Abstract
Lignocellulosic biomass pyrolysis could be an economically feasible option for forest management as it reduces the need to burn litter and helps in fire prevention thus avoiding the release of carbon dioxide and other greenhouse gases into the atmosphere. This study characterises wood vinegar (WV) obtained via a continuous fast pyrolysis process in terms of its composition, ageing and herbicidal properties. The aqueous WV fraction had a moisture content of 84% in weight and contained more than 200 compounds. Acetic acid, hydroxyacetaldehyde and hydroxyacetone were the major components. No significant differences were found in WV composition according to the starting material (poplar, pine, pruning litter, forest waste). No residual aromatic polycyclic compounds that could be harmful to the environment were detected. In a series of climate-controlled glass chamber experiments, the WV proved to be as effective an inhibitor of seed germination and seedling growth as a contact herbicide acting against weeds, especially through aerial contact. Sprayed WV concentrations of 50, 75 and 100 vol. % were effective against all plant species tested. This product could therefore be of commercial interest and help make biomass pyrolysis economically viable, once environmental exposure limits and the safe application for agricultural and urban use of this product have been established.
1. Introduction
Forest fires have a tremendous negative impact on the biodiversity of all Mediterranean regions. Fires may be caused by various factors such as global warming, but their spread is often determined by the build-up of biomass in forest areas. In the past thirty years, fires have led to the yearly burning of an average of 480,000 ha of forests in the European Union. Approximately 85% of the total surface area burnt by forest fires is found in the Mediterranean region. Besides devastating consequences on biodiversity, forest fires also cause the release of thousands of tons of CO2 and other greenhouse gases to the atmosphere, along with soil erosion and a loss in the recovery capacity of ecosystems. It is estimated that during a forest fire, 20% of biomass is transformed into greenhouse gases and 60% of carbon from the lignocellulosic waste fraction is converted into greenhouse gases [1].
Biomass waste pyrolysis was developed as a solution to these environmental problems. Biomass pyrolysis may help reduce the number of forest fires, as forests are better managed and this leads to reduced greenhouse gas emissions [2,3,4]. In addition, the co-products obtained via this process, such as wood vinegar, can be used to replace synthetic crop protection products, whose impacts on the environment and human health are still questionable. As an example, glyphosate, the most used herbicide worldwide, was classified in March 2015 by the International Agency for Research on Cancer (IARC) as “probably carcinogenic” for humans. Therefore, recent controversy over the effects of glyphosate on human and ecosystem health is opening up a market for any environmentally friendly herbicide to replace glyphosate.
Pyrolysis consists of the thermal degradation of waste in the total or partial absence of oxygen. The process consists of two stages described as primary and secondary pyrolysis. Primary pyrolysis involves devolatisation of the main components (dehydration, dehydrogenation and decarboxylation), while secondary pyrolysis includes the thermal or catalytic “cracking” of heavy compounds into gasses such as CO, CO2, CH4 and H2 [5,6,7]. During fast pyrolysis, biomass is rapidly heated at reaction temperatures of around 450–500 °C with short residence times of vapours inside the reactor, traditionally up to 2 s. While slow pyrolysis favours the production of char through the use of low heating rates and high residence times, fast pyrolysis promotes liquid yields [8,9,10,11].
Thus, derived products of pyrolysis have a non-condensable gaseous fraction designated by the term “syngas”; a liquid fraction that contains an organic fraction known as bio-oil and an aqueous fraction containing oxygenated compounds arising from the decomposition of cellulose and hemicellulose (wood vinegar); and a solid fraction composed of carbonised material, or char [12,13,14,15,16,17]. The wood vinegar is separated from the heavy and light organic fractions by decantation. The oil fraction (light organic fraction) is presently a topic of study and its upgrading process to be blended with fuels is being examined, while the heavy organic fraction is being assessed for use as bio-bitumen in the preparation of asphalt mixtures. This study, in turn, focuses on the aqueous fraction.
Pyroligneous acid, or wood vinegar, is composed mainly of water (80–90%) and more than 200 organic compounds, among which acids, alcohols, phenols, aldehydes and esters (10–20%) can be found. The main component is acetic acid. Its composition depends on the pyrolysis process used, on the moisture content of the feedstock, and on the type of biomass used, although, differences in this last case are usually scarce [18,19,20,21,22].
Wood vinegar obtained from biomass pyrolysis has applications in various areas, such as its use as a food additive, anti-inflammatory agent, anti-fungal drug or pest control agent [23] owing to its powerful antioxidant and antimicrobial activity [24,25,26,27,28]. There is also evidence of its use in agriculture as a plant fortifier promoting plant growth [29,30,31,32,33,34,35]. Other studies have shown that wood vinegar improves soil microbiota conditions [36]. However, only a few investigations have assessed the herbicidal properties of wood vinegar and most of these studies have examined effects on a single plant species [37,38,39,40]. The use of wood vinegar against Mediterranean or temperate weed species so far lacks support.
In the present study, the composition of wood vinegar produced by the fast pyrolysis of different raw materials was examined and its ageing over one year assessed. Its properties as a herbicide were also characterised by exploring its effects on the germination and growth of several weeds that are common in the western hemisphere. In addition, this study describes the complex chemical composition of wood vinegar derived from different biomass sources (poplar, pine and urban pruning waste) and assesses its possible use as a herbicide in trials conducted on both seedlings and fully developed plants.
The weed killing capacity of this bio-product of biomass pyrolysis is important as it will confer economic value to the bio-product. Examples of other biomass pyrolysis bio-products with market value are biochar, which is used as a permanent carbon store [41,42,43,44,45,46], bio-oil, used as a fuel [47,48,49], and bio-bitumen, used as an asphalt additive [50].
