Interfacial Charge-Transfer Transitions for Direct Charge-Separation Photovoltaics
Abstract
1. Introduction
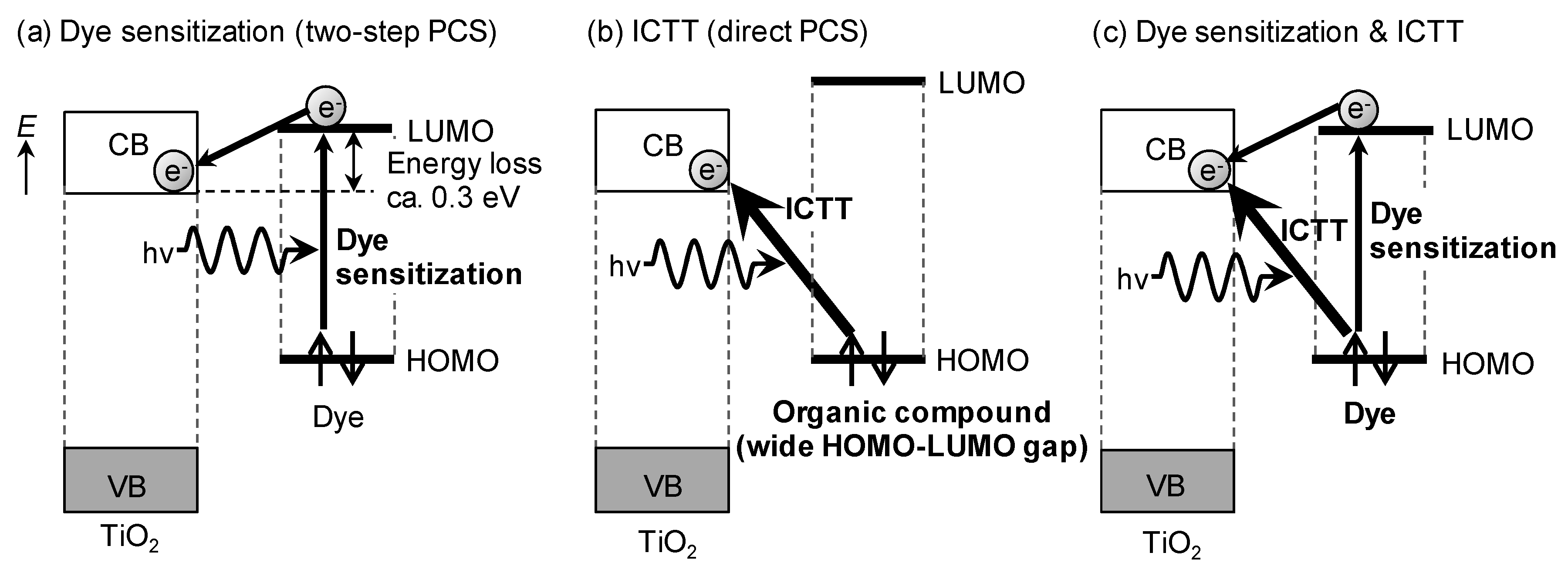
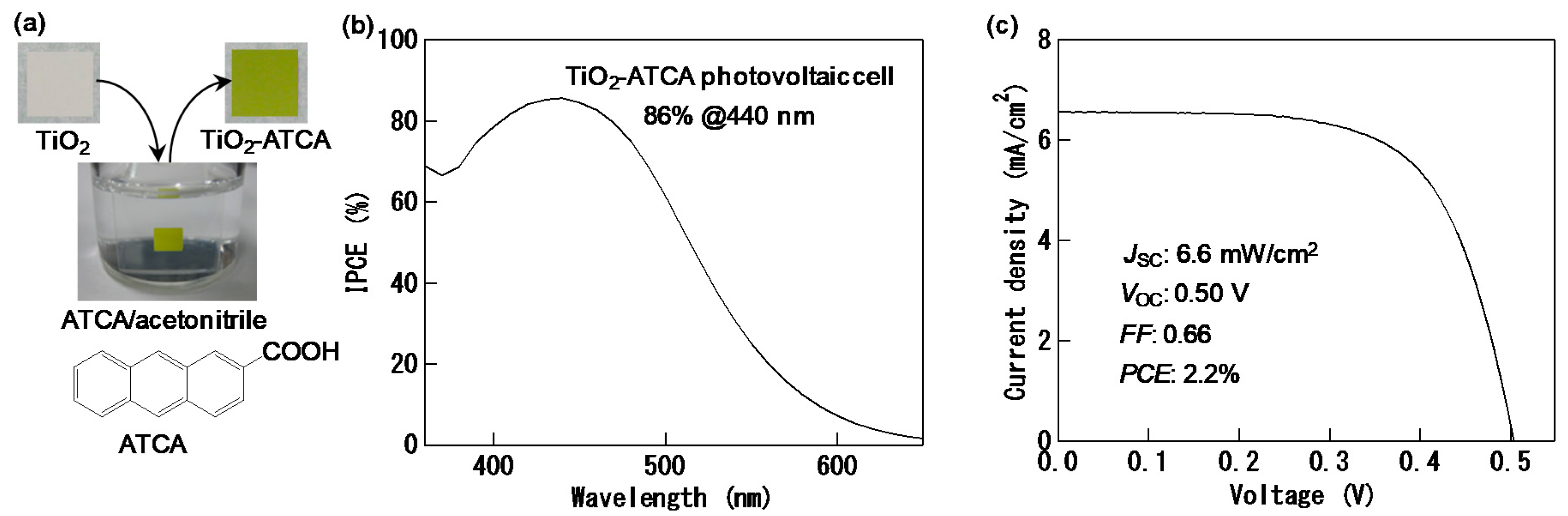
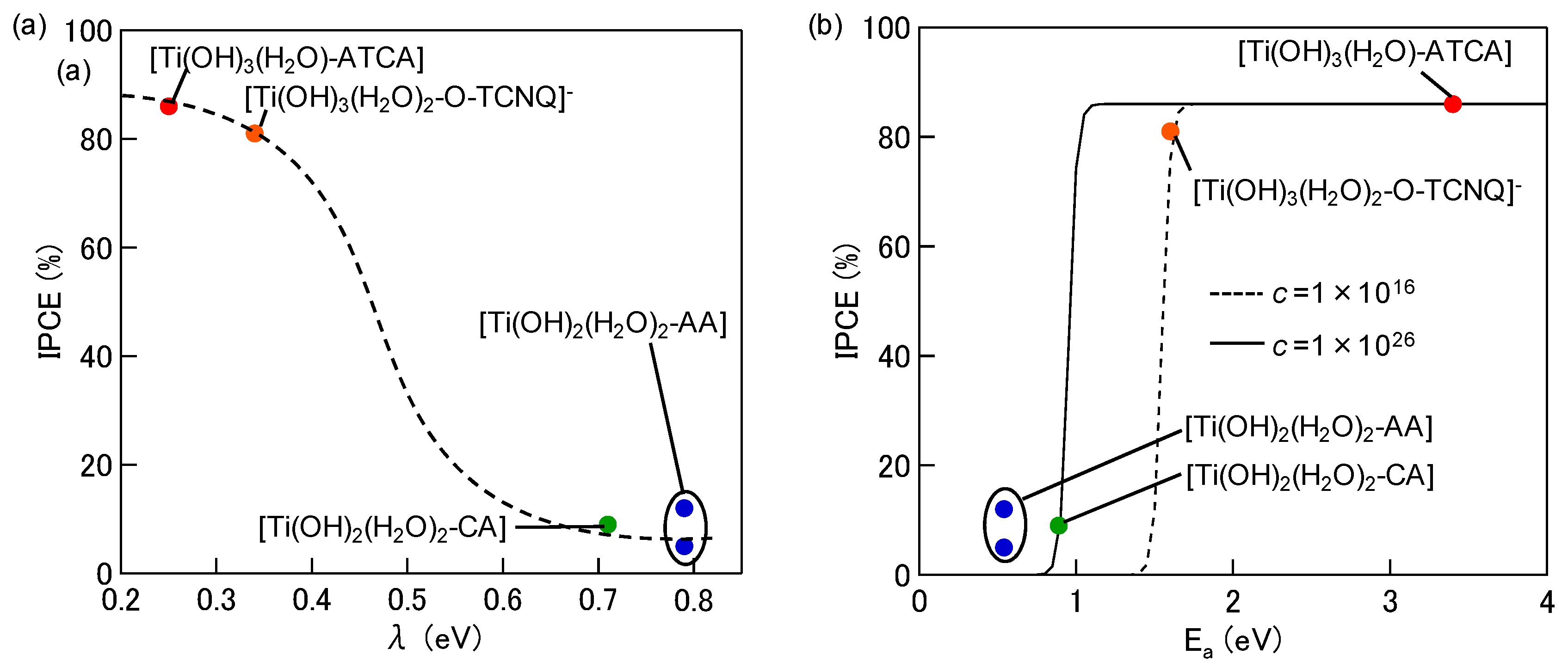
2. Interfacial Charge-transfer Transitions and Their Photovoltaic Conversion Properties
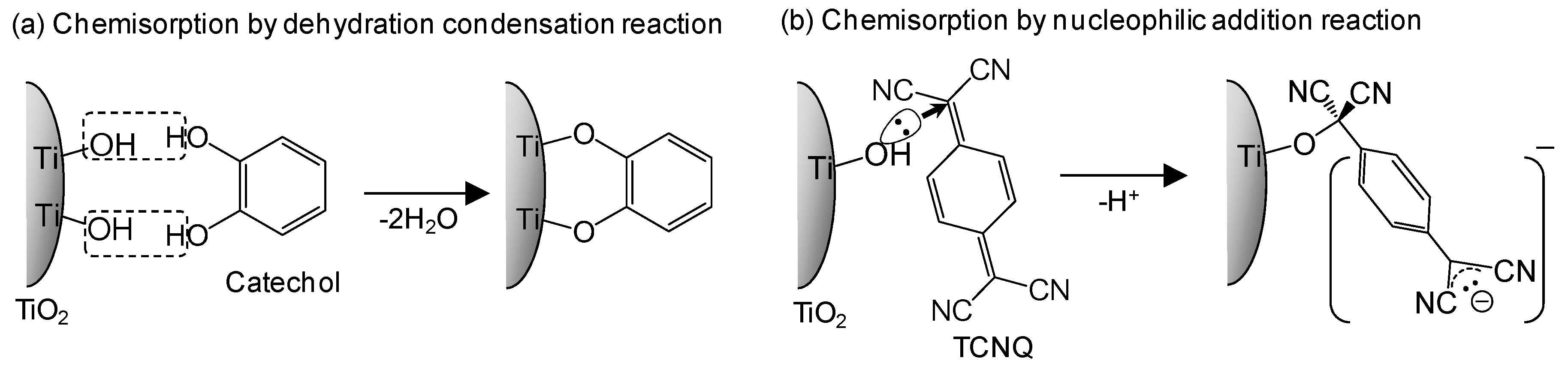
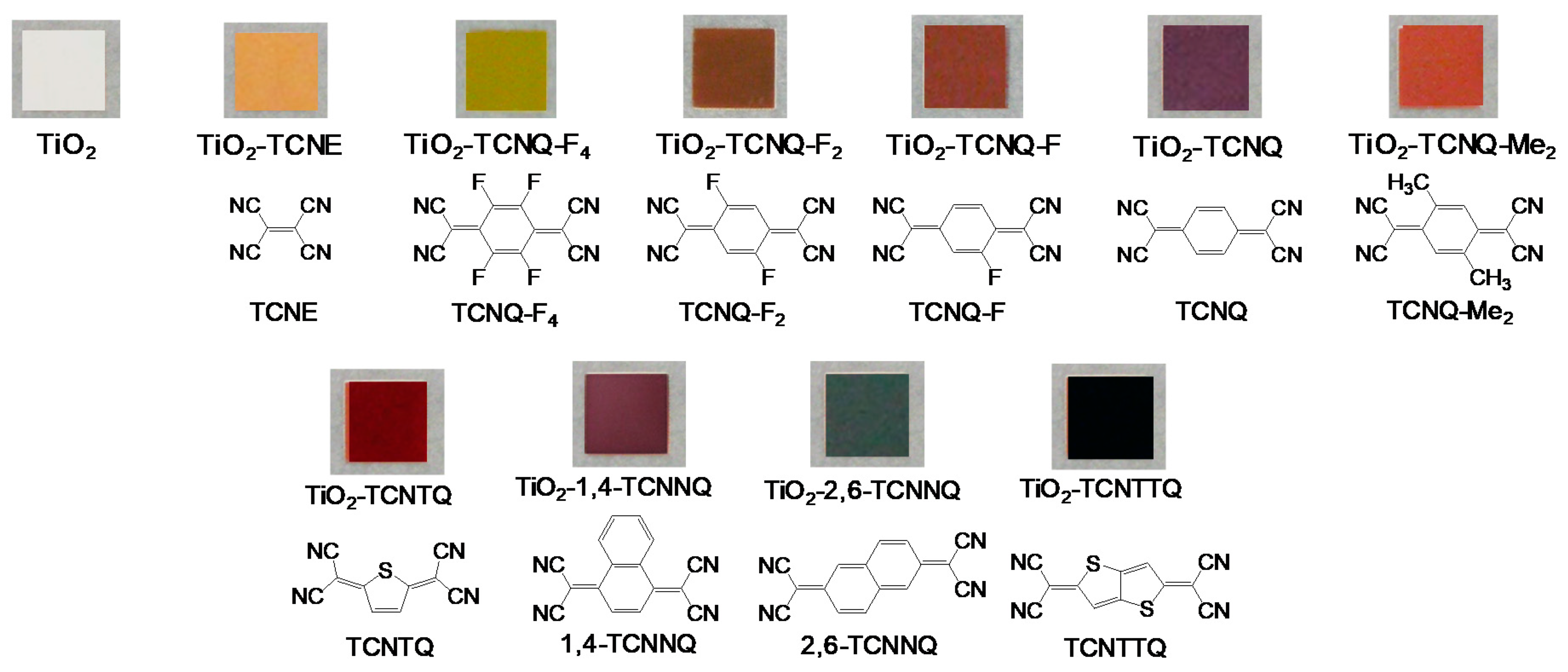
3. Suppression of Charge Recombination for Direct PCS Photovoltaics
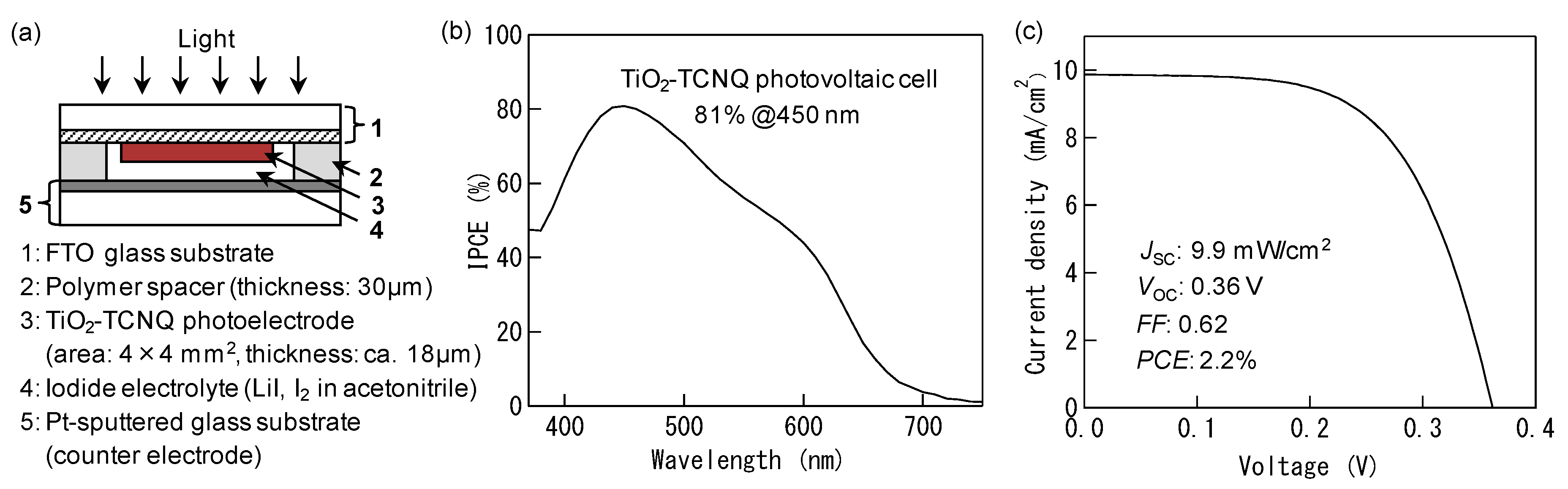
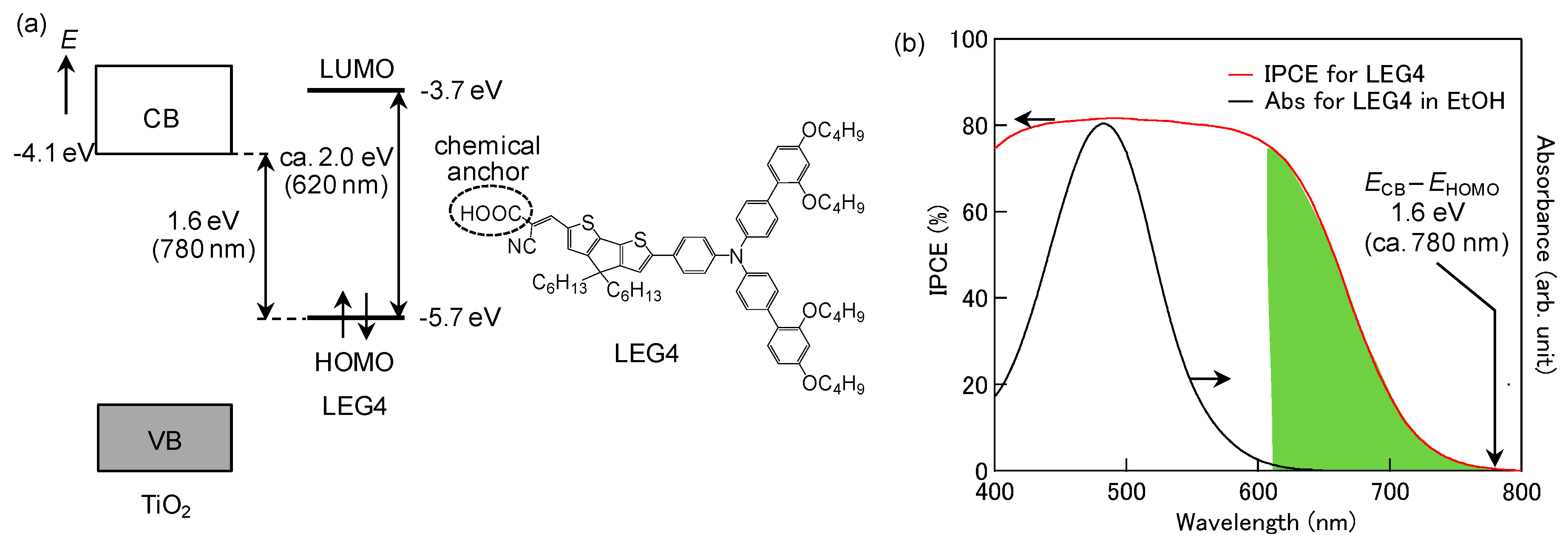
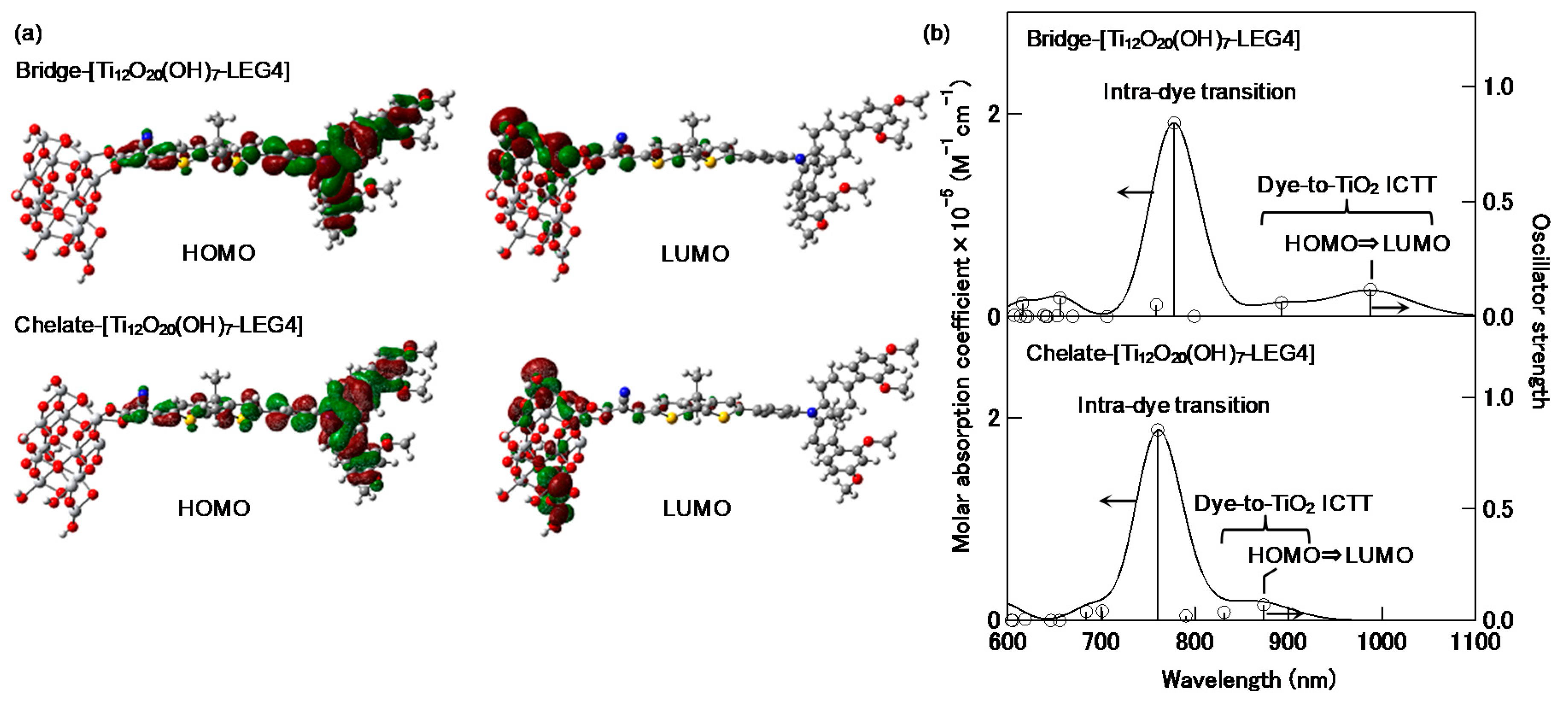
4. Photovoltaic Conversion Based on ICTT in DSSC
5. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Fujisawa, J.; Nagata, M. Efficient Light-to-Current Conversion by Organic−Inorganic Interfacial Charge-Transfer Transitions in TiO2 Chemically Adsorbed with 2-Anthroic Acid. Chem. Phys. Lett. 2015, 619, 180–184. [Google Scholar] [CrossRef]
- Fujisawa, J. Large Impact of Reorganization Energy on Photovoltaic Conversion due to Interfacial Charge-Transfer Transitions. Phys. Chem. Chem. Phys. 2015, 17, 12228–12237. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, J.; Hanaya, M. Light Harvesting and Direct Electron Injection by Interfacial Charge-Transfer Transitions between TiO2 and Carboxy-Anchor Dye LEG4 in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2018, 122, 8–15. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Grätzel, M. Light-Induced Redox Reactions in Nanocrystalline Systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-Sensitized Solar Cells for Efficient Power Generation Under Ambient Lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Desta, M.B.; Vinh, N.S.; Kumar, C.H.P.; Chaurasia, S.; Wu, W.-T.; Lin, J.T.; Wei, T.-C.; Diau, E.W.-G. Pyrazine-Incorporating Panchromatic Sensitizers for Dye Sensitized Solar Cells Under One Sun and Dim Light. J. Mater. Chem. A 2018, 6, 13778–13789. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kuo, T.Y.; Huang, C.W.; Jian, Z.H.; Hsiao, P.T.; Wang, C.L.; Lin, J.C.; Chen, C.Y.; Chen, C.H.; Tung, Y.L.; et al. Thermal and Angular Dependence of Next-Generation Photovoltaics Under Indoor Lighting. Prog. Photovolt. Res. Appl. 2019, 28, 1–11. [Google Scholar] [CrossRef]
