; Wang, Z. Experimental investigation on the mechanical properties of a low-clay shale with different adsorption times in sub-/super-critical CO2. Energy 2018, 147, 1288–1298. [Google Scholar] [CrossRef]
- Liu, F.; Ellett, K.; Xiao, Y.; Rupp, J.A. Assessing the feasibility of CO2 storage in the New Albany Shale (Devonian-Mississippian) with potential enhanced gas recovery using reservoir simulation. Int. J. Greenh. Gas Control 2013, 17, 111–126. [Google Scholar] [CrossRef]
- Heller, R.; Zoback, M. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Unconv. Oil Gas Resour. 2014, 8, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Zhou, J.; Jiang, Y.; Xian, X.; Liu, Q. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 2016, 184, 289–303. [Google Scholar] [CrossRef]
- Luo, X.; Wang, S.; Wang, Z.; Jing, Z.; Lv, M.; Zhai, Z.; Han, T. Adsorption of methane, carbon dioxide and their binary mixtures on Jurassic shale from the Qaidam Basin in China. Int. J. Coal Geol. 2015, 150, 210–223. [Google Scholar] [CrossRef]
- Edwards, R.W.J.; Celia, M.A.; Bandilla, K.W.; Doster, F.; Kanno, C.M. A Model To Estimate Carbon Dioxide Injectivity and Storage Capacity for Geological Sequestration in Shale Gas Wells. Environ. Sci. Technol. 2015, 49, 9222–9229. [Google Scholar] [CrossRef]
- Levine, J.S.; Fukai, I.; Soeder, D.J.; Bromhal, G.; Dilmore, R.M.; Guthrie, G.D.; Rodosta, T.; Sanguinito, S.; Frailey, S.; Gorecki, C.; et al. U.S. DOE NETL methodology for estimating the prospective CO2 storage resource of shales at the national and regional scale. Int. J. Greenh. Gas Control 2016, 51, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Global CCS Institute, The Global Status os CCS 2018. Australia. 2018. Available online: https://www.globalccsinstitute.com (accessed on 26 March 2020).
- Wang, T.U.S. Shale Gas and Tight Oil Plays Production 1999–2050. 2020. Available online: https://www.statista.com/statistics/183740/shale-gas-production-in-the-united-states-since-1999/ (accessed on 7 June 2020).
- Wang, T. Forecast of Carbon Dioxide Emissions Worldwide from 2018 to 2050. 2019. Available online: https://www.statista.com/statistics/263980/forecast-of-global-carbon-dioxide-emissions/ (accessed on 7 June 2020).
- Andrews, R. Global CO2 Emissions Forecast to 2100. 2018. Available online: http://euanmearns.com/global-co2-emissions-forecast-to-2100/ (accessed on 7 June 2020).
- Rochelle, C.A.; Czernichowski-Lauriol, I.; Milodowski, A.E. The impact of chemical reactions on CO2 storage in geological formations: A brief review. Geol. Soc. Spec. Publ. 2004, 233, 87–106. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, D.; Luo, P.; Zhang, Y.; Sun, L.; Wang, K. Experimental Investigation of the Geochemical Interactions between Supercritical CO2 and Shale: Implications for CO2 Storage in Gas-Bearing Shale Formations. Energy Fuels 2018, 32, 1963–1978. [Google Scholar] [CrossRef]
- Wan, J.; Tokunaga, T.K.; Ashby, P.D.; Kim, Y.; Voltolini, M.; Gilbert, B.; DePaolo, D.J. Supercritical CO2 uptake by nonswelling phyllosilicates. Proc. Natl. Acad. Sci. USA 2018, 115, 873–878. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Zhou, J.; Xian, X.; Jiang, Y.; Lu, Z. Experimental study of the effects of sub- and super-critical CO2 saturation on the mechanical characteristics of organic-rich shales. Energy 2017, 132, 84–95. [Google Scholar] [CrossRef]
- Chiquet, P.; Broseta, D.; Thibeau, S. Wettability alteration of caprock minerals by carbon dioxide. Geofluids 2007, 7, 112–122. [Google Scholar] [CrossRef]
- Ao, X.; Lu, Y.; Tang, J.; Chen, Y.; Li, H. Investigation on the physics structure and chemical properties of the shale treated by supercritical CO2. J. CO2 Util. 2017, 20, 274–281. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Luo, Y.; Xian, X.; Liu, H.; Li, Y. Effect of Supercritical Carbon Dioxide Treatment Time, Pressure, and Temperature on Shale Water Wettability. Energ. Fuel 2017, 31, 493–503. [Google Scholar] [CrossRef]
- Roshan, H.; Al-Yaseri, A.Z.; Sarmadivaleh, M.; Iglauer, S. On wettability of shale rocks. J. Colloid Interface Sci. 2016, 475, 104–111. [Google Scholar] [CrossRef]
- Kaveh, N.S.; Barnhoorn, A.; Wolf, K.H. Wettability evaluation of silty shale caprocks for CO2 storage. Int. J. Greenh. Gas Control 2016, 49, 425–435. [Google Scholar] [CrossRef]
- Iglauer, S.; Al-yaseri, A.Z.; Rezaee, R.; Lebedev, M. CO2 wettability of caprocks: Implications for structural storage capacity and containment security. Geophys. Res. Lett. 2015, 42, 9279–9284. [Google Scholar] [CrossRef] [Green Version]
- Iglauer, S. CO2-Water-Rock Wettability: Variability, Influencing Factors, and Implications for CO2 Geostorage. Acc. Chem. Res. 2017, 50, 1134–1142. [Google Scholar] [CrossRef]
- Schoemaker, C.; Barnhoorn, A.; van Hemert, P. Sorption and CT Experiments on the Transport and Sealing Properties of Samples Relevant to Key Sites. CATO2 Program, CATO2-WP3.3-D18; 2011; p. 52. Available online: https://www.CO2-cato.org/publications/library1/sorption-and-ct-experiments (accessed on 29 May 2020).