2. Materials and Methods
2.1. Production of Wood Vinegar
The feedstock used was pine, poplar, forest pine residues and urban pruning waste. 1 cm long wood chips were subjected to pyrolysis at the Universidad de Alcalá (Madrid, Spain) in a laboratory prototype including a screw-type reactor. Three cycles of N2/vacuum were used to generate an inert atmosphere. The corresponding biomass was treated at a temperature of 450 °C to 500 °C. The gases generated during the thermal decomposition of the biomass were passed through an ethanol-cooled system (at 5 °C) to produce liquid fractions (aqueous fraction and light and heavy organic fractions), and a non-condensable gas fraction. The aqueous fraction (wood vinegar) was separated from the light and heavy organic fractions by decantation.
The wood vinegar used in herbicide efficacy tests was prepared from poplar by Neoliquid Advanced Biofuels and Biochemicals S.L. (Guadalajara, Spain). This time, pyrolysis was conducted at an industrial thermal pyrolysis plant equipped with four screw-type reactors in series at a temperature of 450 °C to 500 °C. The gases generated by the thermal decomposition of the biomass were passed through a water-cooled system (at 15 °C) to obtain the liquid fractions (aqueous fraction and light and heavy organic fractions). The wood vinegar was separated from the heavy and light organic fractions by decantation.
2.2. GC/MS
The composition of wood vinegar was analysed by gas chromatography-mass spectrometry (GC-MS) according to Martín et al, 2017 [19]. The instrument used was a gas chromatograph (Agilent 7890 A, Santa Clara, CA, USA) coupled to a single quadrupole mass spectrometer (Agilent 5977 A) equipped with a ZB-624 column. The chromatography conditions were an oven initial temperature at 50 °C for 5 min, increased at 20 °C/min to 220 °C for 5 min. The injector temperature was 225 °C. Helium was the carrier gas at flow rate of 1 mL/min. The compounds with the largest area in wood vinegar samples (acetic acid, furfural, 1-hydroxy-2-propanone, hydroxyacetaldehyde and levoglucosan) were quantified by the GC-MS using the external standard method.
2.3. Moisture Content
The water content of the wood vinegar was calculated by Karl Fischer (KF) analysis using a Metrohm (Madrid, Spain)) apparatus. In the KF titration, a certain amount of sample was dissolved in methanol and titrated against KF reagent.
2.4. Climate-Controlled Glass Chamber
All trials in which seeds, seedlings and adult plants were used were conducted in a growth chamber (Ibercex SL, Madrid, Spain) with an internal circulation fan, illumination and inverter heating/cooling system. The chamber was also equipped with a humidity and temperature system and timer for light exposure control (plants were exposed to 12 h of light from a 58.5 W, 110V FL 6200K cool daylight lamp supplied by Philips, Amsterdam, North Holland, The Netherlands). Chamber temperature ranged from 19 °C to 24 °C, with no significant variations reported and a mean humidity between 50% and 60%.
2.5. Germination Tests
Seed coats of Acacia dealbata were mechanically removed to facilitate their germination. Twenty seeds were then placed in two Petri dishes on filter paper (10 seeds per plate). Next, the filter paper in one of the plates was soaked with water (control). The filter paper of the other plate was soaked with wood vinegar. Both plates were placed in the controlled climate chamber for three days. The effect of the wood vinegar on the seeds was assessed visually by comparing with the control
2.6. Seedling Tests
Seeds of Silibum marianum, Sinapis alba, Cardus tenuiflorus, Bromus madritensis, Bromus maximus and Hordeum murinum were germinated in Petri dishes on moistened filter paper in the controlled climate chamber. When specimens of each species reached an adequate size (30–60 mm), the filter paper in each plate was soaked with the corresponding wood vinegar solution in water (10.0, 5.0, 2.0, 1.0 and 0.5 vol. %) until saturation and introduced again in the chamber. The effects of different wood vinegar solutions on seedlings were visually assessed after 2 and 15 days of incubation. Results were compared by through binary logistic regression with a chi-square test. Differences with p < 0.05 were considered significant. The program used for all statistical tests was Statplus 7.1. (AnalystSoft Inc, Walnut, CA, USA).
2.7. Soil Irrigation Tests
Six trays with plants growing from the seed bank of 6 different soil samples obtained from a natural weed community were incubated in the chamber. These soils were collected from nitrophilous plant community areas at the Alcalá University campus to ensure the presence of a seed bank. These plant communities were identified in a previous study [51]. Some 30 days later when the plants had fully developed, wood vinegar solutions prepared in water were added to the soil (25 and 75 vol. %) using volumes in the range 20 to 60 mL depending on the plant density per pot (Table 1). The effect of the wood vinegar on adult plants was determined visually after 7 and 15 days by comparing with controls (soil irrigated with water).

Table 1.
Volumes of the two dilutions of wood vinegar (WV) used in the soil irrigation tests.
2.8. Spray Tests
Seeds of Anacyclus clavatus, Sinapis alba, Bromus madritensis and Bromus maximus, some of the most representative species of the nitrophilous vegetation of the Mediterranean region [52], were grown directly on soil in 24-pot seedling trays in the growth chamber. Each treatment was repeated in four different pots, with a total of 24 tests (8: control, 4: 25%vw, 4: 50%vw, 4: 75%vw and 4: 100%vw). For the four species, a total of 80 tests were carried out. Additionally, seeds of Acacia dealbata were subjected to mechanical removal of their seed coat before being planted in the soil. Once the plants were well-developed some 30 days after planting, they were sprayed with wood vinegar solutions prepared in water (25, 50, 75, 100 vol. %), using volumes between 10 and 50 mL to ensure that all individuals were soaked (Table 2). Treatments in a spray bottle were evenly applied on leaf surfaces (10–50 mL).

Table 2.
Volumes (mL) of wood vinegar used to spray the different adult plant species.
After spraying, the treated plants were introduced in the climate-controlled glass chamber where the pots received adequate watering and care. At the 10-day time point, mortality rates were calculated as the affected above-ground mass by comparing with controls (plants sprayed with water). Results were compared using non-parametric tests, the Kruskal Wallis test for the whole data set and the Mann Whitney U test for the comparison of paired means. Differences showing a p < 0.05 were considered significant.
