Solid-State Fermentation (SSF) versus Submerged Fermentation (SmF) for the Recovery of Cellulases from Coffee Husks: A Life Cycle Assessment (LCA) Based Comparison
Abstract
1. Introduction
2. Materials and Methods
2.1. Goals and Scope Definition
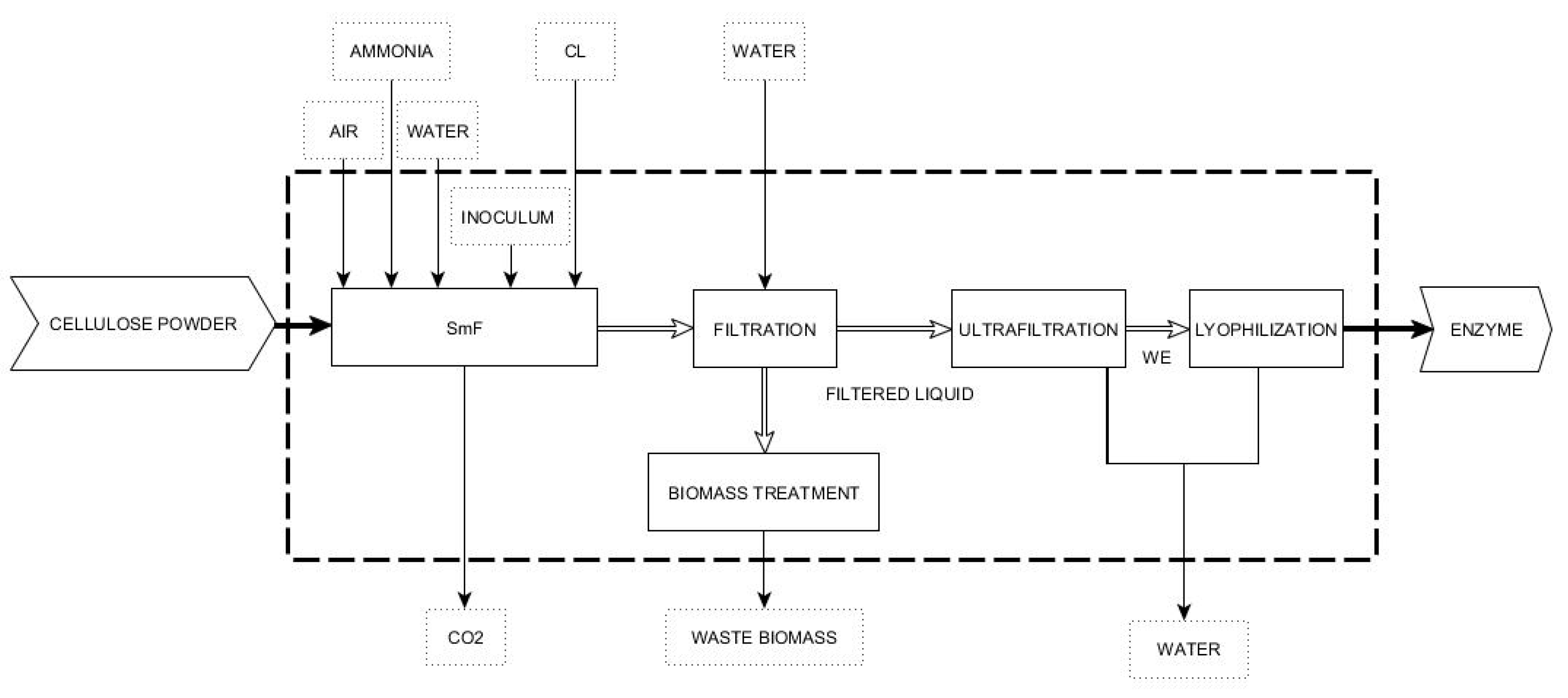
2.1.1. Boundaries and Processes
2.1.2. Submerged Fermentation (SmF)
- (a)
- Inoculum: The inoculum phase was a sequence of growth seeds (3 bioreactors) to provide the necessary amount of inoculum (5% of working volume) and the preparation of the bioreactor media. The specific medium for inoculum included different substances such as ammonium sulphate, potassium phosphate or calcium dichloride, among others [28]. Three growth bioreactors provided inoculum for the production vessel (100 L). These reactors are designed to provide 5% inoculum to each submerged bioreactor to the next scale. The fermentation time of each one of the three growth bioreactors was estimated in 40 h [29].
- (b)
- SmF: The bioreactor working volume was 80% of the total volume and included a compressor and air filter for sterilization to provide the requirements of oxygen to perform the fermentation under optimal conditions. This bioreactor converts raw materials into the desired product: the cellulase enzymes. Ammonium was used to control pH and provide additional nitrogen to the microorganisms. Corn liquor and other trace nutrients were also added to the bioreactors as a carbon source. The bioreactors were aerated with air compressed to provide the oxygen demand and a chilled water system flowing through internal coils to control temperature. The output of fermentation is CO2 released during cellulase production. The microorganism was fed with nutrients to form biomass, a cellulase enzyme (metabolic product), and wastewater. Flow rates were estimated from the amount of raw materials and waste.
- (c)
- Filtration: The first sub-phase was filtration, which consisted of a tubular centrifugation and a 0.22 μm membrane filter where approximately 99.9% of total biomass waste was removed.
- (d)
- Ultrafiltration: The supernatant was ultrafiltrated through tangential filtration to obtain a concentrated liquid without microorganisms. Ultrafiltration (10 kDa) was repeated until the concentration factor was 10.