- Lu, Y.; Chen, X.; Tang, J.; Li, H.; Zhou, L.; Han, S.; Ge, Z.; Xia, B.; Shen, H.; Zhang, J. Relationship between pore structure and mechanical properties of shale on supercritical carbon dioxide saturation. Energy 2019, 172, 270–285. [Google Scholar] [CrossRef]
- Luo, X.; Ren, X.; Wang, S. Supercritical CO2-water-shale interactions and their effects on element mobilization and shale pore structure during stimulation. Int. J. Coal Geol. 2019, 202, 109–127. [Google Scholar] [CrossRef]
- Rezaee, R.; Saeedi, A.; Iglauer, S.; Evans, B. Shale alteration after exposure to supercritical CO2. Int. J. Greenh. Gas Control 2017, 62, 91–99. [Google Scholar] [CrossRef]
- Busch, A.; Alles, S.; Gensterblum, Y.; Prinz, D.; Dewhurst, D.N.; Raven, M.D.; Stanjek, H.; Krooss, B.M. Carbon dioxide storage potential of shales. Int. J. Greenh. Gas Control 2008, 2, 297–308. [Google Scholar] [CrossRef]
- Chareonsuppanimit, P.; Mohammad, S.A.; Robinson, R.L.; Gasem, K.A.M. High-pressure adsorption of gases on shales: Measurements and modeling. Int. J. Coal Geol. 2012, 95, 34–46. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, Y.; Lu, Y.; Qin, C.; Liu, H. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 2016, 97, 173–181. [Google Scholar] [CrossRef]
- Lahann, R.; Mastalerz, M.; Rupp, J.A.; Drobniak, A. Influence of CO2 on New Albany Shale composition and pore structure. Int. J. Coal Geol. 2013, 108, 2–9. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, G.; Wang, Y.; Xing, Y. Experimental investigation on fracture propagation modes in supercritical carbon dioxide fracturing using acoustic emission monitoring. Int. J. Rock Mech. Min. Sci. 2018, 110, 111–119. [Google Scholar] [CrossRef]
- Goodman, A.; Sanguinito, S.; Tkach, M.; Natesakhawat, S.; Kutchko, B.; Fazio, J.; Cvetic, P. Investigating the role of water on CO2-Utica Shale interactions for carbon storage and shale gas extraction activities- Evidence for pore scale alterations. Fuel 2019, 242, 744–755. [Google Scholar] [CrossRef]
- Liu, F.; Lu, P.; Griffith, C.; Hedges, S.W.; Soong, Y.; Hellevang, H.; Zhu, C. CO2-brine-caprock interaction: Reactivity experiments on Eau Claire shale and a review of relevant literature. Int. J. Greenh. Gas Control 2012, 7, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Lan, Q.; Xu, M.; Binazadeh, M.; Dehghanpour, H.; Wood, J.M. A comparative investigation of shale wettability: The significance of pore connectivity. J. Nat. Gas Sci. Eng. 2015, 27, 1174–1188. [Google Scholar] [CrossRef]
- Burke, L.H.; Nevison, G.W.; Peters, W.E. Improved unconventional gas recovery with energized fracturing fluids: Montney example. In SPE Eastern Regional Meeting; Society of Petroleum Engineers: Columbus, OH, USA, 2011; pp. 316–325. [Google Scholar]
- Hsu, S.C.; Nelson, P.P. Characterization of eagle ford shale. Eng. Geol. 2002, 67, 169–183. [Google Scholar] [CrossRef]
- Morsy, S.; Sheng, J.J. Effect of Water Salinity on Shale Reservoir Productivity. Adv. Pet. Explor. Dev. 2014, 8, 9–14. [Google Scholar]
- Li, Z.; Jiang, Z.; Yu, H.; Liang, Z. Organic matter pore characterization of the Wufeng-Longmaxi shales from the fuling gas field, Sichuan Basin: Evidence from organic matter isolation and low-pressure CO2 and N2 adsorption. Energies 2019, 12, 1207. [Google Scholar] [CrossRef] [Green Version]
- Armitage, P.J.; Faulkner, D.R.; Worden, R.H. Caprock corrosion. Nat. Geosci. 2013, 6, 79–80. [Google Scholar] [CrossRef]
- Ozdemir, E.; Schroeder, K. Effect of moisture on adsorption isotherms and adsorption capacities of CO2 on coals. Energy Fuels 2009, 23, 2821–2831. [Google Scholar] [CrossRef] [Green Version]
- Jian, C.; Wenzhong, S.; Yihong, L. Natural Product of Supercritical CO2 Extraction; Chemical Industry Press: Beijing, China, 2005. [Google Scholar]
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Ma, Y.; Zhong, N.; Li, D.; Pan, Z.; Cheng, L.; Liu, K. International Journal of Coal Geology Organic matter/clay mineral intergranular pores in the Lower Cambrian Lujiaping Shale in the north-eastern part of the upper Yangtze area, China: A possible microscopic mechanism for gas preservation. Int. J. Coal Geol. 2015, 137, 38–54. [Google Scholar] [CrossRef]
- Pfeifer, P.; Avnir, D. Chemistry in noninteger dimensions between two and three. J. Chem. Phys. 1983, 79, 3558–3565. [Google Scholar] [CrossRef]
- Jarboe, P.J.; Candela, P.A.; Zhu, W.; Kaufman, A.J. Extraction of Hydrocarbons from High-Maturity Marcellus Shale Using Supercritical Carbon Dioxide. Energy Fuels 2015, 29, 7897–7909. [Google Scholar] [CrossRef]
- Alemu, B.L.; Aagaard, P.; Munz, I.A.; Skurtveit, E. Caprock interaction with CO2: A laboratory study of reactivity of shale with supercritical CO2 and brine. Appl. Geochem. 2011, 26, 1975–1989. [Google Scholar] [CrossRef]