3. Results and Discussion
3.1. Characterisation of Wood Vinegar
The samples analysed were the wood vinegar samples obtained through continuous fast pyrolysis at the Universidad de Alcalá, and the wood vinegar used as a herbicide supplied by Neoliquid Advanced Biofuels and Biochemicals S.L. Moisture contents were 70.66 ± 7.87 wt. % for wood vinegar samples obtained in the laboratory prototype and 89.60% for the industrial wood vinegar herbicide. Moisture values reported in the literature range from 72.4 wt. % for pyroligneous acid derived from pine and forest waste [53] to 81.44–93.48% obtained by Theapparat et al. for bamboo and nimtree, respectively [54].
pH values were 2.04 to 2.50 (2.23 ± 0.21) for the prototype samples and 2.62 for the industrial herbicide. Reported values have been 1.8–2.9 for wood vinegar from birch [20] and 2.2–2.4 for wood vinegar from forest waste and pine [53].
Gas chromatography results revealed scarce composition differences between wood vinegar samples. Although derived from different biomass feedstock (poplar, pine and woody species pruning litter), they all behaved similarly during the pyrolysis process. Cellulose degradation mainly gives rise to hydroxyacetaldehyde and other carboxylic compounds such as acids and ketones through fragmentation reactions, while levoglucosan and primary sugars form via depolymerisation reactions. In turn, hemicellulose decomposes in a similar way to generate volatile organic compounds, levoglucosan and other anhydrohexoses and furans [55,56]. Consequently, wood vinegar contains water-soluble compounds such as polyols, carboxylic acids and phenols, derived from the decomposition of cellulose and hemicellulose. The chromatogram obtained for the sample Poplar 1 is provided in Figure 1.
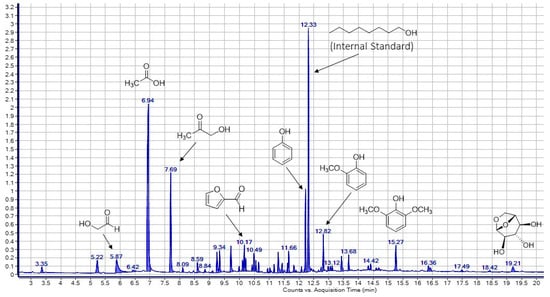
Figure 1.
Chromatogram of a wood vinegar sample derived from poplar.
The main compounds detected in the different samples of wood vinegar including the wood vinegar herbicide used in subsequent experiments assessing its herbicidal behaviour are shown in Table 3.

Table 3.
Chromatographic areas of major compounds identified in wood vinegar samples (given as % area). WV herbicide was that used in the experiments.
Proportions of compounds identified in the different wood vinegar samples were: 95.69% Poplar 1, 95.19% Poplar 2, 85.04% Pine 1, 87.27% Pine 2, 100% Forest pine, 98.36% Pruning litter and 94.75 WV herbicide. Remaining amounts up to 100% were coelutions and/or compounds identified with reverse match factor (RMF) probabilities lower than 700.
Regardless of the biomass feedstock, the main compounds present in all wood vinegar samples analysed were acetic acid, hydroxyacetone and hydroxyacetaldehyde. The presence of phenols, and their greater percentage areas in the samples obtained from poplar should also be mentioned. Fang et al. examined the fractionation of pyroligneous acid obtained from forest litter and pine. GC-MS analysis revealed relative contents of acetic acid and hydroxyacetone expressed as peak areas of 21.21% and 5.75%, respectively [53]. To complete the analysis of the wood vinegar samples, major components were quantified as percentage weights using the corresponding standard (Table 4).

Table 4.
Percentage weights of major compounds detected in the wood vinegar samples examined in this study.
The relative area of the peaks detected by GC/MS allows a quick estimation of the contribution of each compound in the composition of the samples, but it is not a quantitative parameter. For this reason and considering the peaks with the greater area observed, the majority compounds were quantified. No significant differences were detected between the samples obtained from different biomass feeds. Acetic acid concentrations ranged from 2.87 to 4.30 wt. %. Seo et al. reported an acetic acid concentration of 3.52 wt. % and 3.33 wt. % for wood vinegar derived from Quercus sp. and rice husk respectively [57]. For wood vinegar produced from tropical waste biomass, the concentration of this acid ranges between 32.49 and 70.60 mg/mL for eucalyptus E. camaldulensis and Hebea brasiliensis, respectively [54].
Percentage weights were 1.13–2.14 wt. % for hydroxyacetone and 0.70–3.47 wt. % for hydroxyacetaldehyde. Furfural and levoglucosan concentrations were generally below 1 wt. %: 0.08 to 0.31 wt. % and 0.08 to 1.05 wt. %, respectively. The concentration of acetic acid in the industrial wood vinegar herbicide was 2.87 wt. % and its moisture content was 89.60 wt. %, determining that this carboxylic acid accounts for 27.60% in weight of the organic compounds present in the wood vinegar. Although most composition studies work with relative area on the chromatogram [22,58,59,60], this is not an exact measure of the amount of compound present in the sample. Quantification of the components is necessary to determine the exact quantity of each compound [61,62].
3.2. Wood Vinegar Ageing
The wood vinegar produced was kept for one year in 5 litre bottles and then tested for acetic acid concentration, pH and moisture. Results after one year indicated that pH remained under 3, moisture content increased 4.51 wt. % and acetic acid content slightly decreased. Thus, no great changes were produced over time indicating their stability (Table 5).

Table 5.
Ageing of wood vinegar over a 12-month period.
3.3. Germination Test
Seeds of Acacia dealbata were used to examine the impacts of wood vinegar on seed germination. Results after three days of incubation in the growth chamber are provided in Figure 2. After 72 h in the growth chamber, the 10 seeds placed on filter paper soaked with water showed normal growth and development. In contrast, the seeds treated with the wood vinegar herbicide did not germinate, confirming its capacity to inhibit germination (chi squared p < 0.01). This is in line with results reported in the literature for seeds of other species treated with weaker solutions of wood vinegar. Mmojieje and Hornung reported the inhibitory role of a 10 vol. % wood vinegar solution in water on sunflower and tomato seeds [63]. Luo et al. also described this effect on capiscum seeds using higher wood vinegar concentrations and attributed it to the presence of phenols reducing the solution’s pH [23,64].

