Industrial Ceramic Blocks for Buildings: Clay Characterization and Drying Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Clay Characterization
2.1.1. Chemical Analysis
2.1.2. Mineralogical Analysis (by X-ray Diffraction)
2.1.3. Particle Size Analysis
2.1.4. Differential Thermal (DTA) and Thermogravimetric (TGA) Analysis
2.2. Kiln Drying Experiments
2.3. Auxiliary Parameters
- (a)
- Mass of water
- (b)
- Moisture content
- (c)
- Dimensional temperature
- (d)
- Volume
- (e)
- Relative humidity of drying air
2.4. Drying Performance and Specific Energy Consumption
3. Results and Discussion
3.1. Clay Characterization
3.1.1. Chemical Analysis
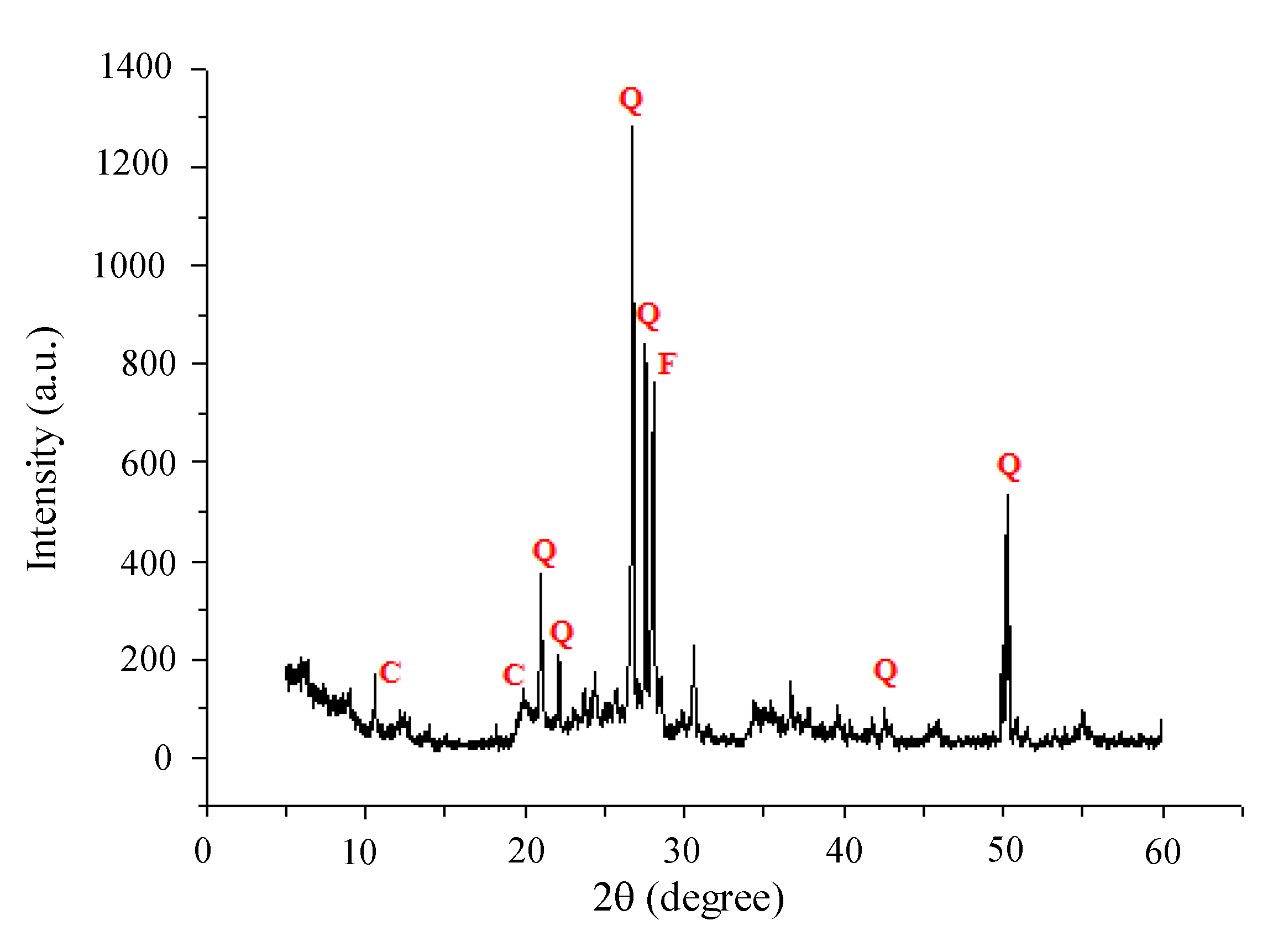
3.1.2. Mineralogical Analysis
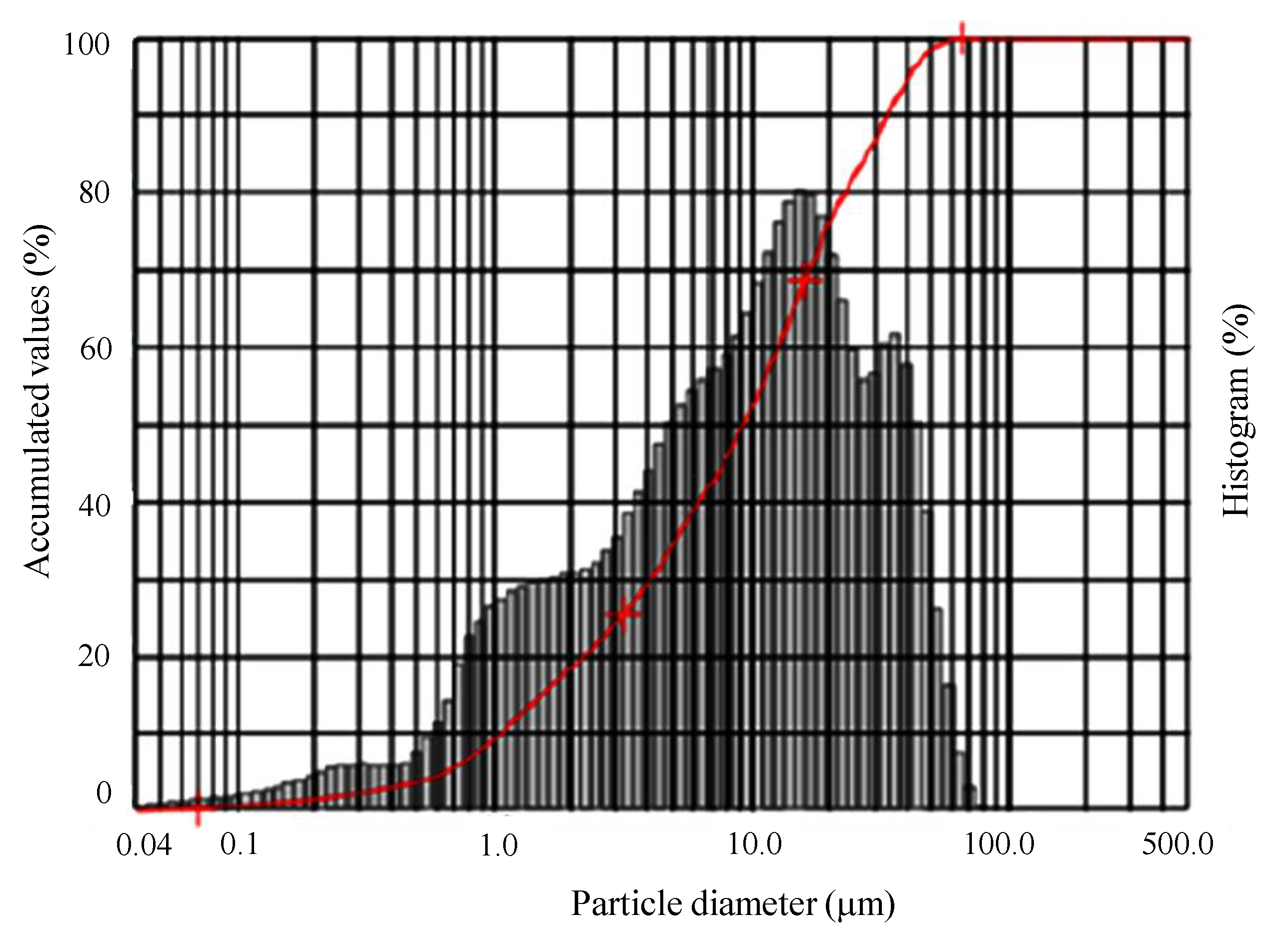
3.1.3. Particle Size Analysis
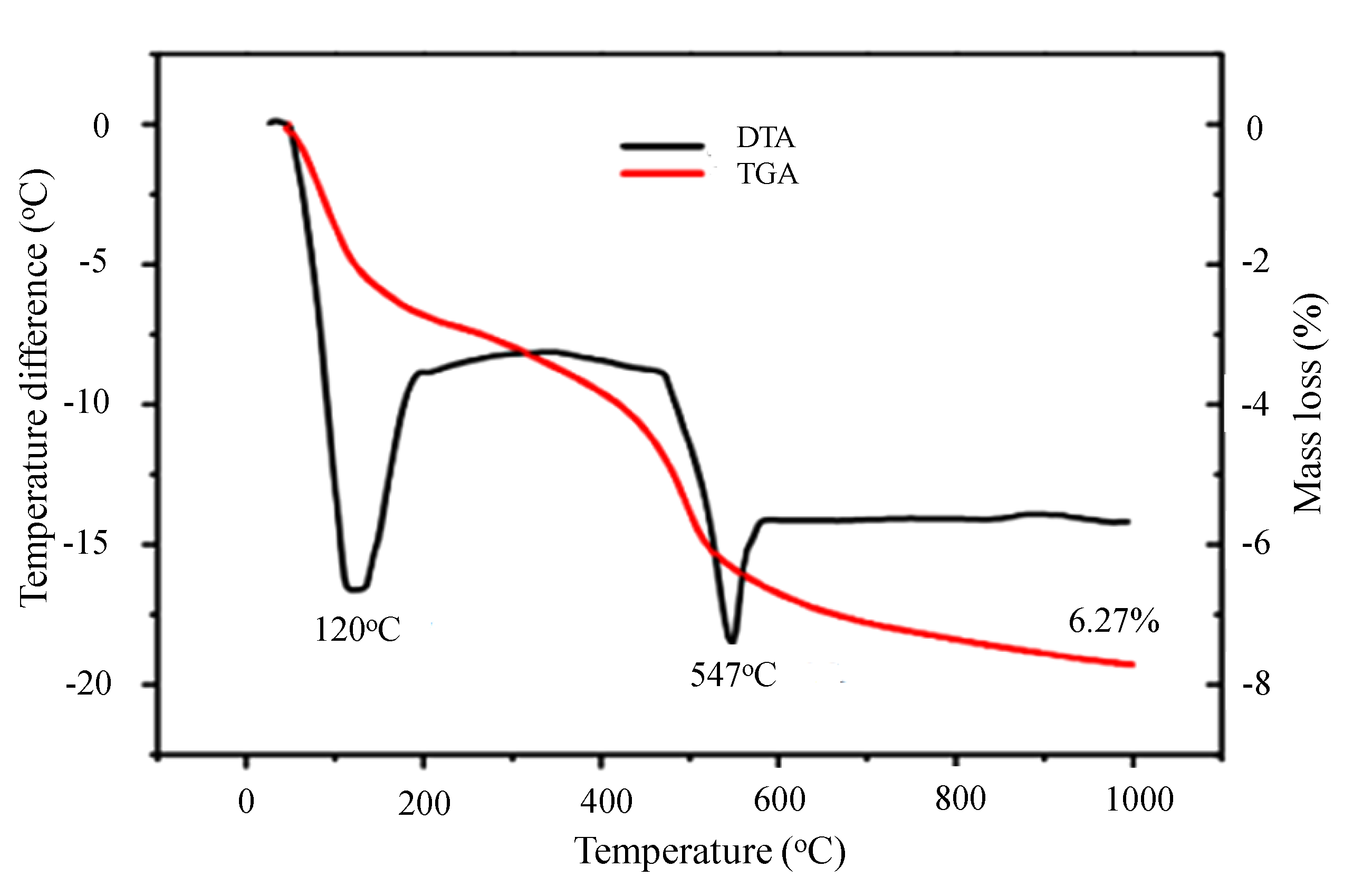
3.1.4. Thermal and Gravimetric Analysis
