Hydrothermal Carbonization as a Strategy for Sewage Sludge Management: Influence of Process Withdrawal Point on Hydrochar Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Characterization
2.2. Hydrothermal Carbonization and Product Analysis
3. Results
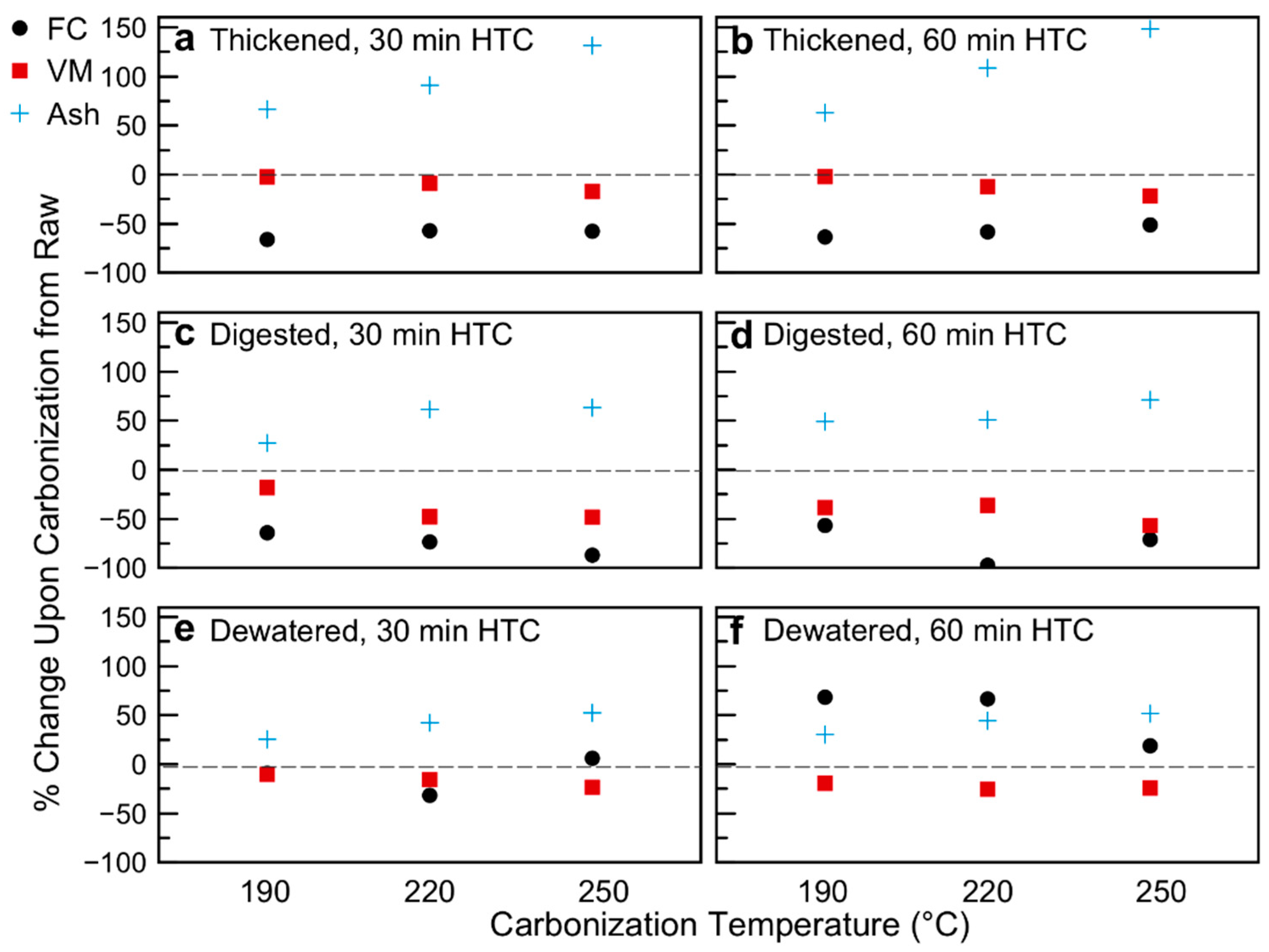
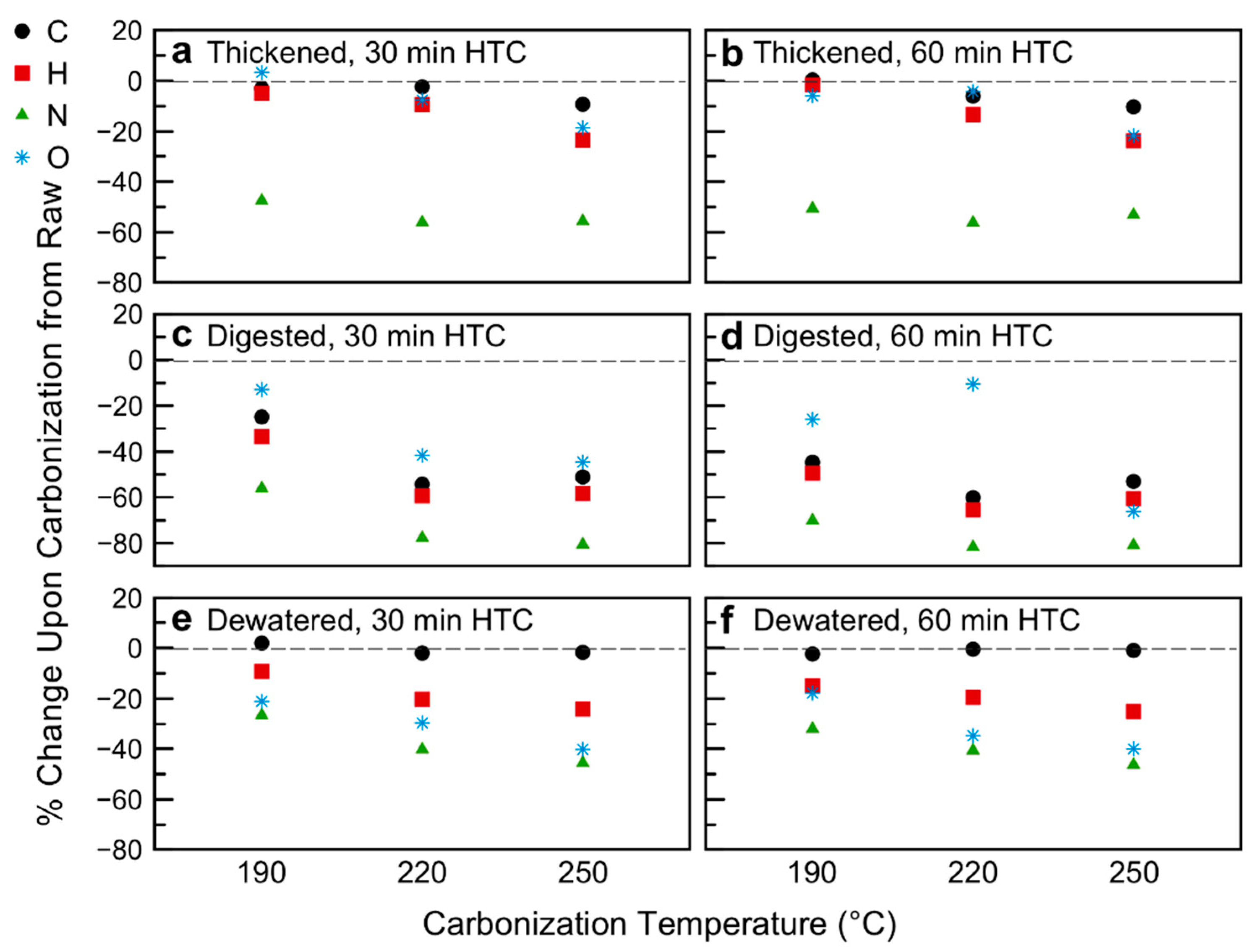
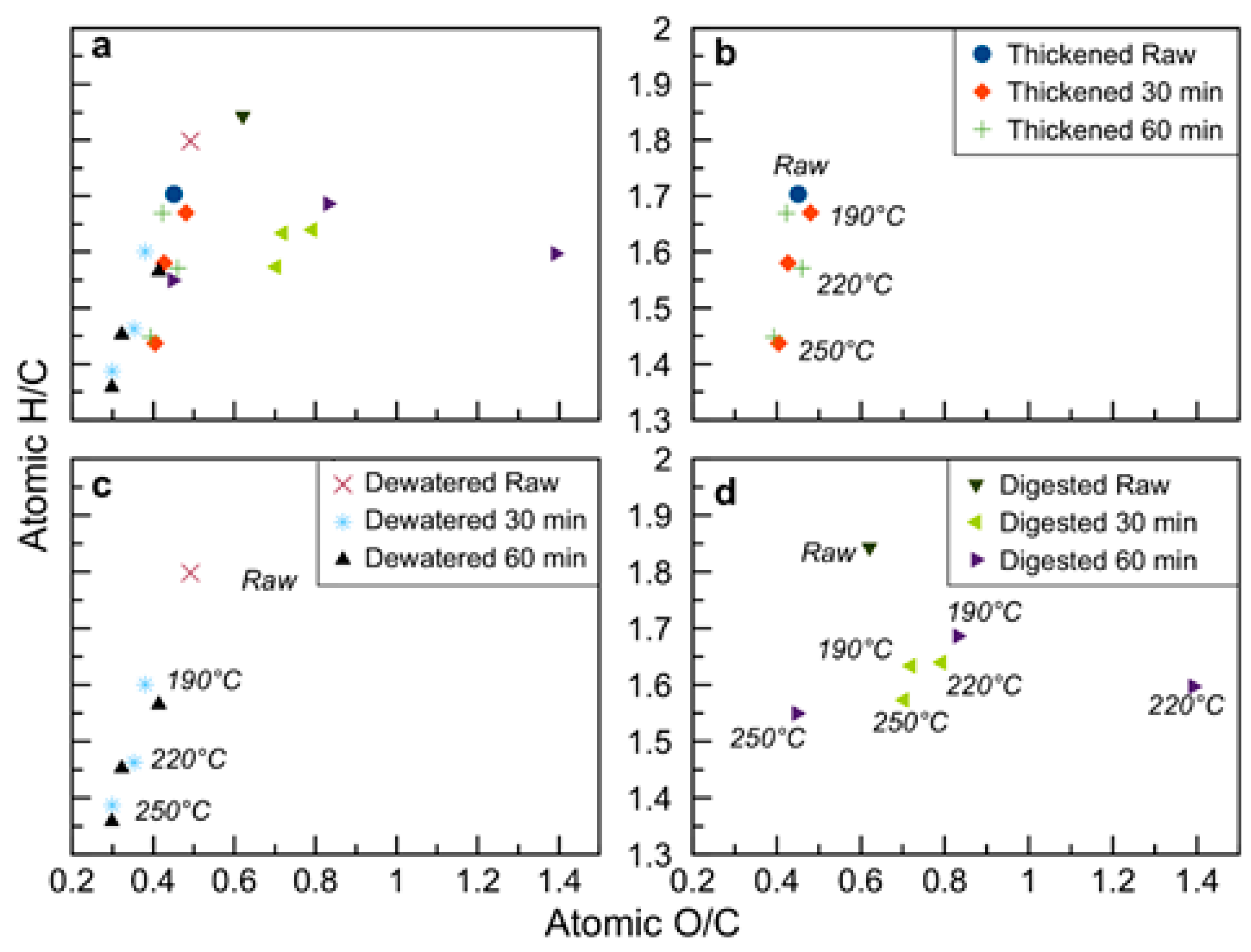
3.1. Hydrochar Properties Depend on Sludge Withdrawal Point and Carbonization Conditions
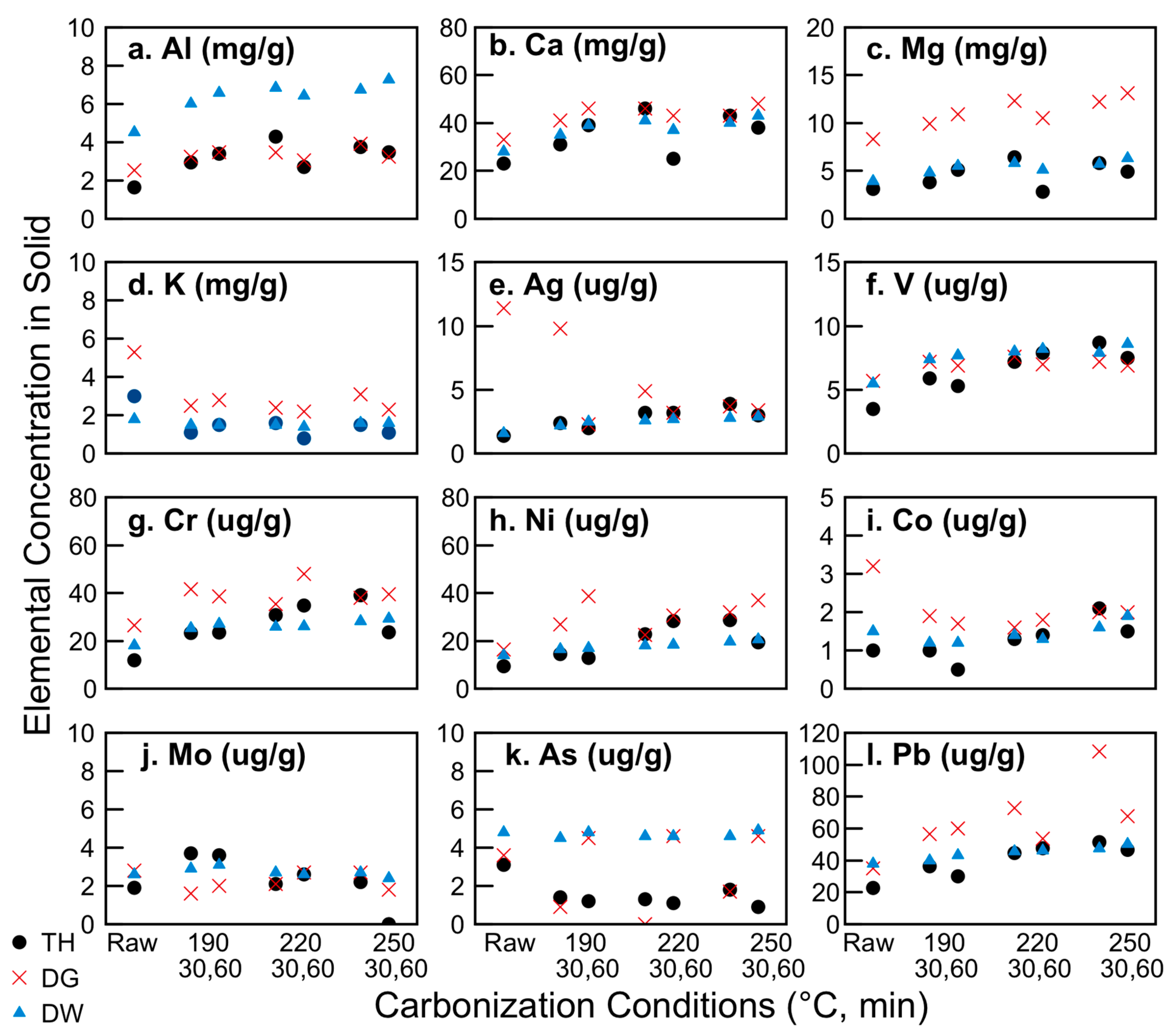
3.2. Distribution of Nutrients and Inorganics: Potential for Use as Soil Amendment
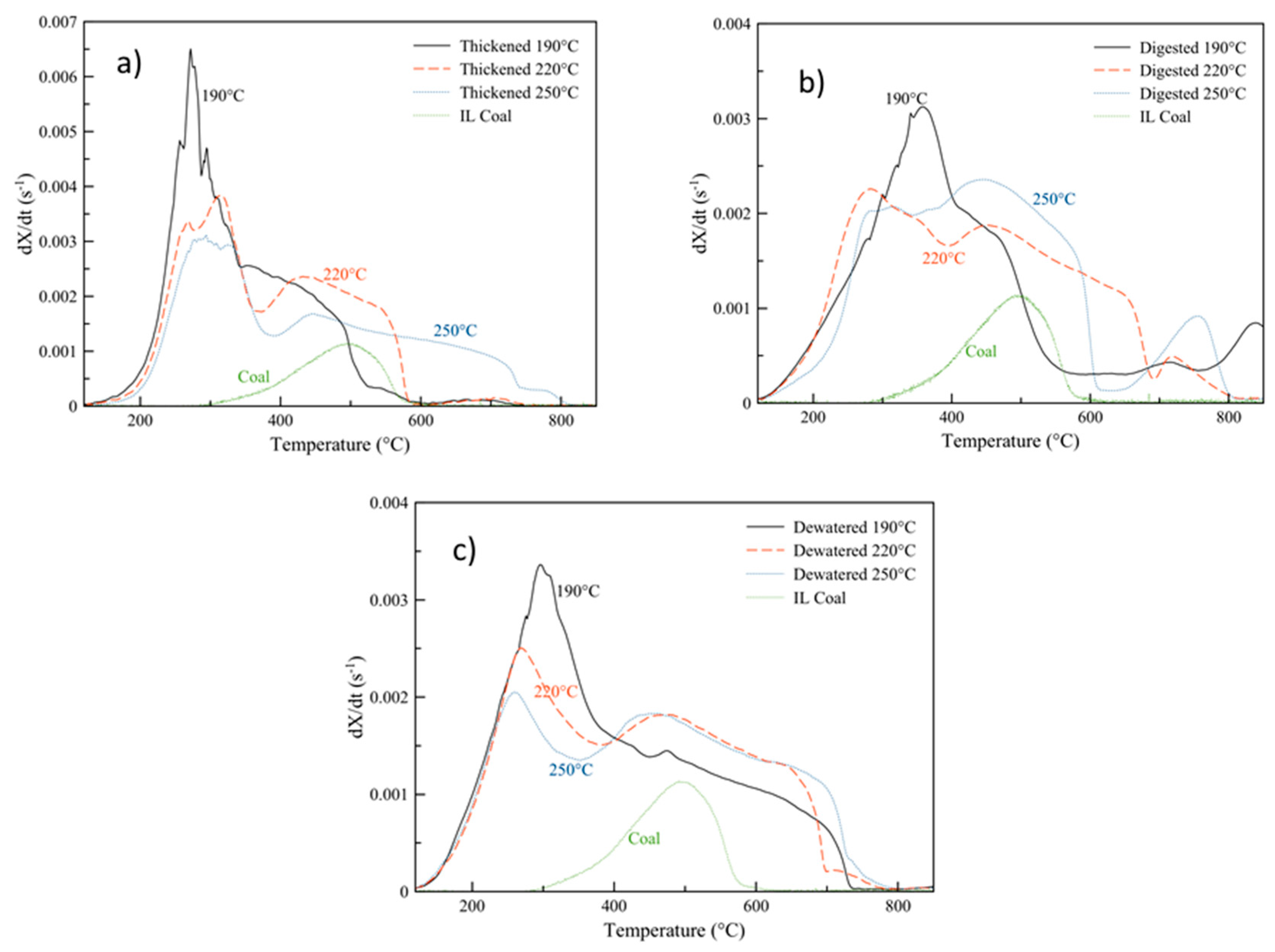
3.3. Energy Content and Oxidative Reactivity: Potential for Use as Solid Fuel
3.4. Further Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steichen, R. Council Directive of 21 May 1991 concerning urban waste water treatment (91/271/EEC). Off. J. Eur. Communities 1991. Available online: https://www.tarimorman.gov.tr/SYGM/Belgeler/ab%20mevzuat%C4%B1/91-271-EEC.pdf (accessed on 4 June 2020).
- Bianchini, A.; Bonfiglioli, L.; Pellegrini, M.; Saccani, C. Sewage sludge management in Europe: A critical analysis of data quality. Int. J. Environ. Waste Manag. 2016, 18, 226. [Google Scholar] [CrossRef]
- EUROSTAT. Sewage Sludge Production and Disposal from Urban Wastewater (in Dry Substance (d.s)). 2017. Available online: https://data.europa.eu/euodp/en/data/dataset/hzWkcfKt5mxEaFijeoA (accessed on 4 June 2020).
- Zhang, Q.; Hu, J.; Lee, D.; Chang, Y.; Lee, Y. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef]
- European Commission. Eighth Report on the Implementation Status and the Programmes for Implementation (as required by Article 17) of Council Directive 91/271/EEC Concerning Urban Waste Water Treatment; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Yanagida, T.; Fujimoto, S.; Minowa, T. Application of the severity parameter for predicting viscosity during hydrothermal processing of dewatered sewage sludge for a commercial PFBC plant. Bioresour. Technol. 2010, 101, 2043–2045. [Google Scholar] [CrossRef]
- Campbell, H.W.; Pacific, C.; Technologies, E. Sludge management—Future issues and trends. Water Sci. Technol. 2000, 41, 1–8. [Google Scholar] [CrossRef]
- European Commission. Disposal and Recycling Routes for Sewage Sludge Part 1—Sludge Use Acceptance Report; European Commission: Brussels, Belgium, 2001; ISBN 9289417986. [Google Scholar]
- Rulkens, W. Sewage Sludge as a Biomass Resource for the Production of Energy: Overview and Assessment of the Various Options. Energy Fuels 2008, 44, 9–15. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Sludge: A waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Cao, Y.; Pawłowski, A. Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: Brief overview and energy efficiency assessment. Renew. Sustain. Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- Merzari, F.; Langone, M.; Andreottola, G.; Fiori, L. Methane production from process water of sewage sludge hydrothermal carbonization. A review. Valorising sludge through hydrothermal carbonization. Crit. Rev. Environ. Sci. Technol. 2019, 49, 947–988. [Google Scholar] [CrossRef]
- Johansson, K.; Perzon, M.; Fröling, M.; Mossakowska, A.; Svanström, M. Sewage sludge handling with phosphorus utilization—Life cycle assessment of four alternatives. J. Clean. Prod. 2008, 16, 135–151. [Google Scholar] [CrossRef]
- Merzari, F.; Lucian, M.; Volpe, M.; Andreottola, G.; Fiori, L. Hydrothermal carbonization of biomass: Design of a bench-Scale reactor for evaluating the heat of reaction. Chem. Eng. Trans. 2018, 65, 43–48. [Google Scholar]
- Akiya, N.; Savage, P.E. Roles of water for chemical reactions in high-temperature water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef] [PubMed]