- Michaels, H.; Rinderle, M.; Freitag, R.; Benesperi, I.; Edvinsson, T.; Socher, R.; Gagliardi, A.; Freitag, M. Dye-Sensitized Solar Cells Under Ambient Light Powering Machine Learning: Towards Autonomous Smart Sensors for the Internet of Things. Chem. Sci. 2020, 11, 2895–2906. [Google Scholar] [CrossRef]
- Horiuchi, T.; Fujisawa, J.; Uchida, S.; Grätzel, M. Data Book on Dye-Sensitized Solar Cells; CMC Publishing Co.: Tokyo, Japan, 2009. [Google Scholar]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)–Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-Sensitized Solar Cells with 13% Efficiency Achieved Through the Molecular Engineering of Porphyrin Sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Otsuka, T.; Kyômen, T.; Unno, M.; Hanaya, M. An Achievement of Over 12 Percent Efficiency in an Organic Dye-Sensitized Solar Cell. Chem. Commun. 2014, 50, 6379–6381. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.; Hanaya, M. Highly-Efficient Dye-Sensitized Solar Cells with Collaborative Sensitization by Silyl-Anchor and Carboxy-Anchor Dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Osada, H.; Aoyama, Y.; Yano, T.; Oya, K.; Iwamoto, S.; Fujisawa, J.; Hanaya, M. Achievement of Over 1.4 V Photovoltage in a Dye-Sensitized Solar Cell by the Application of a Silyl-Anchor Coumarin Dye. Sci. Rep. 2016, 6, 35888. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sun, D.; Cao, Y.; Tsao, H.N.; Yuan, Y.; Zakeeruddin, S.M.; Wang, P.; Grätzel, M. A Stable Blue Photosensitizer for Color Palette of Dye-Sensitized Solar Cells Reaching 12.6% Efficiency. J. Am. Chem. Soc. 2018, 140, 2405–2408. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Wang, W.; Gurzadyan, G.G.; Li, J.; Li, X.; An, J.; Yu, Z.; Wang, H.; Cai, B.; et al. 13.6% Efficient Organic Dye-Sensitized Solar Cells by Minimizing Energy Losses of the Excited State. ACS Energy Lett. 2019, 4, 943–951. [Google Scholar] [CrossRef]
- Ji, J.-M.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% Efficiency Dye-Sensitized Solar Cells by Co-Sensitizing Novel Thieno[3,2-b]indole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10, 2000124. [Google Scholar] [CrossRef]