- Nešić, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Lyu, Q.; Ranjith, P.G.; Long, X.; Ji, B. Experimental investigation of mechanical properties of black shales after CO2-water-rock interaction. Materials 2016, 9, 663. [Google Scholar] [CrossRef]
- Lin, H.; Fujii, T.; Takisawa, R.; Takahashi, T.; Hashida, T. Experimental evaluation of interactions in supercritical CO2/water/rock minerals system under geologic CO2 sequestration conditions. J. Mater. Sci. 2008, 43, 2307–2315. [Google Scholar] [CrossRef]
- Rempel, K.U.; Liebscher, A.; Heinrich, W.; Schettler, G. An experimental investigation of trace element dissolution in carbon dioxide: Applications to the geological storage of CO2. Chem. Geol. 2011, 289, 224–234. [Google Scholar] [CrossRef]
- Jean, J.S.; Wang, C.L.; Hsiang, H.I.; Li, Z.; Yang, H.J.; Jiang, W.T.; Yang, K.M.; Bundschuh, J. Experimental investigation of trace element dissolution in formation water in the presence of supercritical CO2 fluid for a potential geological storage site of CO2 in Taiwan. J. Nat. Gas Sci. Eng. 2015, 23, 304–314. [Google Scholar] [CrossRef]
- Chen, Y.; Furmann, A.; Mastalerz, M.; Schimmelmann, A. Quantitative analysis of shales by KBr-FTIR and micro-FTIR. FUEL 2014, 116, 538–549. [Google Scholar] [CrossRef]
- Lu, X.; Jin, D.; Wei, S.; Zhang, M.; Zhu, Q.; Shi, X.; Deng, Z.; Guo, W.; Shen, W. Competitive adsorption of a binary CO2-CH4 mixture in nanoporous carbons: Effects of edge-functionalization. Nanoscale 2015, 7, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xue, Q.; Li, X.; Tao, Y.; Jin, Y.; Ling, C.; Lu, S. Extraction of kerogen from oil shale with supercritical carbon dioxide: Molecular dynamics simulations. J. Supercrit. Fluids 2016, 107, 499–506. [Google Scholar] [CrossRef]
- Rao, D.N.; Girard, M.G. A new technique for reservoir wettability characterization. J. Can. Pet. Technol. 1996, 35, 31–39. [Google Scholar] [CrossRef]
- Anderson, W.G. Wettability Literature Survey—Part 2: Wettability Measurement. J. Pet. Technol. 1986, 38, 1246–1262. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, M.; Lin, M.; Peng, B.; Sun, L.; Chen, L. Investigation on variations in wettability of reservoir rock induced by CO2-brine-rock interactions. In SPE EUROPEC/EAGE Annual Conference and Exhibition; Society of Petroleum Engineers: Vienna, Austria, 2011; Volume 5, pp. 4027–4038. [Google Scholar]
- Mitchell, A.G.; Hazell, L.B.; Webb, K.J. Wettability Determination: Pore Surface Analysis; Society of Petroleum Engineers: New Orleans, LA, USA, 1990; pp. 351–360. [Google Scholar]
- Siddiqui, M.A.Q.; Ali, S.; Fei, H.; Roshan, H. Current understanding of shale wettability: A review on contact angle measurements. Earth-Sci. Rev. 2018, 181, 1–11. [Google Scholar] [CrossRef]
- Liang, L.; Luo, D.; Liu, X.; Xiong, J. Experimental study on the wettability and adsorption characteristics of Longmaxi Formation shale in the Sichuan Basin, China. J. Nat. Gas Sci. Eng. 2016, 33, 1107–1118. [Google Scholar] [CrossRef]
- Liang, L.; Xiong, J.; Liu, X. Journal of Natural Gas Science and Engineering Experimental study on crack propagation in shale formations considering hydration and wettability. J. Nat. Gas Sci. Eng. 2015, 23, 492–499. [Google Scholar] [CrossRef]
- Xu, M.; Dehghanpour, H. Advances in understanding wettability of tight and shale gas formations. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: Alberta, AB, Canada, 2014; Volume 7, pp. 5161–5179. [Google Scholar]
- Mirchi, V.; Saraji, S.; Goual, L.; Piri, M. Dynamic interfacial tension and wettability of shale in the presence of surfactants at reservoir conditions. Fuel 2015, 148, 127–138. [Google Scholar] [CrossRef]
- Yuan, W.; Li, X.; Pan, Z.; Connell, L.D.; Li, S.; He, J. Experimental investigation of interactions between water and a lower silurian chinese shale. Energy Fuels 2014, 28, 4925–4933. [Google Scholar] [CrossRef]
- Odusina, E.; Sondergeld, C.; Rai, C. An NMR Study on Shale Wettability. In SPE Canadian Unconventional Resources Conference; Society of Petroleum Engineers: Alberta, AB, Canada, 2011. [Google Scholar]
- Sun, Y.; Bai, B.; Wei, M. Microfracture and Surfactant Impact on Linear Cocurrent Brine Imbibition in Gas-Saturated Shale; Energy Fuels: Lakewood, CO, USA, 2015. [Google Scholar]
- Roychaudhuri, B.; Tsotsis, T.T.; Jessen, K. An experimental investigation of spontaneous imbibition in gas shales. J. Pet. Sci. Eng. 2013, 111, 87–97. [Google Scholar] [CrossRef]
- Lan, Q.; Ghanbari, E.; Dehghanpour, H.; Hawkes, R. Water Loss Versus Soaking Time: Spontaneous Imbibition in Tight Rocks. Energy Technol. 2014, 2, 1033–1039. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Lan, Q.; Saeed, Y.; Fei, H.; Qi, Z. Spontaneous imbibition of brine and oil in gas shales: Effect of water adsorption and resulting microfractures. Energy Fuels 2013, 27, 3039–3049. [Google Scholar] [CrossRef]