- Kritzer, P. Corrosion in high-temperature and supercritical water and aqueous solutions: A review. J. Supercrit. Fluids 2004, 29, 1–29. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Lucian, M.; Fiori, L. Hydrothermal carbonization of waste biomass: Process design, modeling, energy efficiency and cost analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Messineo, A.; Mäkelä, M.; Barr, M.R.; Volpe, R.; Corrado, C.; Fiori, L. Reactivity of cellulose during hydrothermal carbonization of lignocellulosic biomass. Fuel Process. Technol. 2020, 206, 106456. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A. Upgrading of Olive Tree Trimmings Residue as Biofuel by Hydrothermal Carbonization and Torrefaction: A Comparative Study. Chem. Eng. Trans. 2016, 50, 13–18. [Google Scholar]
- Mäkelä, M.; Volpe, M.; Volpe, R.; Fiori, L.; Dahl, O. Spatially resolved spectral determination of polysaccharides in hydrothermally carbonized biomass. Green Chem. 2018, 20, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Lucian, M.; Volpe, M.; Fiori, L. Hydrothermal Carbonization Kinetics of Lignocellulosic Agro-Wastes: Experimental Data and Modeling. Energies 2019, 12, 516. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Shen, Y.; Ge, S.; Yoshikawa, K. Energy recycling from sewage sludge by producing solid biofuel with hydrothermal carbonization. Energy Convers. Manag. 2014, 78, 815–821. [Google Scholar] [CrossRef] [Green Version]
- Danso-Boateng, E.; Holdich, R.G.; Wheatley, A.D.; Martin, S.J.; Shama, G. Hydrothermal Carbonization of Primary Sewage Sludge and Synthetic Faeces: Effect of Reaction Temperature and Time on Filterability. Environ. Prog. Sustain. Energy 2015, 34, 1279–1290. [Google Scholar] [CrossRef] [Green Version]
- Khalil, W.A.S.; Shanableh, A.; Rigby, P.; Kokot, S. Selection of hydrothermal pre-treatment conditions of waste sludge destruction using multicriteria decision-making. J. Environ. Manag. 2005, 75, 53–64. [Google Scholar] [CrossRef]
- Catallo, W.J.; Comeaux, J.L. Reductive hydrothermal treatment of sewage sludge. Waste Manag. 2008, 28, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Chen, H.; Ge, S.; Yoshikawa, K. Effect of the hydrothermal pretreatment for the reduction of NO emission from sewage sludge combustion. Appl. Energy 2015, 111, 199–205. [Google Scholar] [CrossRef]
- Becker, G.C.; Wüst, D.; Köhler, H.; Lautenbach, A.; Kruse, A. Novel approach of phosphate-reclamation as struvite from sewage sludge by utilising hydrothermal carbonization. J. Environ. Manag. 2019, 238, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Luo, G.; Rao, Y.; Chen, H.; Zhang, S. Hydrothermal conversion of dewatered sewage sludge: Focusing on the transformation mechanism and recovery of phosphorus. Chemosphere 2019, 228, 619–628. [Google Scholar] [CrossRef]
- Song, E.; Park, S.; Kim, H. Upgrading Hydrothermal Carbonization (HTC) Hydrochar from Sewage Sludge. Energies 2019, 12, 2383. [Google Scholar] [CrossRef] [Green Version]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal carbonisation of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Brookman, H.; Gievers, F.; Zelinski, V.; Ohlert, J.; Loewen, A. Influence of Hydrothermal Carbonization on Composition, Formation and Elimination of Biphenyls, Dioxins and Furans in Sewage Sludge. Energies 2018, 11, 1582. [Google Scholar] [CrossRef] [Green Version]
- Breulmann, M.; Schulz, E.; Van Afferden, M.; Müller, R.A. Hydrochars derived from sewage sludge: Effects of pre-treatment with water on char properties, phytotoxicity and chemical structure. Arch. Agron. Soil Sci. 2017, 64, 860–872. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Z.; Zheng, Q.; Lang, Q.; Xia, Y.; Peng, N. Effect of hydrothermal carbonization on migration and environmental risk of heavy metals in sewage sludge during pyrolysis. Bioresour. Technol. 2018, 247, 282–290. [Google Scholar] [CrossRef]
- Chen, C.; Liu, G.; An, Q.; Lin, L.; Shang, Y.; Wan, C. From wasted sludge to valuable biochar by low temperature hydrothermal carbonization treatment: Insight into the surface characteristics. J. Clean. Prod. 2020, 263, 1–9. [Google Scholar] [CrossRef]
- Zhai, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C. Hydrothermal carbonisation of sewage sludge for char production with different waste biomass: Effects of reaction temperature and energy recycling. Energy 2017, 127, 167–174. [Google Scholar] [CrossRef]
