Surrogate Models for Studying the Wettability of Nanoscale Natural Rough Surfaces Using Molecular Dynamics
Abstract
1. Introduction
2. Methodology
2.1. Ketton Sample
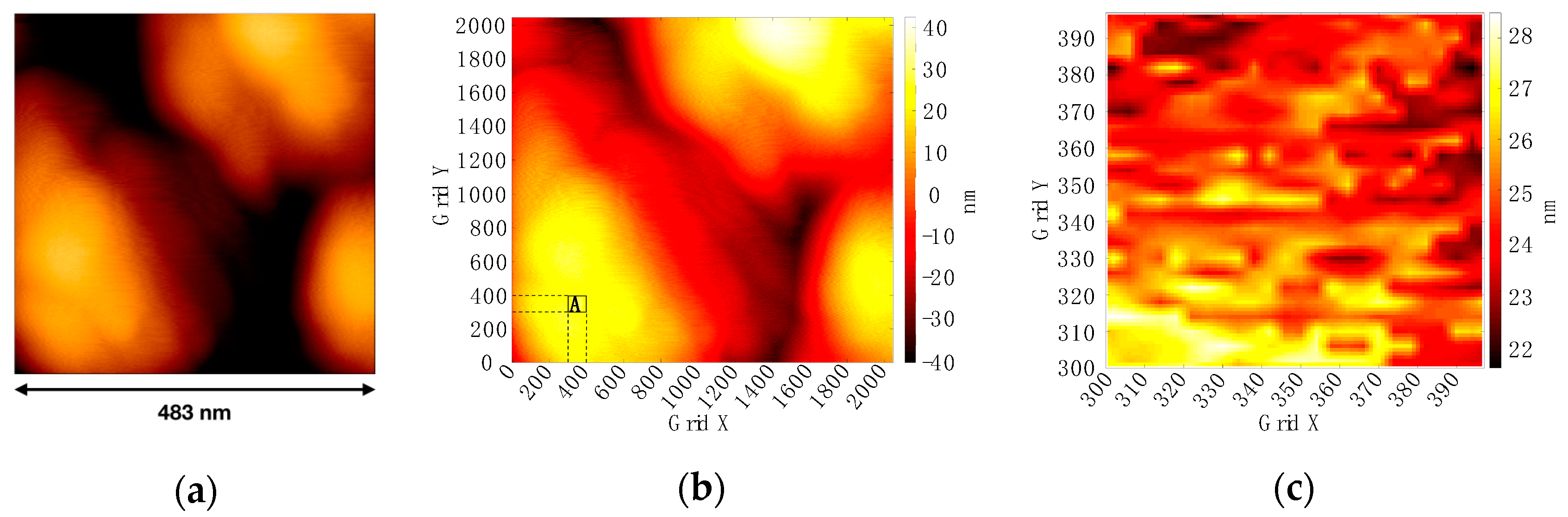
2.2. AFM Imaging
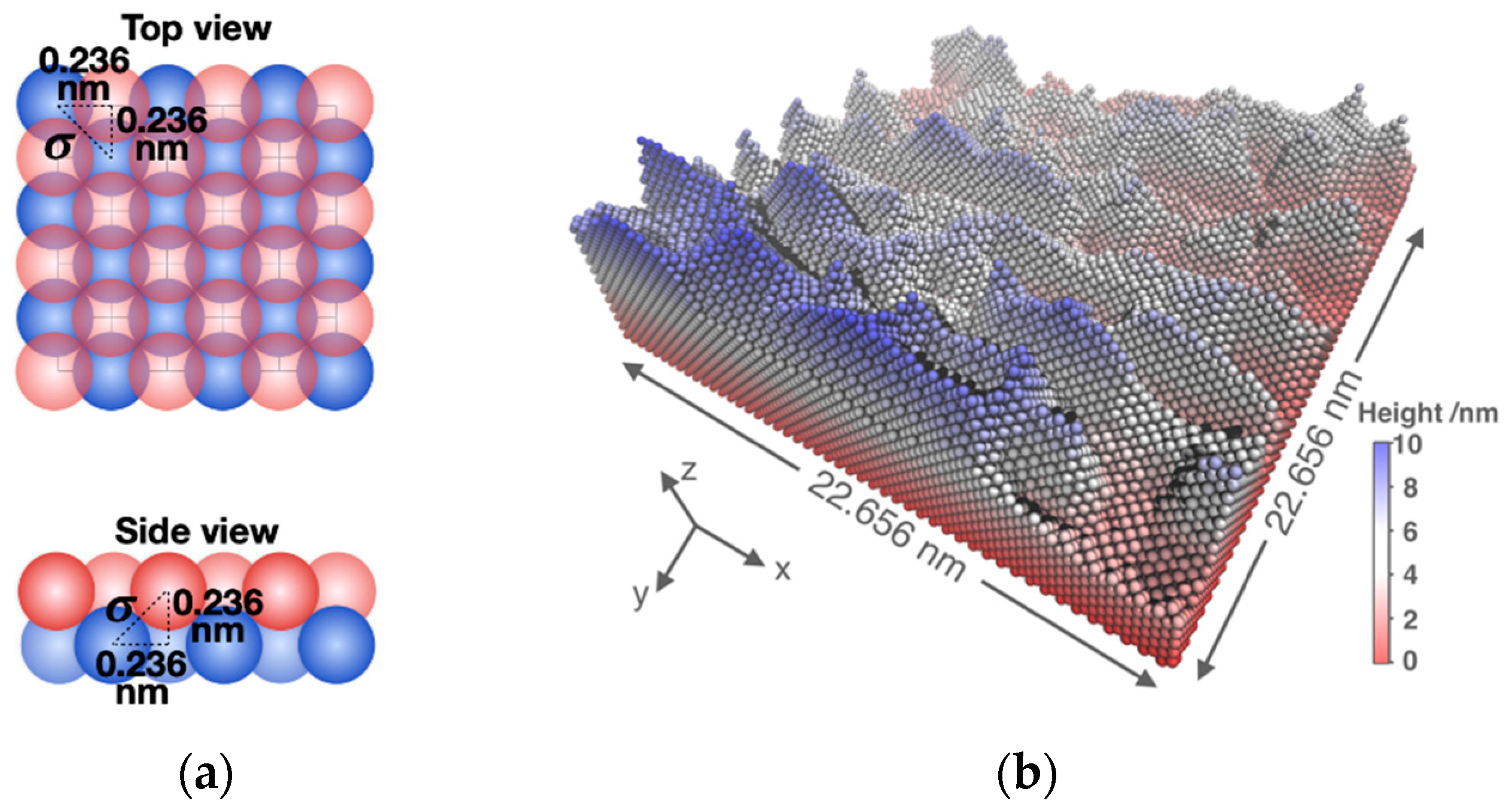
2.3. Surface Molecular Models
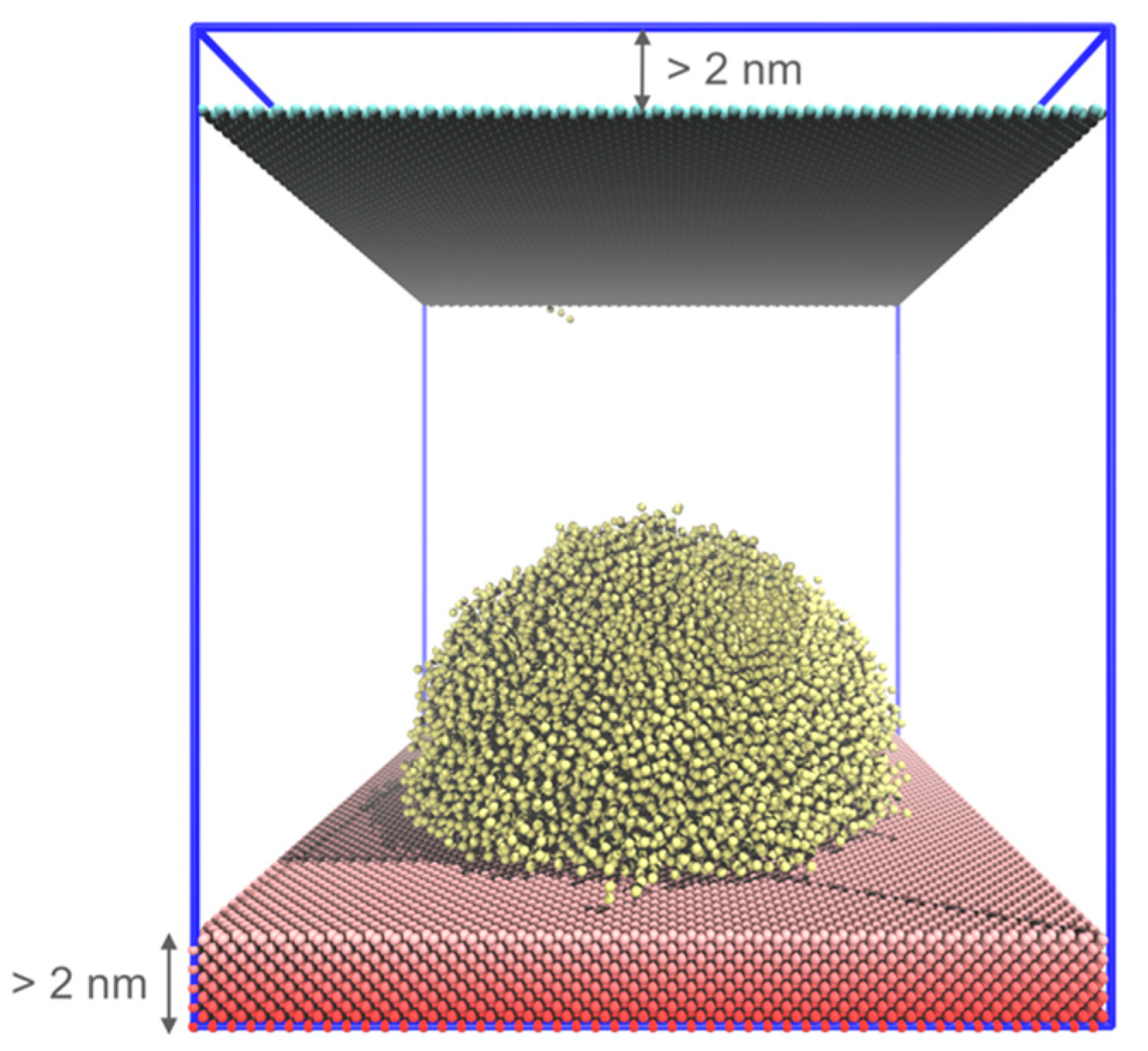
2.4. Coarse-Grained MD Simulation
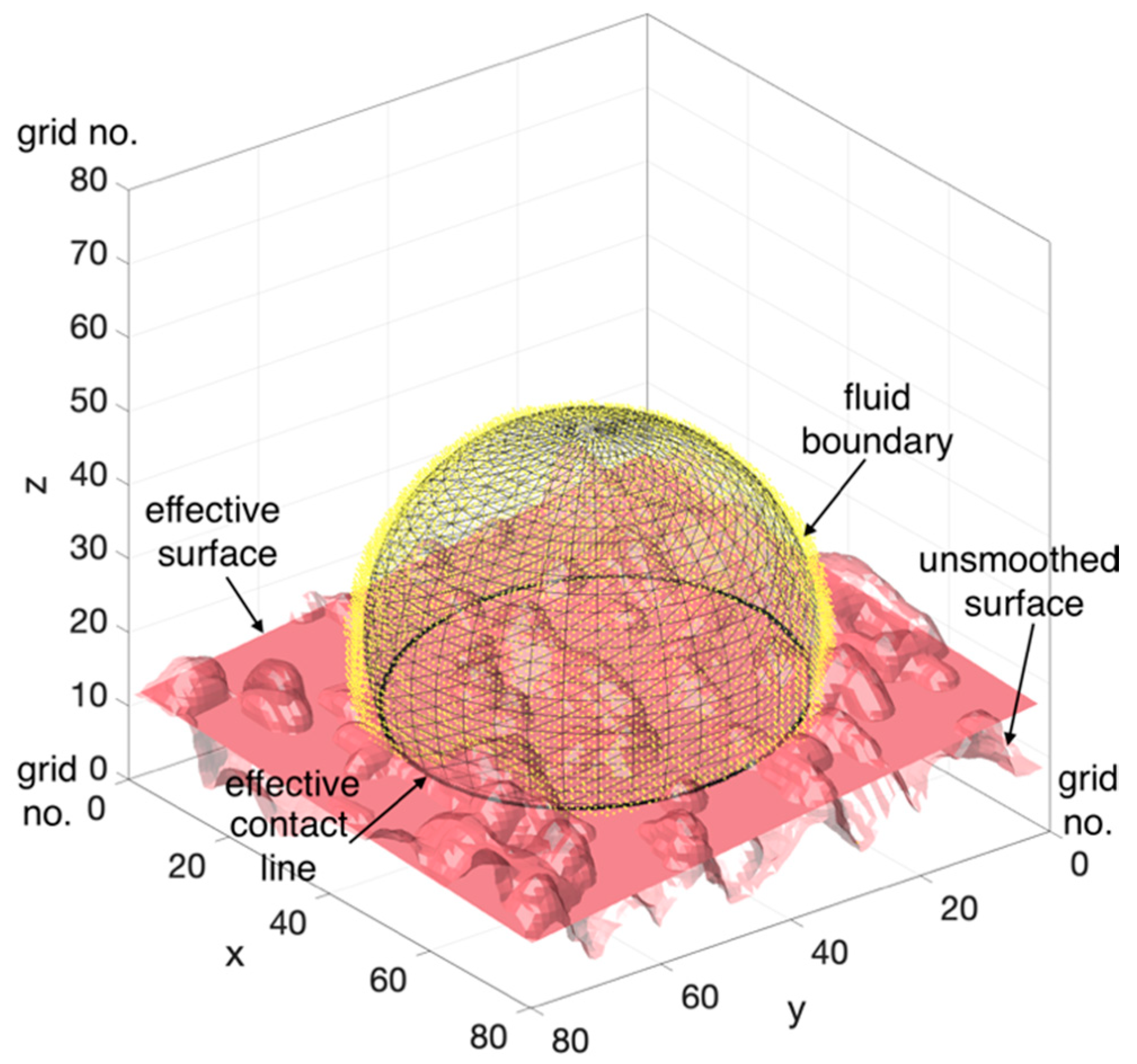
2.5. Computation of the Apparent Contact Angles
3. Results and Discussion
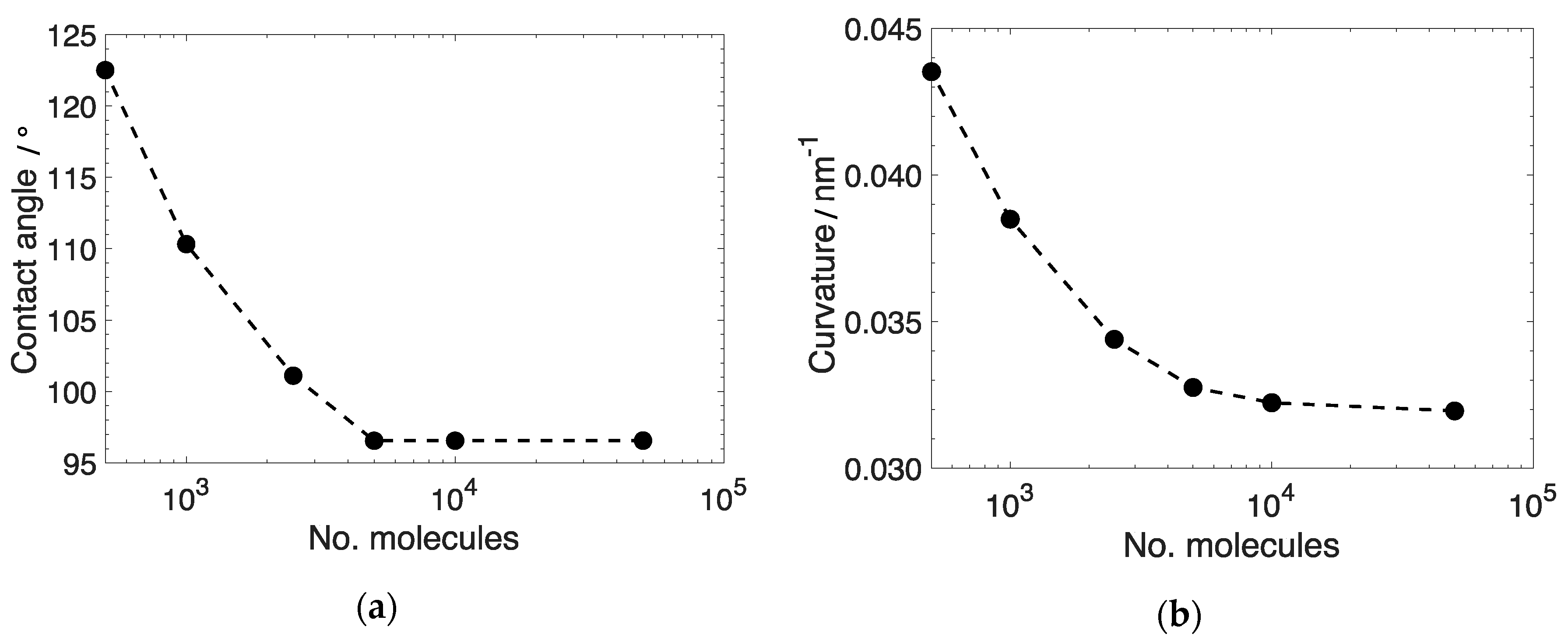
3.1. System Size Effect
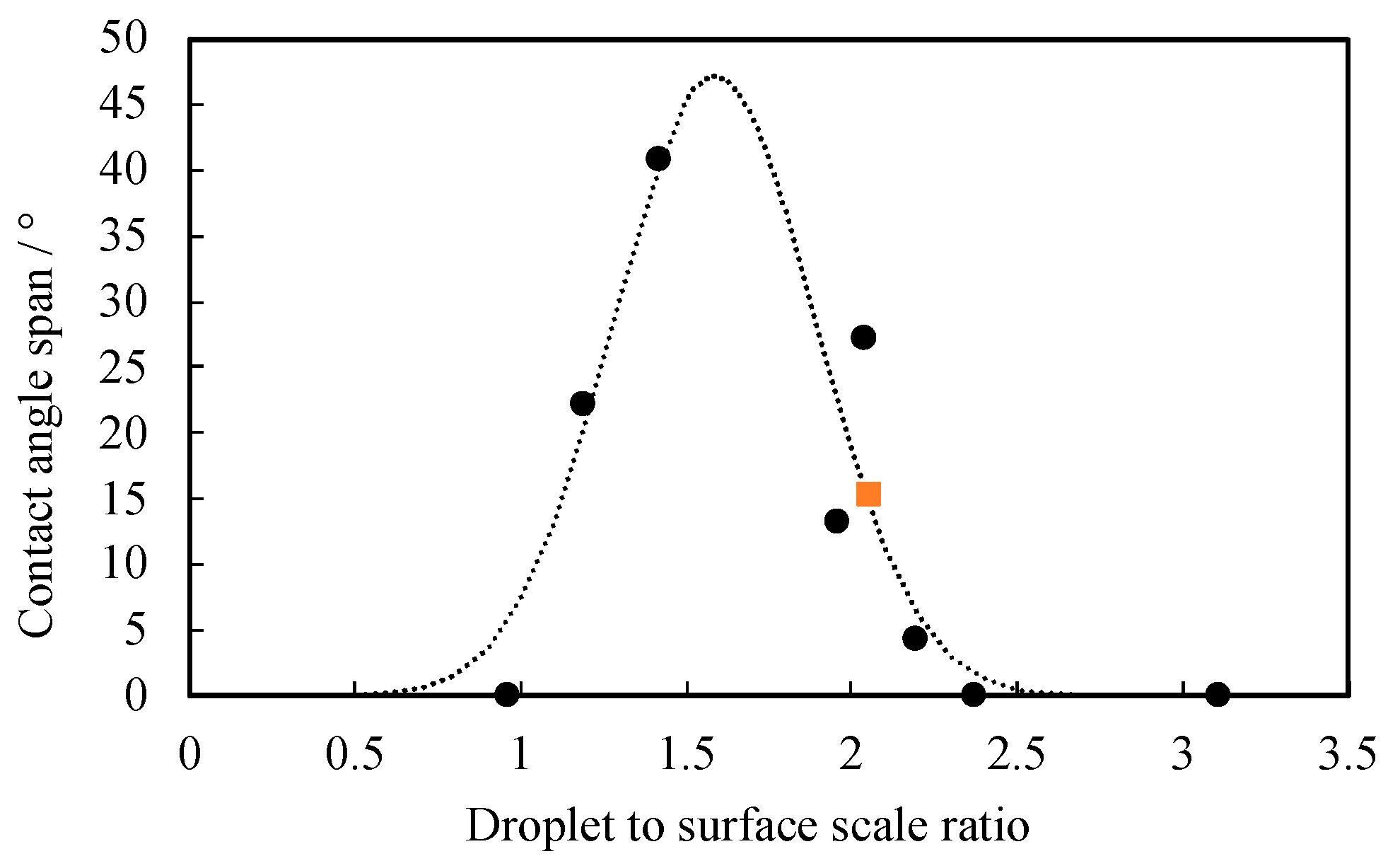
3.2. Contact Angle and Curvature Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliffe, N.J.; McHale, G.; Newton, M.I.; Chabrol, G.; Perry, C.C. Dual-scale roughness produces unusually water-repellent surfaces. Adv. Mater. 2004, 16, 1929–1932. [Google Scholar] [CrossRef]
- Mitik-Dineva, N.; Wang, J.; Truong, V.K.; Stoddart, P.; Malherbe, F.; Crawford, R.J.; Ivanova, E.P. Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr. Microbio. 2009, 58, 268–273. [Google Scholar] [CrossRef]
- Ramiasa, M.; Ralston, J.; Fetzer, R.; Sedev, R. Nanoroughness impact on liquid–liquid displacement. J. Phys. Chem. C 2012, 116, 10934–10943. [Google Scholar] [CrossRef]
- Murison, J.; Semin, B.; Baret, J.C.; Herminghaus, S.; Schröter, M.; Brinkmann, M. Wetting heterogeneities in porous media control flow dissipation. Phys. Rev. Appl. 2014, 2, 034002. [Google Scholar] [CrossRef]
- Herring, A.L.; Sheppard, A.; Andersson, L.; Wildenschild, D. Impact of wettability alteration on 3D nonwetting phase trapping and transport. Int. J. Greenh. Gas Control 2016, 46, 175–186. [Google Scholar] [CrossRef]
- Sajjad Rabbani, H.; Osman, Y.; Almaghrabi, I.; Azizur Rahman, M.; Seers, T. The Control of Apparent Wettability on the Efficiency of Surfactant Flooding in Tight Carbonate Rocks. Processes 2019, 7, 684. [Google Scholar] [CrossRef]
- Bultreys, T.; van Hoorebeke, L.; Cnudde, V. Simulating secondary waterflooding in heterogeneous rocks with variable wettability using an image-based, multiscale pore network model. Water Resour. Res. 2016, 52, 6833–6850. [Google Scholar] [CrossRef]
- Frank, F.; Liu, C.; Scanziani, A.; Alpak, F.O.; Riviere, B. An energy-based equilibrium contact angle boundary condition on jagged surfaces for phase-field methods. J. Coll. Interf. Sci. 2018, 523, 282–291. [Google Scholar] [CrossRef]
- Nakae, H.; Inui, R.; Hirata, Y.; Saito, H. Effects of surface roughness on wettability. Acta Mater. 1998, 46, 2313–2318. [Google Scholar] [CrossRef]
- Al Ratrout, A.; Blunt, M.J.; Bijeljic, B. Wettability in complex porous materials, the mixed-wet state, and its relationship to surface roughness. Proc. Nat. Acad. Sci. USA 2018, 115, 8901–8906. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zhang, Q.; Lv, C.; Tang, Y.; Yin, J. Small degree of anisotropic wetting on self-similar hierarchical wrinkled surfaces. Soft Matter 2018, 14, 1517–1529. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Wang, K. The super hydrophobicity of ZnO nanorods fabricated by electrochemical deposition method. Appl. Surf. Sci. 2011, 257, 6590–6594. [Google Scholar] [CrossRef]