- Cao, Y.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.-E.; Freitag, M.; et al. 11% Efficiency Solid-State Dye-Sensitized Solar Cells with Copper(II/I) Hole Transport Materials. Nat. Commun. 2017, 8, 15390. [Google Scholar] [CrossRef]
- Benesperi, I.; Michaels, H.; Freitag, M. The Researcher’s Guide to Solid-State Dye-Sensitized Solar Cells. J. Mater. Chem. C 2018, 6, 11903–11942. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Bahng, H.W.; Cao, Y.; Yi, C.; Saygili, Y.; Luo, J.; Liu, Y.; Kavan, L.; Moser, J.-E.; et al. Comprehensive Control of Voltage Loss Enables 11.7% Efficient Solid-State Dye-Sensitized Solar Cells. Energy Environ. Sci. 2018, 11, 1779–1787. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Ho-Baillie, A.W.Y. Solar Cell Efficiency Tables (Version 55). Prog. Photovolt. Res. Appl. 2020, 28, 3–15. [Google Scholar] [CrossRef]
- Katoh, R.; Furube, A.; Hara, K.; Murata, S.; Sugihara, H.; Arakawa, H.; Tachiya, M. Efficiencies of Electron Injection from Excited Sensitizer Dyes to Nanocrystalline ZnO Films as Studied by Near-IR Optical Absorption of Injected Electrons. J. Phys. Chem. B 2002, 106, 12957–12964. [Google Scholar] [CrossRef]
- Hara, K.; Sato, T.; Katoh, R.; Furube, A.; Ohga, Y.; Shinpo, A.; Suga, S.; Sayama, K.; Sugihara, H.; Arakawa, H. Molecular Design of Coumarin Dyes for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. B 2003, 107, 597–606. [Google Scholar] [CrossRef]
- Katoh, R.; Furube, A.; Yoshihara, T.; Hara, K.; Fujihashi, G.; Takano, S.; Murata, S.; Arakawa, H.; Tachiya, M. Efficiencies of Electron Injection from Excited N3 Dye into Nanocrystalline Semiconductor (ZrO2, TiO2, ZnO, Nb2O5, SnO2, In2O3) Films. J. Phys. Chem. B 2004, 108, 4818–4822. [Google Scholar] [CrossRef]
- Fujisawa, J.; Osawa, A.; Hanaya, M. A Strategy to Minimize the Energy Offset in Carrier Injection from Excited Dyes to Inorganic Semiconductors for Efficient Dye-Sensitized Solar Energy Conversion. Phys. Chem. Chem. Phys. 2016, 18, 22244–22253. [Google Scholar] [CrossRef]
- Houlding, V.H.; Grätzel, M. Photochemical Hydrogen Generation by Visible Light. Sensitization of Titanium Dioxide Particles by Surface Complexation with 8-Hydroxyquinoline. J. Am. Chem. Soc. 1983, 105, 5695–5696. [Google Scholar] [CrossRef]