3.2. Kiln Drying of Industrial Compensators
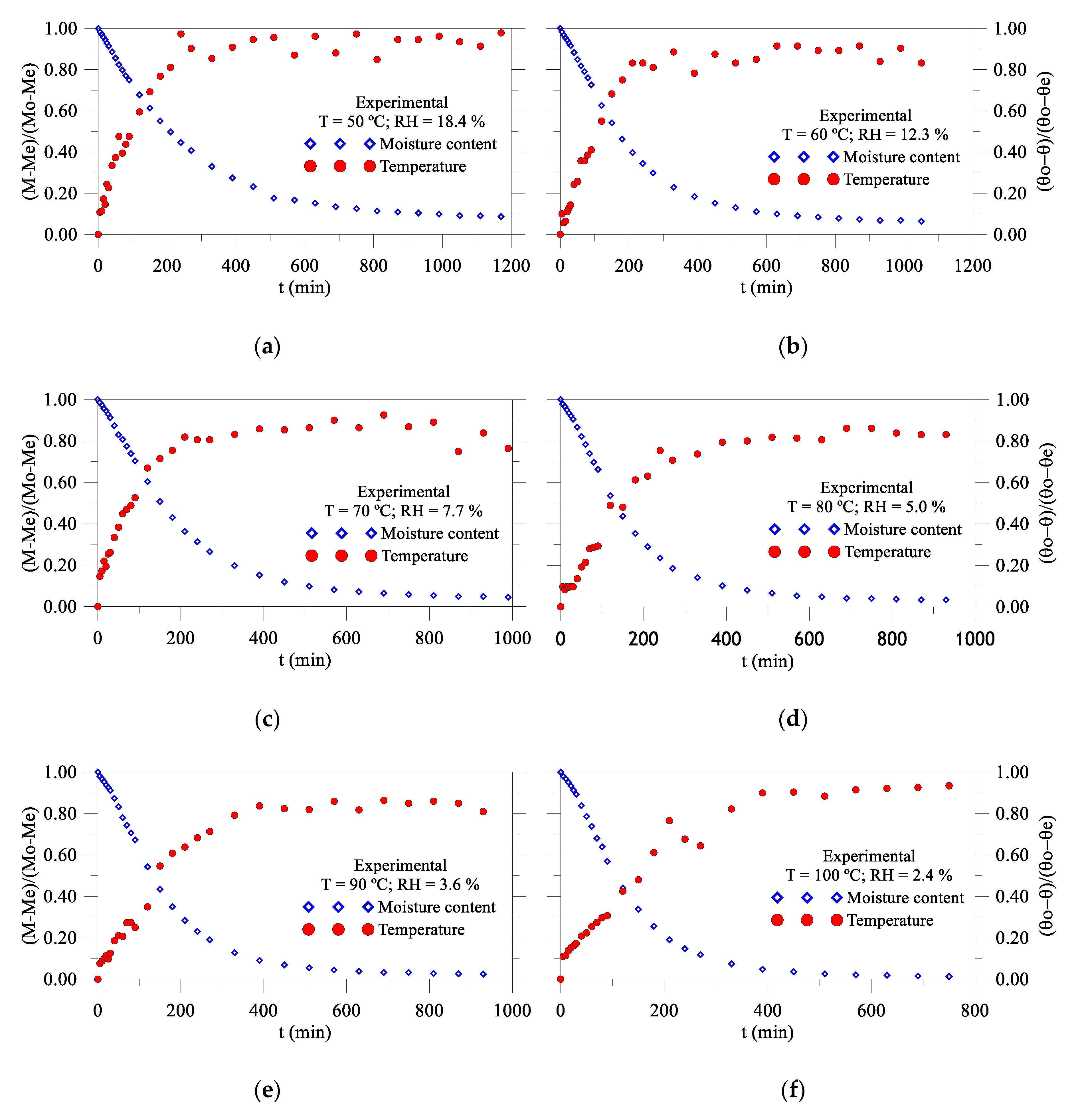
3.2.1. Drying and Heating Kinetics
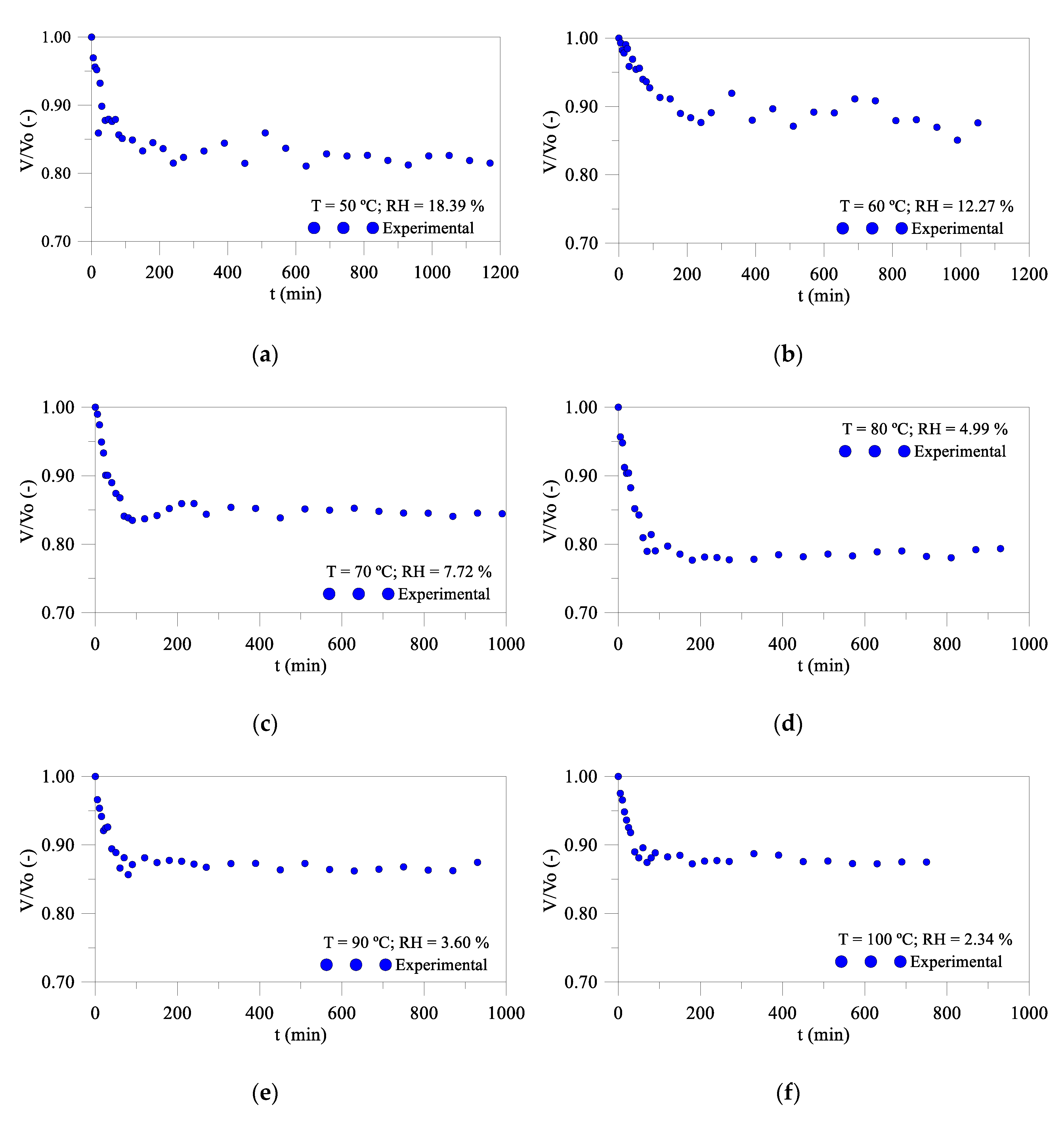
3.2.2. Volumetric Variation Kinetics
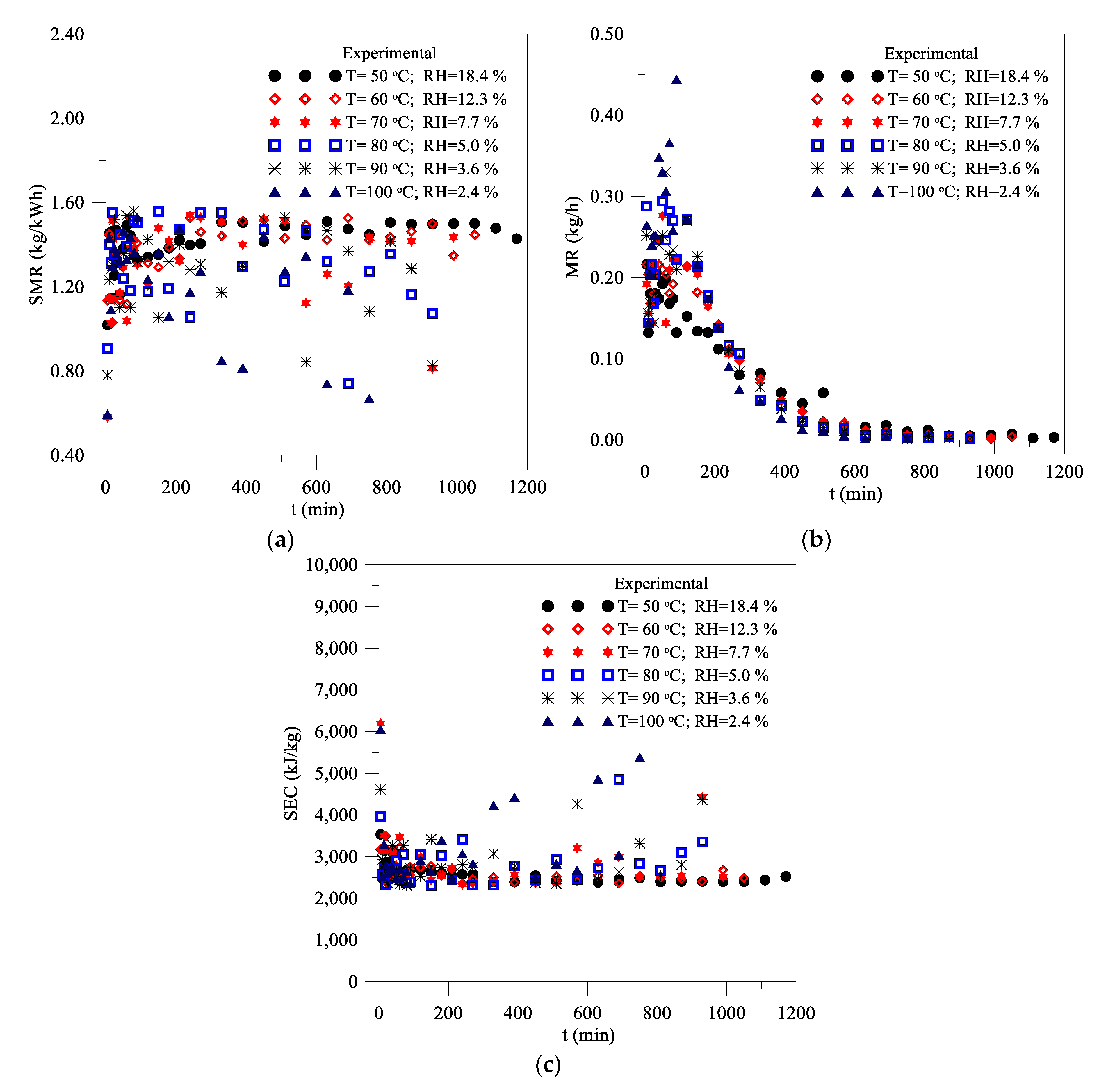
3.2.3. Drying Performance
4. Block Thermal Behavior for Applications in Buildings
- (a)
- Low thermal transmittance and high thermal resistance due to the original shape of the brick, which includes different cavities (holes and rectangular prisms). However, we state that results related to these parameters were not effectively obtained and will be subject of new research;
- (b)
- Low porosity, so, the brick has low water absorbability, thus reducing many troubles due to negative environmental action (for example, rain and wet soil);
- (c)
