Magnetron Sputtered Electron Blocking Layer as an Efficient Method to Improve Dye-Sensitized Solar Cell Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. RF Magnetron Sputtered Electron Blocking Layer Preparation
2.2. Dye-Sensitized Solar Cell Fabrication
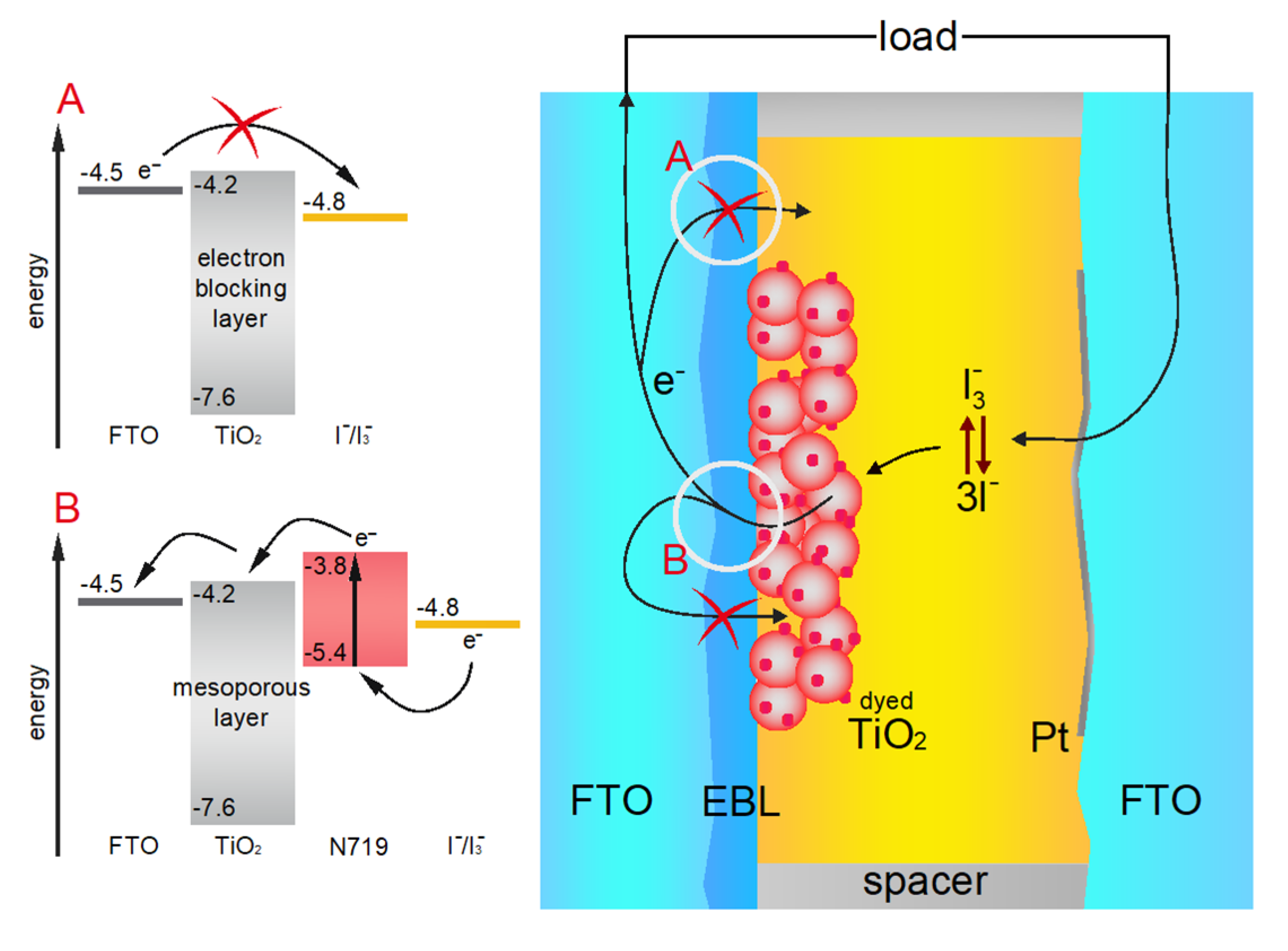
3. Results and Discussion
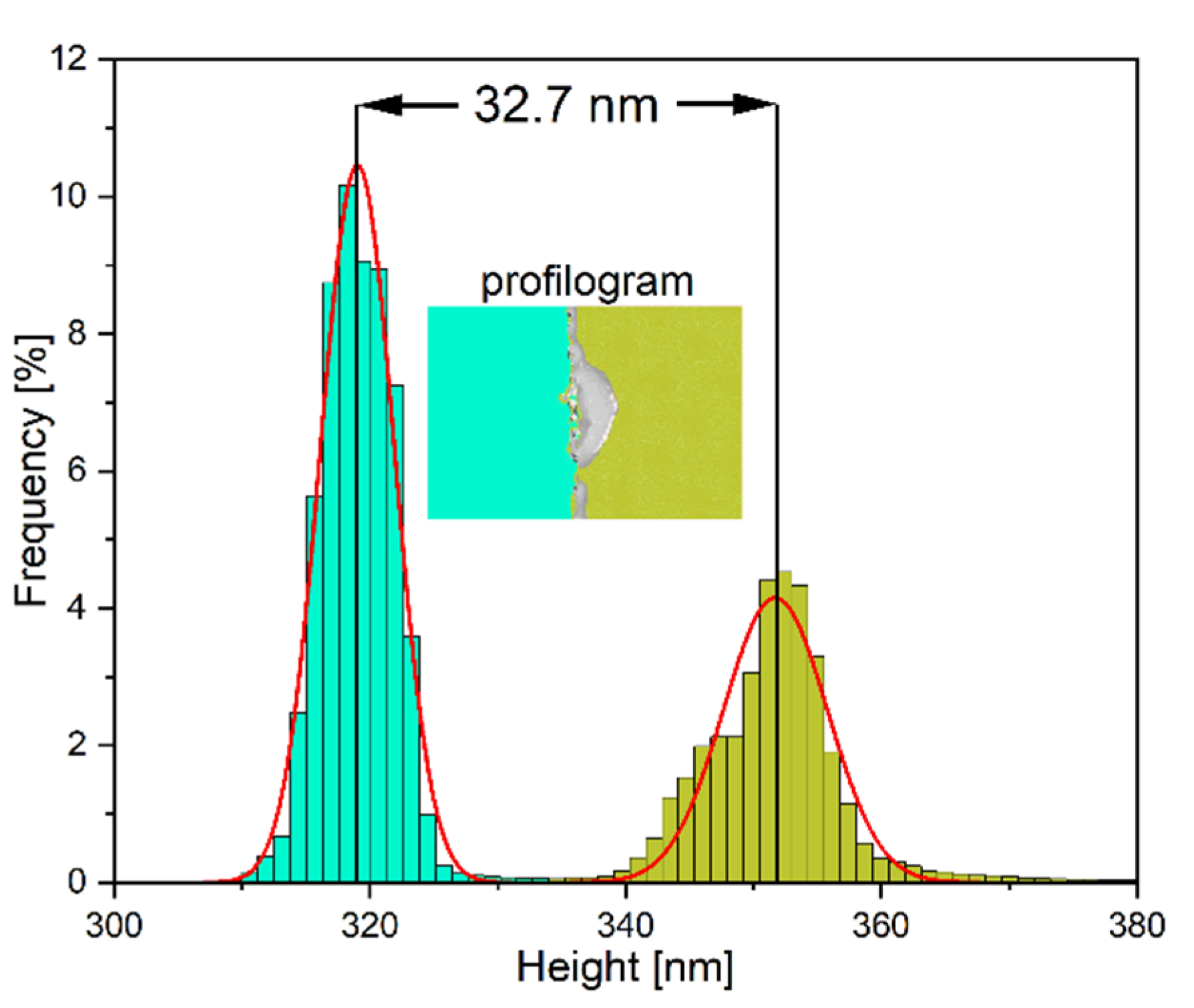
3.1. Measurement of TiO2 Electron Blocking Layer Thickness
3.2. Energy Dispersive Spectroscopy (EDS) Spectrum
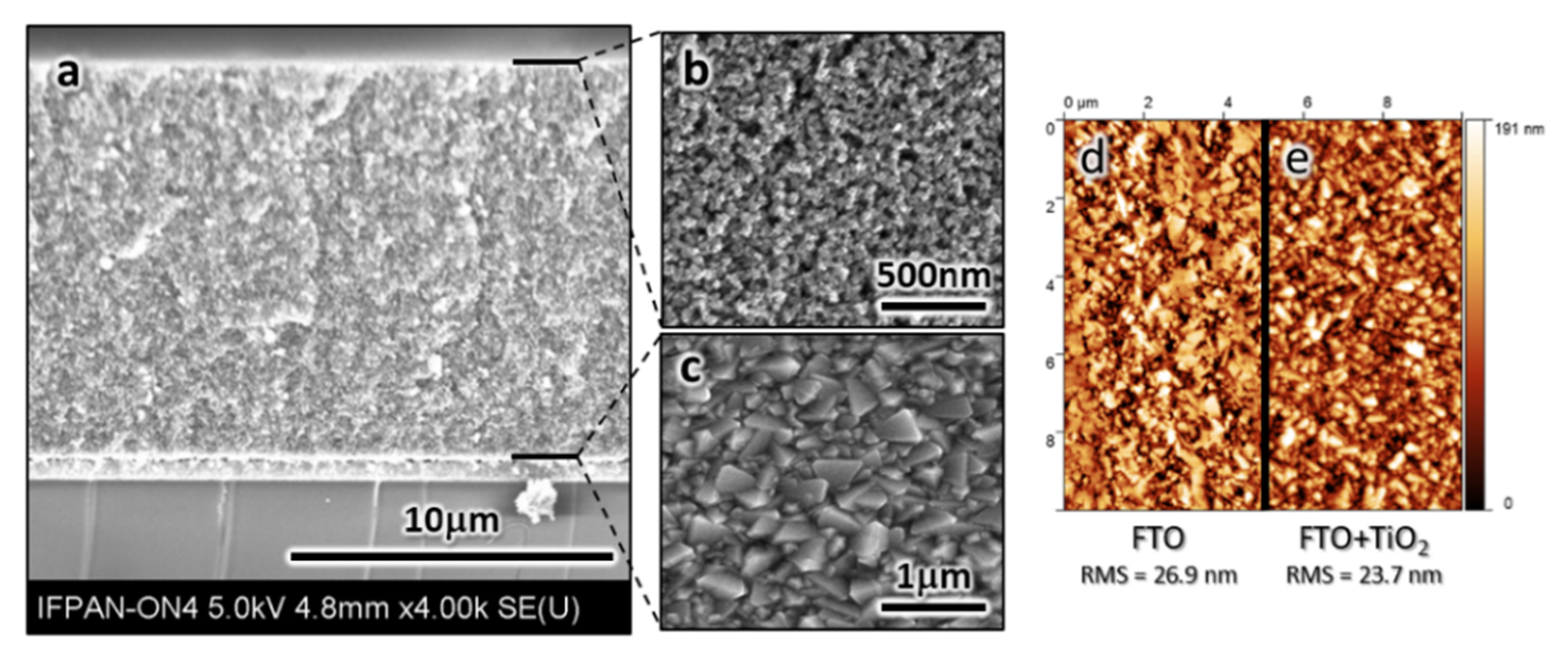
3.3. Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM) Images
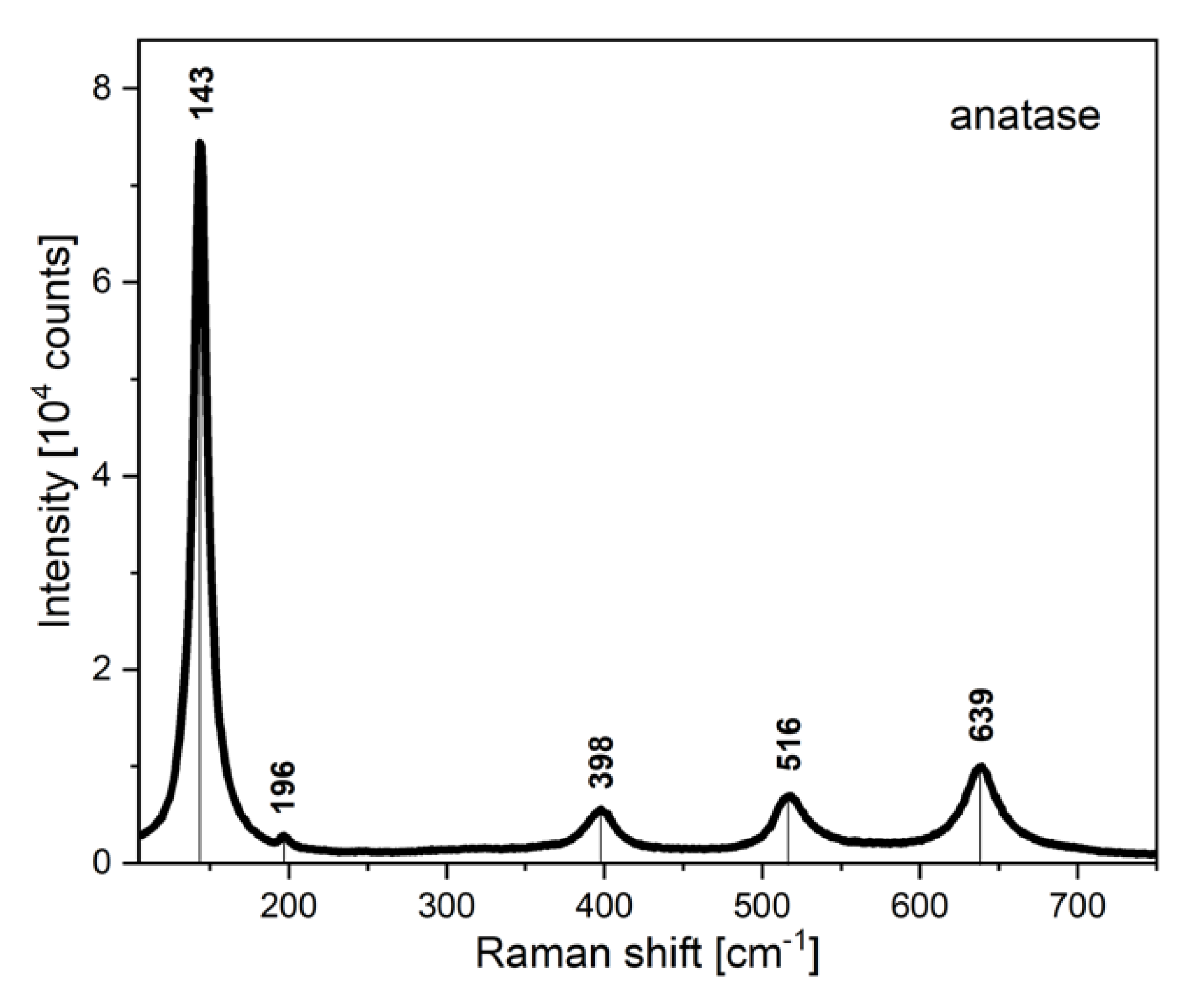
3.4. Raman Spectrum of Electron Blocking Layer
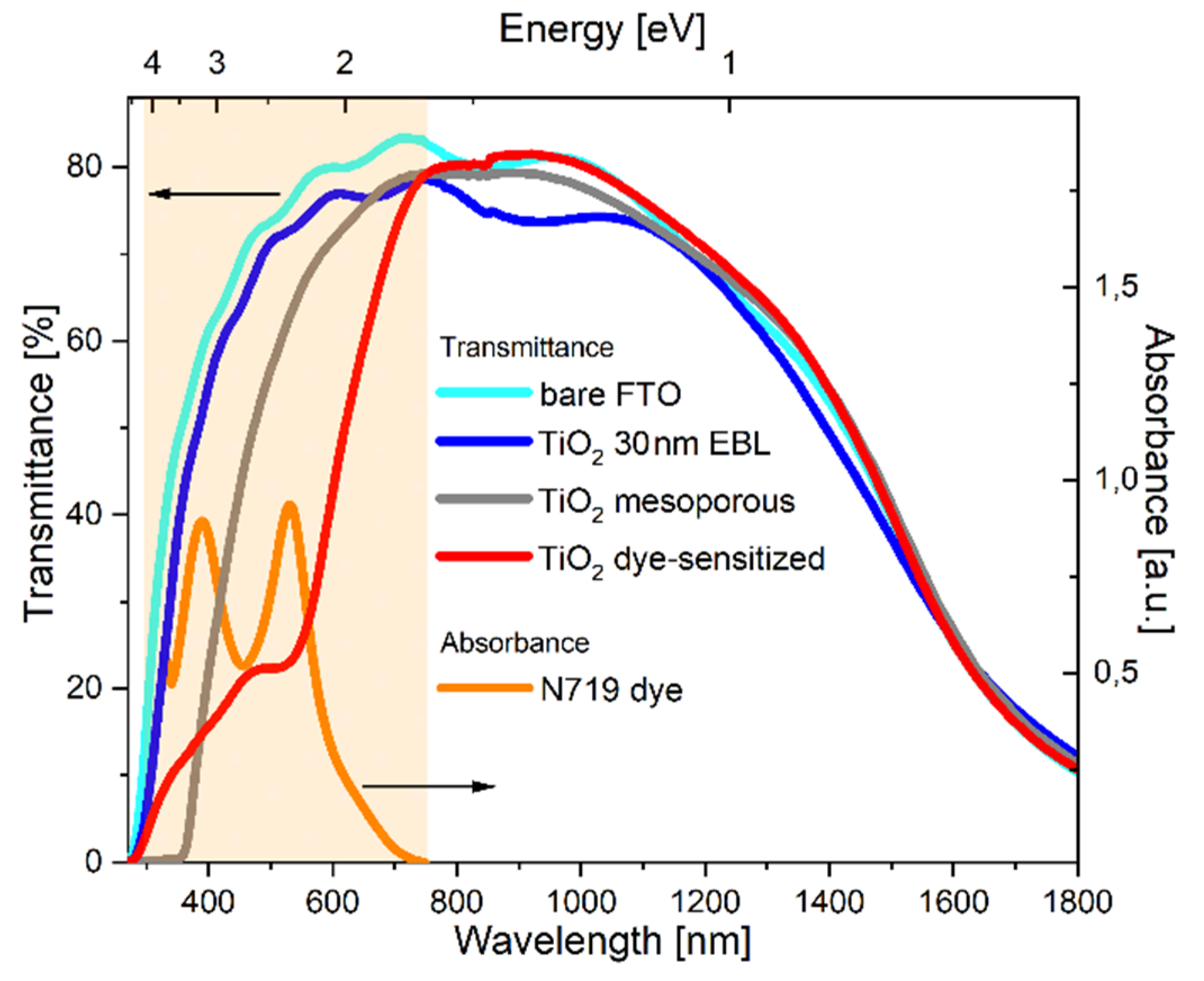
3.5. Transmittance and Absorbance Spectra of the Photoanode
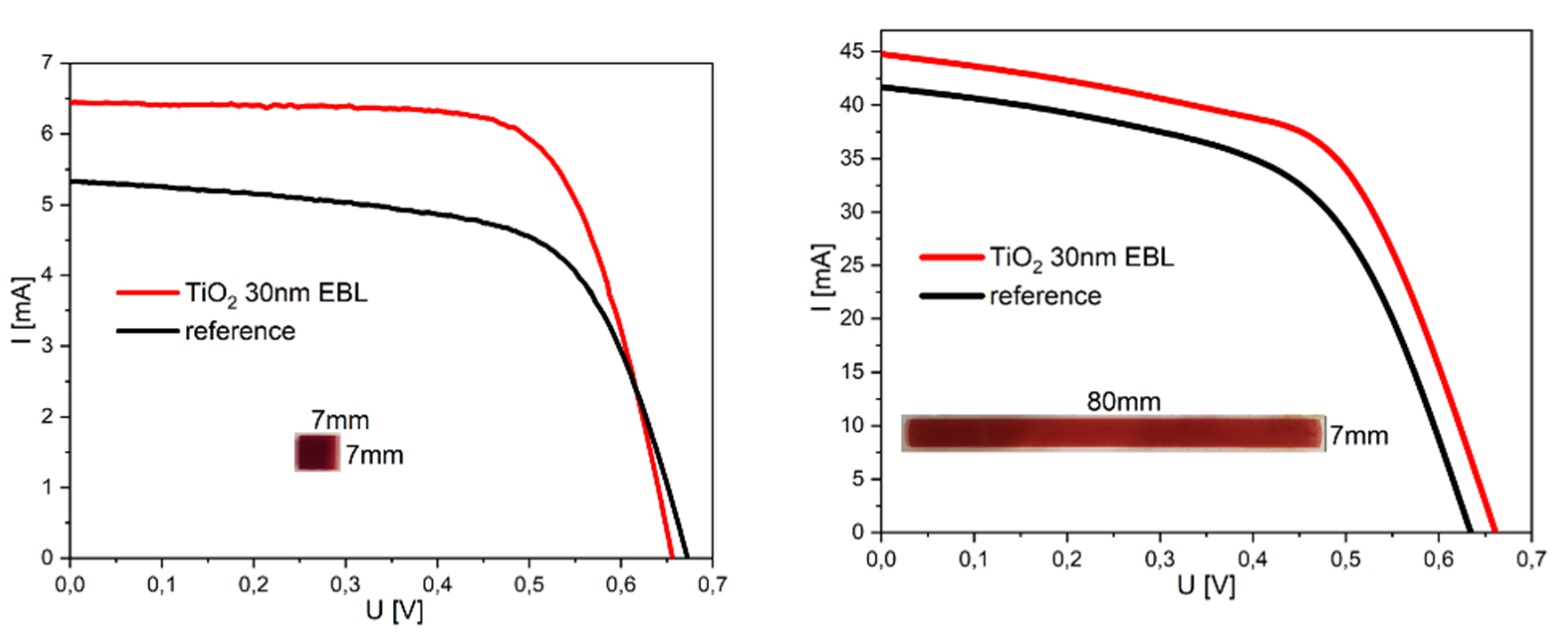
3.6. Performance of the DSSC Devices
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M.; Gr, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Qina, Y.; Peng, Q. Ruthenium Sensitizers and Their Applications in Dye-Sensitized Solar Cells. Int. J. Photoenergy 2012, 2012, 1–21. [Google Scholar] [CrossRef]
- Urbani, M.; Ragoussi, M.-E.; Nazeeruddin, M.K.; Torres, T. Phthalocyanines for dye-sensitized solar cells. Coord. Chem. Rev. 2019, 381, 1–64. [Google Scholar] [CrossRef]
