Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation
Abstract
1. Introduction
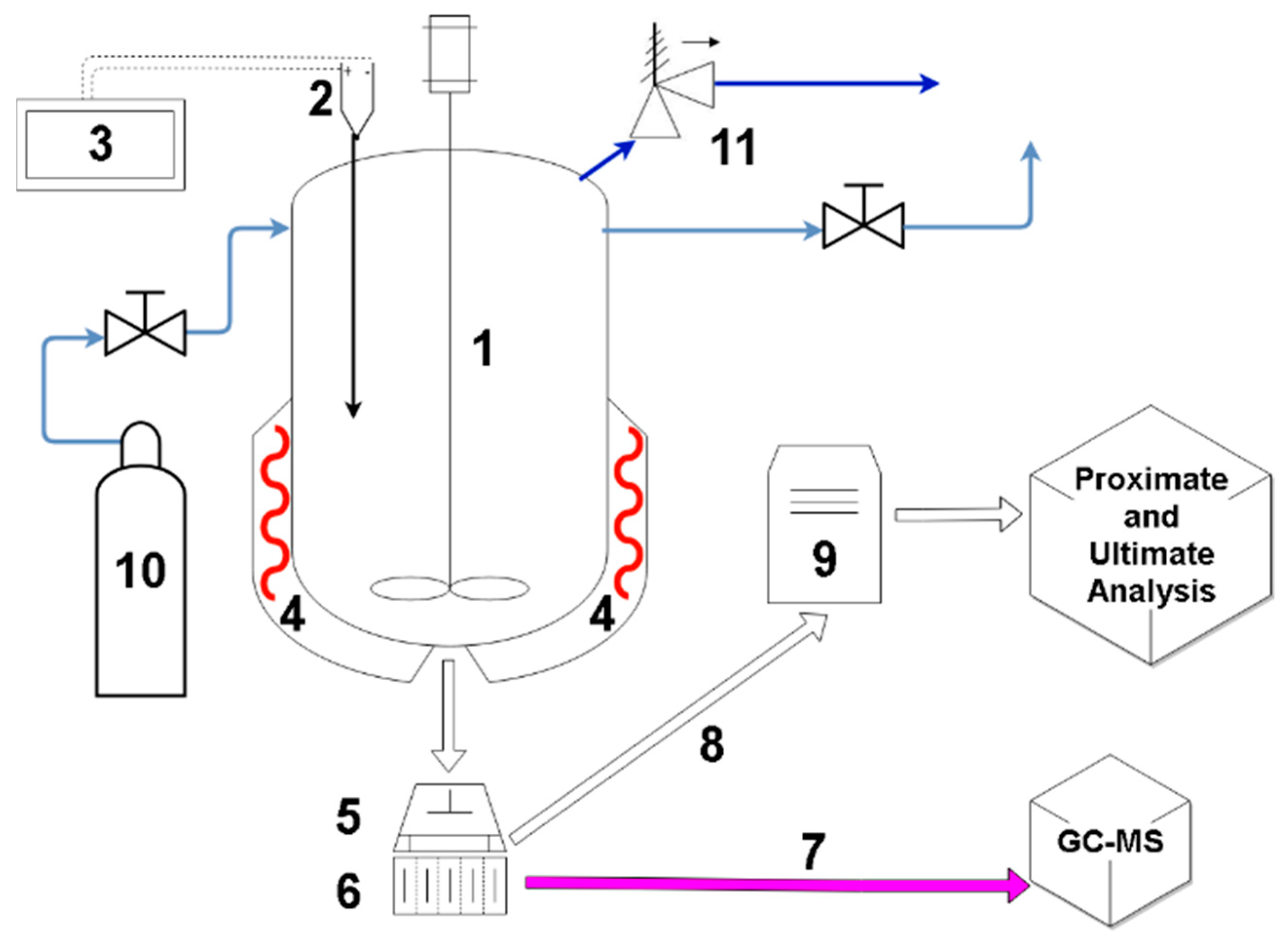