- Makhanov, K.; Habibi, A.; Dehghanpour, H.; Kuru, E. Journal of Unconventional Oil and Gas Resources Liquid uptake of gas shales: A workflow to estimate water loss during shut-in periods after fracturing operations. J. Unconv. Oil GAS Resour. 2014, 7, 22–32. [Google Scholar] [CrossRef]
- Arif, M.; Barifcani, A.; Lebedev, M.; Iglauer, S. Impact of Solid Surface Energy on Wettability of CO2-brine-Mineral Systems as a Function of Pressure, Temperature and Salinity. Energy Procedia 2017, 114, 4832–4842. [Google Scholar] [CrossRef]
- Arif, M.; Abu-Khamsin, S.A.; Iglauer, S. Wettability of rock/CO2/brine and rock/oil/CO2 -enriched-brine systems:Critical parametric analysis and future outlook. Adv. Colloid Interface Sci. 2019, 268, 91–113. [Google Scholar] [CrossRef]
- Broseta, D. Assessing Seal Rock Integrity for CO2 Geological Storage Purposes. In Geomechanics in CO2 Storage Facilities; ISTE: Pau, France, 2013; pp. 3–20. [Google Scholar]
- Pentland, C.H.; El-Maghraby, R.; Iglauer, S.; Blunt, M.J. Measurements of the capillary trapping of super-critical carbon dioxide in Berea sandstone. Geophys. Res. Lett. 2011, 38, L06401. [Google Scholar] [CrossRef] [Green Version]
- Krevor, S.; Blunt, M.J.; Benson, S.M.; Pentland, C.H.; Reynolds, C.; Al-Menhali, A.; Niu, B. Capillary trapping for geologic carbon dioxide storage—From pore scale physics to field scale implications. Int. J. Greenh. Gas Control 2015, 40, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Andrew, M.; Bijeljic, B.; Blunt, M.J. Pore-scale contact angle measurements at reservoir conditions using X-ray microtomography. Adv. Water Resour. 2014, 68, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, K.; Guiltinan, E.J.; Cardenas, M.B.; Maisano, J.A. Wettability measurement under high P-T conditions using X-ray imaging with application to the brine-supercritical CO2 system. Geochem. Geophys. Geosyst. 2015, 16, 2858–2864. [Google Scholar] [CrossRef] [Green Version]
- Bikkina, P.; Shaik, I. Interfacial Tension and Contact Angle Data Relevant to Carbon Sequestration; IntechOpen: London, UK, 2018. [Google Scholar]
- Feng, G.; Kang, Y.; Sun, Z.D.; Wang, X.C.; Hu, Y.Q. Effects of supercritical CO2 adsorption on the mechanical characteristics and failure mechanisms of shale. Energy 2019, 173, 870–882. [Google Scholar] [CrossRef]
- Madland, M.V.; Finsnes, A.; Alkafadgi, A.; Risnes, R.; Austad, T. The influence of CO2 gas and carbonate water on the mechanical stability of chalk. J. Pet. Sci. Eng. 2006, 51, 149–168. [Google Scholar] [CrossRef]
- Bennion, D.B.; Bachu, S. Drainage and imbibition relative permeability relationships for supercritical CO2/brine and H2S/brine systems in intergranular sandstone, carbonate, shale, and anhydrite rocks. SPE Reserv. Eval. Eng. 2008, 11, 487–496. [Google Scholar] [CrossRef]
- Farquhar, S.M.; Pearce, J.K.; Dawson, G.K.W.; Golab, A.; Sommacal, S.; Kirste, D.; Biddle, D.; Golding, S.D. A fresh approach to investigating CO2 storage: Experimental CO2-water-rock interactions in a low-salinity reservoir system. Chem. Geol. 2015, 399, 98–122. [Google Scholar] [CrossRef]
- Rutqvist, J.; Vasco, D.W.; Myer, L. Coupled reservoir-geomechanical analysis of CO2 injection and ground deformations at In Salah, Algeria. Int. J. Greenh. Gas Control 2010, 4, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Rutqvist, J.; Wu, Y.; Tsang, C.; Bodvarsson, G. A modeling approach for analysis of coupled multiphase fluid flow, heat transfer, and deformation in fractured porous rock. Int. J. Rock Mech. Min. Sci. 2002, 39, 429–442. [Google Scholar] [CrossRef]
- Aziz, N.I.; Ming-Li, W. The effect of sorbed gas on the strength of coal—An experimental study. Geotech. Geol. Eng. 1999, 17, 387–402. [Google Scholar] [CrossRef]
- Viete, D.R.; Ranjith, P.G. The effect of CO2 on the geomechanical and permeability behaviour of brown coal: Implications for coal seam CO2 sequestration. Int. J. Coal Geol. 2006, 66, 204–216. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G.; Bui, H. Super-critical CO2 saturation-induced mechanical property alterations in low rank coal: An experimental study. J. Supercrit. Fluids 2016, 109, 134–140. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G. Influence of CO2 adsorption on the strength and elastic modulus of low rank Australian coal under confining pressure. Int. J. Coal Geol. 2016, 167, 148–156. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Viete, D.R. Effects of gaseous and super-critical carbon dioxide saturation on the mechanical properties of bituminous coal from the Southern Sydney Basin. Appl. Energy 2013, 110, 73–81. [Google Scholar] [CrossRef]