- Kim, T.I.; Tahk, D.; Lee, H.H. Wettability-controllable super water-and moderately oil-repellent surface fabricated by wet chemical etching. Langmuir 2009, 25, 6576–6579. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Suh, K.Y.; Lee, H.H. Direct UV-replica molding of biomimetic hierarchical structure for selective wetting. J. Am. Chem. Soc. 2008, 130, 6312–6313. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, L.; Wu, W.; Ge, H.; Chou, S.Y. Fabrication of nanoscale gratings with reduced line edge roughness using nanoimprint lithography. J. Vac. Sci. Tech. B 2003, 21, 2089–2092. [Google Scholar] [CrossRef][Green Version]
- Bogue, R. Nanoscale fabrication: Techniques, limitations and future prospects. Assemb. Autom. 2011, 31, 304–308. [Google Scholar] [CrossRef]
- Doudrick, K.; Liu, S.; Mutunga, E.M.; Klein, K.L.; Damle, V.; Varanasi, K.K.; Rykaczewski, K. Different shades of oxide: From nanoscale wetting mechanisms to contact printing of gallium-based liquid metals. Langmuir 2014, 30, 6867–6877. [Google Scholar] [CrossRef]
- Gerd, B.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930. [Google Scholar]
- Stipp, S.L.S.; Eggleston, C.M.; Nielsen, B.S. Calcite surface structure observed at microtopographic and molecular scales with atomic force microscopy (AFM). Geochim. Cosmochim. Acta 1994, 58, 3023–3033. [Google Scholar] [CrossRef]
- Giessibl, F.J. Advances in atomic force microscopy. Rev. Mod. Phys. 2003, 75, 949. [Google Scholar] [CrossRef]
- Ricci, M.; Spijker, P.; Stellacci, F.; Molinari, J.F.; Voïtchovsky, K. Direct visualization of single ions in the stern layer of calcite. Langmuir 2013, 29, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Mugele, F.; Siretanu, I.; Kumar, N.; Bera, B.; Wang, L.; de Ruiter, R.; Maestro, A.; Duits, M.; van den Ende, D.; Collins, I. Insights from ion adsorption and contact-angle alteration at mineral surfaces for low-salinity waterflooding. SPE J. 2016, 21, 1–204. [Google Scholar] [CrossRef]
- Matthiesen, J.; Bovet, N.; Hilner, E.; Andersson, M.P.; Schmidt, D.A.; Webb, K.J.; Dalby, K.N.; Hassenkam, T.; Crouch, J.; Collins, I.R.; et al. How naturally adsorbed material on minerals affects low salinity enhanced oil recovery. Energy Fuels 2014, 28, 4849–4858. [Google Scholar] [CrossRef]
- Hassenkam, T.; Skovbjerg, L.L.; Stipp, S.L.S. Probing the intrinsically oil-wet surfaces of pores in North Sea chalk at subpore resolution. Proc. Nat. Acad. Sci. USA 2009, 106, 6071–6076. [Google Scholar] [CrossRef]
- Buckley, J.S.; Lord, D.L. Wettability and morphology of mica surfaces after exposure to crude oil. J. Pet. Sci. Eng. 2003, 39, 261–273. [Google Scholar] [CrossRef]
- Kumar, K.; Dao, E.; Mohanty, K.K. AFM study of mineral wettability with reservoir oils. J. Coll. Interf. Sci. 2005, 289, 206–217. [Google Scholar] [CrossRef]
