Direct AC/DC Heating of Oxygen Transport Membranes
Abstract
1. Introduction
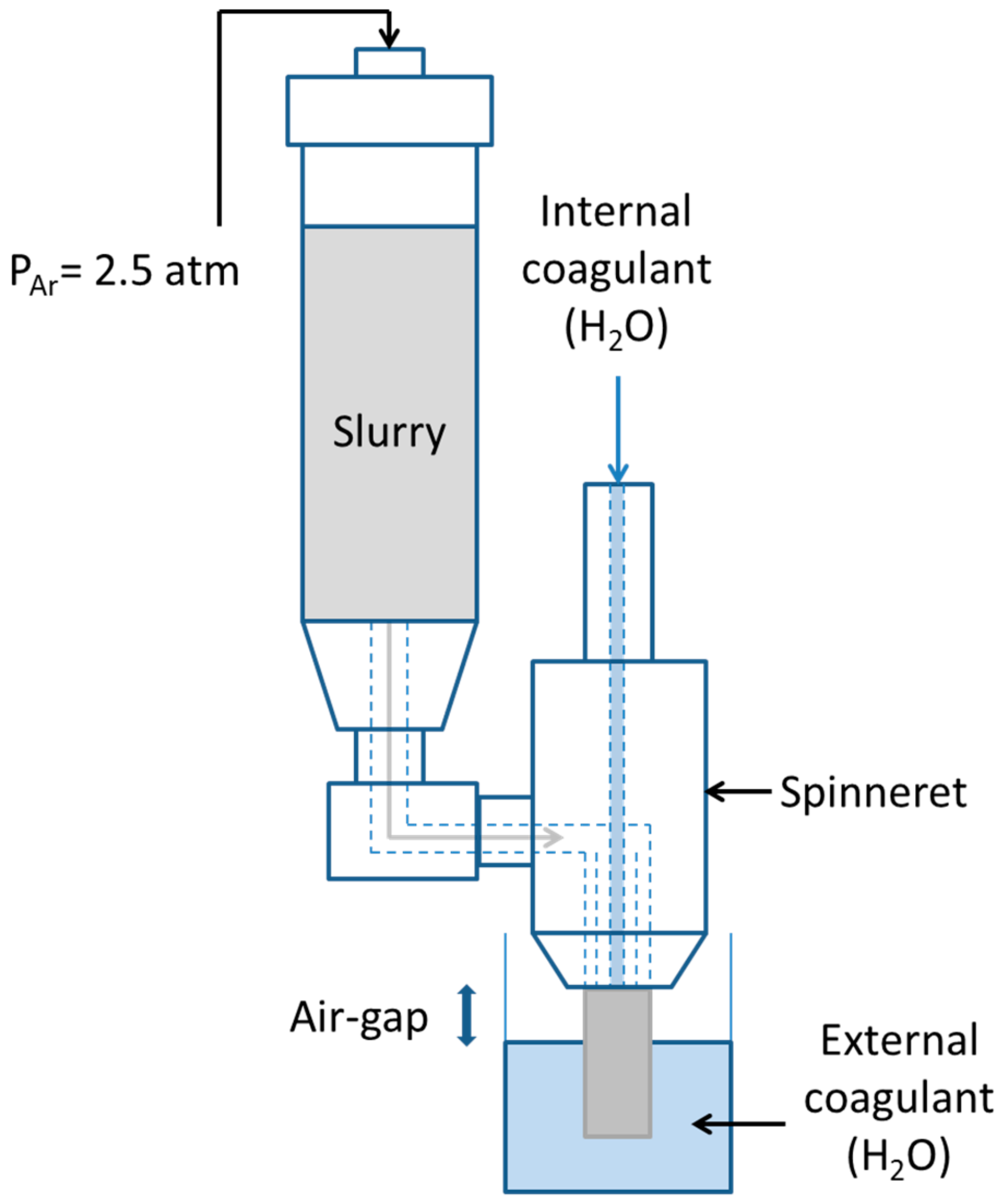
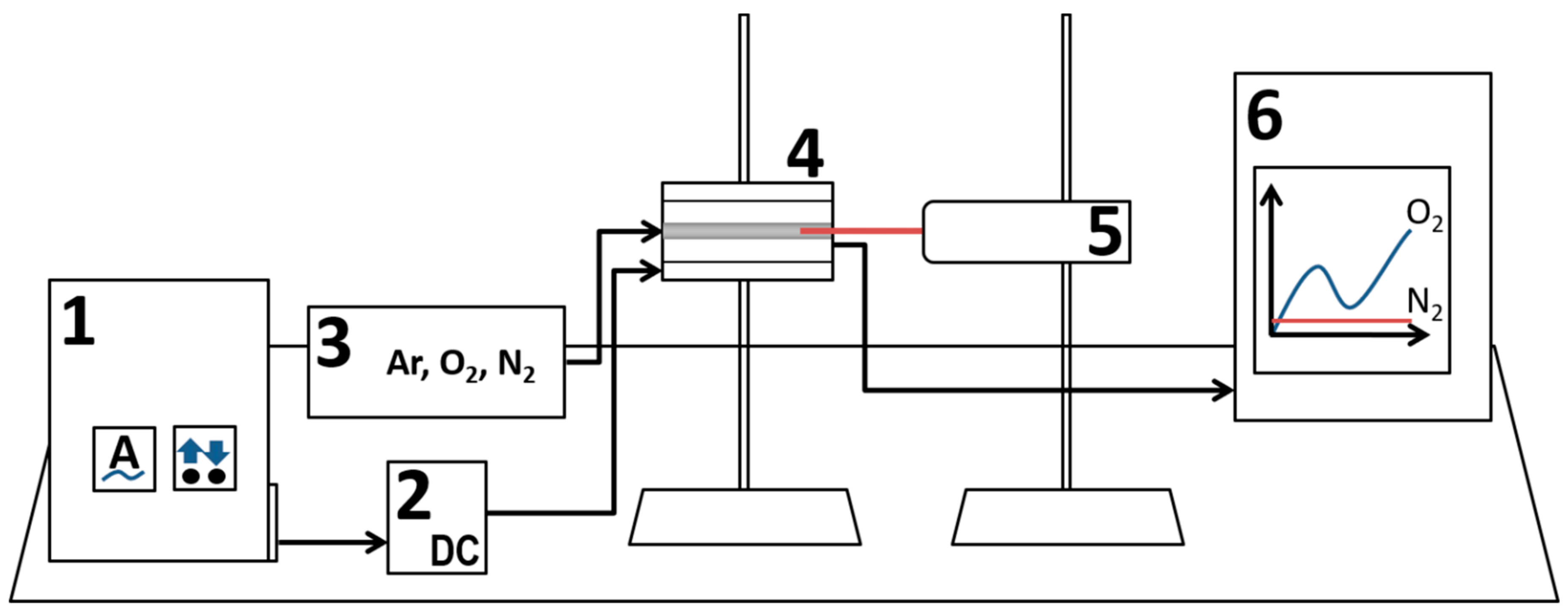
2. Experimental