- De Jong, S.M.; Spiers, C.J.; Busch, A. International Journal of Greenhouse Gas Control Development of swelling strain in smectite clays through exposure to carbon dioxide. Int. J. Greenh. Gas Control 2014, 24, 149–161. [Google Scholar] [CrossRef]
- Zhang, D.; Ranjith, P.G.; Perera, M.S.A. The brittleness indices used in rock mechanics and their application in shale hydraulic fracturing: A review. J. Pet. Sci. Eng. 2016, 143, 158–170. [Google Scholar] [CrossRef]
- Modahl, I.S.; Nyland, C.A.; Raadal, H.L.; Kårstad, O.; Torp, T.A.; Hagemann, R. Life cycle assessment of gas power with CCS—A study showing the environmental benefits of system integration. Energy Procedia 2011, 4, 2470–2477. [Google Scholar] [CrossRef] [Green Version]
- Khoo, H.H.; Tan, R.B.H. Life cycle investigation of CO2 recovery and sequestration. Environ. Sci. Technol. 2006, 40, 4016–4024. [Google Scholar] [CrossRef]
- Bauer, C.; Heck, T.; Dones, R.; Mayer-Spohn, O.; Blesl, M. NEEDS (New Energy Externalities Developments for Sustainability). In Final Report on Technical Data, Costs, and Life Cycle Inventories of Advanced Fossil Power Generation Systems; Paul Scherrer Institut (PSI): Villigen, Switzerland, 2009. [Google Scholar]
- Viebahn, P.; Nitsch, J.; Fischedick, M.; Esken, A.; Schüwer, D.; Supersberger, N.; Edenhofer, O. Comparison of carbon capture and storage with renewable energy technologies regarding structural, economic, and ecological aspects in Germany. Int. J. Greenhouse Gas Control 2007, 1, 121–133. [Google Scholar] [CrossRef]
- Schreiber, A.; Zapp, P.; Kuckshinrichs, W. Environmental assessment of German electricity generation from coal-fired power plants with amine-based carbon capture. Int. J. Life Cycle Assess. 2009, 14, 547–559. [Google Scholar] [CrossRef]
- Korre, A.; Nie, Z.; Durucan, S. Life cycle modelling of fossil fuel power generation with post-combustion CO2 capture. Int. J. Greenh. Gas Control 2010, 4, 289–300. [Google Scholar] [CrossRef]
- Marx, J.; Schreiber, A.; Zapp, P.; Haines, M.; Hake, J.F.; Gale, J. Environmental evaluation of CCS using Life Cycle Assessment—A synthesis report. Energy Procedia 2011, 4, 2448–2456. [Google Scholar] [CrossRef] [Green Version]
- IEA. Greenhouse Gas R&D Programme (IEA GHG): Environmental Impact of Solvent Scrubbing of CO2. 2006. Available online: https://ieaghg.org/docs/General_Docs/Reports/2006-14%20Environmental%20Impact%20of%20Solvent%20Scrubbing%20of%20CO2.pdf (accessed on 29 May 2020).
- Bradshaw, J.; Bachu, S.; Bonijoly, D.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Issues and development of standards. Int. J. Greenh. Gas Control 2007, 1, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y. Recent Advances in Carbon Capture and Storage; Janeza Trdine 9, 51000; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Tayari, F.; Blumsack, S.; Dilmore, R.; Mohaghegh, S.D. Techno-economic assessment of industrial CO2 storage in depleted shale gas reservoirs. J. Unconv. Oil Gas Resour. 2015, 11, 82–94. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Bielicki, J.M.; Langenfeld, J.K.; Tao, Z.; Middleton, R.S.; Menefee, A.H.; Clarens, A.F. The geospatial and economic viability of CO2 storage in hydrocarbon depleted fractured shale formations. Int. J. Greenh. Gas Control 2018, 75, 8–23. [Google Scholar] [CrossRef]
- Hoel, M.; Jensen, S. Cutting costs of catching carbon-Intertemporal effects under imperfect climate policy. Resour. Energy Econ. 2012, 34, 680–695. [Google Scholar] [CrossRef] [Green Version]
- Durmaz, T. The economics of CCS: Why have CCS technologies not had an international breakthrough? Renew. Sustain. Energy Rev. 2018, 95, 328–340. [Google Scholar] [CrossRef]
- Smith, L.A.; Gupta, N.; Sass, B.M.; Bubenik, T.A.; Byrer, C.; Bergman, P. Engineering and Economic Assessment of Carbon Dioxide Sequestration in Saline Formations. In Proceedings of the National Conference on Carbon Sequestration, Washington, DC, USA, 15–17 May 2001. [Google Scholar]
- Heddle, G.; Herzog, H.J.; Klett, M. The Economics of CO2 Storage; Massachusetts Institute of Technology Laboratory for Energy and the Environment: Massachusetts, MA, USA, 2003. [Google Scholar]
- Rubin, E.S.; Rao, A.B.; Chen, C. Comparative assessments of fossil fuel power plants with CO2 capture and storage. Greenh. Gas Control Technol. 2005, 7, 285–293. [Google Scholar]
- Holloway, S. Sequestration—The Underground Storage of Carbon Dioxide. In Climate Change and Energy Pathways for the Mediterranean; Moniz, E.J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 61–88. [Google Scholar]