Figure 2.
Photographs showing the germination test conducted on Acacia dealbata seeds in two Petri dishes exposed to water (a) or wood vinegar (b) at the trial onset (1) and 72 h later (2). Photo: Juan Luis Aguirre.
3.4. Seedling Tests
The objective of these tests was to assess the herbicidal effects of wood vinegar diluted in water on the early development of seedlings and establish the minimum concentration of this effect. Table 6 shows the treatments used on each species and results obtained 2 and 15 days after treatment.

Table 6.
Effects on seedling growth of different concentrations of wood vinegar recorded at 48 h/15 days post-treatment.
In experiment 1, observations 48 h after treatment with the herbicide revealed 100% mortality in response to all three dilutions tested. As an example, Figure 3 shows the appearance of the Silibum marianum seedlings 48 h after treatment with the wood vinegar.
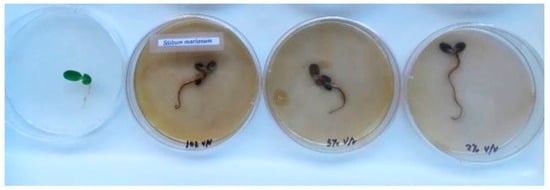
Figure 3.
Silibum marianum 48 h after treatment with (from left to right): 0 (control), 10, 5 and 2 vol. % wood vinegar. Photo: Juan Luis Aguirre.
In view of these findings, in a second experiment, lower concentrations of the herbicide (1.0 and 0.5 vol. %) were tested along with the 2.0 vol. % dilution. Observations 48 h and 15 days after treatment are shown in Table 7.

Table 7.
Effects on seedling growth of different concentrations of wood vinegar recorded at 48 h/15 days post-treatment.
Unlike the previous observations, in Experiment 2, treated seedlings remained alive 48 h after treatment and those subjected to 1.0 vol. % wood vinegar were in worse conditions than those that received the 0.5 vol. % solution of the herbicide.
Fifteen days after treatment, all the plantlets treated with 1.0 vol. % wood vinegar had died while those treated with the 0.5 vol. % dilution were still in relatively good condition (Figure 4). These results are statistically significant, chi-square p ≤ 0.001, between 0.5 vol. % and the other treatments.
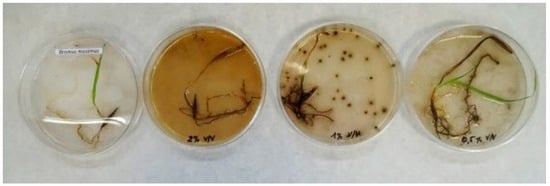
Figure 4.
Bromus maximus 15 days after treatment with (from left to right): 0 (control), 2, 1 and 0.5 % vol. wood vinegar. Photo: Juan Luis Aguirre.
These results indicate the good behaviour of low concentrations of the wood vinegar product in inhibiting early stages of seedling growth. Wood vinegar dilutions of 10, 5 and 2 vol. % induced the death of the seedlings within 48 h. The 1 vol. % solution led to slower plant death. According to these observations, it seems that wood vinegar has herbicidal actions when directly applied to the soil and that low concentrations may help control the development of weeds. Mmojieje and Hornung were also able to confirm the inhibitory effect of pyroligneous acid (10%) on seedlings of Helianthus annuus (sunflower), Solanum lycopersicum (tomato) and Raphanus sativus (radish) [63].
However, when the wood vinegar was used at a concentration of 0.5 vol. %, seedling growth was unaffected. This finding is in agreement with the results of a study by Aisyah et al., who found that a 0.5 vol. % solution of wood vinegar promoted the growth of banana plantlets [65].
Other studies have shown that very low concentrations of the wood acid (0.002% and 0.02%) improve the growth of seedlings and shoots; this has been related to enhanced nutrient availability due to the slow release of active acid and phenol components [64].
It should be noted that the presence of fungi on all filter papers was observed when the 1.0 vol. % wood vinegar solution was used. A possible explanation for this could be that at this low concentration, the vinegar acts as a source of carbon, promoting fungal growth while higher concentrations inhibit their growth. This issue requires further investigation.
3.5. Spray Test
After the tests on the germination and early growth stages of the seeds, the effects of spraying the herbicide directly on the plants were studied. The rationale for this experiment was to assess the product’s behaviour as a contact herbicide.
In the tests performed by Mmojieje and Hornung, no harmful effects on the plants were observed when a 10 vol. % solution of wood vinegar was sprayed on the leaves [63]. Accordingly, we tested the use of 100% wood vinegar and three dilutions in water of this stock solution (25, 50 and 75 vol. %, Table 8).

Table 8.
Mean mortality and standard deviation for each treatment in all species.
Tests were conducted on adult specimens of Anacyclus clavatus, Sinapis alba, Bromus madritensis, and Bromus maximus. The differences between the control and the sprays tests were significant, in all cases, p ≤ 0.01, Kruskal-Wallis test. Between treatments, the means were significantly different, between the 25 vol. % dilution and the rest (Mann-Whitney U test p ≤ 0.001 in all cases). There was no significant difference between dilutions at 50, 75 and 100 vol. %.
The differences between species were significant only between Anacyclus clavatus and the rest (p ≤ 0.05, Kruskal-Wallis test). There was no significant difference between Bromus matritensis, Bromus maximus and Sinapis alba (Table 9).

Table 9.
Mean mortality and standard deviation for each species (n = 16) and all dilutions.
Results at 10 days (Table 10) indicated no significant differences for each dilution and species except in the case of Anacyclus clavatus, where there was a difference between the treatments with 25 vol. % and 50 vol. % and the rest (Mann-Whitney U test p ≤ 0.05).

Table 10.
Mortality rates (MR) of each species recorded 10 days after treatment. Mean mortality and standard deviation.