- Dickinson, L.R.; Suijkerbuijk, B.M.; Berg, S.; Marcelis, F.H.; Schniepp, H.C. Atomic force spectroscopy using colloidal tips functionalized with dried crude oil: A versatile tool to investigate oil-mineral interactions. Energy Fuels 2016, 30, 9193–9202. [Google Scholar] [CrossRef]
- Hassenkam, T.; Pedersen, C.S.; Dalby, K.; Austad, T.; Stipp, S.L.S. Pore scale observation of low salinity effects on outcrop and oil reservoir sandstone. Coll. Surf. A 2011, 390, 179–188. [Google Scholar] [CrossRef]
- Rücker, M.; Bartels, W.-B.; Garfi, G.; Shams, M.; Bultreys, T.; Boone, M.; Pieterse, S.; Maitland, G.C.; Krevor, S.; Cnudde, V.; et al. Relationship between wetting and capillary pressure in a crude oil/brine/rock system: From nano-scale to core-scale. J. Colloid. Interf. Sci. 2020, 562, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Yesufu-Rufai, S.; Rücker, M.; Berg, S.; Lowe, S.F.; Marcelis, F.; Georgiadis, A.; Luckham, P. Assessing the wetting state of minerals in complex sandstone rock in-situ by atomic force microscopy (AFM). Fuel 2020, 273, 117807. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids, 2nd ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Palmer, J.C.; Debenedetti, P.G. Recent advances in molecular simulation: A chemical engineering perspective. AICHE J. 2015, 61, 370–383. [Google Scholar] [CrossRef]
- Tenney, C.M.; Cygan, R.T. Molecular simulation of carbon dioxide, brine, and clay mineral interactions and determination of contact angles. Environ. Sci. Tech. 2014, 48, 2035–2042. [Google Scholar] [CrossRef]
- Lundgren, M.; Allan, N.L.; Cosgrove, T. Modeling of wetting: A study of nanowetting at rough and heterogeneous surfaces. Langmuir 2007, 23, 1187–1194. [Google Scholar] [CrossRef]
- Khan, S.; Singh, J.K. Wetting transition of nanodroplets of water on textured surfaces: A molecular dynamics study. Mol. Simul. 2014, 40, 458–468. [Google Scholar] [CrossRef]
- Nakamura, Y.; Carlson, A.; Amberg, G.; Shiomi, J. Dynamic wetting at the nanoscale. Phys. Rev. E 2013, 88, 33010. [Google Scholar] [CrossRef]
- Yong, X.; Zhang, L.T. Nanoscale wetting on groove-patterned surfaces. Langmuir 2009, 25, 5045–5053. [Google Scholar] [CrossRef]
- Yaghoubi, H.; Foroutan, M. Molecular investigation of the wettability of rough surfaces using molecular dynamics simulation. Phys. Chem. Chem. Phys. 2018, 20, 22308–22319. [Google Scholar] [CrossRef]
- Park, J.Y.; Ha, M.Y.; Choi, H.J.; Hong, S.D.; Yoon, H.S. A study on the contact angles of a water droplet on smooth and rough solid surfaces. J. Mech. Sci. Tech. 2011, 25, 323. [Google Scholar] [CrossRef]
- Amadei, C.A.; Santos, S.; Pehkonen, S.O.; Verdaguer, A.; Chiesa, M. Minimal invasiveness and spectroscopy-like footprints for the characterization of heterogeneous nanoscale wetting in ambient conditions. J. Phys. Chem. C 2013, 117, 20819–20825. [Google Scholar] [CrossRef][Green Version]