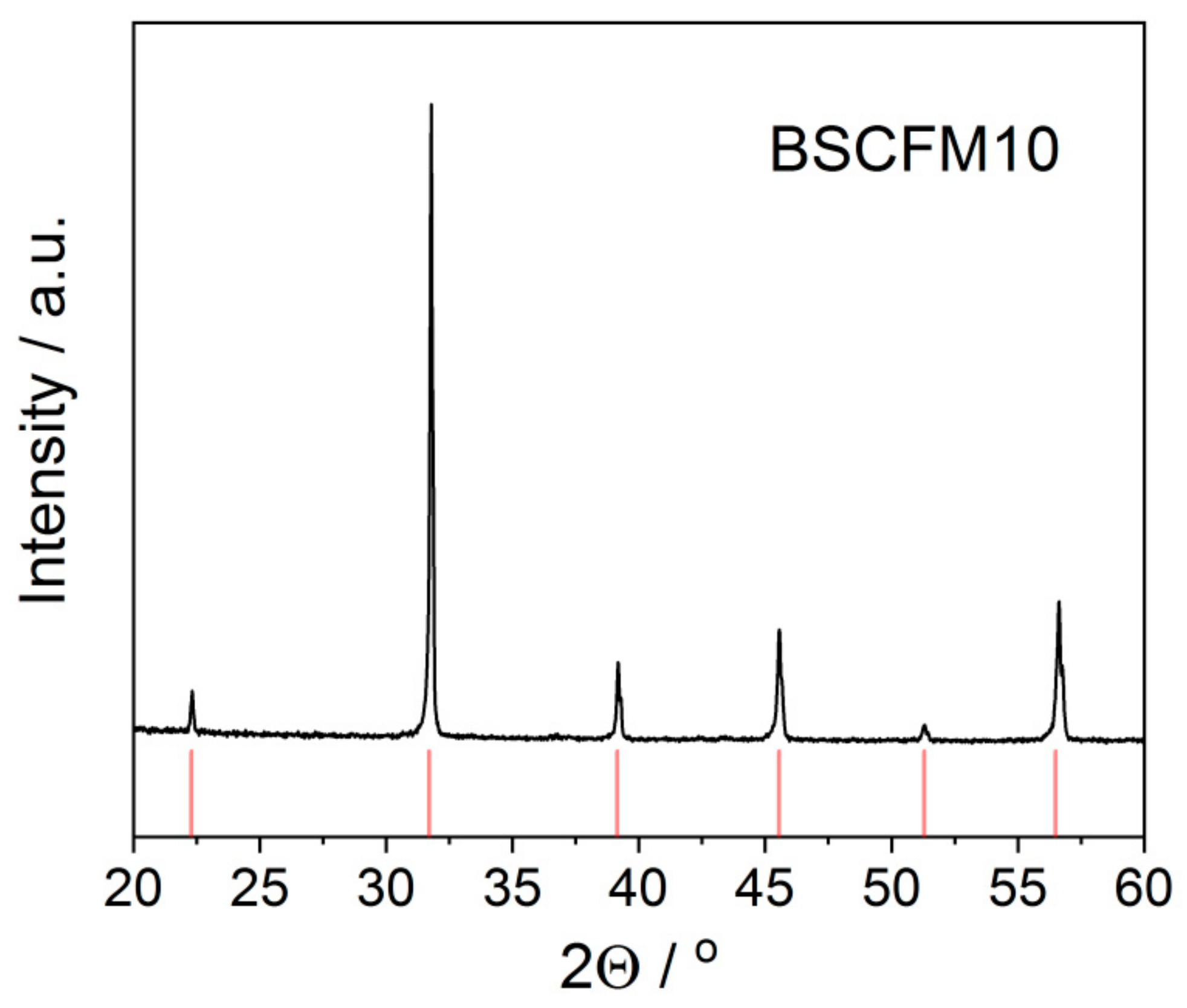
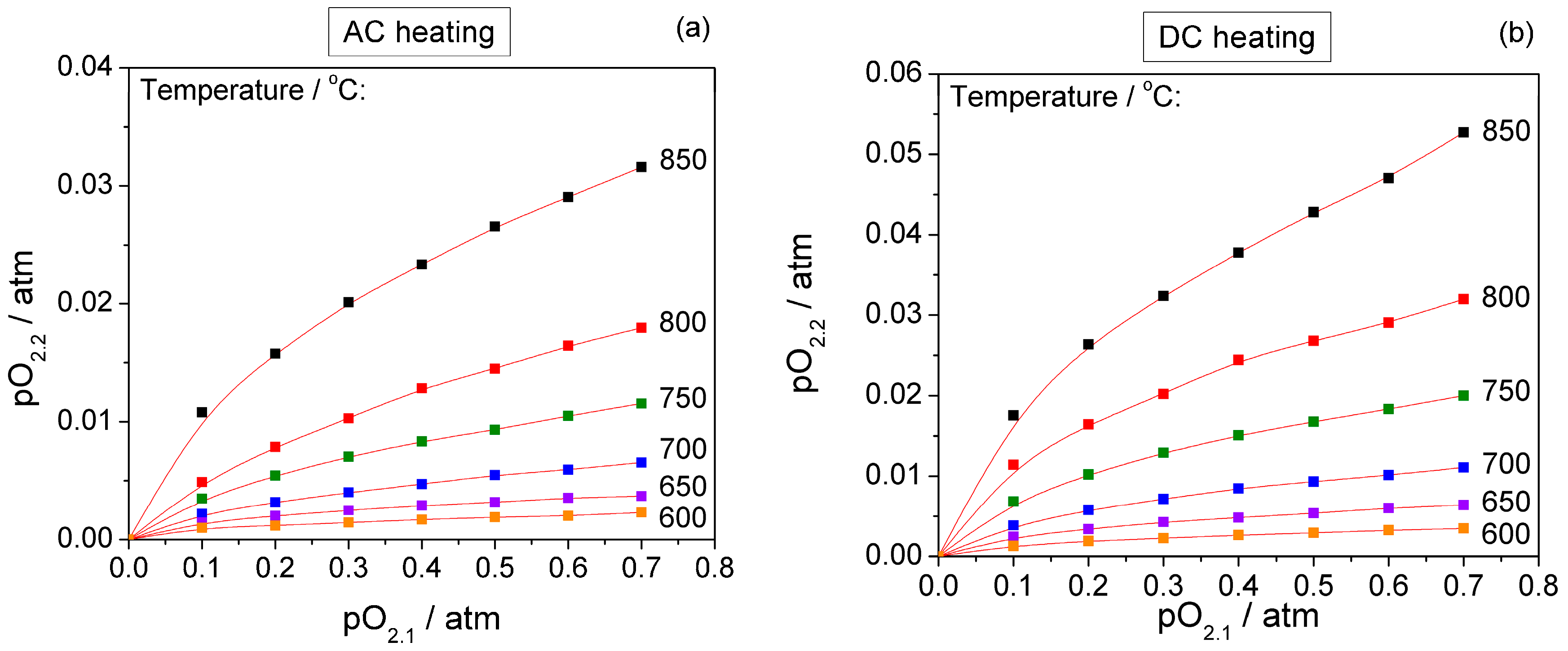