- Moser, J.; Punchihewa, S.; Infelta, P.P.; Grätzel, M. Surface Complexation of Colloidal Semiconductors Strongly Enhances Interfacial Electron-Transfer Rates. Langmuir 1991, 7, 3012–3018. [Google Scholar] [CrossRef]
- Rajh, T.; Nedeljkovic, J.M.; Chen, L.X.; Poluektov, O.; Thurnauer, M.C. Improving Optical and Charge Separation Properties of Nanocrystalline TiO2 by Surface Modification with Vitamin C. J. Phys. Chem. B 1999, 103, 3515–3519. [Google Scholar] [CrossRef]
- Persson, P.; Bergström, R.; Lunell, S. Quantum Chemical Study of Photoinjection Processes in Dye-Sensitized TiO2 Nanoparticles. J. Phys. Chem. B 2000, 104, 10348–10351. [Google Scholar] [CrossRef]
- Tachikawa, T.; Tojo, S.; Fujitsuka, M.; Majima, T. Photocatalytic One-Electron Oxidation of Biphenyl Derivatives Strongly Coupled with the TiO2 Surface. Langmuir 2004, 20, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, W. Visible-Light-Induced Photocatalytic Degradation of 4-Chlorophenol and Phenolic Compounds in Aqueous Suspension of Pure Titania: Demonstrating the Existence of a Surface-Complex-Mediated Path. J. Phys. Chem. B 2005, 109, 5143–5149. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, J.; Matsumura, S.; Hanaya, M. A Single Ti-O-C Linkage Induces Interfacial Charge-Transfer Transitions between TiO2 and a π-Conjugated Molecule. Chem. Phys. Lett. 2016, 657, 172–176. [Google Scholar] [CrossRef]
- Fujisawa, J.; Eda, T.; Giorgi, G.; Hanaya, M. Visible-to-Near-IR Wide-Range Light Harvesting by Interfacial Charge-Transfer Transitions between TiO2 and p-Aminophenol and Evidence of Direct Electron Injection to the Conduction Band of TiO2. J. Phys. Chem. C 2017, 121, 18710–18716. [Google Scholar] [CrossRef]
- Sredojević, D.N.; Kovač, T.; Džunuzović, E.; Ðorđević, V.; Grgur, B.N.; Nedeljković, J.M. Surface-Modified TiO2 Powders with Phenol Derivatives: A Comparative DFT and Experimental Study. Chem. Phys. Lett. 2017, 686, 167–172. [Google Scholar] [CrossRef]
- Rodríguez, R.; Blesa, M.A.; Regazzoni, A.E. Surface Complexation at the TiO2(anatase)/Aqueous Solution Interface: Chemisorption of Catechol. J. Colloid Interface Sci. 1996, 177, 122–131. [Google Scholar] [CrossRef]