- Finkenrath, M. Cost and Performance of Carbon Dioxide Capture from Power Generation. IEA Energy Pap. 2012, 35, 51. [Google Scholar] [CrossRef]
- Lafforgue, G.; Magné, B.; Moreaux, M. Energy substitutions, climate change and carbon sinks. Ecol. Econ. 2008, 67, 589–597. [Google Scholar] [CrossRef] [Green Version]
- REN21. Renewables 2017 Global Status Report; Technical report; Renewable Energy Policy Network for the 21st Century: Paris, France, 2017. [Google Scholar]
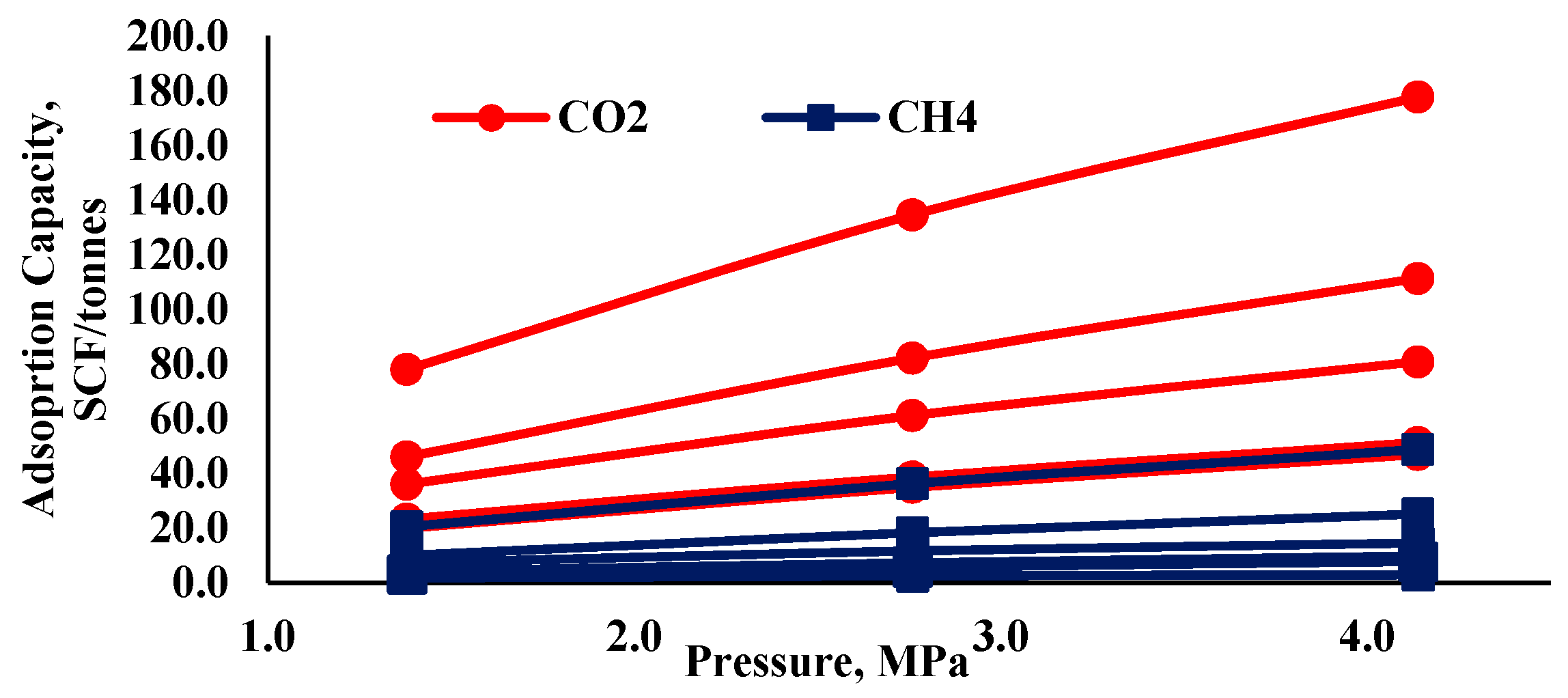

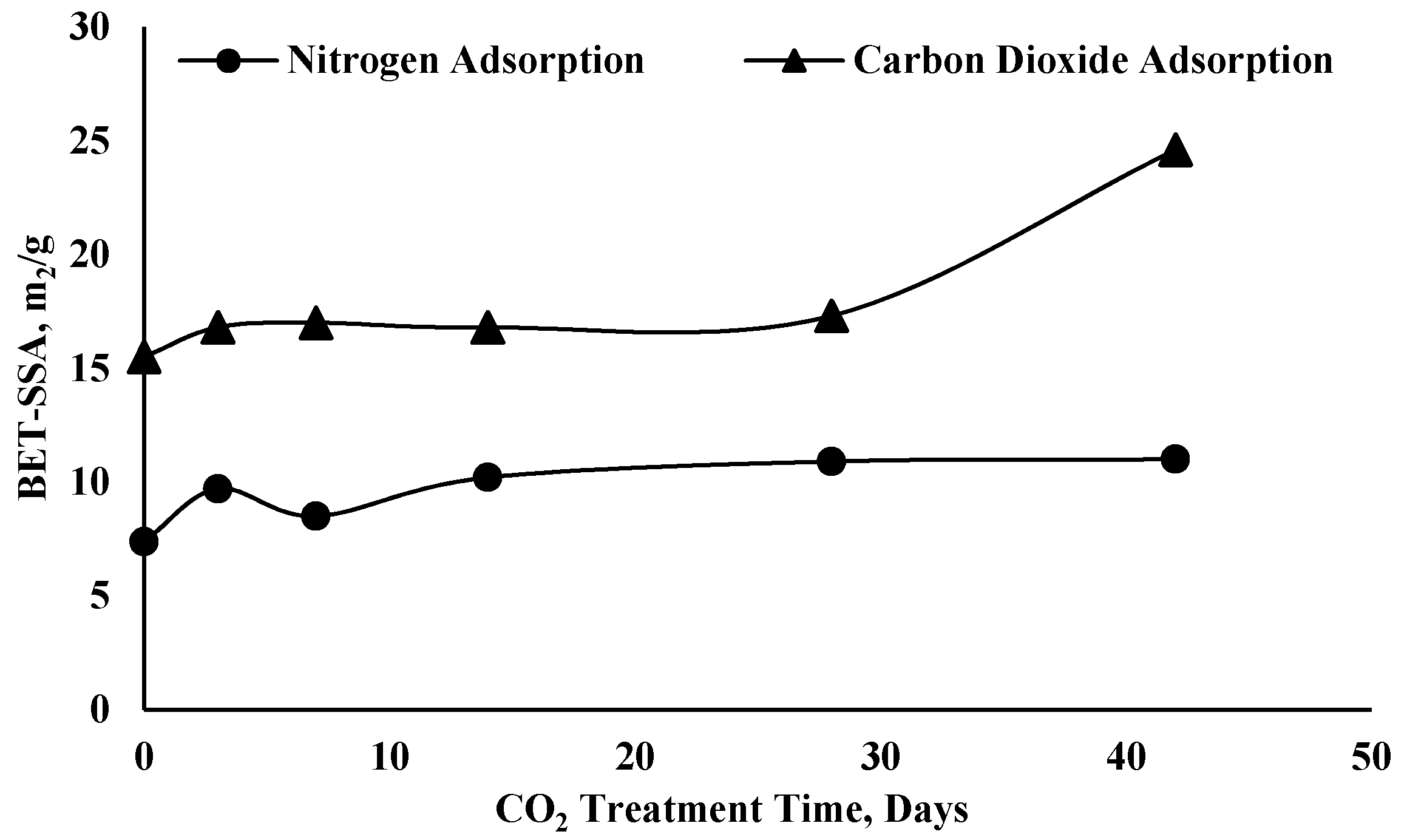
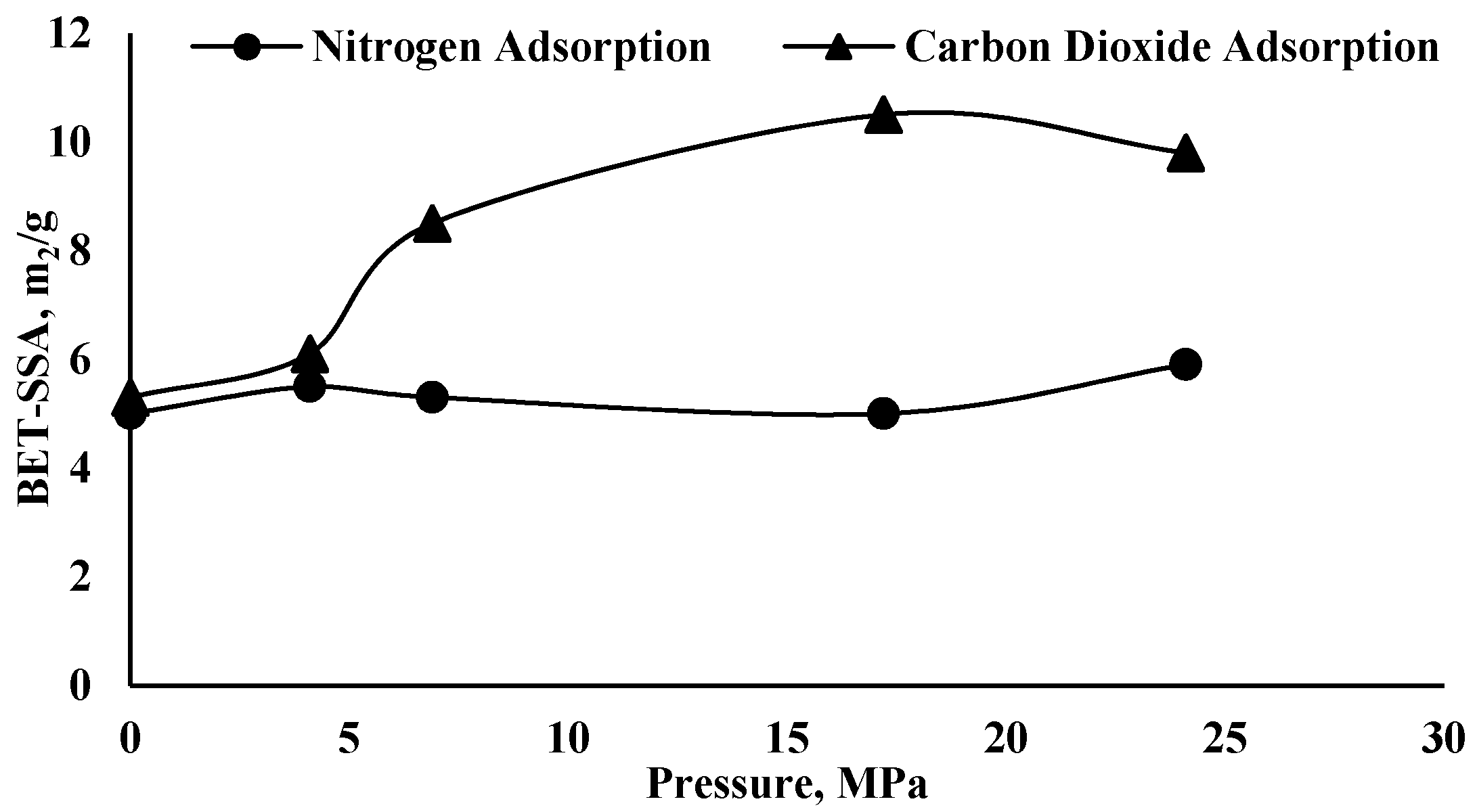
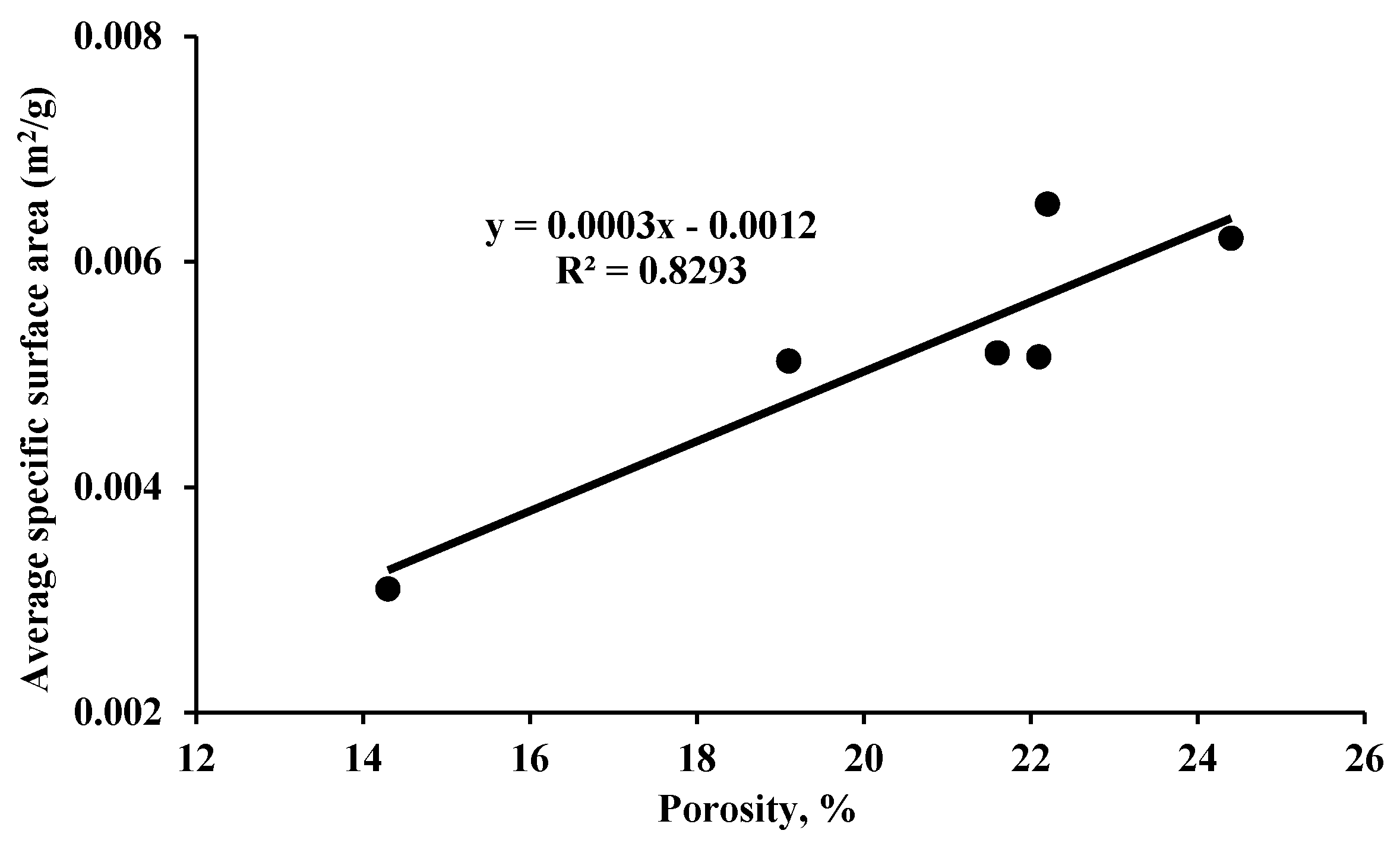
| Author(s) | Alteration % in Pore Structure Parameters | Name and Type of Shale Formation | Exposure Pressure, Temperature and Time | Main Findings |
|---|---|---|---|---|
| Lahann et al. [56] | SSA (49.05%) | New Albany (Organic-rich) | 4–24 MPa 150 °C 3–42 Days | They concluded that at high pressures, CO2 solubility in water increases due to the high dissolution of carbonate minerals. This is the main cause of increasing the SSA of the mesopore and micropore structure. |
| Jiang et al. [55] | SSA (99.39%); Porosity (58.33%) | Longmaxi (silty/organic-rich) | 8–18 MPa 40–90 °C 1–5 Days | This study showed that both shale porosity and SSA are increased with increasing SCCO2 treatment time and pressure, due to the increase in SCCO2 density and dissolving capability to extract organic matter in shale. |
| Yin et al. [30] | SSA (−60.52%); TPV (−28.25%); D (−4.18%); RA (82.01%) | Longmaxi (organic/low thermal maturity) | 16 MPa 40 °C 30 Days | In this study, a significant reduction in SSA, TPV and shale’s fractal dimensions was observed after 30 days of SCCO2 treatment, while the average pore size increased. This behavior is related to organic matter dissolution in micropores by SCCO2. |
| Ao et al. [43] | SSA (−70.27%) | Longmaxi (organic) | 15 MPa 35 °C 5–20 Days | The specific surface area of shale was reduced after SCCO2 treatment. Shale deformation was caused by SCCO2 adsorption and gas pressure, which further affects the strength of shales. |
| Rezaee et al. [51] | Porosity (4.0%); Capillary Pressure (−52.74) | Latrobe Group (mix of mudstone/organic-rich) | 15.17 MPa 66 °C 60 Days | They reported that the increase in pore structure and the reduction in capillary threshold pressure could decrease the caprock seal efficiency, and cause a possible CO2 leakage |
| Pan et al. [39] | For Longmaxi: SSA (−42.91%); D (−5.99%) For Yanchang: SSA (94.09%); D (2.37%) | Yanchang (mix of mudstone/organic-rich) and Longmaxi (organic-rich with low thermal maturity) | 15 MPa 80 °C 10–30 Days | They studied the changes in shale surface morphology and CO2 adsorption. They found that at high temperatures (80 °C), SCCO2 can dissolve and extract organic matter on the surface. Leading to the formation of carbonic acid, which in return causes the alteration of SSA and pore volume in nanopores structure. |
| Hui et al. [18] | For Longmaxi: SSA (−52.05%); TPV (−10.34%) For Yanchang: SSA (23.99%); TPV (−16.67%) | Yanchang (mix of mudstone/organic-rich), Longmaxi (organic-rich) and Wufeng (organic-rich) | 18 MPa 60 °C 10 Days | It was found that the high-pressure CO2 adsorption resulted in a significant reduction in SSA, affecting the structural and geochemical properties of shale. After CO2 treatment, the CO2 adsorption capacity decreased which is not favorable for CO2 sequestration. |
| Luo et al. [50] | SSA (115.1%); TPV (24.78%); D (4.43%) | Yanchang (mudstone) | 10 MPa 50 °C 50 Days | This study confirms that CO2–shale interactions have a strong influence on the micropores of shale, indicating an increase in SSA and pore volume. It was also found that fractal dimensions of shale have increased due to the dissolution of the clay and carbonate minerals. |
| Formation | Treatment Time, Hours | Hydrogen Functional Groups {C-C/C-H}, (%) | Increasing % | Oxygen Functional Groups {C-O, CO3−2, C=O, COO-}, (%) | Reduction % |
|---|---|---|---|---|---|
| Yanchang | 0 | 74.11 | 6.06 | 25.89 | 17.34 |
| 240 | 78.60 | 21.40 | |||
| Marine Longmaxi | 0 | 57.41 | 11.18 | 42.59 | 15.07 |
| 240 | 63.83 | 36.17 | |||
| Marine Wufeng | 0 | 52.36 | 3.53 | 47.64 | 3.88 |
| 240 | 54.21 | 45.79 |
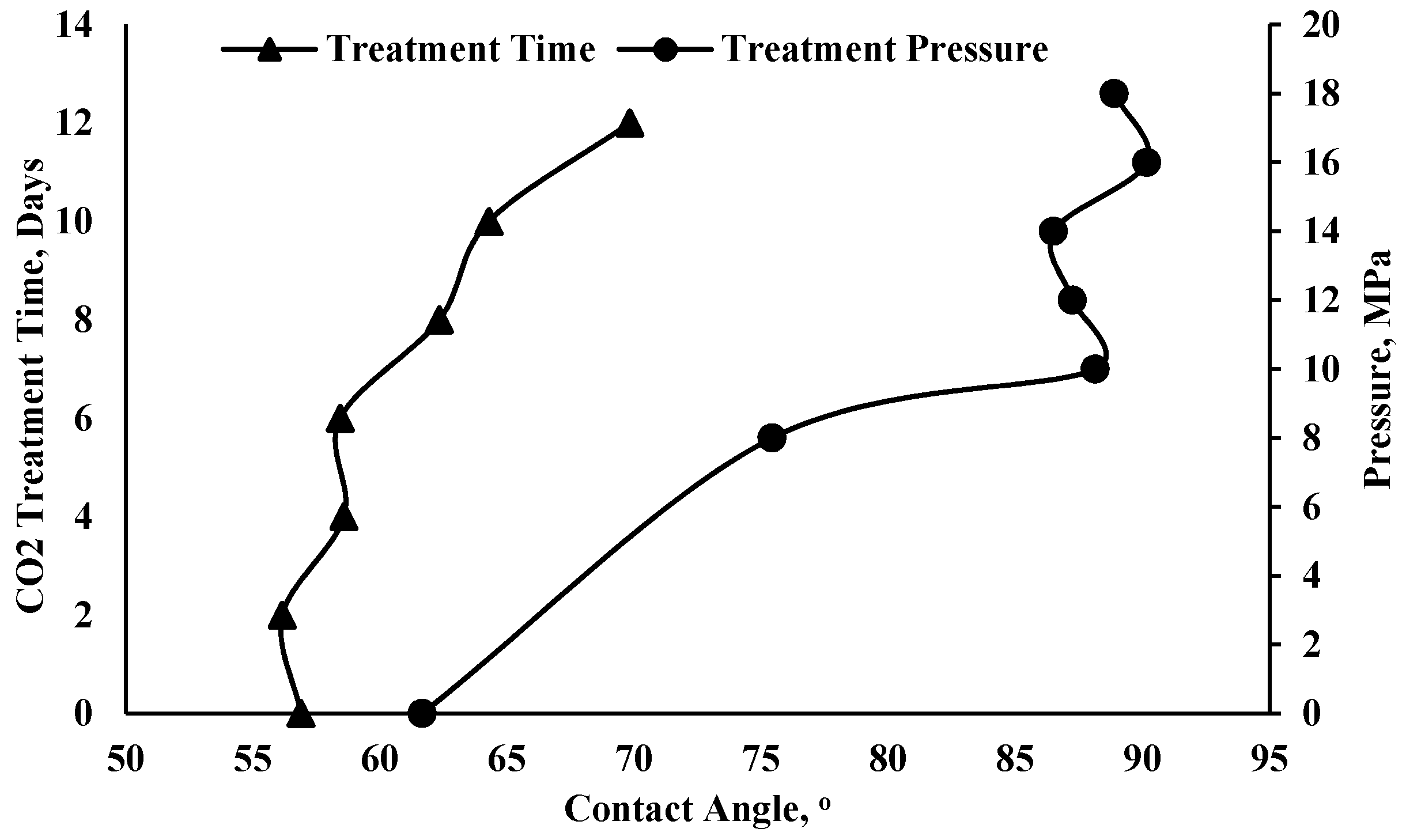
| Author(s) | Wettability System | Contact Angle Measurement Method | Pressure and Temperature | Main Findings | Effect on Storage Capacity and CO2 Leakage Possibility |
|---|---|---|---|---|---|
| Chiquet et al. [42] | CO2/Brine/(Mica-Quartz) | Contact angle (captive-drop) | 3.5–11 MPa Unknown temperature | Contact angle measurements were conducted on shaly caprock minerals (mica and quartz), indicating that the presence of CO2 significantly increases the contact angle. This behavior altered the wettability from water-wet to intermediate-wet at high pressures, caused by the CO2 dissolution and brine pH reduction. | Decreasing in CO2 storage capacity. CO2 leaks more easily due to the increase in contact angles and IFTs. |