- Teppen, B.J.; Rasmussen, K.; Bertsch, P.M.; Miller, D.M.; Schäfer, L. Molecular dynamics modeling of clay minerals. 1. Gibbsite, kaolinite, pyrophyllite, and beidellite. J. Phys. Chem. B 1997, 101, 1579–1587. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Wang, J.; Kirkpatrick, R.J. Molecular dynamics modeling of the structure, dynamics and energetics of mineral–water interfaces: Application to cement materials. Cem. Concr. Res. 2007, 37, 337–347. [Google Scholar] [CrossRef]
- Iglauer, S.; Mathew, M.S.; Bresme, F. Molecular dynamics computations of brine-CO2 interfacial tensions and brine-CO2-quartz contact angles and their effects on structural and residual trapping mechanisms in carbon geo-sequestration. J. Coll. Interf. Sci. 2012, 386, 405–414. [Google Scholar] [CrossRef]
- Yiapanis, G.; Maclaughlin, S.; Evans, E.J.; Yarovsky, I. Nanoscale wetting and fouling resistance of functionalized surfaces: A computational approach. Langmuir 2014, 30, 10617–10625. [Google Scholar] [CrossRef] [PubMed]
- Brekke-Svaland, G.; Bresme, F. Interactions between hydrated calcium carbonate surfaces at nanoconfinement conditions. J. Phys. Chem. C 2018, 122, 7321–7330. [Google Scholar] [CrossRef]
- De Leeuw, N.H.; Parker, S.C. Surface structure and morphology of calcium carbonate polymorphs calcite, aragonite, and vaterite: An atomistic approach. J. Phys. Chem. B 1998, 102, 2914–2922. [Google Scholar] [CrossRef]
- Tribello, G.A.; Bruneval, F.; Liew, C.; Parrinello, M. A molecular dynamics study of the early stages of calcium carbonate growth. J. Phys. Chem. B 2009, 113, 11680–11687. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Micro-and nanoscale characterization of hydrophobic and hydrophilic leaf surfaces. Nanotechnology 2006, 17, 2758. [Google Scholar] [CrossRef]
- Nosonovsky, M. Multiscale roughness and stability of superhydrophobic biomimetic interfaces. Langmuir 2007, 23, 3157–3161. [Google Scholar] [CrossRef]
- Becker, S.; Urbassek, H.M.; Horsch, M.; Hasse, H. Contact angle of sessile drops in Lennard-Jones systems. Langmuir 2014, 30, 13606–13614. [Google Scholar] [CrossRef] [PubMed]
- Law, B.M.; McBride, S.P.; Wang, J.Y.; Wie, H.S.; Paneru, G.; Betelu, S.; Ushijima, B.; Takata, Y.; Flanders, B.; Bresme, F. Line tension and its influence on droplets and particles at surfaces. Progr. Surf. Sci. 2017, 92, 1–39. [Google Scholar] [CrossRef]
- Swain, P.S.; Lipowsky, R. Contact angles on heterogeneous surfaces: A new look at Cassie’s and Wenzel’s laws. Langmuir 1998, 14, 6772–6780. [Google Scholar] [CrossRef]
- Santiso, E.E.; Herdes, C.; Müller, E.A. On the calculation of solid-fluid contact angles from molecular dynamics. Entropy 2013, 15, 3734–3745. [Google Scholar] [CrossRef]
- Theodorakis, P.E.; Müller, E.A.; Craster, R.V.; Matar, O.K. Modelling the superspreading of surfactant-laden droplets with computer simulation. Soft Matter 2015, 11, 9254–9261. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Checco, A.; Guenoun, P.; Daillant, J. Nonlinear dependence of the contact angle of nanodroplets on contact line curvature. Phys. Rev. Lett. 2003, 91, 186101. [Google Scholar] [CrossRef]