- (e)
- Lyophilization: At the last stage, lyophilization, all the remaining water is removed, and a solid, dry enzyme is obtained as the final product.
- (f)
- Biomass treatment: its environmental impact was assessed by the oxygen consumption via the theoretical chemical oxygen demand (ThCOD) consumed in a complete oxidation reaction. The ThCOD was estimated from the approximate molecular composition of biomass. Then, the reaction considered to calculate this parameter is:CH1.8O0.5N0.2 + O2 -> CO2 + NH3 + H2O
2.1.3. Solid-State Fermentation (SSF)
- (a)
- SSF: SSF was performed similarly to a composting process, in discontinuous mode in a 100 L airtight aerated reactor. In a study by Cerda and colleagues [17], the highest cellulase activity was observed after 24 h of SSF. Accordingly, residence time was fixed to this value. Approximately 10% of the fermented solid was the inoculum for the next batch [25,31,32]. Wood chips were used to provide porosity to the mixture [33]. In the same way, all fermented solid (except 10% used as inoculum) was derived to downstream stages.
- (b)
- The downstream processes is composed of four phases as explained below:
- (c)
- Extraction: The fermented solid was mixed with tap water in a 1:2 (v/v) ratio for 30 min [18]. To facilitate further filtration process, the fermented solids were mixed with water, passed through a mesh of 1 mm in the same tank with the objective of removing the biggest solid particles.
- (d)
- Filtration: Then, the liquid obtained from the previous stage (extract) was centrifuged at 15,000 rpm. The resulting liquid was filtered through a 0.22 μm filter. With this operation, all the biomass and suspended solids were removed.
- (e)
- Ultrafiltration: Next, this extract was filtered with an ultrafiltration device (10 kDa of cut-off) to concentrate the cellulase suspension. This step was repeated to reach a concentration ten times higher than the original one.
- (f)
- Lyophilization: Finally, the enzyme concentrated was lyophilized to obtain the main product (dry and concentrated cellulase).
2.2. Inventory Analysis, Functional Unit, Methodology, and Assumptions
2.2.1. Functional Unit
2.2.2. Life Cycle Assessment (LCA) Methodology and Impact Assessment
2.2.3. Technical Assumptions
Overall Assumptions
Submerged Fermentation (SmF)
- (a)
- Cellulose powder was considered with an environmental impact due its production.
- (b)
- The fermentation process was considered to run for 107 h in an airtight packed bed reactor, working under oxygen controlled aeration with 0.58 vvm (Lair·(Lreactor·min)−1) and a constant temperature of 28 °C.
- (c)
- According to Himmel, Biwer, and Wyman [12] and Sáez [26], the principal raw material was cellulose powder. In this study, carboxymethyl cellulose was chosen as a main input (Ecoinvent database). In this case, as carboxymethyl cellulase is a commercial product used to obtain another commercial product (cellulase), its environmental burdens in the production must be considered in this process. Ecoinvent database contains the details of these environmental impacts.