- Wang, R.; Wang, C.; Zhao, Z.; Jia, J.; Jin, Q. Energy recovery from high-ash municipal sewage sludge by hydrothermal carbonization: Fuel characteristics of biosolid products. Energy 2019, 186, 115848. [Google Scholar] [CrossRef]
- Zheng, X.; Jiang, Z.; Ying, Z.; Song, J.; Chen, W.; Wang, B. Role of feedstock properties and hydrothermal carbonization conditions on fuel properties of sewage sludge-derived hydrochar using multiple linear regression technique. Fuel 2020, 271, 1–11. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Song, H.; Li, P.-J.; He, Z.-X.; Wang, Q.; Wang, K.; Duan, P.-G. Hydrothermal carbonization of sewage sludge: Effect of aqueous phase recycling. Chem. Eng. J. 2020, 387, 1–12. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Scott, J.; Yu, G.; Wang, Z.; Schideman, L.; Zhang, Y.; Strathmann, T.J. Chemical properties of biocrude oil from the hydrothermal liquefaction of Spirulina algae, swine manure, and digested anaerobic sludge. Bioresour. Technol. 2011, 102, 8295–8303. [Google Scholar] [CrossRef]
- Hao, X.D.; Li, J.; van Loosdrecht, M.C.M.; Li, T.Y. A sustainability-based evaluation of membrane bioreactors over conventional activated sludge processes. J. Environ. Chem. Eng. 2018, 6, 2597–2605. [Google Scholar] [CrossRef]
- Manser, R.; Gujer, W.; Siegrist, H. Membrane bioreactor versus conventional activated sludge system: Population dynamics of nitrifiers. Water Sci. Technol. 2005, 52, 417–425. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22th ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Fiori, L.; Basso, D.; Castello, D.; Baratieri, M. Hydrothermal carbonization of biomass: Design of a batch reactor and preliminary experimental results. Chem. Eng. Trans. 2014, 37, 55–60. [Google Scholar]
- Basso, D.; Weiss-Hortala, E.; Patuzzi, F.; Castello, D.; Baratieri, M.; Fiori, L. Hydrothermal carbonization of off-specification compost: A byproduct of the organic municipal solid waste treatment. Bioresour. Technol. 2015, 182, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Basso, D.; Patuzzi, F.; Castello, D.; Baratieri, M.; Rada, C.E.; Weiss-Hortala, E.; Fiori, L. Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manag. 2016, 47, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, J.E. Argonne Premium Coal Sample Program; Argonne: Lemont, IL, USA, 2007. Available online: https://publications.anl.gov/anlpubs/2007/04/58856.pdf (accessed on 4 June 2020).
- Celaya, A.A.M.; Lade, A.T.A.; Goldfarb, J.J.L. Co-combustion of brewer’s spent grains and Illinois No. 6 coal: Impact of blend ratio on pyrolysis and oxidation behavior. Fuel Process. Technol. 2015, 129, 39–51. [Google Scholar] [CrossRef]
- Xue, J.; Chellappa, T.; Ceylan, S.; Goldfarb, J.L. Enhancing biomass + coal Co-firing scenarios via biomass torrefaction and carbonization: Case study of avocado pit biomass and Illinois No. 6 coal. Renew. Energy 2018, 122, 152–162. [Google Scholar] [CrossRef]
- Andreottola, G.; Foladori, P.; Ferrai, M.; Ziglio, G. Respirometria Applicata Alla Depurazione Delle Acque; Principi e metodi; Department of Civil, Environmental and Mechanical Enginnering, University of Trento: Trento, Italy, 2002. [Google Scholar]
- Ren, W.; Zhou, Z.; Jiang, L.M.; Hu, D.; Qiu, Z.; Wei, H.; Wang, L. A cost-effective method for the treatment of reject water from sludge dewatering process using supernatant from sludge lime stabilization. Sep. Purif. Technol. 2015, 142, 123–128. [Google Scholar] [CrossRef]
- Al Momani, F.A.; Örmeci, B. Optimization of polymer dose based on residual polymer concentration in dewatering supernatant. Water Air Soil Pollut. 2014, 225. [Google Scholar] [CrossRef]
- Volpe, M.; Goldfarb, J.L.; Fiori, L. Hydrothermal carbonization of Opuntia ficus indica cladodes: Role of process parameters on hydrochar properties. Bioresour. Technol. 2018, 247, 310–318. [Google Scholar] [CrossRef]
- Jomaa, S.; Shanableh, A.; Khalil, W.; Trebilco, B. Hydrothermal decomposition and oxidation of the organic component of municipal and industrial waste products. Adv. Environ. Res. 2003, 7, 647–653. [Google Scholar] [CrossRef]