- Maddah, H.A.; Berry, V.; Behura, S.K. Biomolecular photosensitizers for dye-sensitized solar cells: Recent developments and critical insights. Renew. Sustain. Energy Rev. 2020, 121, 109678. [Google Scholar] [CrossRef]
- Thomas, S.; Kalarikkal, N.; Wu, J.; Sakho, E.H.M.; Oluwafemi, S.O. Nanomaterials for Solar Cell Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-012813337-8. [Google Scholar] [CrossRef]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P. Progress on Electrolytes Development in Dye-Sensitized Solar Cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef]
- Bella, F.; Gerbaldi, C.; Barolo, C.; Grätzel, M. Aqueous dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3431–3473. [Google Scholar] [CrossRef]
- Wu, J.H.; Lan, Z.; Lin, J.M.; Huang, M.L.; Hao, S.C.; Sato, T.; Yin, S. A Novel Thermosetting Gel Electrolyte for Stable Quasi-Solid-State Dye-Sensitized Solar Cells. Adv. Mater. 2007, 19, 4006–4011. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Lu, C.-Y.; Chen, M.-C.; Huang, T.-W.; Wu, C.-C.; Chung, Y.-W. Efficient gel-state dye-sensitized solar cells adopting polymer gel electrolyte based on poly(methyl methacrylate). Org. Electron. 2013, 14, 3131–3137. [Google Scholar] [CrossRef]