- Liu, Y.; Dadap, J.I.; Zimdars, D.; Eisenthal, K.B. Study of Interfacial Charge-Transfer Complex on TiO2 Particles in Aqueous Suspension by Second-Harmonic Generation. J. Phys. Chem. B 1999, 103, 2480–2486. [Google Scholar] [CrossRef][Green Version]
- Rajh, T.; Chen, L.X.; Lukas, K.; Liu, T.; Thurnauer, M.C.; Tiede, D.M. Surface Restructuring of Nanoparticles: An Efficient Route for Ligand−Metal Oxide Crosstalk. J. Phys. Chem. B 2002, 106, 10543–10552. [Google Scholar] [CrossRef]
- de la Garza, L.; Saponjic, Z.V.; Dimitrijevic, N.M.; Thurnauer, M.C.; Rajh, T. Surface States of Titanium Dioxide Nanoparticles Modified with Enediol Ligands. J. Phys. Chem. B 2006, 110, 680–686. [Google Scholar] [CrossRef]
- Murata, Y.; Hori, H.; Taga, A.; Tada, H. Surface Charge-Transfer Complex Formation of Catechol on Titanium(IV) Oxide and the Application to Bio-Sensing. J. Colloid Interface Sci. 2015, 458, 305–309. [Google Scholar] [CrossRef]
- Tennakone, K.; Kumara, G.R.R.A.; Kumarasinghe, A.R.; Sirimanne, P.M.; Wijayantha, K.G.U. Efficient Photosensitization of Nanocrystalline TiO2 Films by Tannins and Related Phenolic Substances. J. Photochem. Photobiol. A 1996, 94, 217–220. [Google Scholar] [CrossRef]
- Xagas, A.P.; Bernard, M.C.; Hugot-Le Goff, A.; Spyrellis, N.; Loizos, Z.; Falaras, P. Surface Modification and Photosensitisation of TiO2 Nanocrystalline Films with Ascorbic Acid. J. Photochem. Photobiol. A 2000, 132, 115–120. [Google Scholar] [CrossRef]
- Sirimanne, P.M.; Soga, T. Fabrication of a Solid-State Cell Using Vitamin C as Sensitizer. Sol. Energy Mater. Sol. Cells 2003, 80, 383–389. [Google Scholar] [CrossRef]
- Tae, E.L.; Lee, S.H.; Lee, J.K.; Yoo, S.S.; Kang, E.J.; Yoon, K.B. A Strategy to Increase the Efficiency of the Dye-Sensitized TiO2 Solar Cells Operated by Photoexcitation of Dye-to-TiO2 Charge-Transfer Bands. J. Phys. Chem. B 2005, 109, 22513–22522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hang, K.; Anderson, N.A.; Lian, T. Comparison of Electron Transfer Dynamics in Molecule-to-Nanoparticle and Intramolecular Charge Transfer Complexes. J. Phys. Chem. B 2003, 107, 9434–9440. [Google Scholar] [CrossRef]
- Rahal, R.; Daniele, S.; Hubert-Pfalzgraf, L.G.; Guyot-Ferréol, V.; Tranchant, J.-F. Synthesis of para-Amino Benzoic Acid–TiO2 Hybrid Nanostructures of Controlled Functionality by an Aqueous One-Step Process. Eur. J. Inorg. Chem. 2008, 980–987. [Google Scholar] [CrossRef]