- Škvára, J.; Škvor, J.; Nezbeda, I. Evaluation of the contact angle from molecular simulations. Mol. Simul. 2018, 44, 190–199. [Google Scholar] [CrossRef]
- Giro, R.; Bryant, P.W.; Engel, M.; Neumann, R.F.; Steiner, M.B. Adsorption energy as a metric for wettability at the nanoscale. Sci. Rep. 2017, 7, 46317. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, P.; Wu, P.; Yang, Q.; Liu, F.; Han, Y.; Xu, F.; Wang, L. Determination of contact angle of droplet on convex and concave spherical surfaces. Chem. Phys. 2015, 457, 63–69. [Google Scholar] [CrossRef]
- Leroy, F.; Müller-Plathe, F. Solid-liquid surface free energy of Lennard-Jones liquid on smooth and rough surfaces computed by molecular dynamics using the phantom-wall method. J. Chem. Phys. 2010, 13, 3044110. [Google Scholar] [CrossRef]
- Saville, G. Computer simulation of the liquid-solid-vapour contact angle. J. Chem. Soc. Faraday Trans. 2 1977, 73, 1122–1132. [Google Scholar] [CrossRef]
- Andrew, M. Reservoir-Condition Pore-Scale Imaging of Multiphase Flow. Ph.D. Thesis, Imperial College, London, UK, 2014. [Google Scholar]
- Hudson, J.D.; Clements, R.G. The middle Jurassic succession at Ketton, Rutland. Proc. Geol. Assoc. 2007, 118, 239–264. [Google Scholar] [CrossRef]
- Flügel, E. Microfacies of Carbonate Rocks: Analysis, Interpretation and Application; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Andrew, M.; Bijeljic, B.; Blunt, M.J. Pore-scale contact angle measurements at reservoir conditions using X-ray microtomography. Adv. Water Resour. 2014, 68, 24–31. [Google Scholar] [CrossRef]
- Singh, K.; Bijeljic, B.; Blunt, M.J. Imaging of oil layers, curvature and contact angle in a mixed-wet and a water-wet carbonate rock. Water Resour. Res. 2016, 52, 1716–1728. [Google Scholar] [CrossRef]
- Rücker, M.; Bartels, W.B.; Singh, K.; Brussee, N.; Coorn, A.; van der Linde, H.A.; Bonnin, A.; Ott, H.; Hassanizadeh, S.M.; Blunt, M.J.; et al. The effect of mixed wettability on pore-scale flow regimes based on a flooding experiment in Ketton limestone. Geophys. Res. Lett. 2019, 46, 3225–3234. [Google Scholar] [CrossRef]
- Okrusch, M. Minralogy: An Introduction to Minerals, Rocks, and Mineral Deposits; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ramrattan, N.S.; Avendaño, C.; Müller, E.A.; Galindo, A. A corresponding-states framework for the description of the Mie family of intermolecular potentials. Mol. Phys. 2015, 113, 932–947. [Google Scholar] [CrossRef]
- Lafitte, T.; Apostolakou, A.; Avendaño, C.; Galindo, A.; Adjiman, C.S.; Müller, E.A.; Jackson, G. Accurate statistical associating fluid theory for chain molecules formed from Mie segments. J. Chem. Phys. 2013, 139, 154504. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, C.; Lafitte, T.; Galindo, A.; Adjiman, C.S.; Jackson, G.; Müller, E.A. SAFT-γ force field for the simulation of molecular fluids. 1. A single-site coarse grained model of carbon dioxide. J. Phys. Chem. B 2011, 115, 11154–11169. [Google Scholar]