Thus, the 25 vol. % wood vinegar treatment was effective in treating Bromus maximus, Sinapis alba and Bromus madritensis, giving rise to mortality rates exceeding 70% in all cases. In contrast, this same treatment used on Anacyclus clavatus caused the death of only 42% in the specimens treated.
Greatest efficiency was observed for the 50 vol. % treatment, which led to a mortality rate higher than 90% for Bromus maximus, Sinapis alba and Bromus madritensis. When used on Anacyclus clavatus, the mortality rate was 52.5%. However, the high value of the standard deviation in the cases of Sinapis alba and Anacyclus clavatus for the 25 vol. % concentration, indicate that at lower concentrations results are much less homogenous than when concentration above 50 vol. % are used.
The response to treatment with the 100 and 75 vol. % dilutions of wood vinegar was similar for Anacyclus clavatus, Bromus maximus, Sinapis alba and Bromus madritensis, provoking the death of all specimens.
The most interesting results were thus those arising from the direct application of the wood vinegar product on the adult plants (Figure 5). The annual weeds selected here to test the effects of the wood vinegar belong to the main botanical families of nitrophilous communities worldwide. The 100% mortality produced by the pure wood vinegar among these weeds indicates its efficiency when undiluted. When diluted, its efficacy diminished but not markedly and depended on the plant treated. Grassland species such as Bromus or cruciferous species such as Sinapis showed high mortality even when treated with the 25 vol. % dilution. The Asteraceae species Anacyclus clavatus was much more resistant. This difference could be related to the presence or absence of compounds with a protective effect against the physical actions of the product in the leaves. Mortality is likely caused by an osmotic reaction whereby severe damage is produced to cell membranes and thylakoid membranes in the leaves, as occurs with other fatty acids [66].

Figure 5.
Anacyclus clavatus (a), Bromus maximus (b), Bromus matritensis (c) Sinapis alba (d), 10 days after treatment with (from left to right) 0% (control), 25%, 50%, 75% and 100 vol. % solutions. Photo: Juan Luis Aguirre.
A woody plant species, Acacia dealbata, which is invasive in the Mediterranean region, was also included (although without replicate trials). The effects observed on Acacia dealbata were transient for all concentrations of the product. Although, initially, there was early leaf damage and the plant lost most of its leaves, after a few weeks, it recovered and started to produce new leaves. This is of interest for its use as a herbicide as the woody nature of the main trunk of Acacia was able to avoid the drying out of the stems that transport fluids in the plant and consequently, although the plant was damaged, it was later able to recover. This means the wood vinegar product will not be effective against woody plants but may be used on most perennial crops with lignified stems. More studies with woody species are necessary to confirm these findings.
Consistent with our observations, Hagner reported that when applied as a spray, wood vinegar behaves as a non-selective or contact foliar herbicide destroying practically all above-ground plant mass [67]. In a study examining the herbicidal capacity of wood vinegar by Kim et al., the product was also found to be effective when leaves were sprayed but not when the vinegar was applied to the soil [68].
The majority of compounds in wood vinegar show very low concentrations, less than 1 wt. % Major components that constitute wood vinegar are used as smoke flavourings, as they impart much-appreciated organoleptic properties to smoked food and are safe to be used as animal feed additives [19]. Although several studies have assessed the possible environmental effects of the product, more work in this area is needed. For example, Hagner found that soil organisms were more tolerant of wood vinegar than aquatic organisms [67]. No long-term effects on soil microbes, nematodes or enchytraeids were found. This author found that initial risk assessment indicated the risks of wood vinegar (to soil and aquatic organisms) were negligible.
However, in a recent study, Goethen de Lima et al. [69] found that wood vinegar may be toxic to Daphnia magna (microcrustacea), while no genotoxicity was detected for Allium cepae. Koc [26] reported that wood vinegar used at different doses to protect plants and/or crops in wheat agro-ecosystems did not have a negative effect on microbial factors determined in the soil. Steiner et al. observed that the application of wood vinegar to Amazonian soils increased the metabolism of soil microorganisms by increasing microbial biomass, population growth and the microbe’s efficiency [36]. Furthermore, Zhang observed that wood vinegar treatments of increasing concentrations significantly increased the quantity of bacteria in soils and led to a significant increase in the total quantity of microbes. It could be that wood vinegar more than directly affecting soil organisms has some impact on aquatic organisms [70]. These data point to a need to determine environmental exposure limits and how to apply this bio-product to promote its safer agricultural use.
3.6. Irrigation Tests
Solutions of the wood vinegar of 25 and 50 vol. % were applied to the soil in the trays containing weeds grown from the seed bank of six different soil samples, and in the trays harbouring single species. Effects of the herbicide when added to the soil were less intense than when the wood vinegar was sprayed (Table 11, Figure 6). When used at concentrations of 25 vol. %, damage was scarce while soil irrigation with a 50 vol. % dilution of the product was harmful mostly to grassland species (mean and SD; 27.5 ± 25.62 for the 25 vol. % solution, and 52.78 ± 35.89 for 50 vol. %). This difference was not significant (U Mann-Whitney test p = 0.18).

Table 11.
Percentage mortality after 7 days/15 days of irrigation with wood vinegar (WV).

Figure 6.
Pots containing Anacyclus clavatus watered with 40 mL of 25 vol. % wood vinegar and 50 mL of 50 vol. % wood vinegar (a) before treatment, (b) after 7 days (25 vol. %) and (c) after 7 days (50 vol. %). Photo: Juan Luis Aguirre.
Under natural conditions, more complex soils will show greater interactions than in the small pots used in this trial. Accordingly, it may be concluded that via the irrigation route, the wood vinegar herbicide is much less effective than when used as a foliar spray. In studies by Mmojieje and Hornung, no adverse effects of adding a 1% wood vinegar solution directly to the soil were detected. However, concentrations of 10% were able to reduce plant growth rates when the wood acid was applied weekly to the soil [63].