2. Materials and Methods
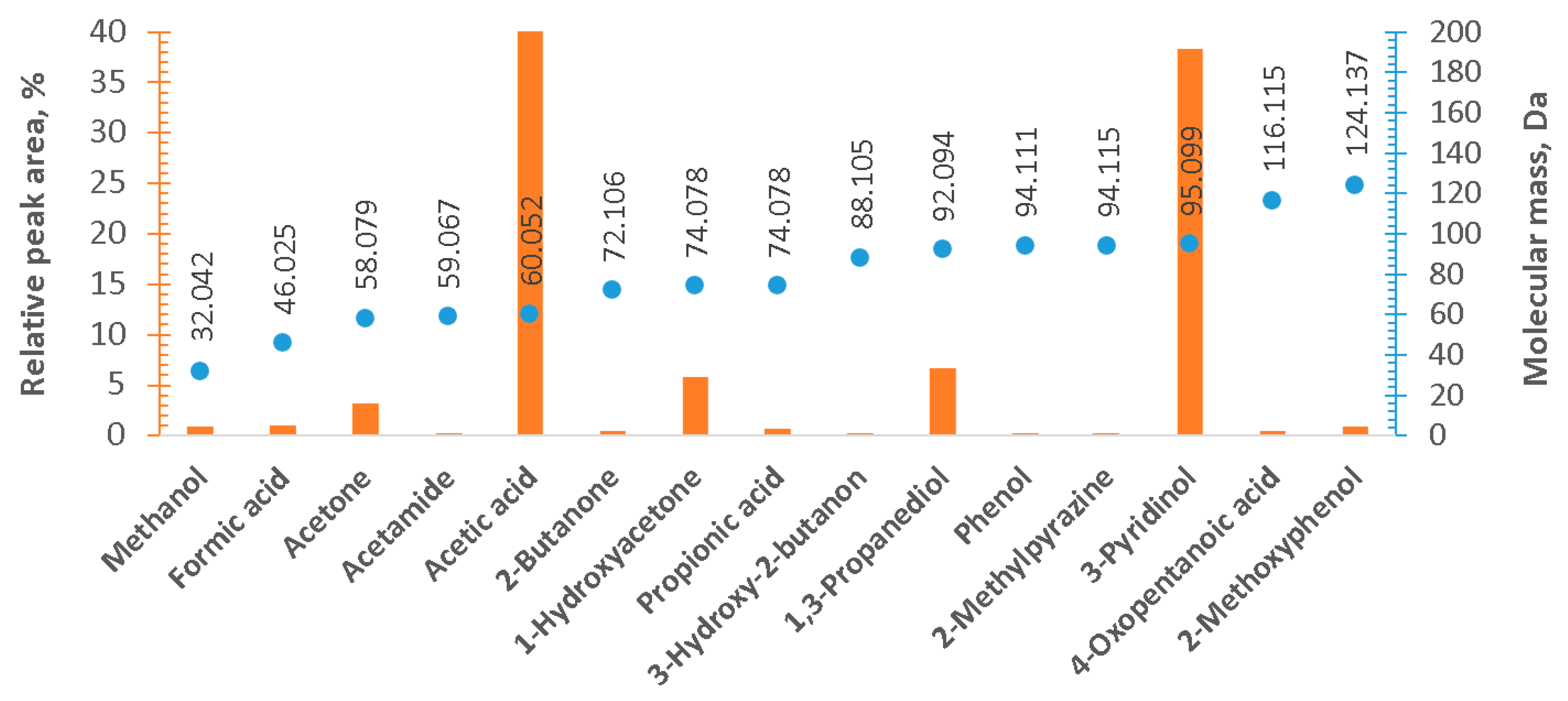
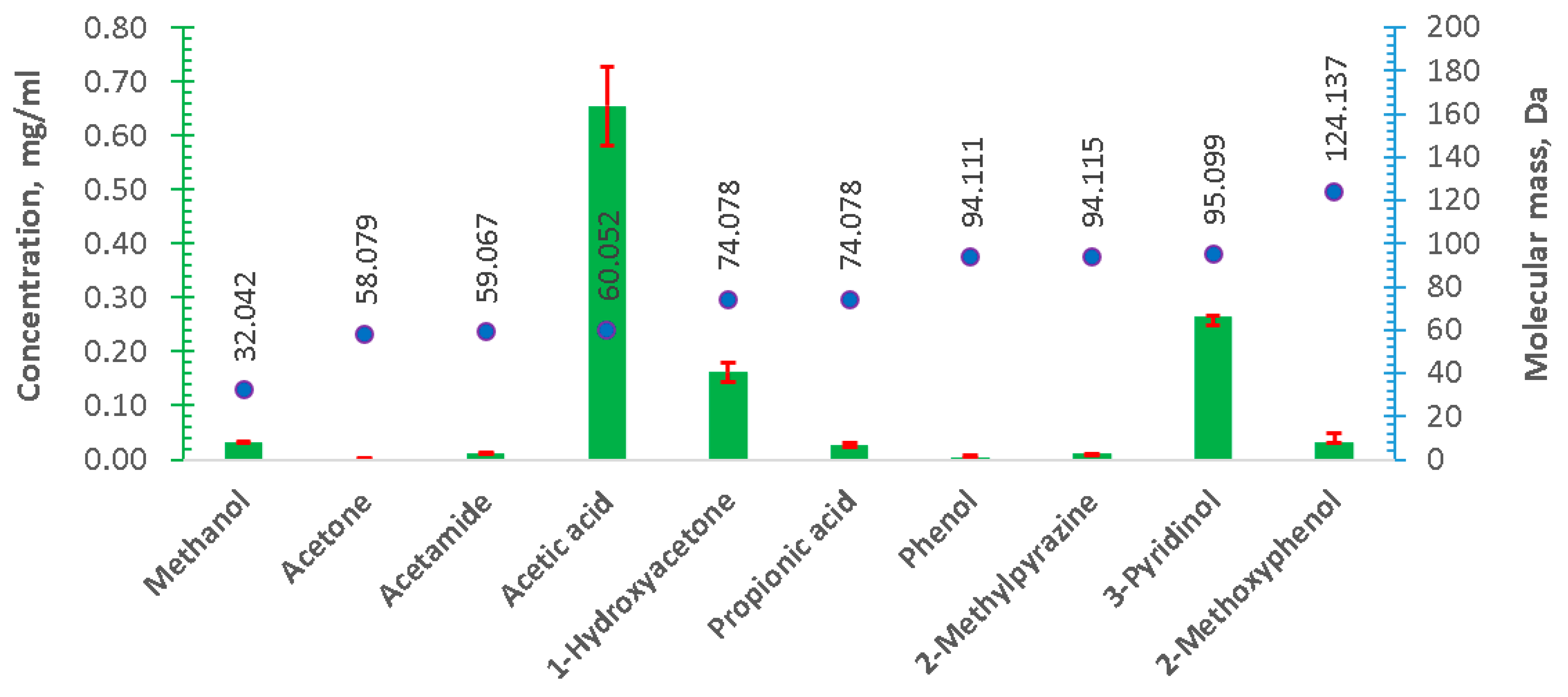