Solid-State Fermentation (SSF)
- (a)
- The main assumption was that the raw material used would not have environmental impacts because it is a waste. In this case, the environmental impacts associated with CH production are associated to the original industry (coffee production) since it is the normal procedure when dealing with waste as raw material for the production of another commercial product.
- (b)
- Exhaust gases emitted in SSF (and further composting) were treated in a biofilter considering that it removes 70% of emitted pollutants, as reported in the literature [41].
2.2.4. Inventory Analysis
Submerged Fermentation
Solid-State Fermentation
2.3. Sensitivity Analysis
3. Results and Discussion (Impact Assessment and Interpretation)
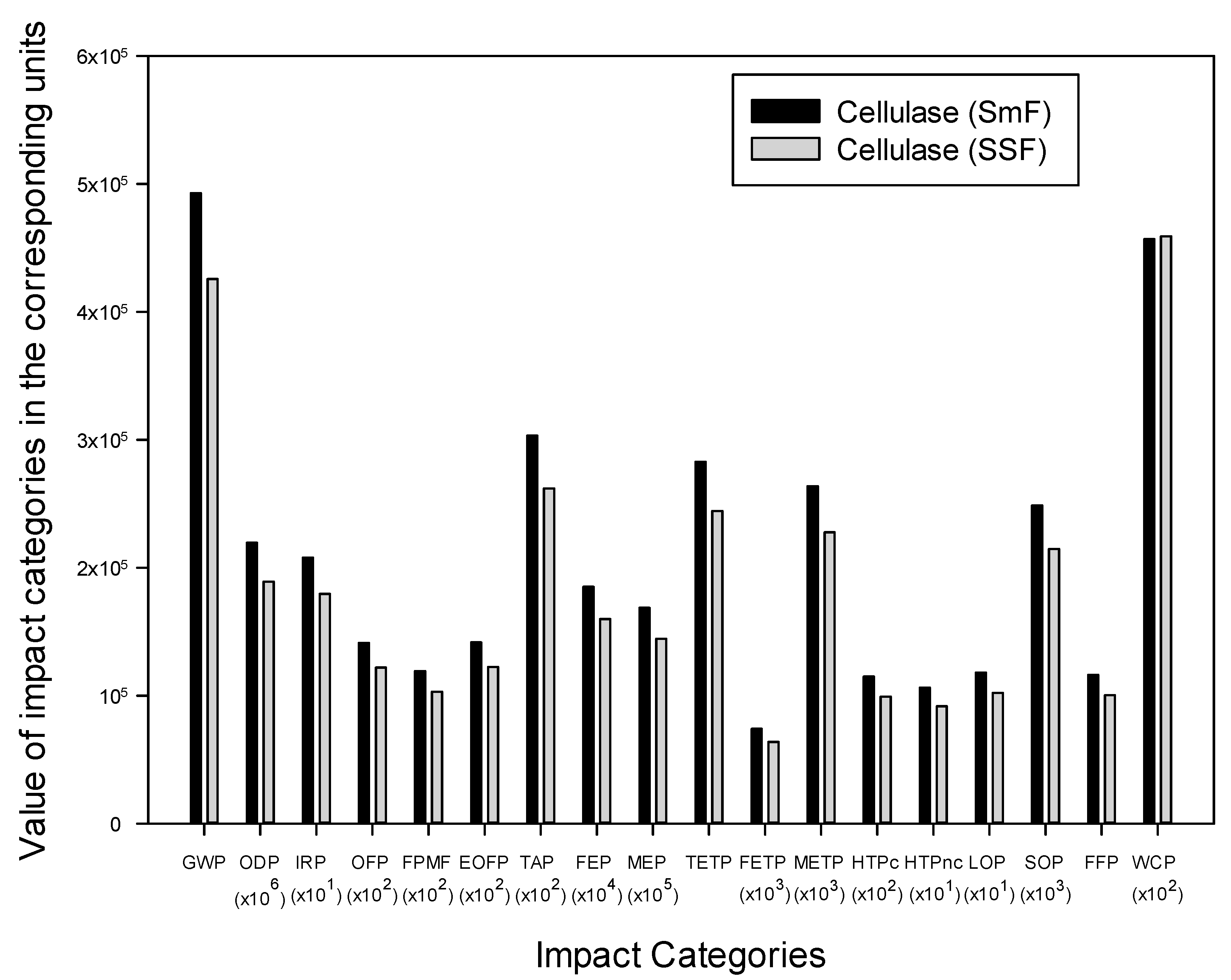
3.1. Overall Results
3.2. Specific Results
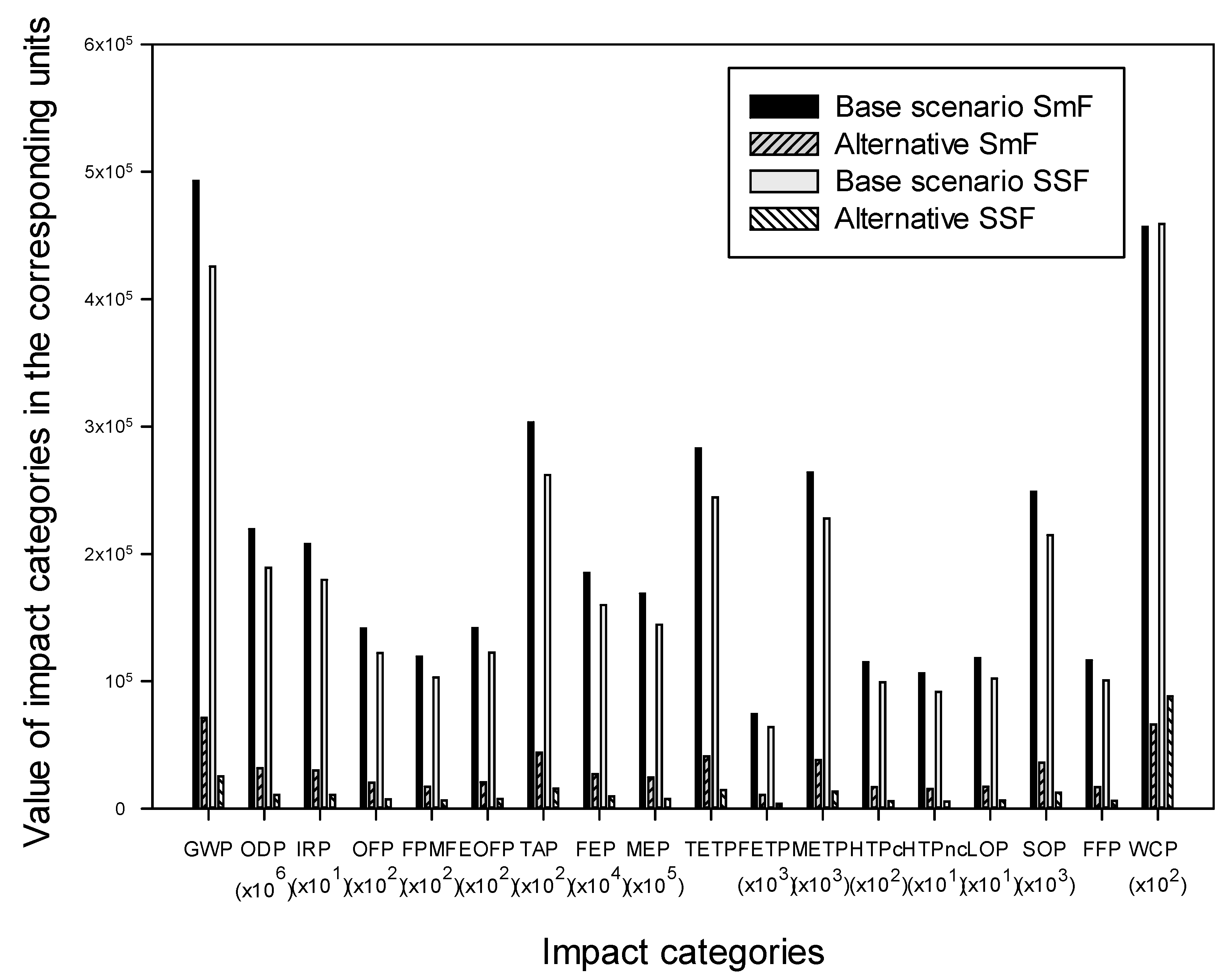
3.3. Sensitivity Analysis
3.4. Preliminary Economic Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parada, M.P.; Osseweijer, P.; Duque, J.A.P. Sustainable bioferineries, an analysis of practices for incorporating sustainability in biorefinery design. Ind. Crops Prod. 2017, 106, 105–123. [Google Scholar] [CrossRef]
- Wooley, R.; Ruth, M.; Glassner, D.; Sheehan, J. Process design and costing of bioethanol technology: A tool for determining the status and direction of research and development. Biotechnol. Prog. 1999, 15, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, J.; Nielsen, J.; Olsson, L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Appl. Microbiol. Biotehnol. 2001, 56, 17–34. [Google Scholar] [CrossRef]
- Pérez, J.; de la Muñoz-Dorado Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Bezerra, T.L.; Ragauskas, A.J. A review of sugarcane bagasse for second generation bioethanol and biopower production. Biofuels Bioprod. Bioref. 2016, 10, 634–647. [Google Scholar] [CrossRef]
- Sindhu, R.; Gnansounou, E.; Binod, P.; Pandey, A. Bioconversion of sugarcane crop residue for value added products—An overview. Renew. Energy 2016, 98, 203–215. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Yang, J.; Bae, H.J. Bioethanol production from individual and mixed agricultural biomass residues. Ind. Crops Prod. 2017, 95, 718–725. [Google Scholar] [CrossRef]
- Doppelbauer, R.; Esterbauer, H.; Steiner, W.; Lafferty, R.; Steinmuller, H. The use of cellulosic wastes for production of cellulose by Trichoderma reesei. Appl. Microbiol. Biotechnol. 1987, 26, 485–494. [Google Scholar] [CrossRef]
- Garcia-Kirchner, O.; Muñoz-Aguilar, M.; Perez-Villalva, R.; Huiltron-Vargas, C. Mixed submerged fermentation with two filamentous fungi for cellulolytic and xylanolytic enzyme production. Appl. Biochem. Biotechnol. 2002, 98, 1105–1114. [Google Scholar] [CrossRef]