- Yang, H.; Huang, M.; Wu, J.-H.; Lan, Z.; Hao, S.; Lin, J. The polymer gel electrolyte based on poly(methyl methacrylate) and its application in quasi-solid-state dye-sensitized solar cells. Mater. Chem. Phys. 2008, 110, 38–42. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Kavan, L. Electrochemistry and dye-sensitized solar cells. Curr. Opin. Electrochem. 2017, 2, 88–96. [Google Scholar] [CrossRef]
- Ramasamy, P.; Kang, M.-S.; Cha, H.-J.; Kim, J. Highly efficient dye-sensitized solar cells based on HfO2 modified TiO2 electrodes. Mater. Res. Bull. 2013, 48, 79–83. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, X.; Chen, S.; Lin, L.; Zhang, B.; Feng, Y. Performance improvement of dye-sensitized solar cells by introducing a hierarchical compact layer involving ZnO and TiO2 blocking films. Ceram. Int. 2014, 40, 15199–15206. [Google Scholar] [CrossRef]
- Shanmugam, M.; Baroughi, M.F.; Galipeau, D. Effect of atomic layer deposited ultra thin HfO2 and Al2O3 interfacial layers on the performance of dye sensitized solar cells. Thin Solid Films 2010, 518, 2678–2682. [Google Scholar] [CrossRef]
- Tan, B.; Toman, E.; Li, Y.; Wu, Y. Zinc Stannate (Zn2SnO4) Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2007, 129, 4162–4163. [Google Scholar] [CrossRef]