- Thomas, A.G.; Jackman, M.J.; Wagstaffe, M.; Radtke, H.; Syres, K.; Adell, J.; Lévy, A.; Martsinovich, N. Adsorption Studies of p-Aminobenzoic Acid on the Anatase TiO2(101) Surface. Langmuir 2014, 30, 12306–12314. [Google Scholar] [CrossRef]
- Manzhos, S.; Kotsis, K. Computational Study of Interfacial Charge Transfer Complexes of 2-Anthroic Acid Adsorbed on a Titania Nanocluster for Direct Injection Solar Cells. Chem. Phys. Lett. 2016, 660, 69–75. [Google Scholar] [CrossRef]
- Kubo, T.; Fujisawa, J.; Segawa, H. 有機無機ハイブリッド太陽電池の新展開. Electrochemistry 2009, 2009, 977–980. [Google Scholar]
- Jono, R.; Fujisawa, J.; Segawa, H.; Yamashita, K. Theoretical Study of the Surface Complex between TiO2 and TCNQ Showing Interfacial Charge-Transfer Transitions. J. Phys. Chem. Lett. 2011, 2, 1167–1170. [Google Scholar] [CrossRef]
- Manzhos, S.; Jono, R.; Yamashita, K.; Fujisawa, J.; Nagata, M.; Segawa, H. Study of Interfacial Charge Transfer Bands and Electron Recombination in the Surface Complexes of TCNE, TCNQ, and TCNAQ with TiO2. J. Phys. Chem. C 2011, 115, 21487–21493. [Google Scholar] [CrossRef]
- Jono, R.; Fujisawa, J.; Segawa, H.; Yamashita, K. The Origin of the Strong Interfacial Charge-Transfer Absorption in the Surface Complex Between TiO2 and Dicyanomethylene Compounds. Phys. Chem. Chem. Phys. 2013, 15, 18584–18588. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, J.; Hanaya, M. Extremely Strong Organic–Metal Oxide Electronic Coupling Caused by Nucleophilic Addition Reaction. Phys. Chem. Chem. Phys. 2015, 17, 16285–16293. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, J.; Nagata, M.; Hanaya, M. Charge-Transfer Complex versus σ-Complex Formed Between TiO2 and Bis(dicyanomethylene) Electron Acceptors. Phys. Chem. Chem. Phys. 2015, 17, 27343–27356. [Google Scholar] [CrossRef]
- Fujisawa, J.; Hanaya, M. Electronic Structures of TiO2-TCNE, -TCNQ, and -2,6-TCNAQ Surface Complexes Studied by Ionization Potential Measurements and DFT Calculations: Mechanism of the Shift of Interfacial Charge-Transfer Bands. Chem. Phys. Lett. 2016, 653, 11–16. [Google Scholar] [CrossRef]