- De Sales, A.N.; De Souza, A.C.; Moutta, R.D.; Ferreira-Leitao, V.S.; Schawan, R.F. Use of lignocellulose biomass for endoxylanase production by Streptomyces termitum. Prep. Biochem. Biotechnol. 2017, 47, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Tolán, J.S.; Foody, B. Cellulase from submerged fermentation. Adv. Biochem. Eng. Biotechnol. 2001, 65, 41–67. [Google Scholar]
- Himmel, M.E.; Biwer, A.; Wyman, C.E. Cellulase for commodity products from cellulosic biomass. Curr. Opin. Biotechnol. 1999, 10, 358–364. [Google Scholar] [CrossRef]
- Sánchez, A.; Gabarrell, X.; Artola, A.; Barrena, R.; Colón, J.; Font, X.; Komilis, D. Composting of wastes. In Resource Recovery to Approach Zero Municipal Waste; Taherzadeh, M.J., Richards, T., Eds.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2016; pp. 77–106. [Google Scholar]
- Chen, H.; Qiu, W. Key technologies for bioethanol production from lignocellulose. Biotechnol. Adv. 2010, 28, 556–562. [Google Scholar] [CrossRef]
- Hong, Y.; Nizami, A.S.; Bafrani, M.P.; Savile, B.A. Impact of cellulase production on environmental and financial metrics for lignocellulosic ethanol. Biofuels Bioprod. Bioref. 2012, 7, 303–313. [Google Scholar] [CrossRef]
- MacLean, H.L.; Spatari, S. The contribution of enzymes and process chemicals to the life cycle of ethanol. Environ. Res. Lett. 2009, 4, 1–10. [Google Scholar] [CrossRef]
- Cerda, A.; Gea, T.; Vargas-Garcia, M.C.; Sánchez, A. Towards a competitive solid state fermentation: Cellulases production from coffee husk by sequential batch operation and role of microbial diversity. Sci. Total Environ. 2017, 589, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.; Artola, A.; Sánchez, A. Optimization of Down-Stream for Cellulases Produced Under Solid-State Fermentation of Coffee Husk. Waste Biomass Valoriz. 2019, 10, 2761–2772. [Google Scholar] [CrossRef]
- Marques, N.P.; Pereira, J.C.; Gomes, E.; Da Silva, R.; Araújo, A.R.; Ferreira, H.; Rodrigues, A.; Dussán, K.J.; Bocchini, D.A. Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind. Crops Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Brand, D.; Mohan, R.; Roussos, S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 2000, 6, 153–162. [Google Scholar] [CrossRef]
- Laurent, A.B.; Menard, J.F.; Lesage, P.; Beauregard, R. Cradle-to-Gate Environmental Life Cycle Assessment of Portfolio of an Innovative Forest Products Manufacturing Unit. Bioresources 2016, 11, 8981–9001. [Google Scholar] [CrossRef]
- ISO. 14040-Environmental Management-Life Cycle Assessment-Principles and Framework; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- ISO. 14044-Environmental Management-Life Cycle Assessment-Requirements and Guidelines; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Goedkoop, M.; Heijungs, R.; Huijbregts, M.; Schryver, A.; Struijs, J.; Van Zelm, R. Recipe 2008: A Life Cycle Assessment Methods Which Comprises Harmonized Category Indicators at the Midpoint and the Endpoint Level, 1st ed.; Report I: Characterisation; 2013. Available online: https://www.leidenuniv.nl/cml/ssp/publications/recipe_characterisation.pdf (accessed on 26 May 2020).