- Kocsisová, T.; Juhasz, J.; Cvengroš, J. Hydrolysis of fatty acid esters in subcritical water. Eur. J. Lipid Sci. Technol. 2006, 108, 652–658. [Google Scholar] [CrossRef]
- Díaz, E.; Pintado, L.; Faba, L.; Ordóñez, S.; González-LaFuente, J.M. Effect of sewage sludge composition on the susceptibility to spontaneous combustion. J. Hazard. Mater. 2019, 361, 267–272. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal Carbonization of Municipal Waste Streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef]
- Kruse, A.; Koch, F.; Stelzl, K.; Wüst, D.; Zeller, M. Fate of Nitrogen during Hydrothermal Carbonization. Energy Fuels 2016, 30, 8037–8042. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonisation: The role of temperature and solid load on secondary char formation and hydrochar energy properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One stage olive mill waste streams valorisation via hydrothermal carbonisation. Waste Manag. 2018, 80, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Williams, P.T. A comparison of product yields and inorganic content in process streams following thermal hydrolysis and hydrothermal processing of microalgae, manure and digestate. Bioresour. Technol. 2016, 200, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, R.; Ceccato, R.; Marchetti, V.; Andreottola, G.; Fiori, L. Sewage Sludge Hydrochar: An Option for Removal of Methylene Blue from Wastewater. Appl. Sci. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Wu, H.; Lai, C.; Zeng, G.; Liang, J.; Chen, J.; Xu, J.; Dai, J.; Li, X.; Liu, J.; Chen, M.; et al. The interactions of composting and biochar and their implications for soil amendment and pollution remediation: A review. Crit. Rev. Biotechnol. 2017, 37, 754–764. [Google Scholar] [CrossRef]
- Melo, T.M.; Bottlinger, M.; Schulz, E.; Leandro, W.M.; Filho, A.M.D.A.; Wang, H.; Ok, Y.S.; Rinklebe, J. Plant and soil responses to hydrothermally converted sewage sludge (sewchar). Chemosphere 2018, 206, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.T.; Zsuzsa, S.; Ahrenfeldt, J.; Henriksen, U.B.; Frandsen, F.J.; Müller-st, D.S. Changes imposed by pyrolysis, thermal gasi fi cation and incineration on composition and phosphorus fertilizer quality of municipal sewage sludge. J. Environ. Manag. 2017, 198, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, A.D.; Dubey, S.P.; Sillanp, M.; Kwon, Y.N.; Lee, C.; Varma, R.S. Fate of engineered nanoparticles: Implications in the environment. Coord. Chem. Rev. 2015, 287, 64–78. [Google Scholar] [CrossRef]
- Wang, Y.; Westerhoff, P.; Hristovski, K.D. Fate and biological effects of silver, titanium dioxide, and C60(fullerene) nanomaterials during simulated wastewater treatment processes. J. Hazard. Mater. 2012, 201–202, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, E.; Lima, A.; Albanese, S.; Birke, M.; Cicchella, D.; Giaccio, L.; Valera, P.; De Vivo, B. Major and trace elements in tap water from Italy. J. Geochem. Explor. 2012, 112, 54–75. [Google Scholar] [CrossRef]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. 2015. Available online: https://www.biochar-international.org/wp-content/uploads/2018/04/IBI_Biochar_Standards_V2.1_Final.pdf (accessed on 4 June 2020).
- Giammanco, S.; Valenza, M.; Pignato, S.; Giammanco, G. Mg, Mn, Fe, and V Concentration in the Ground Waters of Mount Etna (Sicily). Water Res. 1996, 30, 378–386. [Google Scholar] [CrossRef]
- Roccaro, P.; Barone, C.; Mancini, G.; Vagliasindi, F.G.A. Removal of manganese from water supplies intended for human consumption: A case study. Desalination 2007, 210, 205–214. [Google Scholar] [CrossRef]
- Varrica, D.; Tamburo, E.; Dongarrà, G. Sicilian bottled natural waters: Major and trace inorganic components. Appl. Geochem. 2013, 34, 102–113. [Google Scholar] [CrossRef]
- Roccaro, P.; Vagliasindi, F.G.A. Coprecipitation of vanadium with iron(III) in drinking water: A pilot-scale study. Desalin. Water Treat. 2015, 55, 799–809. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Liu, C. Impact of blend ratio on the co-firing of a commercial torrefied biomass and coal via analysis of oxidation kinetics. Bioresour. Technol. 2013, 149, 208–215. [Google Scholar] [CrossRef]