| Zhu et al. [83] | CO2/Brine/Rock | Contact angle (Wilhelm plate method) | Atmospheric pressure 50, 70 °C | This study indicated that tight rocks’ wettability can be altered after CO2–brine–shale interactions, due to the dissolution and precipitation of the minerals. The contact angles increases after the CO2 injection with increasing CO2 treatment pressure at 50 °C. | Continuous dissolution of carbonate minerals may increase the percentage of some elements (O and Si) on the shale surface, which reduces the possibility of CO2 leakage. |
| Iglauer et al. [46] | CO2/Brine/Rock | Tilted plate method | 10–20 MPa 50 °C | In this study, the CO2 wettability of several natural caprock samples was tested at various pressures. They found that CO2 wettability of caprock increased at high pressure of 20Mpa, indicating an intermediate-wet to poor-wet behavior. | Noticeable reduction in the sealing efficiency implies possible CO2 leakage. Structural trapping capacity is reduced significantly. |
| Chaudhary et al. [103] | CO2/brine/Organic Shale | High-resolution X-ray computed tomography (HRXCT) | 13.8–22.8 MPa 60–71 °C | In this study, the authors presented a new method to measure the contact angle and wettability behavior of minerals at reservoir conditions. This method is based on the usage of X-ray imaging and radiography. They reported an increase in the contact angle at a pressure of 22.8 MPa. This confirms the ability of CO2 to alter minerals wettability due to dissolution behavior. | The contact angle in organic-rich shale increased to (59°). However, the shale surface indicated a water-wet behavior. This implies stronger storage capacity and low leakage potential. |
| Kaveh et al. [45] | CO2/water/Silty Shale | Contact angle (pendant-drop) | 0.2–15 MPa 45 °C | They examined the wettability of silty shale with CO2 and water under various temperatures and pressures. The results showed that the silty shale remains hydrophilic at high pressures. They also found that the wettability system slightly increases with decreasing temperature. | Silty shales show strong water-wet behavior, indicating a low possibility of CO2 capillary breakthrough, and high storage capacity. |
| Guiltinan et al. [12] | brine/CO2/organic-rich shale | X-ray computer tomography scanning | 13.79 MPa 20,40,60 °C | This study investigated the effect of organic matter and thermal maturity on the CO2 wettability of shales. The results showed that the wettability system remains strong water-wet despite the changes in concentrations of organic content and thermal maturities. | This behavior has no major influence on the efficiency of structural trapping (low leakage potential) and is favorable for CO2 sequestration. |
| Qin et al. [52] | Water/shale contact angles | Contact angle (pendant-drop) | Atmospheric pressure 25 °C | In this study, treatment time and pressure led to a significant increase the contact angles, due to the decrease in carbonate mineral content and the release of water content from clay minerals during the treatment. | The hydrophilicity of the shale surface decreases, which may result in CO2 leakage and lower capacity. |
| Author(s) | Reduction % in Measured Parameters | Name and Type of Shale Formation | Exposure Pressure, Temperature and Time | Main Findings |
|---|---|---|---|---|
| Lyu et al. [74] | UCS (66.05%); E (56.32%); BI (50%) | Longmaxi (Black shale) | 9 MPa 40 °C 10–30 Days | This study evaluated the effect of different SCCO2 saturation time on UCS and Young’s modulus of black shales. They observed a clear reduction in shale strength and brittleness index, after 10 days of saturation, due to the dissolution of clay minerals, with more reduction after 30 days. |
| Ao et al. [43] | Tensile Strength (22.7%); Tri-axial compressive strength (15.3%); E (29.56%) | Longmaxi (organic) | 15 MPa 35 °C 5–20 Days | They reported a gradual reduction in tensile strength and triaxial compressive strength of the shales with increasing SCCO2 treatment time. |
| Yin et al. [41] | UCS (22.86%); E (23.10%) | Longmaxi (organic-rich) | 4–16 MPa 38 °C 10 Days | This study reported the impact of saturation pressure and CO2 phase on shale mechanical properties. The results showed that SSCO2 has more influence on UCS and E than subCO2, due to the high adsorption and dissolution capacity for SCCO2. Consistently, a noticeable increase was observed in the Crack initiation pressure and a reduction in crack damage stress, which indicates the creation of more micro-cracks by SCCO2. |