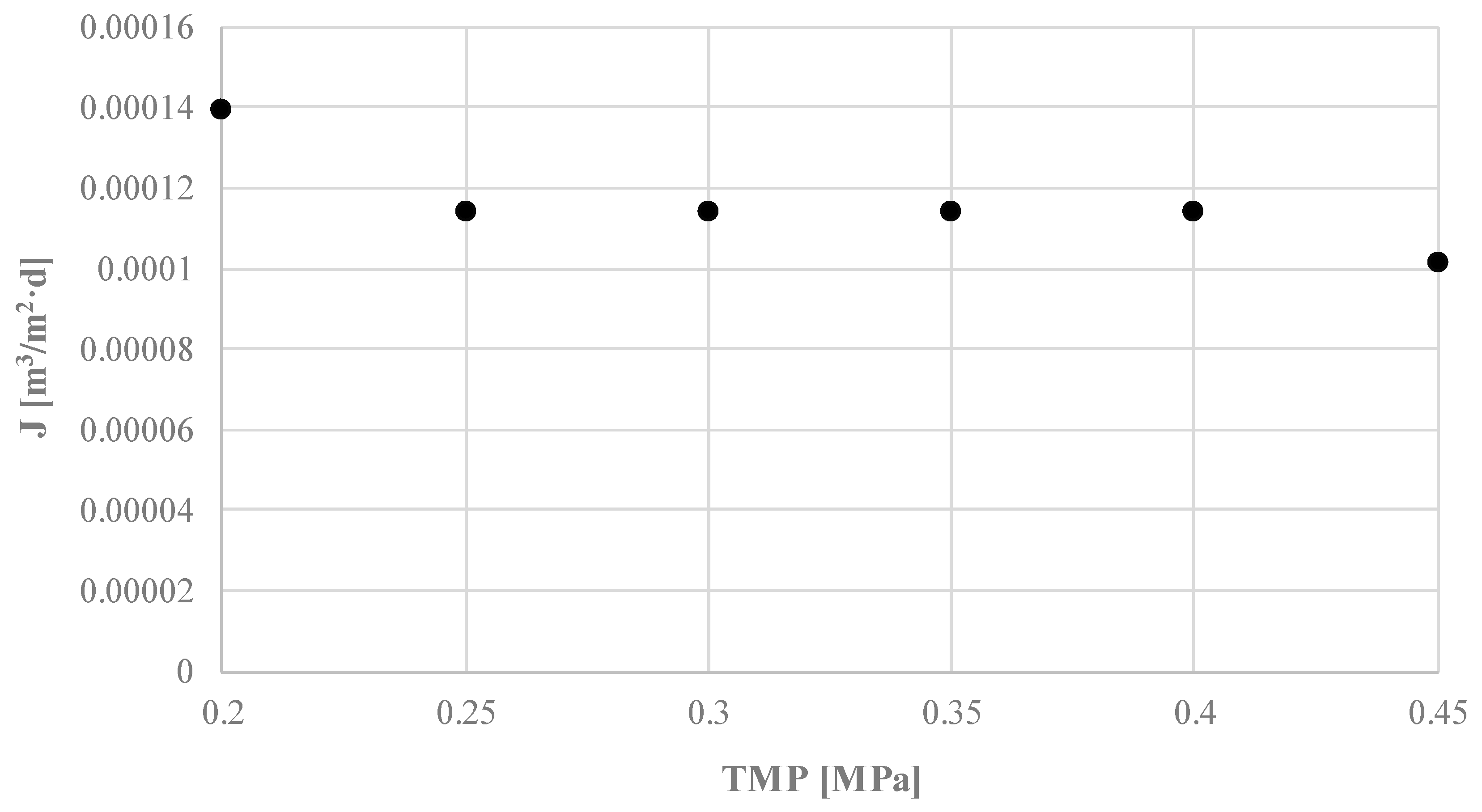
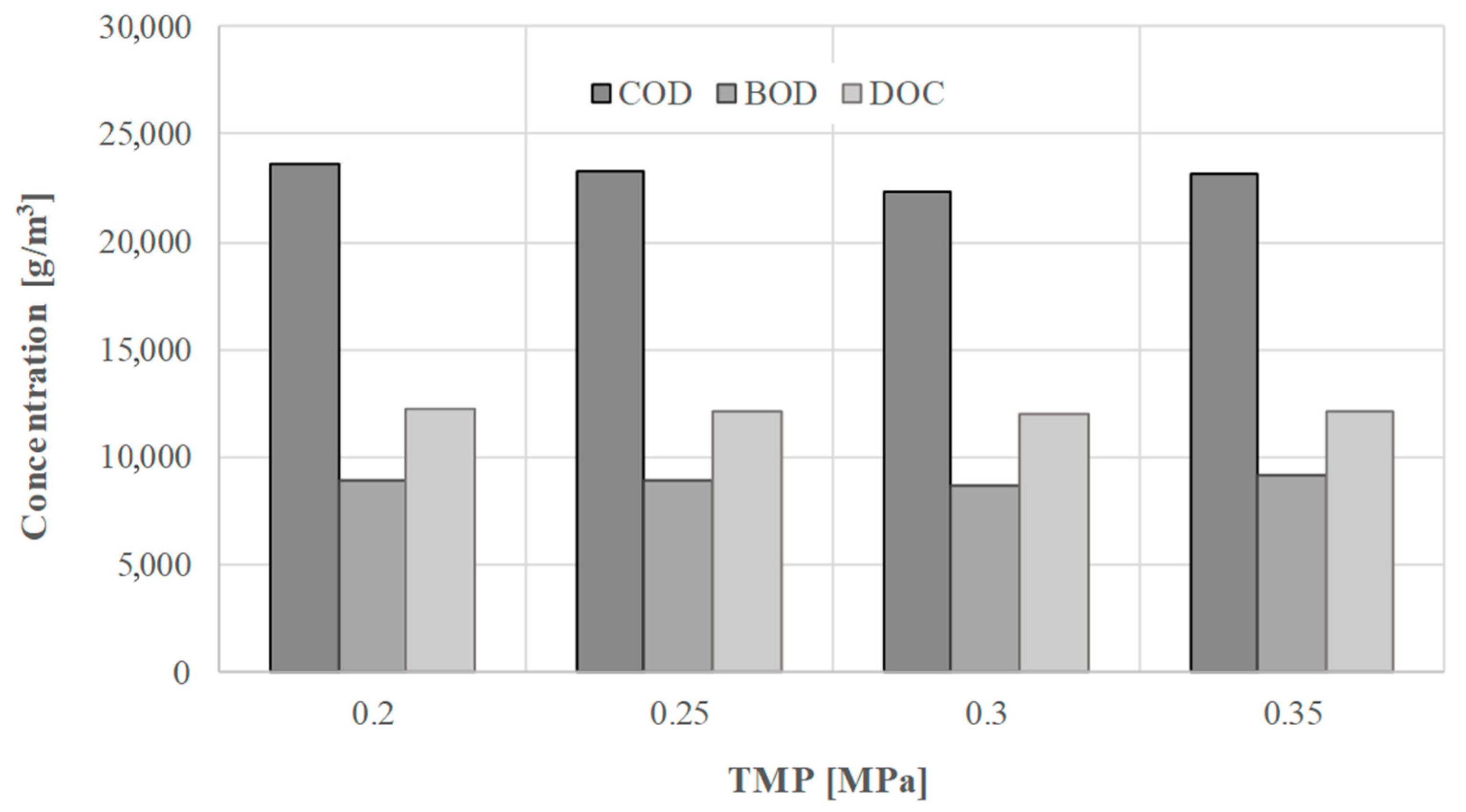