- The brick was constructed by extrusion. Because of the shape of the brick, it is possible, for example, to fill the external void space with air or another isolating material, and thus, to reduce thermal transmittance easily.
5. Conclusions
- (a)
- Regarding the chemical, mineralogical, granulometric, thermal, and gravimetric clay characterizations, it was found that:
- -
- The raw material presents characteristic of red clay, prevailing silica (57.53%), alumina (23.43%), and iron oxide III (8.89%), being basically constituted of kaolinite, quartz, and feldspar, with high silt content (80.09%), a percentage of 1.51% sand, and a fraction of clay of 18.4%.
- -
- Regarding the thermogravimetric curve of the clay, there was a loss of 2% of mass, between 0 and 170 °C, a loss of 4% of organic matter, between 170 °C and 510 °C, and loss of hydroxyl in temperatures above 510 °C.
- -
- Regarding the thermo-differential curve of the clay, endothermic peaks were detected due to water loss, at 120 °C, and the presence of hydroxyl, at a temperature of 547 °C.
- (b)
- Regarding the drying of the ceramic blocks in an oven, it was found that:
- -
- Drying air parameters such as relative humidity and temperature directly affect drying kinetics and the heating of the wet solid. For lower temperatures and higher relative humidity of the drying air, the rates of drying, heating, and dimensional variations are lower, reducing the risk of defects in the product, post-drying.
- -
- Drying at high temperatures and low relative humidity of the air, generate high rates of water loss, temperature rise, and volumetric retraction of the ceramic block, which can generally cause the propagation of cracks, which can compromise the quality and the product’s ability to resist to compression stresses when in operation.