- Khan, A.A.; de Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Does carbonization avoid segregation of biomass and lignite during co-firing? Thermal analysis study. Fuel Process. Technol. 2015, 137, 312–319. [Google Scholar] [CrossRef]
- Cartmell, E.; Gostelow, P.; Riddell-black, D.; Simms, N.; Oakey, J.; Morris, J.O.E.; Jeffrey, P.; Howsam, P. Biosolids-A Fuel or a Waste? An Integrated Appraisal of Five Co-combustion Scenarios with Policy Analysis. Environ. Sci. Technol. 2006, 40, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Ma, X.; Liu, K. Co-combustion kinetics of sewage sludge with coal and coal gangue under different atmospheres. Energy Convers. Manag. 2010, 51, 1976–1980. [Google Scholar] [CrossRef]
- Otero, M.; Calvo, L.F.; Gil, M.V.; Garcia, A.I.; Moran, A. Co-combustion of different sewage sludge and coal: A non-isothermal thermogravimetric kinetic analysis. Bioresour. Technol. 2008, 99, 6311–6319. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal carbonization of sewage sludge for energy production with coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Gao, L.; Volpe, M.; Lucian, M.; Fiori, L.; Goldfarb, J.L. Does hydrothermal carbonization as a biomass pretreatment reduce fuel segregation of coal-biomass blends during oxidation? Energy Convers. Manag. 2019, 181, 93–104. [Google Scholar] [CrossRef]
- Fregolente, L.G.; Miguel, T.B.A.R.; de Castro Miguel, E.; de Almeida Melo, C.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C. Toxicity evaluation of process water from hydrothermal carbonization of sugarcane industry by-products. Environ. Sci. Pollut. Res. 2019, 26, 27579–27589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlindhaug, J.; Ødegaard, H. Thermal hydrolysis for the production of carbon source for denitrification. Water Sci. Technol. 1996, 34, 371–378. [Google Scholar] [CrossRef]
- Friedman, A.A.; Smith, J.E.; DeSantis, J.; Ptak, T.; Ganley, R.C. Characteristics of Residues from Wet Air Oxidation of Anaerobic Sludges. J. (Water Pollut. Control Fed.) 1988, 60, 1971–1978. [Google Scholar]
- Wirth, B.; Reza, T.; Mumme, J. Influence of digestion temperature and organic loading rate on the continuous anaerobic treatment of process liquor from hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2015, 198, 215–222. [Google Scholar] [CrossRef]
- Aragón-briceño, C.; Ross, A.B.; Camargo-valero, M.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Villamil, J.A.; Mohedano, A.F.; Rodriguez, J.J.; de la Rubia, M.A. Valorisation of the liquid fraction from hydrothermal carbonisation of sewage sludge by anaerobic digestion. J. Chem. Technol. Biotechnol. 2018, 93, 450–456. [Google Scholar] [CrossRef]
- Qiao, W.; Peng, C.; Wang, W.; Zhang, Z. Biogas production from supernatant of hydrothermally treated municipal sludge by upflow anaerobic sludge blanket reactor. Bioresour. Technol. 2011, 21, 9904–9911. [Google Scholar] [CrossRef]
- Appels, L.; Dewil, R.; Baeyens, J.; Degrève, J. Ultrasonically enhanced anaerobic digestion of waste activated sludge. Int. J. Sustain. Eng. 2008, 1, 94–104. [Google Scholar] [CrossRef]
- Vavilin, V.A.; Rytov, S.V.; Lokshina, L.Y. A Description of Hydrolysis Kinetics in Aanaerobic Degradation of Particulate Organic Matter. Bioresour. Technol. 1996, 56, 229–237. [Google Scholar] [CrossRef]
- Nuchdang, S.; Frigon, J.; Roy, C.; Pilon, G.; Phalakornkule, C.; Guiot, S.R. Hydrothermal post-treatment of digestate to maximize the methane yield from the anaerobic digestion of microalgae. Waste Manag. 2018, 71, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Stutzenstein, P.; Weiner, B.; Köhler, R.; Pfeifer, C.; Kopinke, F.D. Wet oxidation of process water from hydrothermal carbonization of biomass with nitrate as oxidant. Chem. Eng. J. 2018, 339, 1–6. [Google Scholar] [CrossRef]
- Luz, F.C.; Volpe, M.; Fiori, L.; Manni, A.; Cordiner, S.; Mulone, V.; Rocco, V. Spent Coffee Enhanced Biomethane Potential via an Integrated Hydrothermal Carbonization-Anaerobic Digestion Process. Bioresour. Technol. 2018, 256, 102–109. [Google Scholar]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop. Ecol. 2010, 51, 375–387. [Google Scholar]