4. Conclusions
This study was designed to characterise wood vinegars obtained from different types of biomass and to examine the possible use of this product as a seed germination inhibitor and herbicide in a laboratory-scale experiment. The composition of the different wood vinegars was found to be similar. Main components were acetic acid (2.87–4.30 wt. %) followed by hydroxyacetone (1.13–2.14% wt. %) and hydroxyacetaldehyde (0.70–3.47 wt. %). For all the wood vinegars, pH was 2.04–2.62 owing to their high carboxylic acid contents.
Our findings revealed the efficiency of an industrial wood vinegar herbicide used as a germination inhibitor of Acacia dealbata seeds. In seedling tests, no plant species treated with wood vinegar solutions (2, 5 and 10 vol. %) remained alive after 48 h, while seedlings treated with lower concentrations (0.5 and 1 vol. %) survived the first 48 h only. After 15 days, only plantlets treated with 0.5 vol. % wood vinegar remained alive. In soil irrigation experiments conducted on adult plants, dilutions of the wood vinegar (25 and 50 vol. % did not produce the death of all specimens. Bromus and Sinapis species were most affected by the product, especially when applied at the higher concentration, while Anacyclus (compositae) and cruciferous species were most resistant. The wood vinegar showed good herbicidal properties when solutions were sprayed on adult plants. In general, herbicidal efficacy diminished as its concentration decreased. Best results were obtained for 50, 75 and 100 vol. % solutions applied on Anacyclus clavatus, Sinapis alba, Bromus madritensis and Bromus maximus. In contrast, only scarce and transient damaging effects were produced on Acacia dealbata. While these herbicidal properties and environmental exposure limits still need to be confirmed in the field, once its application procedure is optimized and safety confirmed, the use of wood vinegar could make the industrial pyrolysis of biomass residues economically viable.
Author Contributions
Conceptualization, J.L.A., M.T.M., S.G. and J.B.; methodology, J.L.A., M.T.M. and J.B.; formal analysis, J.L.A., M.T.M., L.N., S.G.; investigation, S.G. and M.P.; resources, S.G. and M.P.; plantations and Climate-controlled glass chamber, J.L.M. and J.L.A.; writing—original draft preparation, J.L.A., M.T.M. and J.B.; writing—review and editing, J.L.A., M.T.M. and J.B.; visualization, J.L.A. and J.B.; supervision, J.L.A.; project administration, J.L.A. and S.G.; funding acquisition, J.L.A., M.P. and S.G. All authors have read and agree to the published version of the manuscript.
Funding
This research was supported by project European funds LIGNOBIOLIFE, LIFE 17 CCM/ES/000051 (www.lignobiolife.com), Matinsa S.A Project (FFC Group), funds from the Junta de Comunidades de Castilla La Mancha (JCCM) and funds from RTC-2016-5823-5, Ministerio de Economía y Competitividad, Spain.
Acknowledgments
The authors thank Neoliquid Advanced Biofuels and Biochemicals S.L. for providing the wood vinegar used in the herbicide experiments and the Centro de Química Aplicada y Biotecnología de la Universidad de Alcalá (CQAB) for the chromatography analyses and moisture content determinations made on the wood vinegar samples. We also thank Ana Burton for language and editorial assistance and anonymous referees who have helped improve the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Inventario Nacional de Emisiones a la atmósfera. 1990–2013. Available online: https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/temas/sistema-espanol-de-inventario-sei-/90-13volumen2_tcm30-179143.pdf (accessed on 11 May 2020).
- Zabaniotou, A.A. Pyrolysis of Forestry Biomass By-Products in Greece Pyrolysis of Forestry Biom ass By-Products in Greece. Energy Sources 1999, 21, 395–403. [Google Scholar] [CrossRef]
- Yang, Q.; Han, F.; Chen, Y.; Yang, H.; Chen, H. Greenhouse gas emissions of a biomass-based pyrolysis plant in China. Renew. Sustain. Energy Rev. 2016, 53, 1580–1590. [Google Scholar] [CrossRef]
- Yang, Y.; Brammer, J.G.; Wright, D.G.; Scott, J.A.; Serrano, C.; Bridgwater, A.V. Combined heat and power from the intermediate pyrolysis of biomass materials: performance, economics and environmental impact. Appl. Energy 2017, 191, 639–652. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Akinola, A.O. Effect of Temperature on Product Yield of Pyrolysis of Seven Selected Wood Species in South West Nigeria. Int. J. Emerg. Res. Manag. Technol. 2016, 9359, 176–181. [Google Scholar]
- Bridgwater, A. V.; Peacocke, G.V.C. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Ward, J.; Rasul, M.G.; Bhuiya, M.M.K. Energy recovery from biomass by fast pyrolysis. Procedia Eng. 2014, 90, 669–674. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Bridgwater, A. V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Lignocellul. Biomass Pyrolysis A Rev. Prod. Prop. Eff. Pyrolysis Parameters 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Wild, P. De; Reith, H.; Heeres, E. Biomass pyrolysis for chemicals. Biofuels 2011, 2, 185–208. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A. V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Chutia, R.S.; Kataki, R.; Bhaskar, T. Characterization of liquid and solid product from pyrolysis of Pongamia glabra deoiled cake. Bioresour. Technol. 2014, 165, 336–342. [Google Scholar] [CrossRef]