- -
- The product showed an almost linear shrinkage in the first 60 min in all drying conditions, with oscillations for long times, due to the successive heating and cooling of the product, during measurements.
- -
- The industrial compensator presented a volumetric variation of 15.37%, with a temperature of 80 °C being the greatest variation (20.7%). On average, its moisture content has decreased by more than 99%.
- (c)
- Regarding the drying performance, it was found that:
- -
- The major portion of the energy received by the clay block is related to latent heat for water evaporation.
- -
- The total energy received by the wet clay block increases as the air temperature is increased.
- -
- Instantaneous SMR and MR parameters have shown decreasing behavior as a function of the time, opposite to the SEC parameter, which increased along the process, in all drying conditions.
- -
- When the temperature ranged from 50 °C to 100 °C, the average values of the SMR, MR, and SEC parameters ranged from 1.408 kg/kWh, 0.096 kg/h, and 2576.905 kJ/kg to 1.212 kg/kWh, 0172 kg/h, and 3172.534 kJ/kg, respectively.
- -
- Based on the visual analysis of the brick at different moments of drying, and the SMR, MR, and SEC performance parameters, the best drying conditions recommended are air temperature 80 °C and relative humidity 5.0%.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomez, R.S.; Porto, T.R.N.; Magalhães, H.L.F.; Moreira, G.; André, A.M.M.C.N.; Melo, R.B.F.; Lima, A.G.B. Natural gas intermittent kiln for the ceramic industry: A transient thermal analysis. Energies 2019, 12, 1568. [Google Scholar]
- Ombaka, O. Characterization and classification of clay minerals for potential applications in Rugi Ward, Kenya. African J. Environm. Sci. Technol. 2016, 10, 415–431. [Google Scholar]
- Kočí, V.; Scheinherrová, L.; Madeřa, J.; Keppert, M.; Suchorab, Z.; Łagód, G.; Černý, R. Experimental and computational study of thermal processes in red clays exposed to high temperatures. Energies 2020, 13, 2211. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Ricchi, A.; Gualtieri, M.L.; Maretti, S.; Tamburini, M. Kinetic study of the drying process of clay bricks. J. Therm. Anal. Calorim. 2016, 123, 153–167. [Google Scholar] [CrossRef]
- Vieira, C.M.F.; Morais, A.S.C.; Monteiro, S.N.; Delaqua, G.C.G. Industrial test of heavy clay ceramic incorporated with fluorescent lamp waste. Cerâmica 2016, 62, 376–385. (In Portuguese) [Google Scholar] [CrossRef]
- Andrade, M.C.; Sampaio, J.A.; Luz, A.B.; Buoso, A. RMIs: Rocks and minerals for ceramic coating. In Industrial Rocks and Minerals in Brazil: Uses and Specifications, 2nd ed.; CETEM/MCTI: Rio de Janeiro, Brazil, 2008; pp. 723–745. (In Portuguese) [Google Scholar]
- Mota Neto, J. Simultaneous control of temperature and stoichiometric applied to a vertical dryer ceramic feeded with natural gas. Master’s Thesis in Mechanical Engineering, Federal University of Rio Grande do Sul, Rio de Janeiro, Brazil, 2008. (In Portuguese). [Google Scholar]
- Silva, W.P.; Silva, C.M.D.P.S.; Silva, L.D.; Farias, V.S.O. Drying of clay slabs: Experimental determination and prediction by two-dimensional diffusion models. Ceram. Int. 2013, 39, 7911–7919. [Google Scholar] [CrossRef]
- Oliveira, B.F.; Silva, M.A.; Freitas, M.S. Drying of Ceramic Materials; Undergraduate Report; State University of the Norte Fluminense Darcy Ribeiro: Campos dos Goytacazes, RJ, Brazil, 2010. (In Portuguese) [Google Scholar]
- Vasić, M.; Radojević1, Z. Non isothermal drying process optimisation—Drying of clay tiles. IOP Conf. Ser. Mater. Sci. Eng. 2015, 95, 012025. [Google Scholar] [CrossRef]
- Silva, L.D.; Silva, W.P.; Silva, C.M.D.P.S.; Farias, V.S.O. Description of drying ceramic plates by a diffusion model. Cerâmica 2013, 59, 409–416. [Google Scholar] [CrossRef][Green Version]
- Lopez, E.M.; Cortes, L.L. Moisture transfer analysis during drying of brick by temperature and relative humidity profiles. Eur. Sci. J. 2012, 9, 109–122. [Google Scholar]