- Tennakone, K.; Bandara, J.; Bandaranayake, P.K.M.; Kumara, G.; Konno, A. Enhanced Efficiency of a Dye-Sensitized Solar Cell Made from MgO-Coated Nanocrystalline SnO2. Jpn. J. Appl. Phys. 2001, 40, L732–L734. [Google Scholar] [CrossRef]
- Arifin, Z.; Suyitno, S.; Hadi, S.; Sutanto, B. Improved Performance of Dye-Sensitized Solar Cells with TiO2 Nanoparticles/Zn-Doped TiO2 Hollow Fiber Photoanodes. Energies 2018, 11, 2922. [Google Scholar] [CrossRef]
- Zhu, K.; Schiff, E.; Park, N.-G.; Van De Lagemaat, J.; Frank, A.J. Determining the locus for photocarrier recombination in dye-sensitized solar cells. Appl. Phys. Lett. 2002, 80, 685–687. [Google Scholar] [CrossRef][Green Version]
- Gu, Z. (Allison); Gao, X.; Li, X.-M.; Jiang, Z.-W.; Huang, Y.-D. Nanoporous TiO2 aerogel blocking layer with enhanced efficiency for dye-sensitized solar cells. J. Alloy. Compd. 2014, 590, 33–40. [Google Scholar] [CrossRef]
- Kim, K.E.; Jang, S.-R.; Park, J.; Vittal, R.; Kim, K.-J. Enhancement in the performance of dye-sensitized solar cells containing ZnO-covered TiO2 electrodes prepared by thermal chemical vapor deposition. Sol. Energy Mater. Sol. Cells 2007, 91, 366–370. [Google Scholar] [CrossRef]