- Bledowski, M.; Wang, L.; Ramakrishnan, A.; Khavryuchenko, O.V.; Khavryuchenko, V.D.; Ricci, P.C.; Strunk, J.; Cremer, T.; Kolbeck, C.; Beranek, R. Visible-light photocurrent response of TiO 2–polyheptazine hybrids: Evidence for interfacial charge-transfer absorption. Phys. Chem. Chem. Phys. 2011, 13, 21511–21519. [Google Scholar] [CrossRef]
- Pitre, S.P.; Yoon, T.P.; Scaiano, J.C. Titanium Dioxide Visible Light Photocatalysis: Surface Association Enables Photocatalysis with Visible Light Irradiation. Chem. Commun. 2017, 53, 4335–4338. [Google Scholar] [CrossRef]
- Fujisawa, J. Interfacial Charge-Transfer Transitions Between TiO2 and Indole. Chem. Phys. Lett. 2020, 739, 136974. [Google Scholar] [CrossRef]
- Fujisawa, J.; Muroga, R.; Hanaya, M. Interfacial Charge-Transfer Transitions in a TiO2-Benzenedithiol Complex with Ti−S−C Linkages. Phys. Chem. Chem. Phys. 2015, 17, 29867–29873. [Google Scholar] [CrossRef]
- Fujisawa, J.; Muroga, R.; Hanaya, M. Interfacial Charge-Transfer Transitions and Reorganization Energies in Sulfur-Bridged TiO2-x-Benzenedithiol Complexes (x: o, m, p). Phys. Chem. Chem. Phys. 2016, 18, 22286–22292. [Google Scholar] [CrossRef]
- Fujisawa, J.; Eda, T.; Hanaya, M. Interfacial Charge-Transfer Transitions in BaTiO3 Nanoparticles Adsorbed with Catechol. J. Phys. Chem. C 2016, 120, 21162–21168. [Google Scholar] [CrossRef]
- Eda, T.; Fujisawa, J.; Hanaya, M. Inorganic Control of Interfacial Charge-Transfer Transitions in Catechol-Functionalized Titanium Oxides Using SrTiO3, BaTiO3, and TiO2. J. Phys. Chem. C 2018, 122, 16216–16220. [Google Scholar] [CrossRef]
- Fujisawa, J.; Kaneko, N.; Hanaya, M. Interfacial Charge-Transfer Transitions in ZnO Induced Exclusively by Adsorption of Aromatic Thiols. Chem. Commun. 2020, 56, 4090–4093. [Google Scholar] [CrossRef] [PubMed]
- Varaganti, S.; Ramakrishna, G. Dynamics of Interfacial Charge Transfer Emission in Small Molecule Sensitized TiO2 Nanoparticles: Is It Localized or Delocalized? J. Phys. Chem. C 2010, 114, 13917–13925. [Google Scholar] [CrossRef]
- Kim, G.; Choi, W. Charge-Transfer Surface Complex of EDTA-TiO2 and its Effect on Photocatalysis Under Visible Light. Appl. Catal. B 2010, 100, 77–83. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.-H.; Choi, W. Glucose–TiO2 Charge Transfer Complex-Mediated Photocatalysis Under Visible Light. Appl. Catal. B 2015, 162, 463–469. [Google Scholar] [CrossRef]