- Shi, L.; Zhang, G.; Wei, D.; Yan, T.; Xue, X.; Shi, S.; Wei, Q. Preparation and utilization of anaerobic granular sludge-based biochar for the adsorption of methylene blue from aqueous solutions. J. Mol. Liq. 2014, 198, 334–340. [Google Scholar] [CrossRef]
- Stark, K.; Plaza, E.; Hultman, B. Phosphorus release from ash, dried sludge and sludge residue from supercritical water oxidation by acid or base. Chemosphere 2006, 62, 827–832. [Google Scholar] [CrossRef]
- Yoshizaki, S.; Tomida, T. Principle and Process of Heavy Metal Removal from Sewage Sludge. Environ. Sci. Technol. 2000, 34, 1572–1574. [Google Scholar] [CrossRef]
- Hotova, G.; Slovak, V.; Soares, O.S.G.P.; Figueiredo, J.L.; Pereira, M.F.R. Oxygen surface groups analysis of carbonaceous samples pyrolysed at low temperature. Carbon 2018, 134, 255–263. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; Skjemstad, J.O.; Thies, J.; Luiza, F.J.; Petersen, J.; Neves, E.G. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Goldfarb, J.L.; Dou, G.; Salari, M.; Grinstaff, M.W. Biomass-Based Fuels and Activated Carbon Electrode Materials: An Integrated Approach to Green Energy Systems. ACS Sustain. Chem. Eng. 2017, 5, 3046–3054. [Google Scholar] [CrossRef]
- Isitan, S.; Ceylan, S.; Topcu, Y.; Hintz, C.; Tefft, J.; Chellappa, T.; Guo, J.; Goldfarb, J.L. Product quality optimization in an integrated biorefinery: Conversion of pistachio nutshell biomass to biofuels and activated biochars via pyrolysis. Energy Convers. Manag. 2016, 127, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Goldfarb, J.L.; Buessing, L.; Gunn, E.; Lever, M.; Billias, A.; Casoliba, E.; Schievano, A.; Adani, F. Novel Integrated Biorefinery for Olive Mill Waste Management: Utilization of Secondary Waste for Water Treatment. ACS Sustain. Chem. Eng. 2017, 5, 876–884. [Google Scholar] [CrossRef]
- Gopu, C.; Gao, L.; Volpe, M.; Fiori, L.; Goldfarb, J.L. Valorizing municipal solid waste: Waste to energy and activated carbons for water treatment via pyrolysis. J. Anal. Appl. Pyrolysis 2018, 133, 48–58. [Google Scholar] [CrossRef]
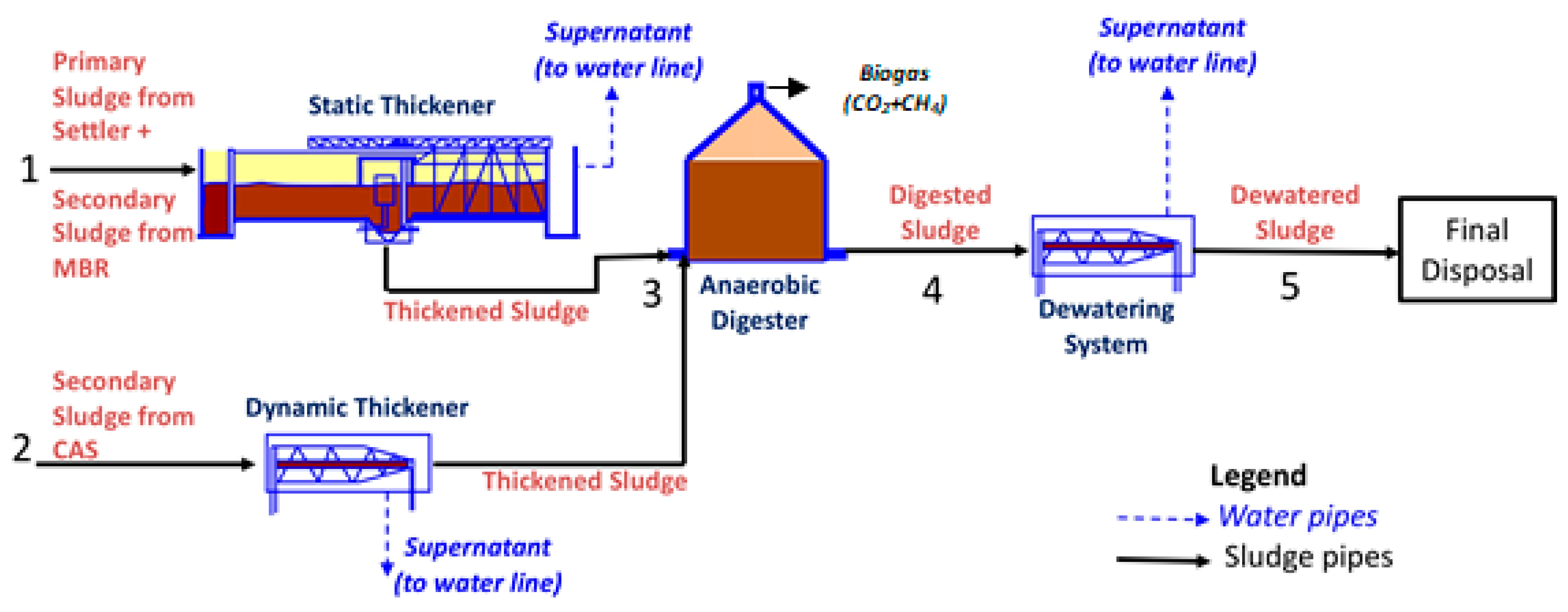
| Property | Thickened Sludge | Digested Sludge | Dewatered Sludge | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture Content (wt%) | 97.1 ± 0.1 | 97.1 ± 0.3 | 74.9 ± 0.1 | ||||||||||||||||
| Total Solids (g/L) | 29.1 ± 0.4 | 29.0 ± 2.3 | n.a. | ||||||||||||||||
| Fixed + Volatile Solids (g/L) | 26.7 ± 0.4 | 15.4 ± 0.9 | n.a. | ||||||||||||||||
| HTC Temp (°C) | 30 min HTC | 60 min HTC | 30 min HTC | 60 min HTC | 30 min HTC | 60 min HTC | |||||||||||||
| Total COD (g/L) | Raw | 28.1 ± 1.4 | 18.0 ± 0.9 | 193.2 ± 0.3 a | |||||||||||||||
| 190 | 14.1 | ± | 2.0 | 15.3 | ± | 0.7 | 9.1 | ± | 0.2 | 9.3 | ± | 0.4 | 55.3 | ± | 1.4 | 56.3 | ± | 2.7 | |
| 220 | 15.4 | ± | 1.4 | 15.1 | ± | 1.1 | 11.3 | ± | 1.2 | 9.9 | ± | 0.1 | 56.7 | ± | 8.2 | 54.1 | ± | 0.3 | |
| 250 | 18.4 | ± | 0.7 | 18.4 | ± | 1.8 | 10.4 | ± | 0.7 | 8.8 | ± | 0.4 | 64.9 | ± | 8.2 | 57.5 | ± | 0.3 | |
| Soluble COD (g/L) | Raw | 2.3 ± 0.1 | 0.4 ± 0.1 | 47.2 ± 2.4 a | |||||||||||||||
| 190 | 10.5 | ± | 1.0 | 13.4 | ± | 0.4 | 6.4 | ± | 0.3 | 6.7 | ± | 0.5 | 49.8 | ± | 0.4 | 55.1 | ± | 2.8 | |
| 220 | 11.9 | ± | 1.5 | 13.5 | ± | 0.5 | 6.7 | ± | 0.3 | 6.7 | ± | 0.5 | 44.8 | ± | 3.4 | 45.7 | ± | 5.5 | |
| 250 | 15.3 | ± | 0.9 | 13.7 | ± | 0.1 | 8.3 | ± | 0.8 | 5.7 | ± | 0.2 | 57.5 | ± | 9.8 | 46.8 | ± | 0.6 | |
| pH | Raw | 7.21 ± 0.1 | 8.42 ± 0.01 | 7.0 ± 0.0 | |||||||||||||||
| 190 | 6.0 | ± | 0.0 | 5.3 | ± | 0.0 | 7.1 | ± | 0.2 | 6.8 | ± | 0.0 | 6.0 | ± | 0.1 | 6.0 | ± | 0.0 | |
| 220 | 6.0 | ± | 0.0 | 6.1 | ± | 0.0 | 6.8 | ± | 0.0 | 6.2 | ± | 0.1 | 6.0 | ± | 0.0 | 5.9 | ± | 0.0 | |
| 250 | 5.8 | ± | 0.1 | 6.0 | ± | 0.0 | 7.3 | ± | 0.6 | 6.9 | ± | 0.1 | 6.0 | ± | 0.0 | 5.5 | ± | 0.0 | |
| Total Phosphorous in Liquid (mg/L) | Raw | 175.00 ± 9.00 | 187.00 ± 9.00 | 10.00 ± 1.00 a | |||||||||||||||
| 190 | 32.5 | ± | 0.1 | 38.4 | ± | 0.1 | 19.6 | ± | 0.3 | 22.7 | ± | 0.1 | 0.3 | ± | 0.0 | 0.4 | ± | 0.0 | |