- Ito, S.; Liska, P.; Comte, P.; Charvet, R.; Pechy, P.; Bach, U.; Schmidt-Mende, L.; Zakeeruddin, S.M.; Kay, A.; Nazeeruddin, M.K.; et al. Control of dark current in photoelectrochemical (TiO2/I--I3-) and dye-sensitized solar cells. Chem. Commun. 2005, 34, 4351–4353. [Google Scholar] [CrossRef]
- Jang, K.-I.; Hong, E.; Kim, J.H. Effect of an electrodeposited TiO2 blocking layer on efficiency improvement of dye-sensitized solar cell. Korean J. Chem. Eng. 2012, 29, 356–361. [Google Scholar] [CrossRef]
- Meng, L.; Li, C. Blocking Layer Effect on Dye-Sensitized Solar Cells Assembled with TiO2 Nanorods Prepared by dc Reactive Magnetron Sputtering. Nanosci. Nanotechnol. Lett. 2011, 3, 181–185. [Google Scholar] [CrossRef][Green Version]
- Kment, Š.; Krýsová, H.; Hubicka, Z.; Kmentová, H.; Kavan, L.; Zbořil, R. Very thin thermally stable TiO 2 blocking layers with enhanced electron transfer for solar cells. Appl. Mater. Today 2017, 9, 122–129. [Google Scholar] [CrossRef]
- Duong, T.-T.; Choi, H.-J.; He, Q.-J.; Le, A.-T.; Gil Yoon, S. Enhancing the efficiency of dye sensitized solar cells with an SnO2 blocking layer grown by nanocluster deposition. J. Alloy. Compd. 2013, 561, 206–210. [Google Scholar] [CrossRef]
- Yeoh, M.-E.; Chan, K.Y. Efficiency Enhancement in Dye-Sensitized Solar Cells with ZnO and TiO2 Blocking Layers. J. Electron. Mater. 2019, 48, 4342–4350. [Google Scholar] [CrossRef]