- Seo, Y.S.; Lee, C.; Lee, K.H.; Yoon, K.B. 1:1 and 2:1 Charge-Transfer Complexes Between Aromatic Hydrocarbons and Dry Titanium Dioxide. Angew. Chem. Int. Ed. 2005, 44, 910–913. [Google Scholar] [CrossRef]
- Haeldermans, I.; Vandewal, K.; Oosterbaan, W.D.; Gadisa, A.; D’Haen, J.; Van Bael, M.K.; Manca, J.V.; Mullens, J. Ground-State Charge-Transfer Complex Formation in Hybrid Poly(3-hexyl thiophene):Titanium Dioxide Solar Cells. Appl. Phys. Lett. 2008, 93, 223302. [Google Scholar] [CrossRef]
- Asahi, R.; Taga, Y.; Mannstadt, W.; Freeman, A.J. Electronic and Optical Properties of Anatase TiO2. Phys. Rev. B 2000, 61, 7459–7465. [Google Scholar] [CrossRef]



| Inorganic Semiconductor | Organic Compound | Bridging Atom | Year | |
|---|---|---|---|---|
| TiO2 | Aromatic hydroxy compound | Mono-hydroxy compound | O | 1983 |
| TiO2 | Catechol | O | 1991 | |
| TiO2 | Ascorbic acid | O | 1999 | |
| TiO2 | Aromatic carboxylic acid | O | 2008 | |
| TiO2 | Bis(dicyanomethylene) compound | O | 2009 | |
| TiO2 | Aromatic amine | N | 2011 | |
| TiO2 | Benzenedithiol | S | 2015 | |
| BaTiO3 | Catechol | O | 2016 | |
| SrTiO3 | Catechol | O | 2018 | |
| ZnO | Benzenethiol | S | 2020 | |
| Organic-Metal Oxide Complex | IPCE Maximum Value |
|---|---|
| TiO2-AA | 5% |
| TiO2-AA | 12% |
| TiO2-CA | 9% |
| TiO2-TCNQ | 81% |
| TiO2-ATCA | 86% |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, J.-i. Interfacial Charge-Transfer Transitions for Direct Charge-Separation Photovoltaics. Energies 2020, 13, 2521. https://doi.org/10.3390/en13102521
Fujisawa J-i. Interfacial Charge-Transfer Transitions for Direct Charge-Separation Photovoltaics. Energies. 2020; 13(10):2521. https://doi.org/10.3390/en13102521
Chicago/Turabian StyleFujisawa, Jun-ichi. 2020. "Interfacial Charge-Transfer Transitions for Direct Charge-Separation Photovoltaics" Energies 13, no. 10: 2521. https://doi.org/10.3390/en13102521
APA StyleFujisawa, J.-i. (2020). Interfacial Charge-Transfer Transitions for Direct Charge-Separation Photovoltaics. Energies, 13(10), 2521. https://doi.org/10.3390/en13102521