| 220 | 20.2 | ± | 0.1 | 16.4 | ± | 0.2 | 19.8 | ± | 0.3 | 17.8 | ± | 0.1 | 0.2 | ± | 0.0 | 0.2 | ± | 0.0 | |
| 250 | 16.1 | ± | 0.1 | 11.1 | ± | 0.2 | 19.3 | ± | 0.3 | 12.2 | ± | 0.1 | 0.2 | ± | 0.0 | 0.2 | ± | 0.0 | |
| Total Phosphorous in Solid (mg/g) | Raw | 5.22 ± 0.10 | 5.81 ± 0.13 | 9.22 ± 0.33 | |||||||||||||||
| 190 | 6.2 | ± | 0.7 | 6.8 | ± | 0.4 | 7.2 | ± | 0.7 | 7.4 | ± | 1.4 | 9.2 | ± | 0.5 | 9.4 | ± | 0.4 | |
| 220 | 7.8 | ± | 0.3 | 9.4 | ± | 0.2 | 7.5 | ± | 0.5 | 7.5 | ± | 1.5 | 10.5 | ± | 0.2 | 10.6 | ± | 0.2 | |
| 250 | 9.9 | ± | 0.5 | 10.2 | ± | 4.6 | 7.4 | ± | 0.9 | 6.7 | ± | 1.2 | 10.8 | ± | 0.8 | 10.9 | ± | 0.9 | |
| Organic Nitrogen in Liquid (g/L) | Raw | 0.470 ± 0.230 | 0.880 ± 0.010 | 11.023 ± 0.551 a | |||||||||||||||
| 190 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 1.3 | ± | 0.0 | 1.2 | ± | 0.0 | 4.5 | ± | 1.0 | 4.1 | ± | 0.2 | |
| 220 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 1.4 | ± | 0.0 | 0.9 | ± | 0.0 | 2.4 | ± | 0.6 | 1.5 | ± | 0.1 | |
| 250 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 1.3 | ± | 0.0 | 1.5 | ± | 0.0 | 1.7 | ± | 0.3 | 1.4 | ± | 0.7 | |
| Ammonia Nitrogen in Liquid (g/L) | Raw | 0.490 ± 0.010 | 0.880 ± 0.010 | 0.598 ± 0.030 a | |||||||||||||||
| 190 | 0.4 | ± | 0.0 | 0.6 | ± | 0.0 | 0.7 | ± | 0.0 | 0.7 | ± | 0.0 | 2.7 | ± | 0.1 | 3.8 | ± | 0.2 | |
| 220 | 0.4 | ± | 0.0 | 0.5 | ± | 0.0 | 0.8 | ± | 0.0 | 1.0 | ± | 0.0 | 4.4 | ± | 0.3 | 4.6 | ± | 0.6 | |
| 250 | 0.6 | ± | 0.0 | 0.6 | ± | 0.0 | 1.0 | ± | 0.0 | 0.8 | ± | 0.0 | 6.9 | ± | 0.4 | 6.5 | ± | 0.4 | |
| Property | HTC Temp (°C) | Thickened Sludge | Digested Sludge | Dewatered Sludge | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 min HTC | 60 min HTC | 30 min HTC | 60 min HTC | 30 min HTC | 60 min HTC | ||||||||||||||
| Process Yields (dry basis) | |||||||||||||||||||
| Solid Yield (wt%) | 190 | 77.2 | ± | 4.7 | 67.0 | ± | 11.0 | 82.8 | ± | 8.9 | 81.5 | ± | 8.9 | 88.2 | ± | 2.0 | 85.6 | ± | 0.0 |
| 220 | 60.6 | ± | 8.8 | 57.2 | ± | 3.4 | 64.6 | ± | 6.4 | 70.6 | ± | 3.4 | 75.1 | ± | 1.0 | 75.2 | ± | 0.3 | |
| 250 | 49.4 | ± | 4.8 | 52.0 | ± | 6.2 | 78.1 | ± | 7.8 | 64.9 | ± | 3.7 | 67.6 | ± | 2.5 | 66.8 | ± | 0.2 | |
| Gas Yield (wt%) | 190 | 2.5 | ± | 0.0 | 2.7 | ± | 0.2 | 3.7 | ± | 0.9 | 4.7 | ± | 0.3 | 2.1 | ± | 1.4 | 2.6 | ± | 0.2 |
| 220 | 4.1 | ± | 0.7 | 4.2 | ± | 0.4 | 5.1 | ± | 1.3 | 5.6 | ± | 1.0 | 3.7 | ± | 0.1 | 4.3 | ± | 0.6 | |
| 250 | 6.3 | ± | 1.6 | 7.2 | ± | 1.3 | 6.1 | ± | 2.1 | 7.6 | ± | 1.9 | 5.1 | ± | 0.2 | 5.9 | ± | 0.1 | |
| Liquid Yield (wt%) | 190 | 20.3 | ± | 2.4 | 34.9 | ± | 5.4 | 43.2 | ± | 4.9 | 43.1 | ± | 4.6 | 45.2 | ± | 1.7 | 44.1 | ± | 0.1 |
| 220 | 35.3 | ± | 4.7 | 30.7 | ± | 1.9 | 34.8 | ± | 3.9 | 38.1 | ± | 2.2 | 39.4 | ± | 0.6 | 39.7 | ± | 0.4 | |
| 250 | 44.3 | ± | 3.2 | 29.6 | ± | 3.8 | 42.1 | ± | 5.0 | 36.3 | ± | 2.8 | 36.4 | ± | 1.3 | 36.4 | ± | 0.2 | |
| Ultimate Analysis (dry basis) | |||||||||||||||||||
| C (wt%) | Raw | 45.96 | ± | 0.20 | 45.96 | ± | 0.20 | 25.60 | ± | 0.33 | 25.60 | ± | 0.33 | 35.91 | ± | 0.25 | 35.91 | ± | 0.25 |
| 190 | 44.56 | ± | 0.39 | 46.11 | ± | 0.39 | 19.22 | ± | 1.37 | 14.16 | ± | 2.57 | 36.61 | ± | 0.02 | 35.07 | ± | 0.23 | |
| 220 | 44.86 | ± | 0.51 | 43.15 | ± | 0.19 | 11.70 | ± | 0.64 | 10.21 | ± | 0.58 | 35.19 | ± | 0.17 | 35.75 | ± | 0.31 | |
| 250 | 41.68 | ± | 0.29 | 41.21 | ± | 0.52 | 12.51 | ± | 0.26 | 12.02 | ± | 0.05 | 35.30 | ± | 0.18 | 35.57 | ± | 0.08 | |
| H (wt%) | Raw | 6.57 | ± | 0.02 | 6.57 | ± | 0.02 | 3.96 | ± | 0.06 | 3.96 | ± | 0.06 | 5.42 | ± | 0.00 | 5.42 | ± | 0.00 |
| 190 | 6.24 | ± | 0.08 | 6.46 | ± | 0.06 | 2.63 | ± | 0.16 | 2.00 | ± | 0.29 | 4.92 | ± | 0.02 | 4.61 | ± | 0.03 | |
| 220 | 5.95 | ± | 0.01 | 5.69 | ± | 0.02 | 1.61 | ± | 0.08 | 1.37 | ± | 0.07 | 4.32 | ± | 0.01 | 4.36 | ± | 0.03 | |
| 250 | 5.03 | ± | 0.05 | 5.01 | ± | 0.03 | 1.65 | ± | 0.01 | 1.56 | ± | 0.02 | 4.11 | ± | 0.00 | 4.05 | ± | 0.01 | |
| N (wt%) | Raw | 4.26 | ± | 0.00 | 4.26 | ± | 0.00 | 3.59 | ± | 0.10 | 3.59 | ± | 0.10 | 5.81 | ± | 0.02 | 5.81 | ± | 0.02 |
| 190 | 2.23 | ± | 0.07 | 2.10 | ± | 0.07 | 1.58 | ± | 0.07 | 1.07 | ± | 0.17 | 4.27 | ± | 0.06 | 3.95 | ± | 0.01 | |