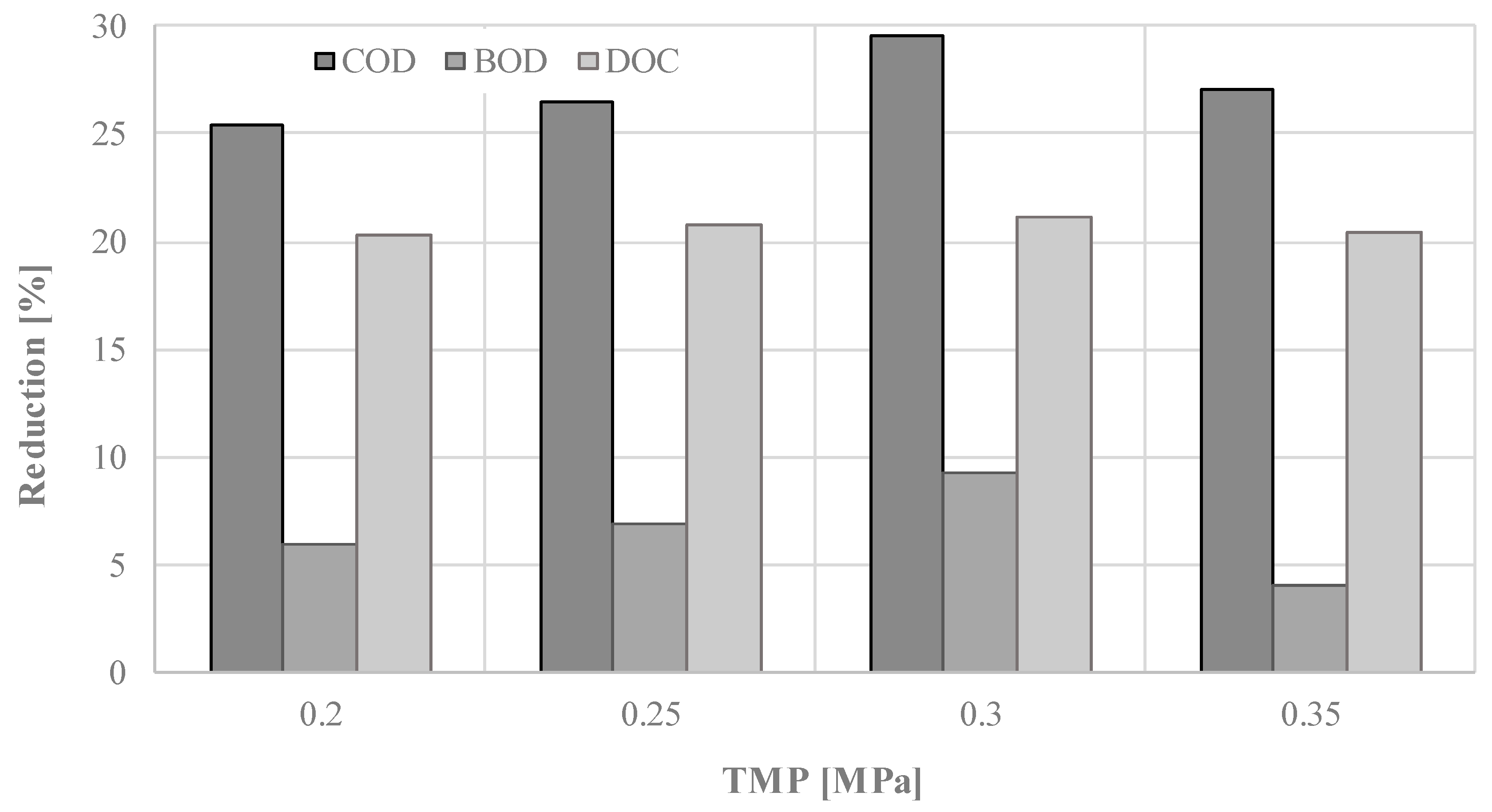
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Connor, R. The United Nations World Water Development Report 2015: Water for a Sustainable World; United Nations: New York, NY, USA, 2015.