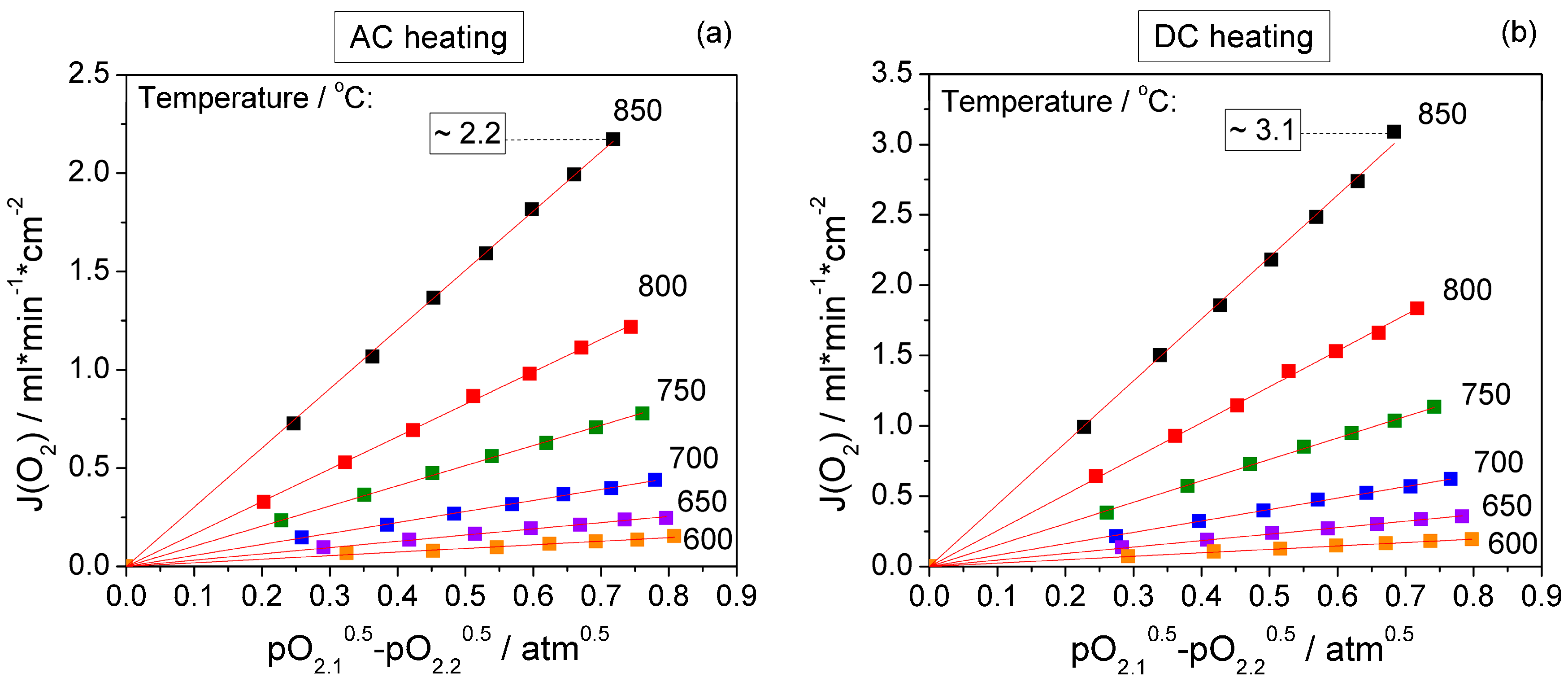
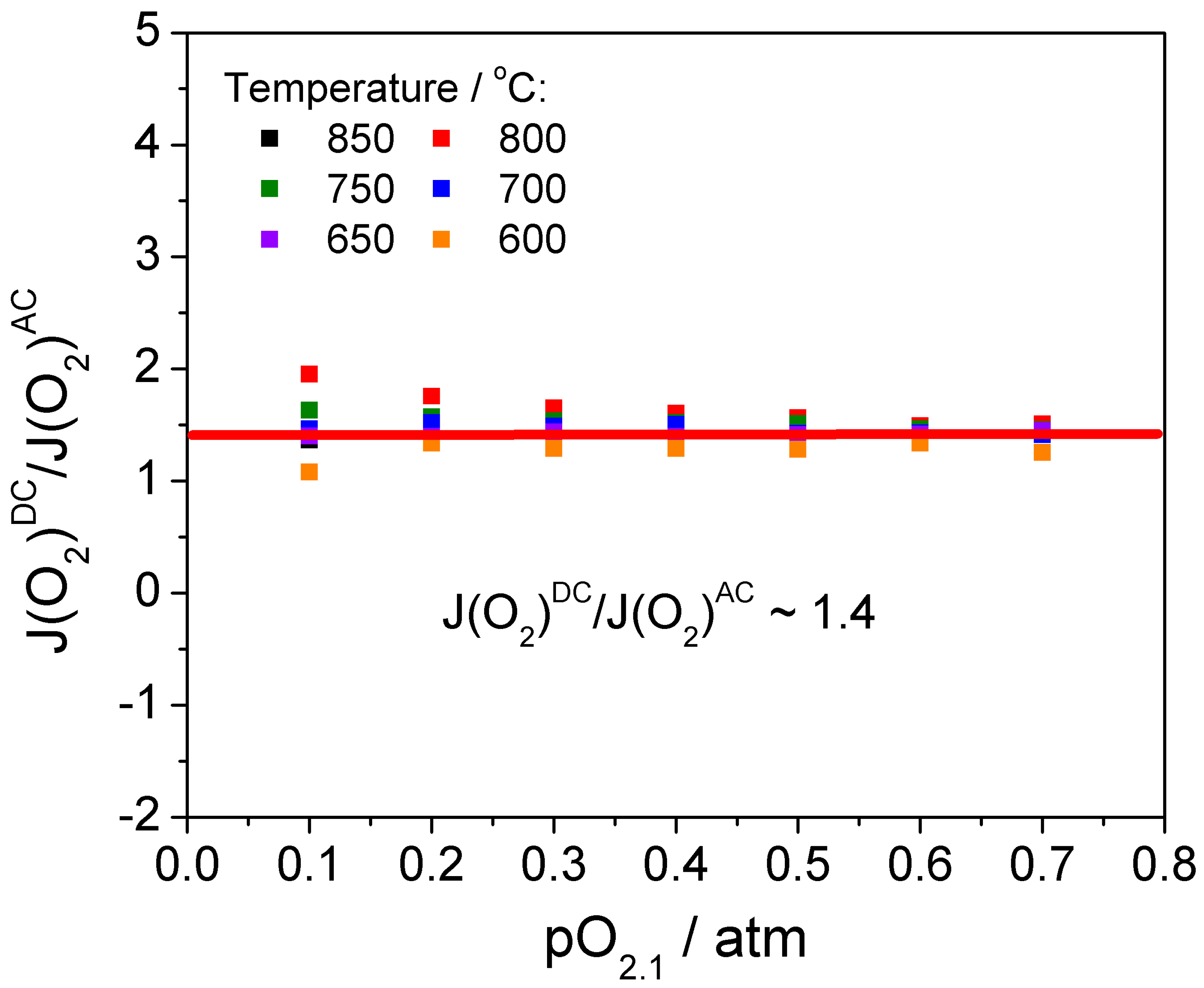
3. Results and Discussion