- Imam, T.; Capareda, S. Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J. Anal. Appl. Pyrolysis 2012, 93, 170–177. [Google Scholar] [CrossRef]
- Martín, M.T.; Sanz, A.B.; Nozal, L.; Castro, F.; Alonso, R.; Aguirre, J.L.; González, S.D.; Matía, M.P.; Novella, J.L.; Peinado, M.; et al. Microwave-assisted pyrolysis of Mediterranean forest biomass waste: Bioproduct characterization. J. Anal. Appl. Pyrolysis 2017, 127, 278–285. [Google Scholar] [CrossRef]
- Mathew, S.; Zakaria, Z.A. Pyroligneous acid—the smoky acidic liquid from plant biomass. Appl. Microbiol. Biotechnol. 2015, 99, 611–622. [Google Scholar] [CrossRef]
- Fagernäs, L.; Kuoppala, E.; Tiilikkala, K.; Oasmaa, A. Chemical composition of birch wood slow pyrolysis products. Energy Fuels 2012, 26, 1275–1283. [Google Scholar]
- Guillén, M.D.; Manzanos, M.J. Characteristics of smoke flavourings obtained from mixtures of oak (Quercus sp.) wood and aromatic plants (Thymus vulgaris L. and Salvia lavandulifolia Vahl.). Flavour Fragr. J. 2005, 20, 676–685. [Google Scholar]
- Wang, Z.; Lin, W.; Song, W.; Yao, J. Preliminary investigation on concentrating of acetol from wood vinegar. Energy Convers. Manag. 2010, 51, 346–349. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Chen, G.; Wu, L.; Liu, B.; Li, Y.; Sun, S.; Zhang, H.; Zhang, Z.; Wang, Z. A new method for comprehensive utilization of wood vinegar by distillationand liquid−liquid extraction. Process Biochem. 2018, 75, 194–201. [Google Scholar] [CrossRef]
- Ratnani, R.D. A Review of Pyrolisis of Eceng Gondok (Water hyacinth) for Liquid Smoke. In Proceedings of the 3rd International Conference on Energy, Environmental and Information System (ICENIS 2018), EDP Sciences, Semarang, Indonesia, 14–15 August 2018; Volume 73, p. 5010. [Google Scholar]
- Koç, I.; Öğün, E.; Namli, A.; Mendeş, M.; Kutlu, E.; Yardim, E.N. The effects of wood vinegar on some soil microorganisms. Appl. Ecol. Environ. Res. 2019, 17, 2437–2447. [Google Scholar] [CrossRef]
- Tiilikkala, K.; Fagernäs, L.; Tiilikkala, J. History and Use of Wood Pyrolysis Liquids as Biocide and Plant Protec- tion Product. Open Agric. J. 2010, 4, 111–118. [Google Scholar] [CrossRef]
- Sayed, A.M.M.; Behle, R.W.; Tiilikkala, K.; Vaughn, S.F. Insecticidal activity of bio-oils and biochar as pyrolysis products and their combination with microbial agents against Agrotis ipsilon (Lepidoptera: Noctuidae). Pesticidi i Fitomedicina 2018, 33, 39–52. [Google Scholar] [CrossRef]
- Mu, J.; Uehara, T.; Furuno, T. Effect of bamboo vinegar on regulation of germination and radicle growth of seed plants II: Composition of moso bamboo vinegar at different collection temperature and its effects. J. Wood Sci. 2004, 50, 470–476. [Google Scholar] [CrossRef]
- Jothityangkoon, D.; Koolachart, R.; Wanapat, S.; Wongkaew, S.; Jogloy, S. Using wood vinegar in enhancing peanut yield and in controlling the contamination of aflatoxin producing fungus. Int. Crop Sci. 2008, 4, 253. [Google Scholar]
- Pan, X.; Zhang, Y.; Wang, X.; Liu, G. Effect of adding biochar with wood vinegar on the growth of cucumber. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 1–5. [Google Scholar] [CrossRef]
- Rahmat, B.; Pangesti, D.; Natawijaya, D.; Sufyadi, D. Generation of wood-waste vinegar and its effectiveness as a plant growth regulator and pest insect repellent. BioResources 2014, 9, 6350–6360. [Google Scholar] [CrossRef]
- Mungkunkamchao, T.; Kesmala, T.; Pimratch, S.; Toomsan, B.; Jothityangkoon, D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hortic. 2013, 154, 66–72. [Google Scholar] [CrossRef]
- Nurhayati, T.; Roliadi, H.; Bermawie, N. Production of Mangium (Acacia mangium) Wood Vinegar and its utilization. Indones. J. For. Res. 2005, 2, 13–25. [Google Scholar] [CrossRef]
- Chung, S.H.C.A.; Ong, K.H.; King, P.J.H. Potential of wood vinegars as bio-fertilizer in promoting seedling growth of Eucalyptus pellita. In Proceedings of the The conference on forestry and forest products research 2013, Kuala Lumpur, Malaysia, 12–13 November 2013; pp. 186–189. [Google Scholar]
- Steiner, C.; Das, K.C.; Garcia, M.; Zech, W. Charcoal and smoke extract stimulate the soil microbial community in a highly weathered. Pedobiologia 2008, 51, 359–366. [Google Scholar] [CrossRef]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Salonen, J.; Tiilikkala, K.; Ruuttunen, P.; Lindqvist, I.; Lindqvist, B. Birch Tar Oil: A Potential Herbicide from the Forests of Finland. In Proceedings of the Abstracts of the 5th International Weed Science Congress. Weeds local problems/global challenge, Vancouver, BC, Canada, 23–27 June 2008. [Google Scholar]
- Ruuttunen, P. Evaluation of Birch Oil Distillate for Weed Control in Potato; Trial Report 2007; MTT Agrifood Research: Jokioinen, Finland, 2007. [Google Scholar]
- Acenas, X.S.; Nunez, J.P.P.; Seo, P.D.; Ultra, V.U.J.; Lee, S.C. Mixing Pyroligneous Acids with Herbicides to Control Barnyardgrass (Echinochloa crus-galli). Weed Turfgrass Sci. 2013, 2, 164–169. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–23. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an Exceptional Bioresource for Energy, Agronomy, Carbon Sequestration, Activated Carbon and Specialty Materials. Waste Biomass Valorization 2016, 7, 201–235. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Ouyang, L.; Wang, F.; Tang, J.; Yu, L.; Zhang, R. Effects of biochar amendment on soil aggregates and hydraulic properties. J. Plant Nutr. Soil Sci. 2013, 13, 991–1002. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, M.; Niu, Y.; Liu, X.; van Zwieten, L.; Chen, D.; Bian, R.; Cheng, K.; Li, L.; Joseph, S.; et al. Is current biochar research addressing global soil constraints for sustainable agriculture? Agric. Ecosyst. Environ. 2016, 226, 25–32. [Google Scholar] [CrossRef]