- Susza w Polsce: Straty Przekroczyły Już Pół Miliarda Złotych—EURACTIV.pl. Available online: https://www.euractiv.pl/section/rolnictwowpr/news/susza-w-polsce-straty-przekroczyly-juz-pol-miliarda-zlotych/ (accessed on 1 August 2018).
- Damkjaer, S.; Taylor, R. The measurement of water scarcity: Defining a meaningful indicator. Ambio 2017, 46, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Urbanowska, A.; Kabsch-Korbutowicz, M. Analysis of the pre-treatment efficiency of digestate liquid fraction from a municipal waste biogas plant. Environ. Prot. Eng. 2019, 45. [Google Scholar] [CrossRef]
- Vázquez-Rowe, I.; Golkowska, K.; Lebuf, V.; Vaneeckhaute, C.; Michels, E.; Meers, E.; Benetto, E.; Koster, D. Environmental assessment of digestate treatment technologies using LCA methodology. Waste Manag. 2015, 43, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Plana, P.V.; Noche, B. A review of the current digestate distribution models: Storage and transport. In Proceedings of the 8 International Conference on Waste Management and The Environment (WM 2016), Valencia, Spain, 7–9 June 2016; Volume 202, pp. 345–357. [Google Scholar]
- Monfet, E.; Aubry, G.; Ramirez, A.A. Nutrient removal and recovery from digestate: A review of the technology. Biofuels 2017, 9, 247–262. [Google Scholar] [CrossRef]
- Choi, O.; Kim, M.; Go, Y.; Hong, M.; Kim, B.; Shin, Y.; Lee, S.; Kim, Y.G.; Joo, J.S.; Jeon, B.S.; et al. Selective Removal of Water Generated during Hydrogenotrophic Methanation from Culture Medium Using Membrane Distillation. Energies 2019, 12, 4130. [Google Scholar] [CrossRef]
- Moscicki, K.J.; Niedzwiecki, L.; Owczarek, P.; Wnukowski, M. Commoditization of wet and high ash biomass: Wet torrefaction—A review. J. Power Technol. 2017, 97, 354–369. [Google Scholar]
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gokalp, I. Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass Bioenergy 2019, 120, 166–175. [Google Scholar] [CrossRef]
- Arauzo, P.; Olszewski, M.; Kruse, A. Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock. Energies 2018, 11, 3226. [Google Scholar] [CrossRef]
- Jackowski, M.; Semba, D.; Trusek, A.; Wnukowski, M.; Niedzwiecki, L.; Baranowski, M.; Krochmalny, K.; Pawlak-Kruczek, H. Hydrothermal Carbonization of Brewery’s Spent Grains for the Production of Solid Biofuels. Beverages 2019, 5, 12. [Google Scholar] [CrossRef]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal carbonization of fruit wastes: A promising technique for generating hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef]
- Broch, A.; Jena, U.; Hoekman, S.K.; Langford, J. Analysis of solid and aqueous phase products from hydrothermal carbonization of whole and lipid-extracted algae. Energies 2014, 7, 62–79. [Google Scholar] [CrossRef]
- Wnukowski, M.; Owczarek, P.; Niedźwiecki, Ł. Wet Torrefaction of Miscanthus—Characterization of Hydrochars in View of Handling, Storage and Combustion Properties. J. Ecol. Eng. 2015, 16, 161–167. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A. Hydrothermal carbonization, torrefaction and slow pyrolysis of Miscanthus giganteus. Energy 2017, 140, 1292–1304. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonisation of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vásquez, V.R. Reaction kinetics of hydrothermal carbonization of loblolly pine. Bioresour. Technol. 2013, 139, 161–169. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Heat of reaction measurements for hydrothermal carbonization of biomass. Bioresour. Technol. 2011, 102, 7595–7598. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B.; Levec, J. Hydrotreatment of solvolytically liquefied lignocellulosic biomass over NiMo/Al2O3 catalyst: Reaction mechanism, hydrodeoxygenation kinetics and mass transfer model based on FTIR. Biomass Bioenergy 2014, 63, 300–312. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B.; Levec, J. Simultaneous Liquefaction and Hydrodeoxygenation of Lignocellulosic Biomass over NiMo/Al2O3, Pd/Al2O3, and Zeolite y Catalysts in Hydrogen Donor Solvents. ChemCatChem 2016, 8, 180–191. [Google Scholar] [CrossRef]