| Lyu et al. [27] | UCS (30%); E (38%) | Sichuan Basin (low-clay) | 9 MPa 40 °C 10–30 Days | The ability of CO2 adsorption in weakening the shale strength and increasing its ductility was addressed. A clear reduction in UCS and E was found on the SCCO2 treated shale samples, with a noticeable increase in crack initiation and the decrease in crack damage. |
| Feng et al. [105] | Brazilian splitting strength (BSS) (46%); Absorbed energy (U) (50%); E (22%) | Sichuan Basin (Black shale) | 10 MPa 40 °C 10–60 Days | This study investigated the effect of SCCO2 saturation time and bedding orientation on shale strength. They concluded that the damage caused by SCCO2 in the pore structure is the key cause of mechanical degradation. Noticeable changes were observed in shale strength with changing the bedding angles (θ); both tensile and shear failure could occur affecting the shale resistance and cracking propagation. |
| Lu et al. [49] | UCS (31%); E (10%) | Yanchang (mudstone) | 12 MPa 50 °C 8 Days | This study found a strong connection between the damage in pore structure and the weakening of shale strength. The possible damage in shale macroscopic structure increases the stress–strain in the initial compaction stage, which results in reducing shale strength. |
| Author(s) | CO2 Capture Cost, $/Metric Tonnes | Plant Type (Source) |
|---|---|---|
| Smith et al. [134] | 21–62 | Coal-based Integrated gasification combined cycle (IGCC) |
| Heddle et al. [135] | 14.55 | N/A |
| Rubin et al. [136] | 29–44 | Pulverized coal combustion (PC) |
| 11–32 | Coal-based Integrated gasification combined cycle (IGCC) | |
| 28–57 | Natural gas combined cycle (NGCC) | |
| Holloway [137] | 18–72 | Power Plant |
| Finkenrath [138] | 43–62 | N/A |
| Environmental Policy | Cost of CCS | Fossil Fuel Energy Costs | Clean Energy Sources |
|---|---|---|---|
| This is the main driver for CCS technology, as it controls the economic market and energy generation. The demand for CCS will depend on the employed strategy that targets carbon emissions through “carbon tax” revenues. When carbon emissions are optimally taxed, this allows for the non-energy cost of CCS to drop, and thus lowers the emissions tax [132]. In this case, a lower carbon tax provides the opportunity for companies to apply CCS projects. | For the CCS project to be cost-effective, the unit cost to capture, transport and storage has to be lower than the emitting CO2 and pay the carbon price. A more advanced CCS technology will lead to an increase in energy generation from fossil fuels and reduce the unit cost of CCS. Moreover, the availability of geological sequestration sites will also result in a higher level of CCS. | Fossil fuel resources are limited in nature, and the increase of generating fossil fuel energy costs will affect the level of fossil fuel energy, carbon emissions, and overall CCS activity. Therefore, due to the exhaustibility and scarcity rent cost, renewable resources should be considered as a possible alternative for fossil fuels, which may help to achieve a higher level of CCS [139]. | There is an approach to utilize carbon-free resources i.e., solar energy, wind and nuclear electric power to replace or at least contribute to energy generated from fossil fuels. It will be ideal to employ clean energy sources only, as generating energy cost is low, which puts CCS in high demand, but the full replacement of fossil fuels is not expected soon. As of today, 80% of the global energy needs are supplied by fossil fuels, however, by combining both sources with optimal timing, the cost of energy generation can be reduced, and thus increases the level of CCS [140]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatah, A.; Bennour, Z.; Ben Mahmud, H.; Gholami, R.; Hossain, M.M. A Review on the Influence of CO2/Shale Interaction on Shale Properties: Implications of CCS in Shales. Energies 2020, 13, 3200. https://doi.org/10.3390/en13123200
Fatah A, Bennour Z, Ben Mahmud H, Gholami R, Hossain MM. A Review on the Influence of CO2/Shale Interaction on Shale Properties: Implications of CCS in Shales. Energies. 2020; 13(12):3200. https://doi.org/10.3390/en13123200
Chicago/Turabian StyleFatah, Ahmed, Ziad Bennour, Hisham Ben Mahmud, Raoof Gholami, and Md. Mofazzal Hossain. 2020. "A Review on the Influence of CO2/Shale Interaction on Shale Properties: Implications of CCS in Shales" Energies 13, no. 12: 3200. https://doi.org/10.3390/en13123200
APA StyleFatah, A., Bennour, Z., Ben Mahmud, H., Gholami, R., & Hossain, M. M. (2020). A Review on the Influence of CO2/Shale Interaction on Shale Properties: Implications of CCS in Shales. Energies, 13(12), 3200. https://doi.org/10.3390/en13123200