- Silva, W.P.; Farias, V.S.O.; Neves, G.A.; Lima, A.G.B. Modeling of water transport in roof tiles by removal of moisture at isothermal conditions. Heat Mass Transf. 2012, 48, 809–821. [Google Scholar] [CrossRef]
- Van der Zanden, A.J.J.; de Wit, M.H. A procedure to measure the diffusion coefficient of water in brick as a function of the water concentration. Dry. Technol. 2012, 30, 526–534. [Google Scholar] [CrossRef]
- Telljohann, U.; Junge, K.; Specht, E. Moisture diffusion coefficients for modeling the first and second drying sections of green bricks. Dry. Technol. 2008, 26, 855–863. [Google Scholar] [CrossRef]
- Silva, J.B.; Almeida, G.S.; Lima, W.C.P.B.; Neves, G.A.; Lima, A.G.B. Heat and Mass Transfer and Volume Variations during Drying of Industrial Ceramic Bricks: An Experimental Investigation. Defect Diff. Forum 2012, 326, 267–272. [Google Scholar] [CrossRef]
- Araújo, M.V.; Santos, R.S.; Silva, R.M.; Nascimento, J.B.S.; Santos, W.R.G.; Lima, A.G.B. Drying of Industrial Hollow Ceramic Brick: A Numerical Analysis Using CFD. Defect Diffus. Forum 2019, 391, 48–53. [Google Scholar] [CrossRef]
- Araújo, M.V.; Pereira, A.S.; Oliveira, J.L.; Brandão, V.A.A.; Brasileiro Filho, F.; De, A.; Silva, R.M.; Lima, A.G.B. Industrial Ceramic Brick Drying in Oven by CFD. Materials 2019, 12, 1612. [Google Scholar] [CrossRef]
- Araújo, M.V.; Correia, B.R.B.; Brandão, V.A.A.; Oliveira, I.R.; Santos, R.S.; Oliveira Neto, G.L.; Silva, L.P.L.; Lima, A.G.B. Convective Drying of Ceramic Bricks by CFD: Transport Phenomena and Process Parameters Analysis. Energies 2020, 13, 2073. [Google Scholar] [CrossRef]
- Muñoz, P.; Morales, M.P.; Mendívil, M.A.; Juárez, M.C.; Muñoz, L. Using of waste pomace from winery industry to improve thermal insulation of fired clay bricks. Eco-friendly way of building construction, Constr. Build. Mater. 2014, 71, 181–187. [Google Scholar] [CrossRef]
- Zukowski, M.; Haese, G. Experimental and numerical investigation of a hollow brick filled with perlite insulation. Energy Build. 2010, 42, 1402–1408. [Google Scholar] [CrossRef]
- Morales, M.P.; Juárez, M.C.; López-Ochoa, L.M.; Doménech, J. Study of the geometry of a voided clay brick using rectangular perforations to optimize its thermal properties. Appl. Thermal Eng. 2011, 31, 2063–2065. [Google Scholar] [CrossRef]
- Bai, G.-L.; Du, N.-J.; Xu, Y.-Z.; Qin, C.-G. Study on the Thermal Properties of Hollow Shale Blocks as Self-Insulating Wall Materials. Adv. Mater. Sci. Eng. 2017, 2017, 9432145. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Mourad, A.-H.; Hittini, W.; Hassan, M.; Hameedi, S. Traditional, state-of-the-art and renewable thermal building insulation materials: An overview. Constr. Build. Mater. 2019, 214, 709–735. [Google Scholar] [CrossRef]
- Meng, X.; Yan, B.; Gao, Y.; Wang, J.; Zhang, W.; Long, E. Factors affecting the in situ measurement accuracy of the wall heat transfer coefficient using the heat flow meter method. Energy Build. 2015, 86, 754–765. [Google Scholar] [CrossRef]
- Meng, X.; Luo, T.; Gao, Y.; Zhang, L.; Shen, Q.; Long, E. A new simple method to measure wall thermal transmittance in situ and its adaptability analysis. Appl. Thermal Eng. 2017, 122, 747–757. [Google Scholar] [CrossRef]
- Gualtieri, M.L.; Gualtieri, A.F.; Gagliardi, S.; Ruffini, P.; Ferrari, R.; Hanuskova, M. Thermal conductivity of fired clays: Effects of mineralogical and physical properties of the raw materials. Appl. Clay Sci. 2010, 49, 269–275. [Google Scholar] [CrossRef]
- Jain, M.; Pathak, K.K. Thermal modelling of insulator for energy saving in existing residential building. J. Build. Eng. 2018, 19, 62–68. [Google Scholar] [CrossRef]
- Ozturk, S.; Sutcu, M.; Erdogmus, E.; Gencel, O. Influence of tea waste concentration in the physical, mechanical and thermal properties of brick clay mixtures. Constr. Build. Mater. 2019, 217, 592–599. [Google Scholar] [CrossRef]