- Faussone, G.C.; Grilc, M.; Likozar, B. Removal of inorganics from sludge and digestate: The BiAR process. Water Pract. Technol. 2017, 12, 937–941. [Google Scholar] [CrossRef]
- Gao, N.; Li, Z.; Quan, C.; Miskolczi, N.; Egedy, A. A new method combining hydrothermal carbonization and mechanical compression in-situ for sewage sludge dewatering: Bench-scale verification. J. Anal. Appl. Pyrolysis 2019, 139, 187–195. [Google Scholar] [CrossRef]
- He, C.; Tang, C.; Li, C.; Yuan, J.; Tran, K.Q.; Bach, Q.V.; Qiu, R.; Yang, Y. Wet torrefaction of biomass for high quality solid fuel production: A review. Renew. Sustain. Energy Rev. 2018, 91, 259–271. [Google Scholar] [CrossRef]
- Zubrowska-Sudol, M.; Walczak, J. Enhancing combined biological nitrogen and phosphorus removal from wastewater by applying mechanically disintegrated excess sludge. Water Res. 2015, 76, 10–18. [Google Scholar] [CrossRef]
- Zubrowska-Sudol, M.; Walczak, J. Effects of mechanical disintegration of activated sludge on the activity of nitrifying and denitrifying bacteria and phosphorus accumulating organisms. Water Res. 2014, 61, 200–209. [Google Scholar] [CrossRef]
- Svensson, K.; Kjørlaug, O.; Higgins, M.J.; Linjordet, R.; Horn, S.J. Post-anaerobic digestion thermal hydrolysis of sewage sludge and food waste: Effect on methane yields, dewaterability and solids reduction. Water Res. 2018, 132, 158–166. [Google Scholar] [CrossRef]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef]
- Funke, A.; Mumme, J.; Koon, M.; Diakité, M. Cascaded production of biogas and hydrochar from wheat straw: Energetic potential and recovery of carbon and plant nutrients. Biomass Bioenergy 2013, 58, 229–237. [Google Scholar] [CrossRef]
- Wirth, B.; Mumme, J. Anaerobic Digestion of Waste Water from Hydrothermal Carbonization of Corn Silage. Appl. Bioenergy 2014, 1, 1–10. [Google Scholar] [CrossRef]
- Seyedsadr, S.; Al Afif, R.; Pfeifer, C. Hydrothermal carbonization of agricultural residues: A case study of the farm residues -based biogas plants. Carbon Resour. Convers. 2018, 1, 81–85. [Google Scholar] [CrossRef]
- Usman, M.; Chen, H.; Chen, K.; Ren, S.; Clark, J.H.; Fan, J.; Luo, G.; Zhang, S. Characterization and utilization of aqueous products from hydrothermal conversion of biomass for bio-oil and hydro-char production: A review. Green Chem. 2019, 21, 1553–1572. [Google Scholar] [CrossRef]
- Parmar, K.R.; Ross, A.B. Integration of hydrothermal carbonisation with anaerobic digestion; Opportunities for valorisation of digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Wang, L.; Li, A.; Chang, Y. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: Heavy metals, volatile organic compounds and combustion characteristics of hydrochar. Chem. Eng. J. 2016, 297, 1–10. [Google Scholar] [CrossRef]
- Luz, F.C.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Anaerobic digestion of coffee grounds soluble fraction at laboratory scale: Evaluation of the biomethane potential. Appl. Energy 2017, 207, 166–175. [Google Scholar] [CrossRef]
- Kabsch-Korbutowicz, M.; Wisniewski, J.; Lakomska, S.; Urbanowska, A. Application of UF, NF and ED in natural organic matter removal from ion-exchange spent regenerant brine. Desalination 2011, 280, 428–431. [Google Scholar] [CrossRef]
- Yan, W.; Hastings, J.T.; Acharjee, T.C.; Coronella, C.J.; Vásquez, V.R. Mass and energy balances of wet torrefaction of lignocellulosic biomass. Energy Fuels 2010, 24, 4738–4742. [Google Scholar] [CrossRef]