| 220 | 1.87 | ± | 0.24 | 1.86 | ± | 0.07 | 0.80 | ± | 0.08 | 0.66 | ± | 0.01 | 3.48 | ± | 0.02 | 3.45 | ± | 0.02 | |
| 250 | 1.89 | ± | 0.05 | 1.99 | ± | 0.04 | 0.70 | ± | 0.00 | 0.69 | ± | 0.02 | 3.16 | ± | 0.04 | 3.12 | ± | 0.02 | |
| O (wt%) | Raw | 27.61 | ± | 4.86 | 27.61 | ± | 4.86 | 21.16 | ± | 0.79 | 21.16 | ± | 0.79 | 23.52 | ± | 0.52 | 23.52 | ± | 0.52 |
| 190 | 28.52 | ± | 6.69 | 25.97 | ± | 4.63 | 18.43 | ± | 3.50 | 15.67 | ± | 6.89 | 18.55 | ± | 0.17 | 19.33 | ± | 0.58 | |
| 220 | 25.51 | ± | 6.34 | 26.43 | ± | 5.06 | 12.35 | ± | 1.03 | 18.93 | ± | 1.10 | 16.53 | ± | 0.33 | 15.34 | ± | 0.49 | |
| 250 | 22.47 | ± | 6.44 | 21.59 | ± | 3.83 | 11.72 | ± | 2.00 | 7.17 | ± | 2.53 | 14.07 | ± | 0.44 | 14.12 | ± | 2.18 | |
| Proximate Analysis (dry basis) | |||||||||||||||||||
| Fixed Carbon (wt%) | Raw | 12.56 | ± | 1.43 | 12.56 | ± | 1.43 | 4.76 | ± | 3.78 | 4.76 | ± | 3.78 | 5.90 | ± | 0.62 | 5.90 | ± | 0.62 |
| 190 | 4.25 | ± | 2.50 | 4.58 | ± | 1.75 | 1.70 | ± | 0.09 | 2.06 | ± | 0.34 | 5.37 | ± | 0.23 | 9.94 | ± | 6.93 | |
| 220 | 5.37 | ± | 4.00 | 5.23 | ± | 3.97 | 1.26 | ± | 0.07 | 0.13 | ± | 0.01 | 4.04 | ± | 2.06 | 9.83 | ± | 5.58 | |
| 250 | 5.31 | ± | 5.85 | 6.12 | ± | 5.93 | 0.62 | ± | 0.05 | 1.38 | ± | 0.09 | 6.27 | ± | 0.61 | 7.02 | ± | 0.03 | |
| Volatile Matter (wt%) | Raw | 72.48 | ± | 3.19 | 72.48 | ± | 3.19 | 50.25 | ± | 3.51 | 50.25 | ± | 3.51 | 65.67 | ± | 0.50 | 65.67 | ± | 0.50 |
| 190 | 70.84 | ± | 6.65 | 71.03 | ± | 5.36 | 41.09 | ± | 3.22 | 30.84 | ± | 4.20 | 58.98 | ± | 0.29 | 53.02 | ± | 6.61 | |
| 220 | 66.05 | ± | 5.56 | 63.57 | ± | 7.81 | 26.17 | ± | 1.59 | 31.99 | ± | 0.90 | 55.48 | ± | 2.19 | 49.07 | ± | 5.72 | |
| 250 | 60.06 | ± | 2.19 | 56.70 | ± | 3.95 | 25.97 | ± | 1.68 | 21.65 | ± | 4.68 | 50.37 | ± | 0.39 | 49.85 | ± | 2.03 | |
| Ash (Inorganic) (wt%) | Raw | 14.96 | ± | 2.31 | 14.96 | ± | 2.31 | 44.99 | ± | 3.65 | 44.99 | ± | 3.65 | 28.43 | ± | 0.56 | 28.43 | ± | 0.56 |
| 190 | 24.91 | ± | 4.57 | 24.40 | ± | 3.56 | 57.21 | ± | 1.61 | 67.10 | ± | 2.27 | 35.66 | ± | 0.26 | 37.05 | ± | 6.77 | |
| 220 | 28.58 | ± | 4.78 | 31.20 | ± | 5.89 | 72.57 | ± | 0.80 | 67.88 | ± | 0.45 | 40.48 | ± | 2.13 | 41.10 | ± | 5.65 | |
| 250 | 34.63 | ± | 4.02 | 37.18 | ± | 4.94 | 73.42 | ± | 0.86 | 76.97 | ± | 2.34 | 43.36 | ± | 0.50 | 43.14 | ± | 1.03 | |
| Combustion Analysis | |||||||||||||||||||
| HHV (MJ/kg) | Raw | 20.50 | ± | 0.14 | 20.50 | ± | 0.14 | 10.66 | ± | 1.79 | 10.66 | ± | 1.79 | 16.02 | ± | 0.09 | 16.02 | ± | 0.09 |
| 190 | 19.45 | ± | 0.64 | 20.71 | ± | 0.03 | 9.27 | ± | 1.73 | 7.97 | ± | 1.77 | 16.30 | ± | 0.19 | 15.96 | ± | 0.07 | |
| 220 | 20.06 | ± | 0.90 | 18.72 | ± | 0.03 | 8.96 | ± | 1.77 | 7.86 | ± | 0.39 | 15.70 | ± | 0.09 | 15.47 | ± | 0.37 | |
| 250 | 18.66 | ± | 0.26 | 19.17 | ± | 0.61 | 8.59 | ± | 1.78 | 9.37 | ± | 1.39 | 15.98 | ± | 0.11 | 15.33 | ± | 0.26 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merzari, F.; Goldfarb, J.; Andreottola, G.; Mimmo, T.; Volpe, M.; Fiori, L. Hydrothermal Carbonization as a Strategy for Sewage Sludge Management: Influence of Process Withdrawal Point on Hydrochar Properties. Energies 2020, 13, 2890. https://doi.org/10.3390/en13112890
Merzari F, Goldfarb J, Andreottola G, Mimmo T, Volpe M, Fiori L. Hydrothermal Carbonization as a Strategy for Sewage Sludge Management: Influence of Process Withdrawal Point on Hydrochar Properties. Energies. 2020; 13(11):2890. https://doi.org/10.3390/en13112890
Chicago/Turabian StyleMerzari, Fabio, Jillian Goldfarb, Gianni Andreottola, Tanja Mimmo, Maurizio Volpe, and Luca Fiori. 2020. "Hydrothermal Carbonization as a Strategy for Sewage Sludge Management: Influence of Process Withdrawal Point on Hydrochar Properties" Energies 13, no. 11: 2890. https://doi.org/10.3390/en13112890
APA StyleMerzari, F., Goldfarb, J., Andreottola, G., Mimmo, T., Volpe, M., & Fiori, L. (2020). Hydrothermal Carbonization as a Strategy for Sewage Sludge Management: Influence of Process Withdrawal Point on Hydrochar Properties. Energies, 13(11), 2890. https://doi.org/10.3390/en13112890