- Chun, J.H.; Kim, J.S. Comparison of different structures of niobium oxide blocking layer for dye-sensitized solar cells. J. Nanosci. Nanotechnol. 2014, 14, 6226–6230. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Nahm, C.; Moon, J.; Kim, C.; Nam, S.; Jung, D.-R.; Park, B. The effect of a blocking layer on the photovoltaic performance in CdS quantum-dot-sensitized solar cells. J. Power Sources 2011, 196, 10526–10531. [Google Scholar] [CrossRef]
- Dziedzic, J. Deposition methods of TiO2 on the glass and characterisation of conductive layer in DSSC. Elektronika Konstr. Technol. Zastos. 2018, 1, 8–10. [Google Scholar] [CrossRef]
- Choi, H.; Nahm, C.; Kim, J.; Moon, J.; Nam, S.; Jung, D.-R.; Park, B. The effect of TiCl4-treated TiO2 compact layer on the performance of dye-sensitized solar cell. Curr. Appl. Phys. 2012, 12, 737–741. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Katoh, R.; Yaguchi, K.; Furube, A. Effect of dye concentration on electron injection efficiency in nanocrystalline TiO2 films sensitized with N719 dye. Chem. Phys. Lett. 2011, 511, 336–339. [Google Scholar] [CrossRef]