4. Conclusions
- -
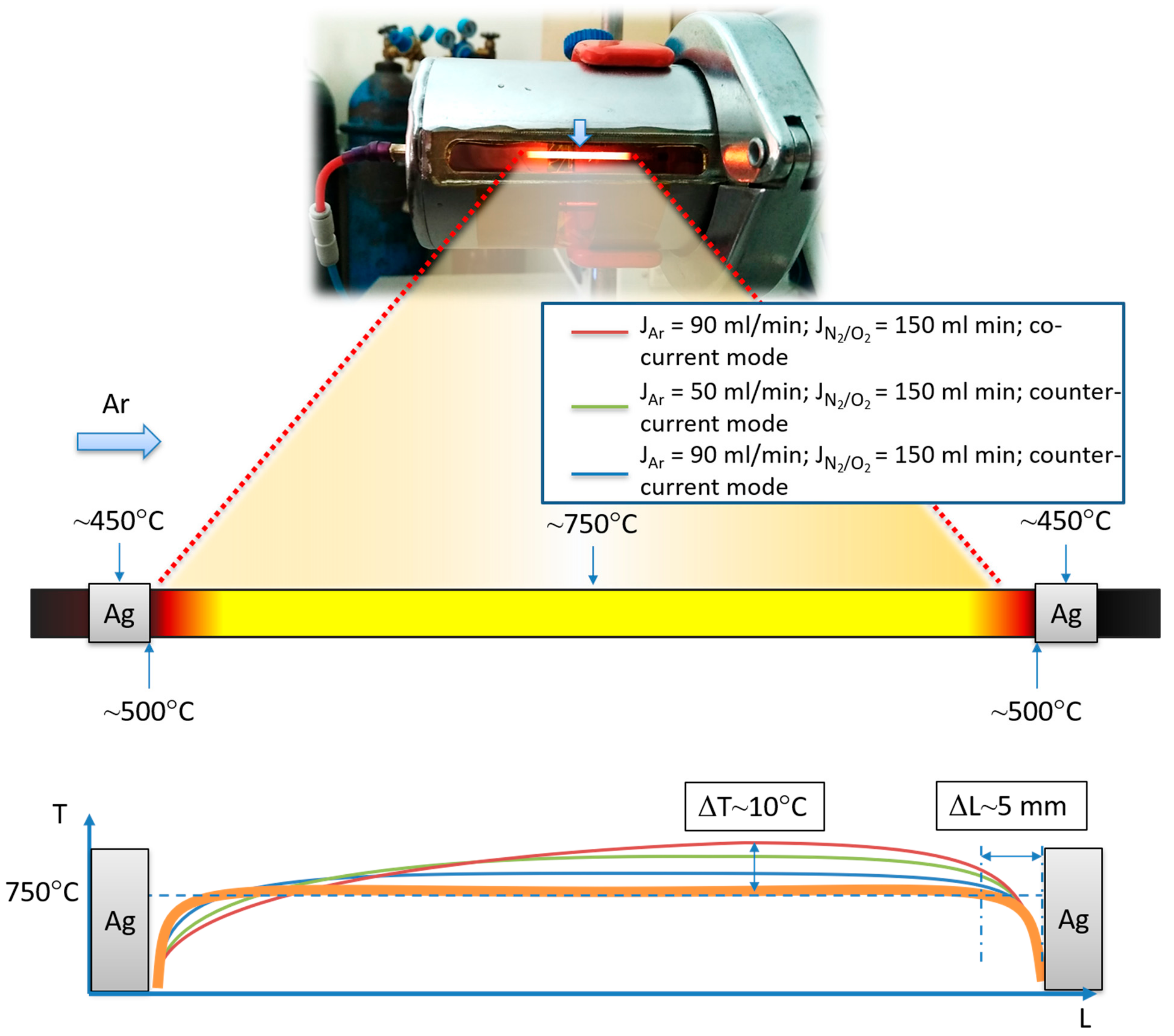
- The direct heating technique allowed us to study the longitudinal temperature gradient of the operating HFMs.