- Siebeneichler, E.A.; Figueredo, N.A.; Costa, L.M.; Tronto, J. Recycling of Eucalipto cloeziana Under Biochar Different Pyrolisis Temperatures. FLORESTA 2019, 49, 199–208. [Google Scholar] [CrossRef]
- Mushtaq, F.; Abdullah, T.A.T.; Mat, R.; Ani, F.N. Optimization and characterization of bio-oil produced by microwave assisted pyrolysis of oil palm shell waste biomass with microwave absorber. Bioresour. Technol. 2015, 190, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kirtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Kolokolova, O. Biomass Pyrolysis and Optimisation for Bio-Bitumen. Ph.D. Thesis, University of Canterbury, Canterbury, New Zealand, 2013. [Google Scholar]
- Bartolomé Esteban, C.; Díaz Santiago, G. Flora y vegetación espontánea del campus externo de la Universidad de Alcalá. Cuad. Campus Nat. y Medio Ambient. 2006, 1, 1–23. [Google Scholar]
- Bartolomé Esteban, C.; de la Cruz Rot, M.; Álvarez Jiménez, J. La vegetacion nitrofila de la Campiña de Guadalajara. Acta Botánica Barcelona 1988, 37, 17–23. [Google Scholar]
- Fang, Y.; Li, Y.; Yi, W.; Liu, S.; Bai, X. Fractionation of Pyroligneous Acid: The First Step for the Recovery of Levoglucosan. BioResources 2017, 12, 981–991. [Google Scholar] [CrossRef]
- Theapparat, Y.; Chandumpai, A.; Faroongsarng, D. Physicochemistry and Utilization of Wood Vinegar from Carbonization of Tropical Biomass Waste. In Tropical Forests—New Edition; InTech: London, UK, 2018. [Google Scholar]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energies Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Seo, P.D.; Ultra, V.U.; Rubenecia, M.R.U.; Lee, S.C. Influence of herbicides-pyroligneous acids mixtures on some soil properties, growth and grain quality of paddy rice. Int. J. Agric. Biol. 2015, 17, 499–506. [Google Scholar] [CrossRef]
- Pimsuta, M.; Sosa, N.; Deekamwong, K.; Keawkumay, C.; Thathong, Y.; Rakmae, S.; Junpirom, S.; Prayoonpokarach, S.; Wittayakun, J. Charcoal and wood vinegar from pyrolysis of lead tree wood and activated carbon from physical activation. Suranaree J. Sci. Technol. 2018, 25, 177–190. [Google Scholar]
- Bilehal, D.; Li, L.; Kim, Y.H. Gas Chromatography-Mass Spectrometry Analysis and Chemical Composition of the Bamboo-Carbonized Liquid. Food Anal. Methods 2012, 5, 109–112. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, C.H.; Liang, M.T.; Gao, Z.J.; Wu, Y.W.; Chuang, L.Y. Chemical composition, antioxidant, and antibacterial activity of wood vinegar from litchi chinensis. Molecules 2016, 21, 1150. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Wu, L.; Zhang, H.; Wang, Z. Characterization of Five Kinds of Wood Vinegar Obtained from Agricultural and Forestry Wastes and Identification of Major Antioxidants in Wood Vinegar. Chem. Res. Chinese Univ. 2019, 35, 12–20. [Google Scholar] [CrossRef]
- Li, R.; Narita, R.; Nishimura, H.; Marumoto, S.; Yamamoto, S.P.; Ouda, R.; Yatagai, M.; Fujita, T.; Watanabe, T. Antiviral Activity of Phenolic Derivatives in Pyroligneous Acid from Hardwood, Softwood, and Bamboo. ACS Sustain. Chem. Eng. 2018, 6, 119–126. [Google Scholar] [CrossRef]
- Mmojieje, J.; Hornung, A. The Potential Application of Pyroligneous Acid in the UK Agricultural Industry. J. Crop Improv. 2015, 29, 228–246. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Z.; Meki, K.; Wang, X.; Liu, B.; Zheng, H.; You, X.; Li, F. Effect of co-application of wood vinegar and biochar on seed germination and seedling growth. J. Soils Sediments 2019, 19, 3934–3944. [Google Scholar] [CrossRef]
- Aisyah, I.; Sinaga, M.S.; Nawangsih, A.A.; Giyanto; Pari, G. Utilization of liquid smoke to suppress blood diseases on bananas and its effects on the plant growth. J. Agric. Sci. 2018, 40, 453–460. [Google Scholar] [CrossRef]
- Fukuda, M.; Tsujino, Y.; Fujimori, T.; Wakabayashi, K.; Böger, P. Phytotoxic activity of middle-chain fatty acids I: Effects on cell constituents. Pestic. Biochem. Physiol. 2004, 80, 143–150. [Google Scholar] [CrossRef]
- Hagner, M. Potential of the Slow Pyrolysis Products Birch Tar Oil, Wood Vinegar and Biochar in Sustainable Plant Protection - Pesticidal Effects, Soil Improvement and Environmental Risks; University of Helsinki: Lahti, Finland, 2013; ISBN 9789521091681. [Google Scholar]
- Kim, S.M.; Kim, Y.H.; Kim, J.S.; Ahn, M.S.; Heo, S.J.; Hur, J.H.; Han, D.S. Herbicidal Activity of Wood Vinegar from Quercus mongolica Fisch. Korean J. Pestic. Sci. 2000, 4, 82–88. [Google Scholar]
- de Lima, G.G.; Mendes, C.; de Marchi, G.; Vicari, T.; Cestari, M.M.; Gomes, M.F.; Ramsdorf, W.A.; Magalhães, W.L.E.; Hansel, F.A.; Leme, D.M. The evaluation of the potential ecotoxicity of pyroligneous acid obtained from fast pyrolysis. Ecotoxicol. Environ. Saf. 2019, 180, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, D.; Yao, Z.; Chao, Z.; Xiaojuan, A. Effects of wood vinegar on the soil microbial characteristics. J. Chem. Pharm. Res. 2014, 6, 1254–1260. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).