- Avendaño, C.; Lafitte, T.; Adjiman, C.S.; Galindo, A.; Müller, E.A.; Jackson, G. SAFT-γ force field for the simulation of molecular fluids: 2. Coarse-grained models of greenhouse gases, refrigerants, and long alkanes. J. Phys. Chem. B 2013, 117, 2717–2733. [Google Scholar]
- Lafitte, T.; Avendaño, C.; Papaioannou, V.; Galindo, A.; Adjiman, C.S.; Jackson, G.; Müller, E.A. SAFT-γ force field for the simulation of molecular fluids: 3. Coarse-grained models of benzene and hetero-group models of n-decylbenzene. Mol. Phys. 2012, 110, 1189–1203. [Google Scholar] [CrossRef]
- Müller, E.A.; Jackson, G. Force-field parameters from the SAFT-γ equation of state for use in coarse-grained molecular simulations. Annu. Rev. Chem. Biomolec. Eng. 2014, 5, 405–427. [Google Scholar] [CrossRef]
- Mejía, A.; Herdes, C.; Müller, E.A. Force fields for coarse-grained molecular simulations from a corresponding states correlation. Ind. Eng. Chem. Res. 2014, 53, 4131–4141. [Google Scholar] [CrossRef]
- Jiménez-Serratos, G.; Totton, T.S.; Jackson, G.; Müller, E.A. Aggregation behavior of model asphaltenes revealed from large-scale coarse-grained molecular simulations. J. Phys. Chem. B 2019, 123, 2380–2396. [Google Scholar] [CrossRef]
- Herdes, C.; Totton, T.S.; Müller, E.A. Coarse grained force field for the molecular simulation of natural gases and condensates. Fluid Phase Equilib. 2015, 406, 91–100. [Google Scholar] [CrossRef]
- Herdes, C.; Petit, C.; Mejía, A.; Müller, E.A. Combined experimental, theoretical, and molecular simulation approach for the description of the fluid-phase behavior of hydrocarbon mixtures within shale rocks. Energy Fuels 2018, 32, 5750–5762. [Google Scholar] [CrossRef]
- Herdes, C.; Ervik, Å.; Mejía, A.; Müller, E.A. Prediction of the water/oil interfacial tension from molecular simulations using the coarse-grained SAFT-γ Mie force field. Fluid Phase Equilib. 2018, 476, 9–15. [Google Scholar] [CrossRef]
- Herdes, C.; Forte, E.; Jackson, G.; Müller, E.A. Predicting the adsorption of n-perfluorohexane in BAM-P109 standard activated carbon by molecular simulation using SAFT-γ Mie coarse-grained force fields. Adsorpt. Sci. Tech. 2016, 34, 64–78. [Google Scholar] [CrossRef]
- Theodorakis, P.E.; Müller, E.A.; Craster, R.V.; Matar, O.K. Superspreading: Mechanisms and molecular design. Langmuir 2015, 31, 2304–2309. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Lobanova, O.; Jiménez-Serratos, G.; Braga, C.; Raptis, V.; Müller, E.A.; Jackson, G.; Avendaño, C.; Galindo, A. SAFT-γ force field for the simulation of molecular fluids. 5. Hetero-group coarse-grained models of linear alkanes and the importance of intramolecular interactions. J. Phys. Chem. B 2018, 122, 9161–9177. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Haslam, A.J.; Jackson, G.; Müller, E.A. Effective coarse-grained solid-fluid potentials and their application to model adsorption of fluids on heterogeneous surfaces. Phys. Chem. Chem. Phys. 2014, 16, 19165–19180. [Google Scholar] [CrossRef]