- Cerda, A.; Mejias, L.; Gea, T.; Sánchez, A. Cellulase and xylanase production at pilot scale by solid-state fermentation from coffee husk using specialized consortia: The consistency of the process and the microbial communities involved. Bioresour. Technol. 2017, 243, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.C.; Schell, D.J.; Tholudur, A.; Farmer, J.; Hamilton, J.; Colucci, J.A.; McMillan, J.D. Carbon mass balance evaluation of cellulase production on soluble and insoluble substrates. Biotechnol. Progr. 2002, 18, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.; Harrison, S. Life cycle assessment (LCA) and comparison of various cellulase production methods. In Proceedings of the Life Cycle Management Conference (LCM 2011), Berlin, Germany, 28–31 August 2011. [Google Scholar]
- Heinzle, E.; Biwer, A.P.; Cooney, C.L. Development of Sustainable Bioprocesses: Modeling and Simulation of Bioprocesses; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Wooley, R.; Ruth, M.; Sheehan, J.; Ibsen, K. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis Current and Futuristic Scenarios; No. NREL/TP-580-26157; National Renewable Energy Laboratory: Golden, CO, USA, 1999.
- Catalán, E.; Komilis, D.; Sánchez, A. Environmental impact of cellulase production from coffee husks by solid-state fermentation: A life-cycle assessment. J. Clean. Prod. 2019, 233, 954–962. [Google Scholar] [CrossRef]
- Zulkeflee, Z.; Sánchez, A. Solid-state fermentation of soybean residues for bioflocculant production in a pilot-scale bioreactor system. Water Sci. Technol. 2014, 70, 1032–1039. [Google Scholar] [CrossRef][Green Version]
- Ruggieri, L.; Gea, T.; Artola, A.; Sánchez, A. Air filled porosity measurements by air pycnometry in the composting process: A review and a correlation analysis. Bioresour. Technol. 2009, 100, 2655–2666. [Google Scholar] [CrossRef]
- Weidema, B.; Wenzel, H.; Petersen, C. The Product, Functional Unit and Reference Flows in LCA; Environmental Protection Agency Nº 70; Danish Ministry of the Environment: Copenhagen, Denmark, 2004.
- Fiorentino, G.; Ripa, M.; Protano, G.; Hornsby, C.; Ulgiati, S. Life cycle assessment of mixed municipal solid waste: Multi-input versus multi-output perspective. Waste Manag. 2015, 46, 599–611. [Google Scholar] [CrossRef]
- Finnveden, G.; Hauschild, M.Z.; Ekvail, T.; Guinee, J.; Heijungs, R.; Hellwege, S.; Koehler, A.; Pennington, D.; Suh, S. Recent developments in life cycle assessment. J. Environ. Manag. 2009, 91, 1–21. [Google Scholar] [CrossRef]
- Puyuelo, B.; Gea, T.; Sánchez, A. GHG emissions during the high-rate production of compost using standard and advanced aeration strategies. Chemosphere 2014, 109, 64–70. [Google Scholar] [CrossRef]
- Sánchez, A.; Ferrer, P.; Serrano, A.; Valero, F.; Solà, C.; Pernas, M.; Rúa, M.L.; Fernández-Lafuente, R.; Guisán, J.M.; De la Casa, R.M.; et al. A controlled fed-batch cultivation for the production of new crude lipases from Candida rugosa with improved properties in fine chemistry. J. Biotechnol. 1999, 69, 169–182. [Google Scholar] [CrossRef]
- Sánchez, A.; Ferrer, P.; Serrano, A.; Pernas, M.; Valero, F.; Rúa, M.; Casas, C.; Solà, C. Characterization of the lipase and esterase multiple froms in an enzyme preparation from a Candida rugosa pilot-plant scale fed-batch fermentation. Enzyme Microb. Technol. 1999, 25, 214–223. [Google Scholar] [CrossRef]
- IEA. The International Energy Agency. 2017. Available online: https://www.iea.org/statistics (accessed on 15 April 2020).
- Colón, J.; Martínez-Blanco, J.; Gabarrell, X.; Font, X.; Artola, A.; Sánchez, A. Performance of an industrial biofilter from a composting plant in the removal of ammonia and VOCs after material replacement. J. Chem. Technol. Biotechnol. 2009, 84, 1111–1117. [Google Scholar] [CrossRef]
- Guo, M.; Murphy, R.J. LCA data quality: Sensitivity and uncertainty analysis. Sci. Total Environ. 2012, 435, 230–243. [Google Scholar] [CrossRef]
- Nizami, A.S.; Ismail, I.M. Life-Cycle Assessment of Biomethane from Lignocellulosic Biomass; Green Energy and Technology; Singh, A., Pant, D., Olse, S.I., Eds.; Springer Nature: Basel, Switzerland, 2013; pp. 79–94. [Google Scholar]
- Maulini-Duran, C.; Abraham, J.; Rodríguez-Perez, S.; Cerda, A.; Jimenez-Peñalvez, P.; Gea, T.; Barrena, R.; Artola, A.; Font, X.; Sánchez, A. Gaseous emissions during the solid state fermentation of different wastes for enzyme production at pilot scale. Bioresour. Technol. 2015, 179, 211–218. [Google Scholar] [CrossRef]
- EPA. Sources of Greenhouse Gas Emissions; United States Environmental Protection Agency: Washington, DC, USA, 2017. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (accessed on 10 April 2020).