- -
- Increasing the sweep gas flow up to 70 ml/min did not significantly affect the gradient along the membrane.
- -
- The activation energies determined from Arrhenius plot were 89 ± 4 and 94 ± 2 kJ/mol for the AC- and DC-heated HFMs, respectively.
- -
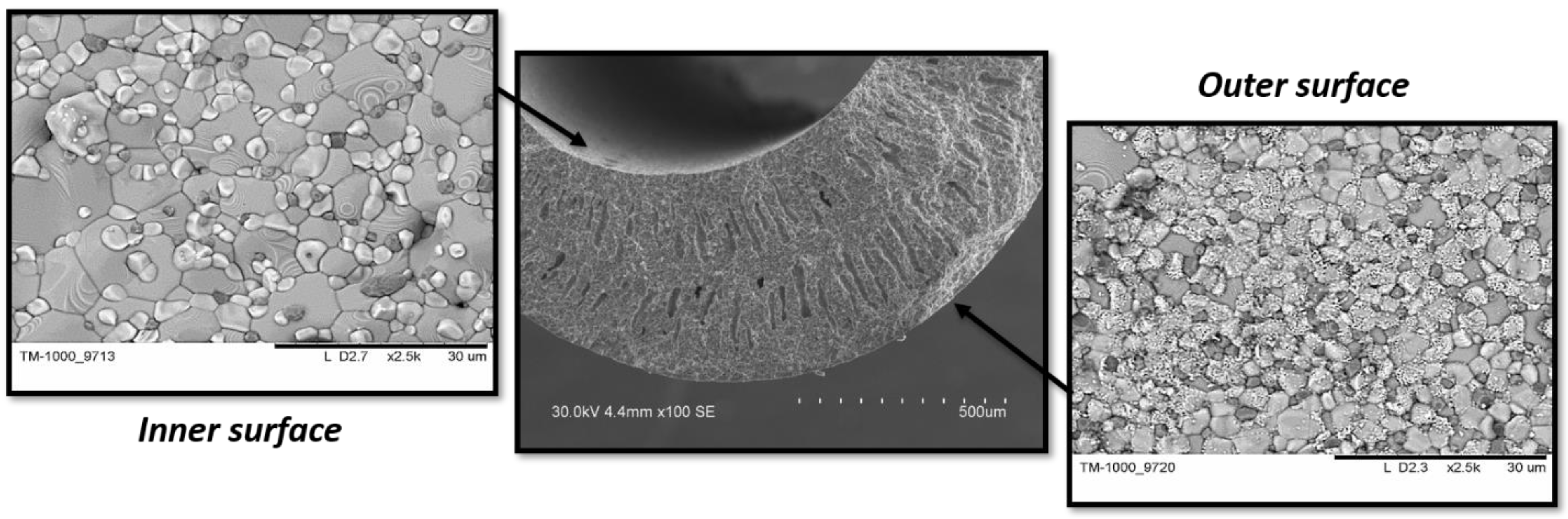
- In the short-term experiment with direct current, a significant positive effect on the oxygen transport was found, whereas the sample exposure under direct current for a long period of time had a significant negative effect on the microstructure of the membrane.