- Scott, P.W. Introduction to Analytical Gas Chromatography, 2nd ed.; Marcel Dekker: New York, NY, USA, 1998. [Google Scholar]
- Wang, S.; Persson, H.; Yang, W.; Jönsson, P.G. Pyrolysis study of hydrothermal carbonization-treated digested sewage sludge using a Py-GC/MS and a bench-scale pyrolyzer. Fuel 2019, 262. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal carbonisation of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef]
- Villamil, J.A.; Mohedano, A.F.; Rodríguez, J.J.; Borja, R.; De la Rubia, M.A. Anaerobic Co-digestion of the Organic Fraction of Municipal Solid Waste and the Liquid Fraction From the Hydrothermal Carbonization of Industrial Sewage Sludge Under Thermophilic Conditions. Front. Sustain. Food Syst. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Chiumenti, A.; Da Borso, F.; Teri, F.; Chiumenti, R.; Piaia, B. Full-scale membrane filtration system for the treatment of digestate from a co-digestion plant. Appl. Eng. Agric. 2013, 29, 985–990. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Dubey, B.K. Hydrothermal carbonization of yard waste for solid bio-fuel production: Study on combustion kinetic, energy properties, grindability and flowability of hydrochar. Waste Manag. 2019, 91, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Dutta, A. Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers. Manag. 2015, 105, 746–755. [Google Scholar] [CrossRef]
| Sample | MCwet | MCpress | VM | A | HHV | LHV | C | H | O |
|---|---|---|---|---|---|---|---|---|---|
| % wb | % wb | % db | % db | MJ/kg | MJ/kg | % daf | % daf | % daf | |
| Raw digestate | 91.34 | 59.67 | 63.6 | 8.1 | 19.74 | 5.97 | 44.63 | 6.99 | 44.52 |
| Digestate after HTC | 84.49 | 51.32 | 53.9 | 9.7 | 23.63 | 9.63 | 57.69 | 7.38 | 30.94 |
| Index | Value |
|---|---|
| pH | 6.3 |
| Conductivity, mS/cm | 8.11 |
| Total suspended solids, mg/dm3 | 230.4 |
| Volatile suspended solids, mg/dm3 | 139.6 |
| Chemical oxygen demand (COD), mg O2/dm3 | 31,650 |
| Biochemical oxygen demand (BOD), mg O2/dm3 | 9520 |
| Dissolved organic carbon (DOC), mg C/dm3 | 15,281 |
| N-NH4+, mg/dm3 | 700 |
| N-NO2−, mg/dm3 | 0.1 |
| N-NO3−, mg/dm3 | below the limit of detection |
| PO43−, mg/dm3 | 950 |
| Na, mg/dm3 | 293.6 |
| K, mg/dm3 | 688.9 |
| Ca, mg/dm3 | 28.2 |
| Mg, mg/dm3 | 449.7 |
| Cl−, mg/dm3 | 85.7 |
| Fe, mg/dm3 | 22.13 |
| Mn, mg/dm3 | 1.68 |
| Cu, mg/dm3 | 0.71 |
| Zn, mg/dm3 | 1.28 |
| Hg, mg/dm3 | 0.0036 |
| Co, mg/dm3 | 0.137 |
| Ni, mg/dm3 | 0.359 |
| Ba, mg/dm3 | 0.260 |
| As, mg/dm3 | 0.0005 |
| Cr, mg/dm3 | 0.00911 |
| Pb, mg/dm3 | 0.211 |
| Cd, mg/dm3 | 0.0008 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowska, A.; Kabsch-Korbutowicz, M.; Wnukowski, M.; Seruga, P.; Baranowski, M.; Pawlak-Kruczek, H.; Serafin-Tkaczuk, M.; Krochmalny, K.; Niedzwiecki, L. Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation. Energies 2020, 13, 262. https://doi.org/10.3390/en13010262
Urbanowska A, Kabsch-Korbutowicz M, Wnukowski M, Seruga P, Baranowski M, Pawlak-Kruczek H, Serafin-Tkaczuk M, Krochmalny K, Niedzwiecki L. Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation. Energies. 2020; 13(1):262. https://doi.org/10.3390/en13010262
Chicago/Turabian StyleUrbanowska, Agnieszka, Małgorzata Kabsch-Korbutowicz, Mateusz Wnukowski, Przemysław Seruga, Marcin Baranowski, Halina Pawlak-Kruczek, Monika Serafin-Tkaczuk, Krystian Krochmalny, and Lukasz Niedzwiecki. 2020. "Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation" Energies 13, no. 1: 262. https://doi.org/10.3390/en13010262
APA StyleUrbanowska, A., Kabsch-Korbutowicz, M., Wnukowski, M., Seruga, P., Baranowski, M., Pawlak-Kruczek, H., Serafin-Tkaczuk, M., Krochmalny, K., & Niedzwiecki, L. (2020). Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation. Energies, 13(1), 262. https://doi.org/10.3390/en13010262