- Gilpin, G.S.; Andrae, A.S.G. Comparative attributional life cycle assessment of European cellulase enzyme production for use in second-generation lignocellulosic bioethanol production. Int. J. Life Cycle Assess. 2017, 22, 1034–1053. [Google Scholar] [CrossRef]
- Silalertruksa, T.; Gheewala, S.H. A comparative LCA of rice straw utilization for fuels and fertilizer in Thailand. Bioresour. Technol. 2013, 150, 412–419. [Google Scholar] [CrossRef]
- IEA. The International Energy Agency Oil Market Report. 2018. Available online: https://www.iea.org/oilmarketreport/omrpublic (accessed on 15 April 2020).
- IEA. The International Energy Agency, Energy and Air Pollution. 2016. Available online: https://www.iea.org/publications/freepublications/publication/WorldEnergyOutlookSpecialReport2016EnergyandAirPollution.pdf (accessed on 15 April 2020).
- González-García, S.; Morales, P.C.; Gullón, B. Estimating the environmental impacts of a brewery waste-based biorefinery: Bio-ethanol and xylooligosaccharides joint production case study. Ind. Crops Prod. 2018, 123, 331–340. [Google Scholar] [CrossRef]
- Doric, J.; Mirjana, M.; Maja, T.S.; Radonic, J. BTEX in the exhaust emissions of motor vehicles. In Proceedings of the Global conference on Global Warning (GCGW), Istanbul, Turkey, 8–12 July 2012. [Google Scholar]
- Ferreira, S.L.; Dos Santos, A.M.; Souza, G.R.; Polito, W.L. Analysis of the emissions of volatile organic compounds from the compression ignition engine fuelled by diesel-biodiesel blend and diesel oil using gas chromatography. Energy 2008, 33, 1801–1806. [Google Scholar] [CrossRef]
- Rao, P.H.; Zhang, W.Q.; Yao, W.; Zhu, A.Y.; Xia, J.L.; Tan, Y.F.; Li, Y.J.; Liu, T.Z. Chemical compounds recover in Carboxymethyl Cellulose wastewater treatment. Chemistry in industry. J. Chem. Chem. Eng. 2015, 64, 247–254. [Google Scholar]
- Wyman, V.; Henríquez, J.; Palma, C.; Carvajal, A. Lignocellulosic waste valorisation strategy through enzyme and biogas production. Bioresour. Technol. 2018, 247, 402–411. [Google Scholar] [CrossRef]
- Schweier, J.; Schnitzler, J.P.; Becker, G. Selected environmental impacts of the technical production of wood chips from poplar short rotation coppice on marginal land. Biomass Bioenerg. 2016, 85, 235–242. [Google Scholar] [CrossRef]
- Gassara, F.; Brar, S.K.; Pelletier, F.; Verma, M.; Godbout, S.; Tyagi, R.D. Pomace waste management scenarios in Québec. Impact on greenhouse gas emissions. J. Hazard. Mater. 2011, 192, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Hujibregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.D.M.; Hollander, A.; Zijp, M.; van Zelm, R. ReciPe 2016 v1.1. A harmonized Life Cycle Assessment Method at Midpoint and Endpoint Level. Report I: Characterization; National Institute for Public Health and Environmental, Ministry of Health, Welfare and Sport: Bilthoven, The Netherlands, 2017.
- Garret, P.; Ronde, K. Life Cycle Assessment of Electricity Production from V90-2.0 MW Gridstreamer Wind Plant; Vestas Wind Systems A/S: Randers, Denmark, 2011; Available online: https://www.vestas.com/~/media/vestas/about/sustainability/pdfs/lca_v902mw_version1.pdf (accessed on 15 April 2020).
- Bonilla-Hermosa, V.A.; Duarte, W.F.; Shwan, R.F. Utilization of coffee by-products obtained from semi-washed process for production of value-added compounds. Bioresour. Technol. 2014, 166, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Mejías, L.; Cerda, A.; Barrena, R.; Gea, T.; Sánchez, A. Microbial Strategies for Cellulase and Xylanase Production through Solid-State Fermentation of Digestate from Biowaste. Sustainability 2018, 10, 2433. [Google Scholar] [CrossRef]
- Colón, J.; Cadena, E.; Pognani, M.; Barrena, R.; Sánchez, A.; Font, A. Determination of the energy and environmental burdens associated with the biological treatment of source-separated Municipal Solid Waste. Energy Environ. Sci. 2011, 5, 5731–5741. [Google Scholar] [CrossRef]

| Impact Category | Unit of the Results |
|---|---|
| Global warming (GWP) | kg CO2eq |
| Stratospheric ozone depletion (ODP) | kg CFC−11eq |