- Jiménez-Serratos, G.; Cárdenas, H.; Müller, E.A. Extension of the effective solid-fluid Steele potential for Mie force fields. Mol. Phys. 2019, 117, 3840–3851. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; Van Der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comp. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Jover, J.; Haslam, A.J.; Galindo, A.; Jackson, G.; Müller, E.A. Pseudo hard-sphere potential for use in continuous molecular-dynamics simulation of spherical and chain molecules. J. Chem. Phys. 2012, 137, 144505. [Google Scholar] [CrossRef]
- Jennings, A. Least-Squared Sphere Fit (Software), Matlab Central 2013. Available online: https://www.mathworks.com/matlabcentral/fileexchange/34129-sphere-fit-least-squared (accessed on 6 March 2020).
- Peng, H.; Nguyen, A.V.; Birkett, G.R. Determination of contact angle by molecular simulation using number and atomic density contours. Mol. Sim. 2012, 38, 945–952. [Google Scholar] [CrossRef]
- Sumith, Y.D. An efficient algorithm for contact angle estimation in molecular dynamics simulations. Int. J. Eng. 2015, 9, 1–8. [Google Scholar]
- Scocchi, G.; Sergi, D.; D’Angelo, C.; Ortona, A. Wetting and contact-line effects for spherical and cylindrical droplets on graphene layers: A comparative molecular-dynamics investigation. Phys. Rev. E 2011, 84, 61602. [Google Scholar] [CrossRef]
- Bresme, F.; Oettel, M. Nanoparticles at fluid interfaces. J. Phys. Condens. Matt. 2007, 19, 413101. [Google Scholar] [CrossRef] [PubMed]
- Whyman, G.; Bormashenko, E.; Stein, T. The rigorous derivation of young, Cassie-Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon. Chem. Phys. Lett. 2008, 450, 355–359. [Google Scholar] [CrossRef]
- Scanziani, A.; Singh, K.; Blunt, M.J.; Guadagnini, A. Automatic method for estimation of in situ effective contact angle from X-ray micro tomography images of two-phase flow in porous media. J. Colloid Interf. Sci. 2017, 496, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Koishi, T.; Yasuoka, K.; Fujikawa, S.; Ebisuzaki, T.; Cheng, X. Coexistence and transition between Cassie and Wenzel state on pillared hydrophobic surface. Proc. Natl. Acad. Sci. USA 2009, 206, 8435–8440. [Google Scholar] [CrossRef]
- Koishi, T.; Yasuoka, K.; Zeng, X.C. Molecular dynamics simulation of water nanodroplet bounce back from flat and nanopillared surface. Langmuir 2017, 33, 10184–10192. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Emoto, M.; Konno, A.; Ito, S. Molecular dynamics simulation of the influence of the nanoscale structure on water wetting and condensation. Micromachines 2019, 10, 587. [Google Scholar] [CrossRef]
- Song, F.; Ma, L.; Fan, J.; Chen, Q.; Zhang, L.; Li, B.Q. Wetting behaviors of a nano-droplet on a rough solid substrate under perpendicular electric field. Nanomaterials 2018, 8, 340. [Google Scholar] [CrossRef]