- Sousa, H.; Sousa, L.; António, C.; Castro, C. Optimization of the geometry of blocks for masonry in a thermal perspective. In Proceedings of the National Construction Conference 2004—2nd National Construction Congress, Porto, Portugal, 13–15 December 2004; Faculty of Engineering, University of Porto: Porto, Portugal, 2004; pp. 479–484. [Google Scholar]
- Coutinho, N.C.; Vieira, C.M.F. Characterization and incorporation of MSWI ash in red ceramic. Cerâmica 2016, 62, 249–255. [Google Scholar] [CrossRef][Green Version]
- Silva, V.S. Heat and Mass Transfer in Materials with Complex Shape via Lumped Analysis Method. Case Study: Drying of Ceramic Materials. Ph.D. Thesis, Federal University of Campina Grande, Campina Grande, Brazil, 2016; p. 198f. [Google Scholar]
- Nascimento, J.J.S.; Lima, A.G.B. Experimental Drying of Ceramics Bricks Including Shrinkage. Defect Diffus. Forum 2015, 365, 106–111. [Google Scholar] [CrossRef]
- Silva, J.B.; Almeida, G.S.; Lima, W.C.P.B.; Neves, G.A.; Lima, A.G.B. Heat and mass diffusion including shrinkage and hygrothermal stress during drying of holed ceramics bricks. Defect Diffus. Forum 2011, 312, 971–976. [Google Scholar] [CrossRef]
- Murmu, A.L.; Patel, A. Towards sustainable bricks production: An overview. Constr. Build. Mater. 2018, 165, 112–125. [Google Scholar] [CrossRef]

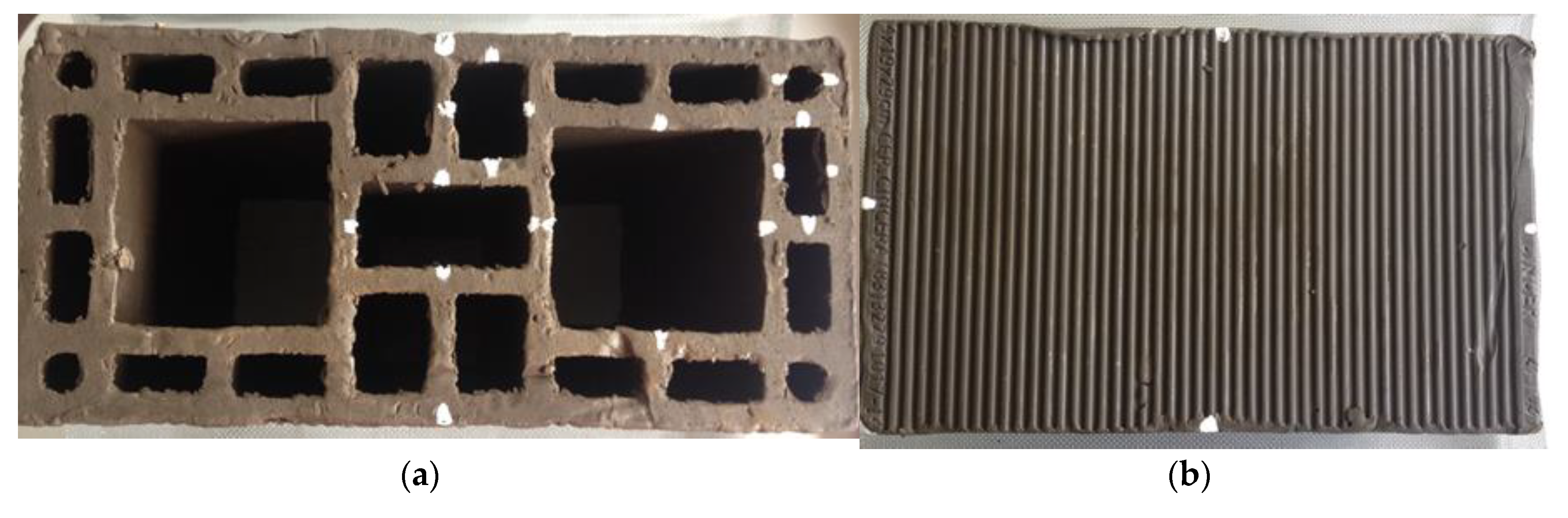
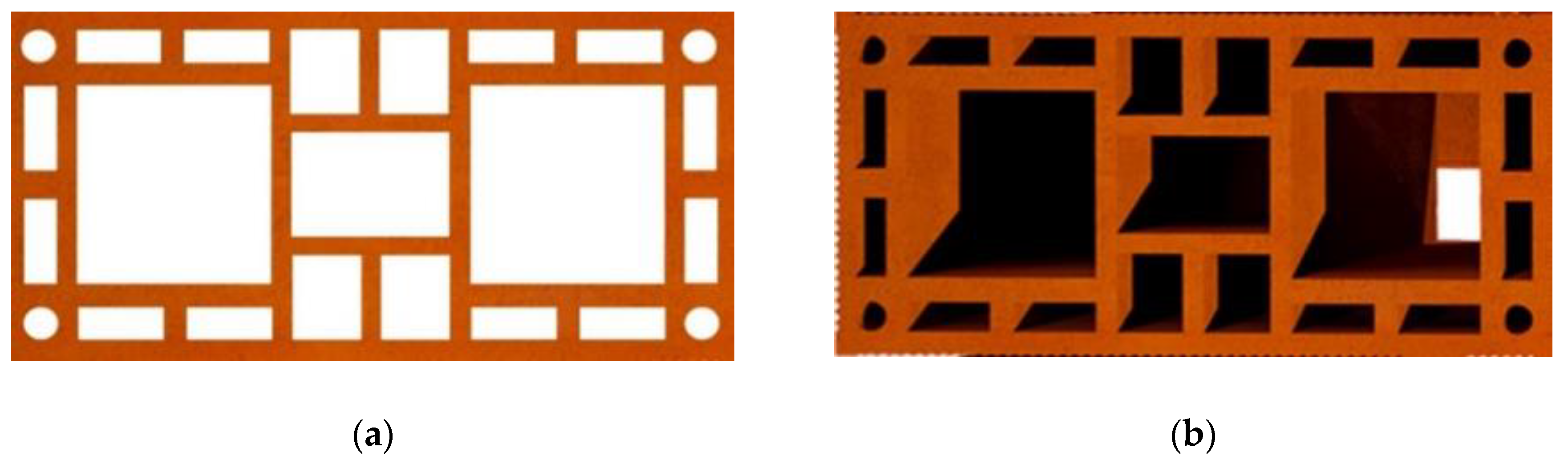

| Test | Air | Compensator 07 | ||||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | RH (%) | v (m/s) | M0 (d.b.) | Me (d.b.) | θ0 (°C) | θe (°C) | t (min) | |
| 1 | 50 | 18.39 | 1.0 | 0.1723191 | 0.002685 | 31.5 | 49.5 | 1170 |
| 2 | 60 | 12.27 | 1.0 | 0.173163 | 0.001834 | 32.0 | 58.0 | 1050 |
| 3 | 70 | 7.72 | 1.0 | 0.170186 | 0.001189 | 29.8 | 64.7 | 990 |
| 4 | 80 | 4.99 | 1.0 | 0.172723 | 0.000826 | 30.5 | 68.6 | 930 |
| 5 | 90 | 3.56 | 1.0 | 0.167900 | 0.000511 | 27.6 | 76.2 | 930 |
| 6 | 100 | 2.34 | 1.0 | 0.169366 | 0.000054 | 27.5 | 95.1 | 750 |
| Sample | Clay (<2 µm) | Silt (2 ≤ X ≤ 50 µm) | Sand (50 ≤ X ≤ 100 µm) | Average Diameter (µm) |
|---|---|---|---|---|
| Clay | 18.4% | 80.09% | 1.51% | 13.36 |
| V (mm3) | A (mm) | C (mm) | L (mm) | M (kg/kg) | V (mm3) | A (mm) | C (mm) | L (mm) | M (kg/kg) | |
|---|---|---|---|---|---|---|---|---|---|---|
| T= 50 °C | T= 60 °C | |||||||||
| Before drying | 3,956,538 | 143 | 303 | 199 | 0.172319 | 3,538,313 | 140 | 302 | 200 | 0.173163 |
| After drying | 3,224,142 | 136 | 289 | 190 | 0.002685 | 3,099,442 | 135 | 289 | 190 | 0.001834 |
| Abs. variation | 732,396 | 7 | 14 | 9 | 0.169634 | 438,871 | 5 | 13 | 10 | 0.171329 |
| Variation (%) | 18.5% | 4.9% | 4.6% | 4.5% | 98.4% | 12.4% | 3.6% | 4.3% | 5% | 98.9% |
| T= 70 °C | T= 80 °C | |||||||||
| Before drying | 3,682,082 | 142 | 300 | 200 | 0.170186 | 3,842,866 | 144 | 300 | 200 | 0.172723 |
| After drying | 3,109,589 | 137 | 288 | 191 | 0.001189 | 3,049,143 | 138 | 287 | 191 | 0.000826 |
| Abs. variation | 572,493 | 5 | 12 | 9 | 0.168997 | 793,723 | 6 | 13 | 9 | 0.171897 |
| Variation (%) | 15.6% | 3.5% | 4% | 4.5% | 99.3% | 20.7% | 4.2% | 4.3% | 4.5% | 99.5% |
| T= 90 °C | T= 100 °C | |||||||||
| Before drying | 3,732,089 | 143 | 300 | 199 | 0.167900 | 3,869,290 | 142 | 301 | 201 | 0.169366 |
| After drying | 3,264,174 | 139 | 288 | 190 | 0.000511 | 3,385,790 | 138 | 291 | 192 | 0.000054 |
| Abs. variation | 467,915 | 4 | 12 | 9 | 0.167389 | 483,500 | 4 | 10 | 9 | 0.169312 |
| Variation (%) | 12.5% | 2.8% | 4% | 4.5% | 99.7% | 12.5% | 2.8% | 3.3% | 4.5% | 100.0% |
| D (mm) | CF1 (mm) | LF1 (mm) | CF2 (mm) | LF2 (mm) | CF3 (mm) | LF3 (mm) | CF4 (mm) | LF4 (mm) | |
|---|---|---|---|---|---|---|---|---|---|
| T = 50 °C | |||||||||
| Before drying | 14.32 | 15.21 | 33.03 | 73.08 | 75.90 | 37.89 | 24.65 | 30.92 | 62.90 |
| After drying | 14.03 | 15.54 | 33.00 | 70.11 | 73.04 | 36.02 | 24.44 | 29.81 | 60.38 |
| Absolute variation | 0.29 | 0.33 | 0.03 | 2.97 | 2.86 | 1.87 | 0.21 | 1.11 | 2.52 |