| Ionizing radiation (IRP) | kBq Co-60eq |
| Ozone formation, human health (HOFP) | kg NOxeq |
| Fine particulate matter formation (FPMF) | kg PM2.5eq |
| Ozone formation, terrestrial ecosystems (EOFP) | kg NOxeq |
| Terrestrial acidification (TAP) | kg SO2 |
| Freshwater eutrophication (FEP) | kg Peq |
| Marine eutrophication (MEP) | kg Neq |
| Terrestrial ecotoxicity (TETP) | kg 1.4-DCB |
| Freshwater ecotoxicity (FETP) | kg 1.4-DCB |
| Marine ecotoxicity (METP) | kg 1.4-DCB |
| Human carcinogenic toxicity (HTPc) | kg 1.4-DCB |
| Human non-carcinogenic toxicity (HTPnc) | kg 1.4-DCB |
| Land use (LOP) | m2 year |
| Mineral resource scarcity (SOP) | kg Cueq |
| Fossil resource scarcity (FFP) | kg oileq |
| Water consumption (WCP) | m3 |
| Material | Value | Comments and Assumptions | |
|---|---|---|---|
| INPUTS | Cellulose powder1 (kg/FU) | 3.62 | In SimaPro the chosen input was directly carboxymethyl cellulose because it is prepared from pure cellulose [42]. |
| Ammonia2 (kg/FU) | 0.10 | Heinzle et al. [28]. | |
| Corn liquor3 (kg/FU) | 0.75 | The carbon source necessary for the microorganism growth was assumed to be corn residue, although it is typically a nitrogen source [28]. | |
| Water (L/FU) | 77.4 | Heinzle et al. [28]. | |
| Nutrients4 (kg/FU) | 0.55 | The nutrients required during fermentation were: 32.5 g/L ammonium sulphate ((NH4)2SO4), 46.5 g/L monopotassium phosphate (KH2PO4), 7.0 g/L magnesium sulphate heptahydrate (MgSO4·7H2O), 9.3 g/L calcium chloride dihydrate (CaCl2·2H2O), and 4.7 g/L Tween 80 [2,29]. | |
| Energy (kJ/FU) | 1,413,935 | Taking into account that the energy was the sum for each equipment power and the total air consumption. The air consumption from fermentation and the stabilization biomass was 372,701 L air/kg cellulase. | |
| OUTPUTS | Biomass waste (kg/FU) | 1.5 | The required oxygen for biomass oxidation produced in 1 kg of cellulase was 102 kg. Theoretical chemical oxygen demand (ThCOD) was calculated through theory reaction of oxidation microorganism with Equation (1): CH1.8O0.5N0.2 + O2 + → CO2 + NH3 + H20 |
| Cellulase (kg/FU) | 0.35 | Heinzle et al. [28]. | |
| Wastewater (L/FU) | 65.2 | Heinzle et al. [28]. | |
| Corn liquor (kg/FU) | 0.15 | Heinzle et al. [28]. | |
| CO2 (kg/FU) | 0.13 | This value was taken and calculated according to the study from Sáez et al. [26] where it was demonstrated that during the fermentation 18 g/L of CO2 in terms of bioreactor volume can be produced. |
| Materials | Value | Comments and Assumptions | |
|---|---|---|---|
| INPUTS | Coffee husk1 (kg/FU) | 84.3 | Coffee husk (CH) waste coming from Marcilla S.L, Barcelona (Spain). This CH is not associated with environmental impacts as it is attributed to coffee production. |
| Wood chips2 (kg/FU) | 55 | Cerda et al. [17]. | |
| Water from extraction (L/FU) | 678.8 | Cerda et al. [17]. | |
| Energy3 (kJ/FU) | 1,407,873 | Taking into account that the energy was the sum for each equipment power and the total air consumption. The sum total (air required in SSF and the air required in composting step) of air consumption to obtain 1 kg of cellulase (FU) was 357,053 L. | |
| OUTPUTS | Compost | 186.8 | Composting process was performed in the same reactor of SSF with aeration for 15 days. In this study it was assumed that the compost obtained avoided the production of fertilizer. It was considered that 50% of the total nitrogen of compost are available to plants and therefore, can replace an equivalent amount of N in the form of ammonium nitrate. This entails that 5.6 kg of fertilizer production were avoided per 1 kg cellulase (FU). |
| COD4 (kg/FU) | 0.03 | Estimated | |
| Wastewater (L/FU) | 601.5 | Cerda et al. [17]. | |
| Methane3 (kg/FU) | 0.02 | Maulini-Duran et al. [43] | |
| Nitrous oxide3 (kg/FU) | 0.06 | Maulini-Duran et al. [43] | |
| VOCs3 (kg/FU) | 0.13 | Maulini-Duran et al. [43] | |
| Ammonia3 (kg/FU) | 0.06 | Maulini-Duran et al. [43] |
| Impact Category | Units | Cellulase Production | Carboxymethyl * Cellulose | Ammonia | Nutrients | Electricity |
|---|---|---|---|---|---|---|
| GWP | kg CO2eq | 1.8 | 16.32 | 0.20 | 1.03 | 492,695 |