- Smith, E.R.; Müller, E.A.; Craster, R.V.; Matar, O.K. A Langevin model for fluctuating contact angle behaviour parametrised using molecular dynamics. Soft Matter 2016, 12, 9604–9615. [Google Scholar] [CrossRef]
- Sun, C.; McClure, J.E.; Mostaghimi, P.; Herring, A.L.; Berg, S.; Armstrong, R.T. Probing effective wetting in subsurface systems. Geophys. Res. Lett. 2020, 4, e2019GL086151. [Google Scholar] [CrossRef]
- Svoboda, M.; Malijevsky, A.; Lísal, M. Wetting properties of molecularly rough surfaces. J. Chem. Phys. 2015, 143, 104701. [Google Scholar] [CrossRef]
- Kumar, V.; Sridhar, S.; Errington, J.R. Monte Carlo simulation strategies for computing the wetting properties of fluids at geometrically rough surfaces. J. Chem. Phys. 2011, 135, 184702. [Google Scholar] [CrossRef] [PubMed]
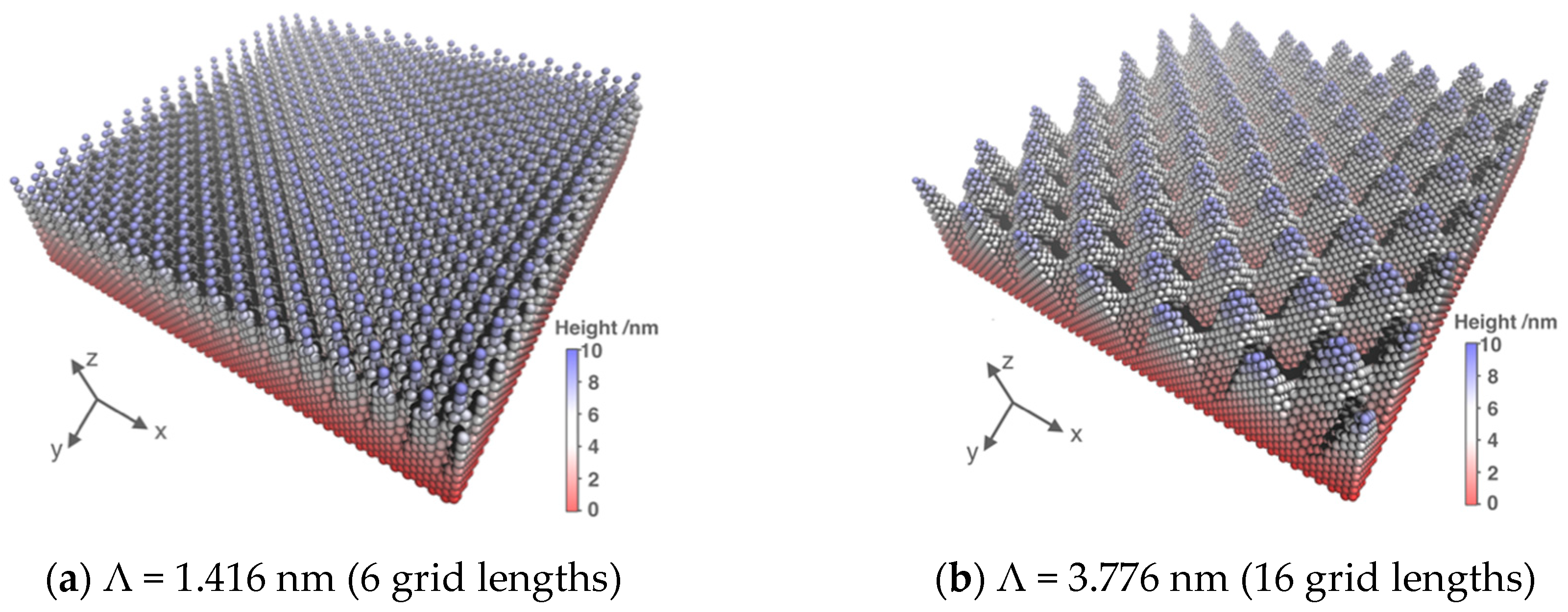
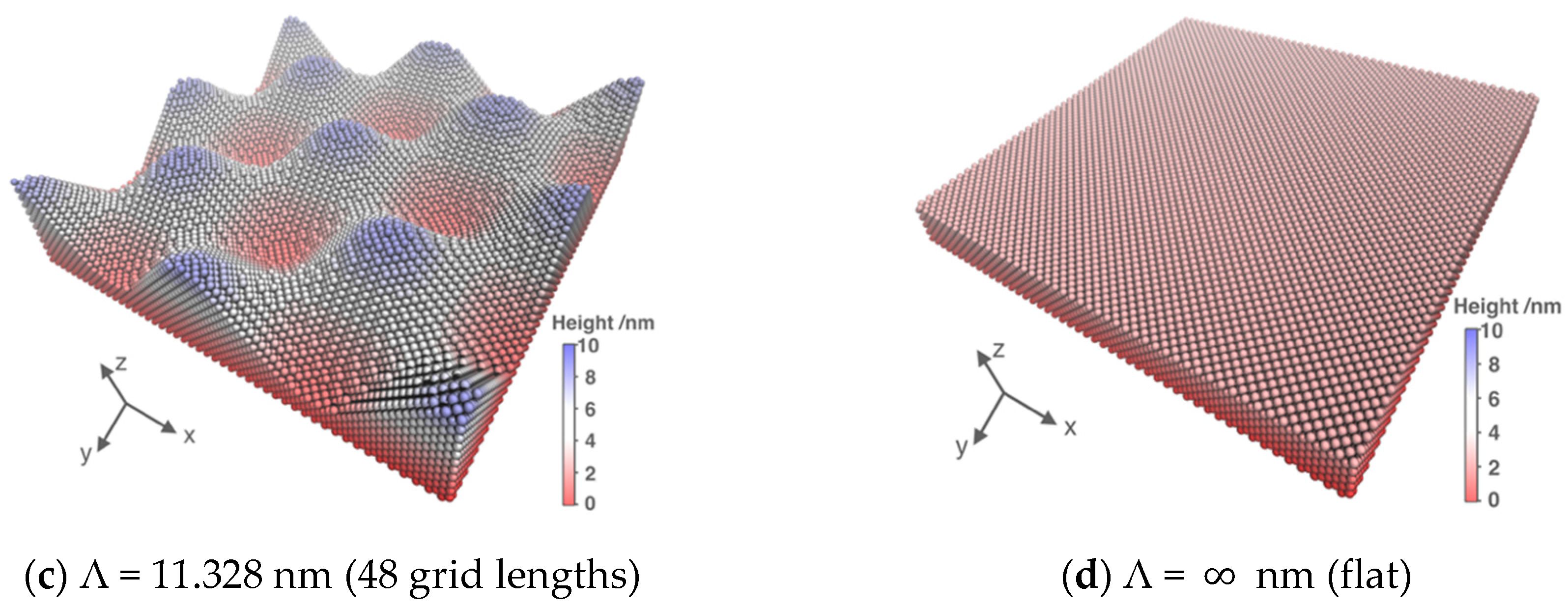

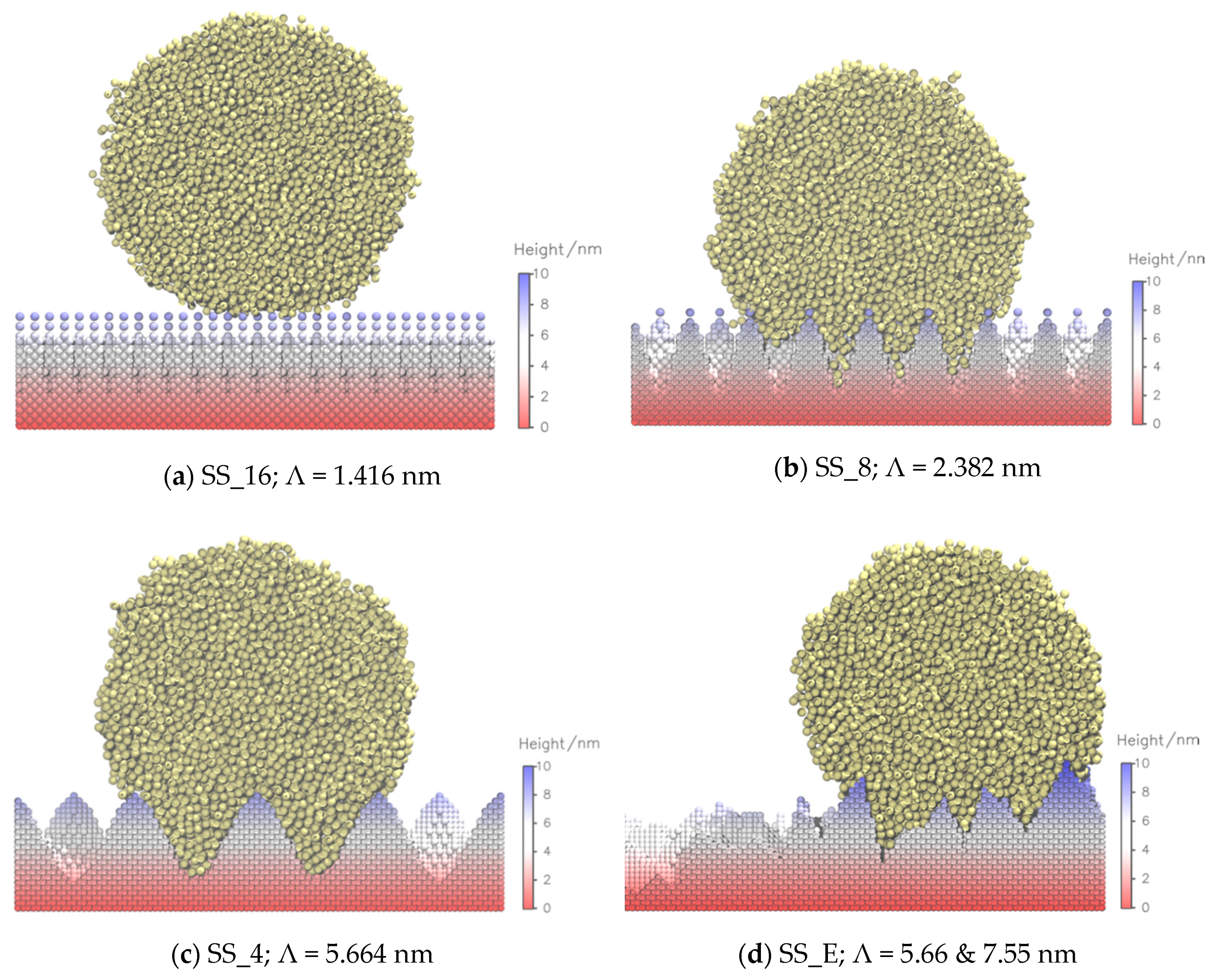
| Surface | Λ/nm | R | θ |
|---|---|---|---|
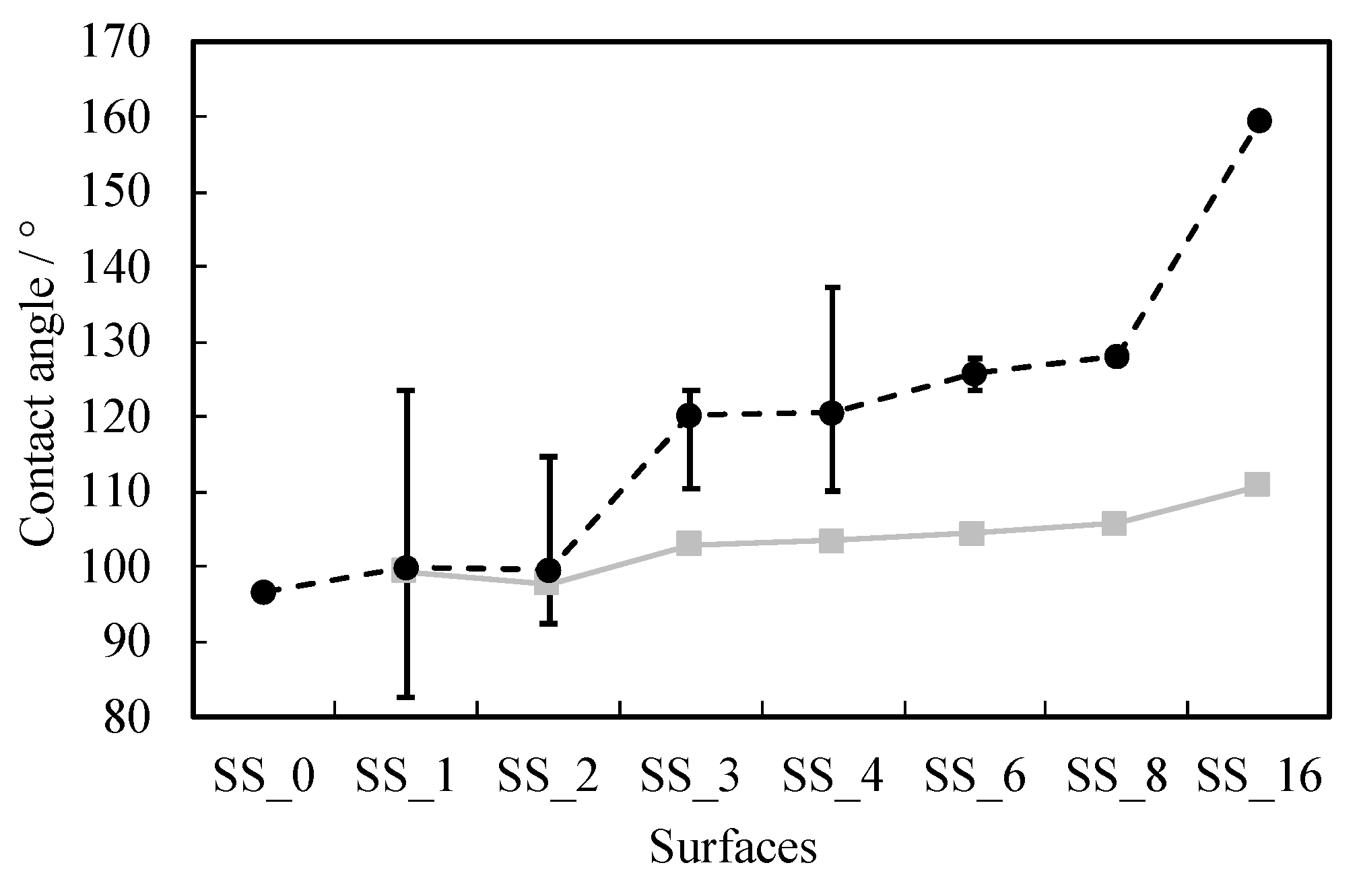
| SS_0 (flat) | ∞ | 1.00 | (97 ± 1)° |
| SS_1 | 22.656 | 1.07 | (100 ± 24)° |
| SS_2 | 11.328 | 1.26 | (100 ± 15)° |
| SS_3 | 7.552 | 1.51 | (120 ± 10)° |
| SS_4 | 5.664 | 1.80 | (121 ± 17)° |
| SS_6 | 3.776 | 2.40 | (126 ± 2)° |
| SS_8 | 2.382 | 3.00 | (128 ± 1)° |
| SS_16 | 1.416 | 5.00 | (160 ± 1)° |
| SS_E | 5.66, 7.55 | 1.74 | (116 ± 9)° |
| Intermolecular Parameters | ||||
| Interaction | σ/nm | (ε/kB)/K | λ | m |
| fluid–fluid | 0.4584 | 415.19 | 20.92 | 3 |
| fluid–solid | 0.3957 | 125 | 15.70 | 1 |
| Intramolecular Parameters | ||||
| Component | kangle/(kJ·mol−1·rad−2) | kbond/(kJ·mol−1·nm−2) | /nm | |
| fluid | 22.18 | 157.6° | 6309.5 | 0.4584 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Rücker, M.; Bultreys, T.; Georgiadis, A.; Mooijer-van den Heuvel, M.M.; Bresme, F.; Trusler, J.P.M.; Müller, E.A. Surrogate Models for Studying the Wettability of Nanoscale Natural Rough Surfaces Using Molecular Dynamics. Energies 2020, 13, 2770. https://doi.org/10.3390/en13112770
Zheng L, Rücker M, Bultreys T, Georgiadis A, Mooijer-van den Heuvel MM, Bresme F, Trusler JPM, Müller EA. Surrogate Models for Studying the Wettability of Nanoscale Natural Rough Surfaces Using Molecular Dynamics. Energies. 2020; 13(11):2770. https://doi.org/10.3390/en13112770
Chicago/Turabian StyleZheng, Lingru, Maja Rücker, Tom Bultreys, Apostolos Georgiadis, Miranda M. Mooijer-van den Heuvel, Fernando Bresme, J. P. Martin Trusler, and Erich A. Müller. 2020. "Surrogate Models for Studying the Wettability of Nanoscale Natural Rough Surfaces Using Molecular Dynamics" Energies 13, no. 11: 2770. https://doi.org/10.3390/en13112770
APA StyleZheng, L., Rücker, M., Bultreys, T., Georgiadis, A., Mooijer-van den Heuvel, M. M., Bresme, F., Trusler, J. P. M., & Müller, E. A. (2020). Surrogate Models for Studying the Wettability of Nanoscale Natural Rough Surfaces Using Molecular Dynamics. Energies, 13(11), 2770. https://doi.org/10.3390/en13112770