Author Contributions
Funding
Conflicts of Interest
References
- Ten Elshof, J.E.; van Hassel, B.A.; Bouwmeester, H.J.M. Activation of methane using solid oxide membranes. Catal. Today 1995, 25, 397. [Google Scholar] [CrossRef][Green Version]
- Marques, F.M.B.; Kharton, V.V.; Naumovich, E.N.; Shaula, A.L.; Kovalevsky, A.V.; Yaremchenko, A.A. Oxygen ion conductors for fuel cells and membranes: Selected developments. Solid State Ion. 2006, 177, 1697. [Google Scholar] [CrossRef]
- Sunarso, J.; Baumann, S.; Serra, J.M.; Meulenberg, W.A.; Liu, S.; Lin, Y.S. Mixed ionic–electronic conducting (MIEC) ceramic-based membranes for oxygen separation. J. Membr. Sci. 2008, 320, 13. [Google Scholar] [CrossRef]
- Leo, A.; Liu, S.; Diniz da Costa, J.C. Development of mixed conducting membranes for clean coal energy delivery. Int. J. Greenh. Gas Con. 2009, 3, 357. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141. [Google Scholar] [CrossRef]
- Popov, M.P.; Bychkov, S.F.; Nemudry, A.P. Direct AC heating of oxygen transport membranes. Solid State Ion. 2017, 312, 73. [Google Scholar] [CrossRef]
- Popov, M.P.; Gainutdinov, I.I.; Bychkov, S.F.; Nemudry, A.P. New approaches for enhancement of oxygen fluxes on hollow fiber membranes. Mat. Today Proc. 2017, 4, 11381. [Google Scholar] [CrossRef]
- Hancke, R.; Larsen, T.V.; Xing, W.; Li, Z.; Fontaine, M.-L.; Norby, T. Ohmically heated ceramic asymmetric tubular membranes for gas separation. J. Membr. Sci. 2018, 564, 598. [Google Scholar] [CrossRef]
- Tong, J.; Yang, W.; Cai, R.; Zhu, B.; Xiong, G.; Lin, L. Investigation on the structure stability and oxygen permeability of titanium-doped perovskite-type oxides of BaTi0.2CoxFe0.8−xO3−δ (x = 0.2 − 0.6). Sep. Pur. Tech. 2003, 32, 289–299. [Google Scholar] [CrossRef]
- Savinskaya, O.A.; Nemudry, A.P. Oxygen permeability and structural features of SrFe1−xWxO3−δ membranes. J. Membr. Sci. 2014, 459, 45–51. [Google Scholar] [CrossRef]
- Liu, S.; Gavalas, G.R. Oxygen selective ceramic hollow fiber membranes. J. Membr. Sci. 2005, 246, 103–108. [Google Scholar] [CrossRef]
- Artimonova, E.V.; Savinskaya, O.A.; Nemudry, A.P. Effect of B-site tungsten doping on structure and oxygen permeation properties of SrCo0.8Fe0.2O3−δ perovskite membranes. J. Eur. Ceram. Soc. 2015, 35, 2343–2349. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; He, Y.; Yang, W. Oxygen permeability and stability of BaCe0.1Co0.4Fe0.5O3−δ oxygen permeable membrane. Sep. Pur. Tech. 2010, 73, 38–43. [Google Scholar] [CrossRef]
- Popov, M.P.; Starkov, I.A.; Bychkov, S.F.; Nemudry, A.P. Improvement of Ba0.5Sr0.5Co0.8Fe0.2O3−δ functional properties by partial substitution of cobalt with tungsten. J. Membr. Sci. 2014, 469, 88–94. [Google Scholar] [CrossRef]
- Geankoplis, C.J. Transport Processes and Unit Operations, 3rd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1993. [Google Scholar]
- Shubnikova, E.V.; Popov, M.P.; Chizhik, S.A.; Bychkov, S.F.; Nemudry, A.P. The modeling of oxygen transport in MIEC oxide hollow fiber membranes. Chem. Eng. J. 2019, 372, 251–259. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalev, I.; Vorobyev, A.; Bagishev, A.; Popov, M.; Sharafutdinov, M.; Titkov, A.; Bychkov, S.; Nemudry, A. Direct AC/DC Heating of Oxygen Transport Membranes. Energies 2020, 13, 30. https://doi.org/10.3390/en13010030
Kovalev I, Vorobyev A, Bagishev A, Popov M, Sharafutdinov M, Titkov A, Bychkov S, Nemudry A. Direct AC/DC Heating of Oxygen Transport Membranes. Energies. 2020; 13(1):30. https://doi.org/10.3390/en13010030
Chicago/Turabian StyleKovalev, Ivan, Alexander Vorobyev, Artem Bagishev, Mikhail Popov, Marat Sharafutdinov, Alexander Titkov, Sergey Bychkov, and Alexander Nemudry. 2020. "Direct AC/DC Heating of Oxygen Transport Membranes" Energies 13, no. 1: 30. https://doi.org/10.3390/en13010030
APA StyleKovalev, I., Vorobyev, A., Bagishev, A., Popov, M., Sharafutdinov, M., Titkov, A., Bychkov, S., & Nemudry, A. (2020). Direct AC/DC Heating of Oxygen Transport Membranes. Energies, 13(1), 30. https://doi.org/10.3390/en13010030