| ODP | kg CFC−11eq | 0 | 7.50 × 10−6 | 7.62 × 10−8 | 3.33 × 10−7 | 0.22 |
| IRP | kBq Co-60eq | 0 | 0.11 | 1.01 × 10−3 | 0.01 | 20,775 |
| OFP | kg NOxeq | 0 | 0.03 | 1.96 × 10−4 | 1.85 × 10−3 | 1412 |
| FPMF | kg PM2.5eq | 0 | 0.03 | 1.88 × 10−4 | 1.73 × 10−3 | 1191 |
| EOFP | kg NOxeq | 0 | 0.03 | 2.023 × 10−4 | 1.88 × 10−3 | 1417 |
| TAP | kg SO2 | 0 | 0.06 | 4.77 × 10−4 | 3.73 × 10−3 | 3032 |
| FEP | kg Peq | 0 | 9.52 × 10−4 | 8.67 × 10−7 | 3.87 × 10−5 | 18.50 |
| MEP | kg Neq | 0 | 8.15 × 10−5 | 1.39 × 10−7 | 4.81 × 10−6 | 1.69 |
| TETP | kg 1.4-DCB | 0 | 31.77 | 0.87 | 1.31 | 282,712 |
| FETP | kg 1.4-DCB | 1.70 × 10−17 | 6.37 | 6.61 × 10−5 | 3.01 × 10−4 | 74.12 |
| METP | kg 1.4-DCB | 0.02 | 0.03 | 6.86 × 10−4 | 8.14 × 10−4 | 263.72 |
| HTPc | kg 1.4-DCB | 3.49 × 10−4 | 0.09 | 3.85 × 10−4 | 2.08 × 10−3 | 1149 |
| HTPnc | kg 1.4-DCB | 0 | 1.07 | 7.70 × 10−3 | 0.03 | 10,617 |
| LOP | m2 year | 0 | 2.61 | 2.04 × 10−4 | 0.01 | 11,804 |
| SOP | kg Cueq | 0 | 0.03 | 1.00 × 10−4 | 1.07 × 10−3 | 248.62 |
| FFP | kg oileq | 0 | 4.59 | 0.09 | 0.27 | 116,232 |
| WCP | m3 | 0.08 | 0.20 | 5.17 × 10−3 | 0.01 | 4566 |
| Impact Category | Units | Cellulase | Wood Chips | Electricity | Nitrogen Fertilizer as N |
|---|---|---|---|---|---|
| GWP | kg CO2eq | 17.2 | 7.1 | 425,758 | −60.5 |
| ODP | kg CFC−11eq | 6.27 × 10−4 | 6.20 × 10−6 | 0.19 | −1.26 × 10−3 |
| IRP | kBq Co-60eq | 0 | 8.78 × 10−2 | 17953 | −0.27 |
| HOFP | kg NOxeq | 0 | 3.08 × 10−2 | 1221 | −0.11 |
| FPMF | kg PM2.5eq | 0 | 2.18 × 10−2 | 1029 | −6.22 × 10−2 |
| EOFP | kg NOxeq | 0 | 3.29 × 10−2 | 1224 | −0.11 |
| TAP | kg SO2 | 0 | 3.27 × 10−2 | 2620 | −0.26 |
| FEP | kg Peq | 0 | 2.88 × 10−4 | 16.0 | −1.46 × 10−3 |
| MEP | kg Neq | 0 | 1.16 × 10−4 | 1.46 | 1.35 × 10−2 |
| TETP | kg 1.4-DCB | 0 | 28.1 | 244,303 | −82.3 |
| FETP | kg 1.4-DCB | 0 | 6.00 × 10−3 | 64.1 | −0.12 |
| METP | kg 1.4-DCB | 0 | 2.23 × 10−2 | 228 | −0.15 |
| HTPc | kg 1.4-DCB | 0 | 0.12 | 993 | −5.43 × 10−2 |
| HTPnc | kg 1.4-DCB | 0 | 2.2 | 9174 | −5.4 |
| LOP | m2 year | 0 | 19.6 | 10,200 | −8.2 |
| SOP | kg Cueq | 0 | 3.18 × 10−3 | 215 | −0.14 |
| FFP | kg oileq | 0 | 2.7 | 100,441 | −7.4 |
| WCP | m3 | 645 | 0.19 | 3946 | −1.2 |
| Impact Category | Units | Cellulase Production by SSF: Base Scenario | Cellulase Production by SSF: Sub-Alternative |
|---|---|---|---|
| GWP | kg CO2eq | 425,722 | 423,714 |
| ODP | kg CFC−11eq | 0.19 | 0.19 |
| IRP | kBq Co-60eq | 17,953 | 17,868 |
| HOFP | kg NOxeq | 1220 | 1215 |
| FPMF | kg PM2.5eq | 1029 | 1024 |
| EOFP | kg NOxeq | 1224 | 1219 |
| TAP | kg SO2 | 2620 | 2607 |
| FEP | kg Peq | 16.0 | 15.9 |
| MEP | kg Neq | 1.4 | 1.4 |
| TETP | kg 1.4-DCB | 244,249 | 243,096 |
| FETP | kg 1.4-DCB | 63.9 | 63.6 |
| METP | kg 1.4-DCB | 228 | 227 |
| HTPc | kg 1.4-DCB | 993 | 988 |
| HTPnc | kg 1.4-DCB | 9171 | 9128 |
| LOP | m2 year | 10,212 | 10,164 |
| SOP | kg Cueq | 215 | 214 |
| FFP | kg oileq | 100,436 | 99,963 |
| WCP | m3 | 4590 | 4571 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalán, E.; Sánchez, A. Solid-State Fermentation (SSF) versus Submerged Fermentation (SmF) for the Recovery of Cellulases from Coffee Husks: A Life Cycle Assessment (LCA) Based Comparison. Energies 2020, 13, 2685. https://doi.org/10.3390/en13112685
Catalán E, Sánchez A. Solid-State Fermentation (SSF) versus Submerged Fermentation (SmF) for the Recovery of Cellulases from Coffee Husks: A Life Cycle Assessment (LCA) Based Comparison. Energies. 2020; 13(11):2685. https://doi.org/10.3390/en13112685
Chicago/Turabian StyleCatalán, Eva, and Antoni Sánchez. 2020. "Solid-State Fermentation (SSF) versus Submerged Fermentation (SmF) for the Recovery of Cellulases from Coffee Husks: A Life Cycle Assessment (LCA) Based Comparison" Energies 13, no. 11: 2685. https://doi.org/10.3390/en13112685
APA StyleCatalán, E., & Sánchez, A. (2020). Solid-State Fermentation (SSF) versus Submerged Fermentation (SmF) for the Recovery of Cellulases from Coffee Husks: A Life Cycle Assessment (LCA) Based Comparison. Energies, 13(11), 2685. https://doi.org/10.3390/en13112685