- Katoh, R.; Fuke, N.; Furube, A.; Koide, N. Effect of dye coverage on photo-induced electron injection efficiency in N719-sensitized nanocrystalline TiO2 films. Chem. Phys. Lett. 2010, 489, 202–206. [Google Scholar] [CrossRef]
- Seo, H.; Son, M.-K.; Park, S.; Chebrolu, V.T.; Shiratani, M. The blocking effect of charge recombination by sputtered and acid-treated ZnO thin film in dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2012, 248, 50–54. [Google Scholar] [CrossRef]
- Voloshin, M.V.R.R.A. Components of Natural Photosynthetic Apparatus in Solar Cells. Appl. Photosynth. New Prog. 2016, 9, 161–188. [Google Scholar] [CrossRef]
- Kavan, L.; Zivcova, Z.V.; Zlamalova, M.; Zakeeruddin, S.M.; Grätzel, M. Electron-Selective Layers for Dye-Sensitized Solar Cells Based on TiO2 and SnO2. J. Phys. Chem. C 2020, 124, 6512–6521. [Google Scholar] [CrossRef]

| Element | C | O | Si | Sn | Ti | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Line | Kα1,2 | Kα1,2 | Kα1,2 | Ll | Lα1 | Lβ1 | Lβ2 | Lγ1 | Lα1,2 | Kα1,2 | Kβ1,3 |
| Energy | 0.27 | 0.52 | 1.74 | 3.05 | 3.44 | 3.67 | 3.91 | 4.13 | 0.39 | 4.51 | 4.93 |
| Sample Size [mm2] | Uoc [V] | Isc [mA] | Pmax [mW] | FF | η [%] |
|---|---|---|---|---|---|
| 7 × 7 | 0.67 | 5.33 | 2.29 | 0.64 | 4.67 |
| ±0.03 | ±0.06 | ±0.05 | ±0.02 | ±0.12 | |
| 7 × 7 with EBL | 0.66 | 6.44 | 2.97 | 0.70 | 6.07 |