| Percentage variation | 2.0% | 2.2% | 0.1% | 4.1% | 3.8% | 4.9% | 0.9% | 3.6% | 4.0% |
| T= 60 °C | |||||||||
| Before drying | 15.77 | 15.20 | 33.75 | 72.94 | 78.00 | 37.26 | 28.91 | 30.58 | 64.23 |
| After drying | 15.67 | 14.62 | 32.77 | 69.99 | 75.17 | 35.50 | 27.45 | 28.60 | 61.60 |
| Absolute variation | 0.10 | 0.60 | 0.98 | 2.95 | 2.83 | 1.76 | 1.46 | 1.98 | 2.63 |
| Percentage variation | 0.6% | 4.0% | 2.9% | 4.0% | 3.6% | 4.7% | 5.1% | 6.5% | 4.1% |
| T= 70 °C | |||||||||
| Before drying | 15.40 | 15.97 | 33.41 | 74.00 | 75.61 | 36.75 | 26.54 | 31.04 | 62.84 |
| After drying | 15.57 | 16.29 | 32.72 | 71.32 | 73.33 | 35.78 | 25.85 | 30.45 | 61.04 |
| Absolute variation | 0.17 | 0.32 | 0.69 | 2.68 | 2.28 | 0.97 | 0.69 | 0.59 | 1.8 |
| Percentage variation | 1.1% | 2.0% | 2.1% | 3.6% | 3.0% | 2.6% | 2.6% | 1.9% | 2.9% |
| T= 80 °C | |||||||||
| Before drying | 15.19 | 15.52 | 33.91 | 74.38 | 74.91 | 35.89 | 26.54 | 31.57 | 63.10 |
| After drying | 15.13 | 15.27 | 33.04 | 71.64 | 71.85 | 35.58 | 25.77 | 30.09 | 61.24 |
| Absolute variation | 0.06 | 0.25 | 0.87 | 2.74 | 3.06 | 0.31 | 0.77 | 1.48 | 1.86 |
| Percentage variation | 0.4% | 1.6% | 2.6% | 3.7% | 4.1% | 0.9% | 2.9% | 4.7% | 3.0% |
| T= 90 °C | |||||||||
| Before drying | 15.27 | 15.57 | 34.82 | 73.94 | 75.13 | 36.81 | 26.17 | 30.77 | 63.19 |
| After drying | 14.97 | 15.30 | 33.64 | 71.28 | 72.77 | 36.17 | 25.77 | 30.60 | 61.10 |
| Absolute variation | 0.30 | 0.27 | 1.18 | 2.66 | 2.36 | 0.64 | 0.40 | 0.17 | 2.09 |
| Percentage variation | 2.0% | 1.7% | 3.4% | 3.6% | 3.1% | 1.7% | 1.5% | 0.6% | 3.3% |
| T= 100 °C | |||||||||
| Before drying | 14.49 | 15.35 | 31.70 | 73.85 | 75.67 | 36.16 | 26.86 | 30.29 | 63.78 |
| After drying | 14.47 | 15.29 | 32.78 | 71.25 | 71.98 | 35.39 | 26.21 | 30.12 | 62.56 |
| Absolute variation | 0.02 | 0.06 | 1.08 | 2.6 | 3.69 | 0.77 | 0.65 | 0.17 | 1.22 |
| Percentage variation | 0.1% | 0.4% | 3.4% | 3.5% | 4.9% | 2.1% | 2.4% | 0.6% | 1.9% |
| Test | T (°C) | t (min) | QS (J) | QL (J) | QT (J) | SMR (kg/kWh) | MR (kg/h) | SEC (kJ/kg) |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 1170 | 5514.759 | 67,211.797 | 72,726.556 | 1.408 | 0.096 | 2576.905 |
| 2 | 60 | 1050 | 8492.932 | 73,992.917 | 82,485.850 | 1.388 | 0.112 | 2619.426 |
| 3 | 70 | 990 | 12,737.089 | 75,943.850 | 88,680.938 | 1.300 | 0.120 | 2891.546 |
| 4 | 80 | 930 | 15,049.773 | 79,920.573 | 94,970.345 | 1.313 | 0.137 | 2819.680 |
| 5 | 90 | 930 | 18,863.588 | 77,187.028 | 96,050.616 | 1.276 | 0.130 | 2915.302 |
| 6 | 100 | 750 | 26,155.155 | 87,008.158 | 113,163.313 | 1.212 | 0.172 | 3172.534 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos da Silva, A.M.; Delgado, J.M.P.Q.; Guimarães, A.S.; Barbosa de Lima, W.M.P.; Soares Gomez, R.; Pereira de Farias, R.; Santana de Lima, E.; Barbosa de Lima, A.G. Industrial Ceramic Blocks for Buildings: Clay Characterization and Drying Experimental Study. Energies 2020, 13, 2834. https://doi.org/10.3390/en13112834
Vasconcelos da Silva AM, Delgado JMPQ, Guimarães AS, Barbosa de Lima WMP, Soares Gomez R, Pereira de Farias R, Santana de Lima E, Barbosa de Lima AG. Industrial Ceramic Blocks for Buildings: Clay Characterization and Drying Experimental Study. Energies. 2020; 13(11):2834. https://doi.org/10.3390/en13112834
Chicago/Turabian StyleVasconcelos da Silva, A.M., J.M.P.Q. Delgado, A.S. Guimarães, W.M.P. Barbosa de Lima, R. Soares Gomez, R. Pereira de Farias, E. Santana de Lima, and A.G. Barbosa de Lima. 2020. "Industrial Ceramic Blocks for Buildings: Clay Characterization and Drying Experimental Study" Energies 13, no. 11: 2834. https://doi.org/10.3390/en13112834
APA StyleVasconcelos da Silva, A. M., Delgado, J. M. P. Q., Guimarães, A. S., Barbosa de Lima, W. M. P., Soares Gomez, R., Pereira de Farias, R., Santana de Lima, E., & Barbosa de Lima, A. G. (2020). Industrial Ceramic Blocks for Buildings: Clay Characterization and Drying Experimental Study. Energies, 13(11), 2834. https://doi.org/10.3390/en13112834