| ±0.04 | ±0.09 | ±0.05 | ±0.02 | ±0.16 | |
| 7 × 80 | 0.63 | 41.67 | 14.75 | 0.55 | 2.62 |
| ±0.04 | ±2.18 | ±0.93 | ±0.05 | ±0.34 | |
| 7 × 80 with EBL | 0.66 | 44.80 | 17.30 | 0.58 | 3.06 |
| ±0.04 | ±2.28 | ±0.69 | ±0.04 | ±0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augustowski, D.; Kwaśnicki, P.; Dziedzic, J.; Rysz, J. Magnetron Sputtered Electron Blocking Layer as an Efficient Method to Improve Dye-Sensitized Solar Cell Performance. Energies 2020, 13, 2690. https://doi.org/10.3390/en13112690
Augustowski D, Kwaśnicki P, Dziedzic J, Rysz J. Magnetron Sputtered Electron Blocking Layer as an Efficient Method to Improve Dye-Sensitized Solar Cell Performance. Energies. 2020; 13(11):2690. https://doi.org/10.3390/en13112690
Chicago/Turabian StyleAugustowski, Dariusz, Paweł Kwaśnicki, Justyna Dziedzic, and Jakub Rysz. 2020. "Magnetron Sputtered Electron Blocking Layer as an Efficient Method to Improve Dye-Sensitized Solar Cell Performance" Energies 13, no. 11: 2690. https://doi.org/10.3390/en13112690
APA StyleAugustowski, D., Kwaśnicki, P., Dziedzic, J., & Rysz, J. (2020). Magnetron Sputtered Electron Blocking Layer as an Efficient Method to Improve Dye-Sensitized Solar Cell Performance. Energies, 13(11), 2690. https://doi.org/10.3390/en13112690






